Abstract
Aims
Danon disease (DD) is a rare X‐linked disorder caused by mutations in the lysosomal‐associated membrane protein type 2 gene (LAMP2). DD is difficult to distinguish from other causes of dilated or hypertrophic cardiomyopathy (HCM) in female patients. As DD female patients regularly progress into advanced heart failure (AHF) aged 20–40 years, their early identification is critical to improve patient survival and facilitate genetic counselling. In this study, we evaluated the prevalence of DD among female patients with non‐ischemic cardiomyopathy, who reached AHF and were younger than 40 years.
Methods and results
The study cohort comprised 60 female patients: 47 (78%) heart transplant recipients, 2 (3%) patients treated with ventricular assist device, and 11 (18%) patients undergoing pre‐transplant assessment. Aetiology of the cardiomyopathy was known in 15 patients (including two DD patients). LAMP2 expression in peripheral white blood cells (WBC) was tested by flow cytometry (FC) in the remaining 45 female patients. Whole exome sequencing was used as an alternative independent testing method to FC. Five additional female DD patients (two with different novel LAMP2 mutations) were identified by FC. The total prevalence of DD in this cohort was 12%. HCM phenotype (57% vs. 9%, * P = 0.022) and delta waves identified by electrocardiography (43% vs. 0%, ** P = 0.002) were significantly more frequent in DD female patients.
Conclusions
Danon disease is an underdiagnosed cause of AHF in young female patients. LAMP2 expression testing in peripheral WBCs by FC can be used as an effective screening/diagnostic tool to identify DD in this patient population.
Keywords: Advanced heart failure, Danon disease, Lysosomal‐associated membrane protein type 2, Screening, White blood cells
Introduction
Danon disease (DD, OMIM 300257) is a rare X‐linked disorder caused by mutations in the lysosomal‐associated membrane protein type 2 gene (LAMP2, Xq24). 1 All three LAMP2 isoforms (B, A, and C) contribute to lysosomal processing of autophagic substrates. 2 Almost all LAMP2 mutations result in the absence of the protein [LAMP2 deficiency (LAMP2def)]. X‐hemizygous male DD patients present with a complex phenotype that is dominated by cardiomyopathy with massive left ventricular hypertrophy, delta waves by electrocardiography, muscle weakness, and mild cognitive disability. Importantly, male DD patients have extremely poor prognosis due to end‐stage congestive heart failure and malignant ventricular arrhytmias. Their mean age at heart transplantation or death is 18–19 years. 3 , 4
Unless modified by processes such as formation of syncytia, 5 expression of the mutant LAMP2 allele is mosaic (mosaic LAMP2def) because of X‐chromosome inactivation (XCI) in the tissues of the X‐heterozygous female DD patients. As a likely consequence, females present with a milder cardiac DD phenotype with an equal prevalence of (on many occasions isolated) dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM). DD female patients also become symptomatic approximately 15 years later than male patients, have an average survival ~35 years, and are less affected by skeletal myopathy and cognitive defects. 3 However, as shown by the latest meta‐analysis of published DD studies, similar proportions of DD females and males progress into end‐stage heart failure. 6 Some DD females are also at risk of sudden cardiac death. 7 Timely identification of DD female patients is therefore important for their prognostic stratification and family counselling.
Peripheral white blood cells (WBCs) can be used to assess LAMP2 expression/LAMP2 deficiency in suspect male and female DD patients. LAMP2 western blotting in WBCs homogenates was described by Fanin et al. 8 who documented the protein deficiency in male DD patients. In their single female DD patient, however, the latter authors showed nearly normal LAMP2 levels and highlighted the inefficiency of this approach due to problematic interpretation of the results in this particular patient group. Flow cytometric (FC) detection of LAMP2 in WBCs was first reported by Regelsberger et al. 9 Expanding these seminal studies, we optimized and presented a polychromatic FC protocol that allows quantitation of LAMP2def WBC populations not only in male DD patients but also in female DD patients and somatic mosaic carriers of LAMP2 mutations. 10 , 11 , 12
Even though female patients are frequently the first affected individuals in DD families, many are identified retrospectively or even post‐mortem after the diagnosis is established in their affected male relative(s) (brother or son). To the best of our knowledge, no large‐scale study(ies) evaluating prevalence of DD among female patients with cardiologic pathologies has been presented. To fill this unfortunate information gap, we assessed the prevalence and evaluated the clinical characteristics of DD in female patients who reached advanced heart failure (AHF) due to non‐ischemic cardiomyopathy and were younger than 40 years of age. LAMP2 FC in WBCs was used as a screening diagnostic method. Whole‐exome sequencing (WES) served as an alternative independent testing approach.
Methods
Study design and patient cohort
This was a two‐centre cohort study. The study group was recruited from patients of the two Czech heart transplant centres. From November 2016 to October 2018, 60 female patients with AHF due to non‐ischemic cardiomyopathy were identified and agreed to inclusion in the study (Figure 1A ). All patients were younger than 40 years at the time of heart transplantation or at pre‐transplant assessment and were either living female heart transplant recipients, female patients on mechanical circulatory support or female patients referred to pre‐transplant assessment. The selected cut‐off age of 40 years was based on previously presented data of mean age of cardiac transplant (32.3 ± 14.5 years) 3 and median age of end‐stage cardiomyopathy [28 years (18.0–50.0)] 6 in female DD patients.
Figure 1.
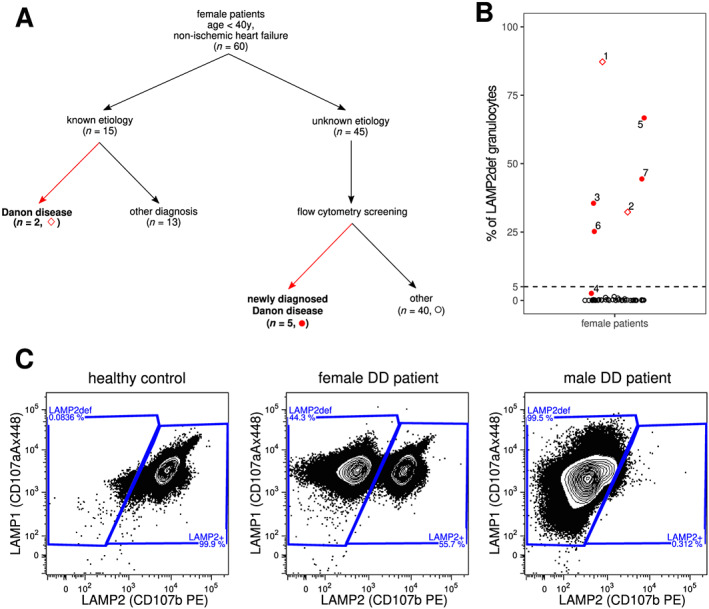
LAMP2 flow cytometric screening in the patient cohort. (A) Schematic summary of the findings in the patient cohort. Symbols correspond to panel B. Summary of the whole‐exome sequencing findings is provided in the supporting information. (B) % fractions of LAMP2 deficiency (LAMP2def) granulocytes identified by the LAMP2 flow cytometry screening in 45 female patients. The numbers correspond to individual female DD patients as listed in Table 2. Values shown for Patients #1 10 and #2 13 were measured prior the start of this screening study. The threshold of 5% of LAMP2def granulocytes is highlighted by the dashed line. The minute fraction of LAMP2def granulocytes (2.6%) in Patient #4 is a result of her unique Xq24 molecular pathology resulting in extremely skewed X‐chromosome inactivation ratios in white blood cells (Table 2 and also Majer et al. 14 for further details including the LAMP1/LAMP2 scatterplots in monocytes and granulocytes of patient #4). (C) LAMP1/LAMP2 flow cytometry scatterplots demonstrating the typical profiles seen healthy control, female DD patient (Patient #7 is shown), and male DD patient (patient III.3 from Majer et al. 10 is shown). LAMP2def and LAMP2+ granulocytes are gated. The deficit is mosaic (LAMP2def and LAMP2+ cells are found) and corresponds to white blood cell X‐chromosome inactivation ratios in the X‐heterozygous female DD patient, whereas it is uniform (only LAMP2def cells are found) in X‐hemizygous male DD patient. DD, Danon disease.
The study was approved by the Ethics Committee of the authors' home institution and was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided a written informed consent prior to participating in the study.
Study protocol
Medical records of the 60 female patients were reviewed, and their clinical data, electrocardiographic, and echocardiographic findings were collected. Abnormal electrocardiograms suggesting pre‐excitation were reviewed by an electrophysiologist blinded to the aetiology of the cardiomyopathy, to confirm/exclude the presence of delta waves. Among the 60 patients (Figure 1A , Table 1 ), 15 female patients (25%) had a known aetiology of the cardiomyopathy (for details, see supporting information). Two patients (#1 and #2, Table 2 ) who were diagnosed with DD prior the start of this study were among these 15 patients. The remaining 45 patients (75%) underwent LAMP2 FC in WBCs and WES analyses. For these analyses, 5 ml of peripheral venous blood were collected into an EDTA‐containing tube(s). Samples for FC were maintained at room temperature and tested within 24 h of collection.
Table 1.
Study group characteristics and comparison of clinical and instrumental findings between individuals with Danon disease and other aetiologies of non‐ischemic heart failure
| Characteristic | Danon disease (n = 7) | Non‐Danon disease (n = 53) | Overall (n = 60) | P value |
|---|---|---|---|---|
| Age at first symptoms (year) | 16 (15–24) | 24 (12–32) | 22 (12–31) | 0.398 |
| Age at progression (year) | 25 (21–28) | 30 (19–36) | 28 (20–35) | 0.228 |
| Cardiomypathy type | 0.022 * | |||
| Hypertrophic | 4 (57%) | 5 (9%) | 9 (15%) | |
| Dilated | 3 (43%) | 43 (81%) | 46 (77%) | |
| Restrictive | 0 (0%) | 4 (8%) | 4 (6%) | |
| Left‐ventricular non‐compaction | 0 (0%) | 1 (2%) | 1 (2%) | |
| NYHA functional class | (n = 7) | (n = 45) | (n = 52) | 0.754 |
| I | 1 (14%) | 3 (7%) | 4 (8%) | |
| II | 1 (14%) | 10 (22%) | 11 (21%) | |
| III | 4 (58%) | 21 (47%) | 25 (48%) | |
| IV | 1 (14%) | 11 (24%) | 12 (23%) | |
| Arrhythmia | 0.052 | |||
| Atrial fibrillation | 3 (43%) | 4 (8%) | 7 (12%) | |
| Ventricular tachyarrhythmia | 0 (0%) | 5 (10%) | 5 (8%) | |
| Electrocardiogram | (n = 7) | (n = 42) | (n = 49) | |
| PR duration (ms) | 154 (152–160) | 166 (159–184) | 162 (154–184) | 0.268 |
| QRS duration (ms) | 144 (137–187) | 96 (80–110) | 102 (83–121) | 0.001 ** |
| Delta‐waves | 3 (43%) | 0 (0%) | 3 (6%) | 0.002 ** |
| LBBB | 3 (43%) | 5 (12%) | 8 (16%) | 0.068 |
| Echocardiography | (n = 7) | (n = 46) | (n = 53) | |
| LVEDD (mm) | 62 (49–67) | 61 (56–69) | 62 (56–68) | 0.537 |
| LVEF (mm) | 20 (20–39) | 25 (20–30) | 24 (20–30) | 0.749 |
| Interventricular septum (mm) | 14 (9–14) | 8 (7–9) | 8 (7–9) | 0.001 ** |
| Posterior wall (mm) | 12 (10–14) | 8 (7–9) | 8 (7–9) | 0.002 ** |
| Mitral regurgitation | (n = 7) | (n = 50) | (n = 57) | 0.899 |
| None or trace | 4 (58%) | 20 (40%) | 24 (42%) | |
| Mild | 1 (14%) | 10 (20%) | 11 (19%) | |
| Moderate | 1 (14%) | 12 (24%) | 13 (23%) | |
| Severe | 1 (14%) | 8 (16%) | 9 (16%) |
LBBB, left bundle branch block; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
P ˂ 0.05.
P ˂ 0.01.
Table 2.
LAMP2 flow cytometry, LAMP2 molecular genetic analyses, and LAMP2 myocardial expression in Danon disease female patients
| Patient | LAMP2def granulocytes (%) | LAMP2def monocytes (%) | XCI in WBCs (HUMARA) | LAMP2 mutation/ LAMP2 cardiac IHC | Note |
| #1 b | 87.2 | 84.9 | Not informative | p.[Ala314Glnfs*32];[=] / mosaic expression | III.2 in Majer et al. 10 |
| #2 b | 32.3 | 38.0 | 30:70 | g.19925_45401[del25477];[=], deletion of LAMP2 exons 4–9C/no tissue available | II.1 (family 2) in reference Majer et al. 13 |
| #3 c | 35.5 | 49.1 | 40:60 | g.17916_29069[del11154];[=], deletion of LAMP2 exons 4‐8/no tissue available | II.1 (family 1) in Majer et al. . 13 |
| #4 c | 2.6 | 3.9 | 96:4 a | Heterozygous deletion of CUL4B, LAMP2, ATP1B4, TMEM255A, and ZBTB33 genes/mosaic expression | II.1 in Majer et al. 14 |
| #5 c | 66.7 | 70.8 | 57:44 | p.[Asp149Phefs*2];[=] /mosaic expression | Novel LAMP2 mutation (Supporting Information, Figure S1A ) |
| #6 c | 25.2 | 28.9 | 20:80 | p.[Gln240*];[=] / mosaic expression | Known LAMP2 mutation (Figure S1B ) 17 |
| #7 c | 44.3 | 52.7 | 46:54 | p.[Leu139Phefs*8];[=] / mosaic expression | Novel LAMP2 mutation (Figure S1C ) |
DD, Danon disease; HUMARA, human androgen receptor assay; IHC, immunohistochemistry
Skewed WBC XCI ratios are an effect of the parallel mutation in the CUL4B gene.
DD diagnosis established prior the LAMP2 FC screening study.
DD diagnosis established by the LAMP2 FC screening study.
Table 3.
Clinical findings in Danon disease female patients
| Patient | Age at disease onset (years) | Age at HTx (years) | Age at DD diagnosis (years) | Cardiac phenotype | QRS width (ms) | Delta waves | LVEDD (mm) | LVEF (%) | IVS (mm) | PW (mm) |
| #1 | 15 | 29 | 33 | DCM | 140 | I, aVL, V4–6 | 67 | 15 | 10 | 10 |
| #2 | 11 | — | 17 | HCM | 134 | I, II, III, aVF, V5–6 | 33 | 60 | 22 | 21 |
| #3 | 12 | 21 | 42 | HCM | 208 | Absent | 68 | 20 | 15 | 15 |
| #4 | 25 | 26 | 36 | DCM | 112 | Absent | 62 | 20 | 8 | 7 |
| #5 | 25 | 28 | 48 | HCM | 200 | Absent | 68 | 20 | 14 | 14 |
| #6 | 16 | 27 | 25 | HCM | 174 | I, II, III, aVF, V4–6 | 40 | 55 | 14 | 12 |
| #7 | 23 | 24 | 23 | DCM | 144 | Absent | 59 | 23 | 9 | 10 |
DCM, dilated cardiomyopathy; DD, Danon disease; HCM, hypertrophic cardiomyopathy; HTx, heart transplantation; IVS, interventricular septum end‐diastolic thickness; LVEDD, left‐ventricular end‐diastolic diameter; LVEF; left‐ventricular ejection fraction; PW, left‐ventricular posterior wall end‐diastolic thickness.
LAMP2 flow cytometry in white blood cells
Intracellular LAMP1 and LAMP2 protein content in peripheral WBCs was assessed by polychromatic FC based on previously reported protocols. 11 In short, leukocytes were isolated by sedimentation over 2% dextran, fixed and permeabilized (BD FACS Lyse and Perm solution (BD Bioscience, San Jose, CA, USA)), and then incubated with fluorochrome‐conjugated antibodies anti‐LAMP2 (CD107b, clone H4B4) phycoerythrin, anti‐LAMP1 (lysosomal‐associated membrane protein type 1, CD107a, clone H4A3) Alexa Fluor 488, anti‐CD45 PerCP, anti‐CD19 APC, anti‐CD3 Alexa Fluor 700 and anti‐CD14 Pacific Blue (all from Exbio Praha, Vestec, Czech Republic), anti‐CD15 Brilliant Violet 510 (BioLegend, Inc, San Diego, CA, USA), for 30 min, washed twice, and measured using BD LSRII flow cytometer (BD Biosciences) equipped with 405, 488, and 633 nm lasers. Technical details (sample processing/staining protocol and gating strategy), interpretation, and representative examples of positive results in female and male DD patients are summarized in a Standard Operating Protocol, which is included in the supporting information (pages 7–9).
Populations of LAMP2def granulocytes and LAMP2def monocytes were analysed by FC specialists (T.K. and O.P.) blinded to the aetiology of cardiomyopathy and results of the WES analyses. Anti‐LAMP1 staining served as a permeabilization control. A threshold of 5% of LAMP2def granulocytes (or monocytes) was set to calculate the sensitivity and specificity of the FC assay. This value was selected because ~98.3% of healthy adult females have WBC XCI ratios within the >5:95/<95:5 range. 15
Molecular genetic analyses
To detect causal genetic variants, WES was performed according to internationally accepted guidelines. 16 Full technical details are provided in the supporting information. Analyses of the LAMP2 genomic DNA, full‐length isoform LAMP2 messenger RNAs/complementary DNAs and HUMARA XCI assays in DD patients #5, #6, and #7 were performed as reported previously. 10 , 11
Myocardial LAMP2 immunohistochemistry
In DD patients #5, #6 and #7, myocardial LAMP2 immunohistochemistry (IHC) was performed in endomyocardial biopsies, explanted hearts or excisions from the left ventricle obtained at implantation of ventricular assist devices. The staining protocol followed previous studies. 10 , 14
Statistical analyses of the clinical, electrocardiographic, and echocardiographic data
Categorical data were expressed as percentages and compared using a χ2‐test or Fisher's exact test. Normally distributed continuous variables were expressed as a mean and standard deviation. Abnormally distributed continuous variables were given as a median and interquartile range. Continuous variables were compared using the Student's t‐tests or by the non‐parametric Mann–Whitney U test, where appropriate. For all tests, a probability value of P < 0.05 was considered significant. All analyses were performed using the statistical software SPSS, version 17.0 (Chicago, Illinois, USA).
Results
Clinical findings in the patient cohort
The study group of 60 female patients included 47 (78%) heart transplant recipients, 2 individuals (3%) treated by a ventricular assist device, and 11 patients (18%) in the pre‐transplant phase. Table 1 summarizes the clinical findings. Median ages at disease onset, surgery or pre‐transplant assessment, and the LAMP2 FC screening were 22, 28, and 37 years, respectively. DCM was found in 46 patients (77%), HCM in nine patients (15%), restrictive cardiomyopathy in three patients (5%), and left ventricular non‐compaction cardiomyopathy in one patient (2%).
LAMP2 flow cytometry in white blood cells
Patients #1 10 and #2, 13 who were diagnosed with DD prior the start date of this study, had fractions of LAMP2def granulocytes larger than 5% (Figure 1A,B , Table 2 ).
Of the 45 female patients tested by FC in this study, populations of LAMP2def granulocytes larger than 5% were identified in four female patients (Patients #3, #5, #6, and #7) (Figure 1A,B , Table 2 ). Additional five female patients had distinct populations of LAMP2def granulocytes that were smaller than 5% (0.17–2.6%). The most suspect of DD was the values of 2.6% LAMP2def granulocytes and 3.9% LAMP2def monocytes in a single patient. This result was later explained by identifying a very complex LAMP2 molecular pathology 14 that skewed WBC XCI ratios in DD patient #4 (Figure 1B and Table 2 ). No LAMP2 mutation was detected in the remaining four patients harbouring the very small LAMP2def cellular subsets. The remaining 36 samples did not contain any distinct populations of LAMP2def granulocytes.
Including patients #1 and #2, the total prevalence of DD in this cohort of female patients with non‐ischemic heart failure was 12% (7/60). At a threshold of 5% LAMP2def granulocytes or monocytes, DD female patients were identified with a sensitivity of 86% and specificity of 100%. Positive predictive value (PPV) and negative predictive value (NPV) of the test were 100% and 98%, respectively. The presence of any distinct subpopulation of LAMP2def granulocytes identified DD female patients with a sensitivity of 100%, specificity of 92%, PPV 63%, and NPV 100%.
Whole‐exome sequencing analyses, LAMP2 mutations, HUMARA X‐chromosome inactivation ratios, and immunohistochemistry assessment of the myocardial samples
Whole‐exome sequencing results are summarized in the supporting information. ACMG class 3–5 variants in disease‐related genes were found in 24 patients (53%) including the five newly identified DD patients (Figure 1A,B ). Importantly, characterization of the LAMP2 mutations in DD patients #3 and #4 mandated an individualized methodological approach that was far beyond the standard WES analytics in both patients. 13 , 14 WES testing was negative or inconclusive in 21 patients (47%).
Table 2 summarizes the molecular genetic analyses, WBC XCI, and myocardial LAMP2 IHC findings in the female DD patients. Patient #1 was heterozygous for a frame‐shift c.del940G LAMP2 mutation. 10 Patients #2 and #3 carried heterozygous deletion exon copy number variants encompassing LAMP2 exons 4‐9C and 4‐8, respectively. 13 Patient #4 had an Xq24 re‐arrangement that caused a heterozygous deletion of several C‐terminal exons of CUL4B and the complete deletion of LAMP2, ATP1B4, TMEM255A, and ZBTB33 genes. 14 Patient #5 was heterozygous for a novel frameshift c.445_449delGACCT mutation in the LAMP2 exon 4 (Supporting Information, Figure S1A ). A previously reported 17 heterozygous c.718C>T non‐sense mutation (Figure S1B ) in the LAMP2 exon 5 was found in patient #6. Patient #7 was heterozygous for a novel frameshift c.418delC mutation in the LAMP2 exon 4 (Figure S1C ).
White blood cell XCI ratios were within the >5:95/<95:5 range and corresponded to percentages of LAMP2def granulocytes and monocytes in patients #2, #3, #5, #6, and #7. The extremely skewed WBC XCI ratios in patient #4 (96:4) were an effect of the parallel mutation in the CUL4B gene. 14 LAMP2 expression in cardiomyocytes was mosaic in all DD patients from whom tissue samples were available (Table 2 ).
Family screening in newly identified Danon disease patients
Patients #3, 13 #4, 14 and #7 (Figure S1C ) were the only affected individuals in their families. Patient #5 transmitted the LAMP2 mutation to her daughter (III.3, Figure S1A ). At 30 years of age, the daughter had a sinus rhythm with a short PQ interval (98 ms) and discrete delta waves in leads I, II, aVF, and V4–V6. Her echocardiography showed a borderline systolic function of the non‐dilated left ventricle (end‐diastolic diameter 49 mm, ejection fraction 50–55%, and interventricular septum thickness 10 mm). The populations of her LAMP2def granulocytes and monocytes were 22.1% and 27.2%, respectively. XCI ratios by HUMARA were 33:67. Patient #6 had the most complex family history (Figure S1B ). Her mother (an obligatory heterozygote for the LAMP2 mutation) had a DCM and died at 27 years of age. Hypertrophic cardiomyopathy was diagnosed in the patient's brother who carried the same LAMP2 mutation as Patient #6 and died aged 18 years. For additional clinical details see supporting information.
Clinical characteristics of Danon disease female patients
Hypertrophic cardiomyopathy phenotype (57% vs. 9%, * P = 0.022) and delta waves by electrocardiograms (43% vs. 0%, ** P = 0.002) were significantly more frequent in DD female patients in comparison with the rest of the patients in the cohort. The presence of HCM identified DD female patients with a sensitivity of 57%, specificity of 91%, PPV of 44%, and NPV of 94%. Delta waves identified DD female patients with a sensitivity of 43%, a specificity of 100%, PPV of 100%, and NPV of 93%. The sensitivity of these clinical variables was thus much lower than sensitivity of the LAMP2 FC test. The increased thickness of the left ventricular walls and broader QRS complexes in DD female patients corresponded to higher prevalence of HCM and delta waves in these individuals (Table 1 ). Electrocardiograms of DD female patients are shown in Supporting Information, Figure S2 .
Discussion
To the best of our knowledge, we present the first study specifically assessing the prevalence of DD among female patients with AHF. This is also the first large‐scale study that uses LAMP2 flow cytometry as a primary screening tool in this particular patient group.
Our key findings are (i) the prevalence of DD among young female patients (≤40 years of age) with AHF due to non‐ischemic cardiomyopathy is relatively high (12%), (ii) LAMP2 FC in WBCs is an effective screening/diagnostic tool to detect female (and also male) DD patients, and (iii) despite more frequent than in non‐DD patients, DD female patients cannot be reliably identified based on the presence of hypertrophic cardiomyopathy and/or delta waves in their electrocardiograms.
Danon disease screening studies
The overall population frequency as well as population ratio of male and female DD patients is, to the best of our knowledge, not known. The recent extensive review of the available DD literature documented 90 male and only 56 female patients. 6 It is very likely that female patients heterozygous for pathogenic LAMP2 variants remain an underdiagnosed DD patient group despite they are often the first affected family members. Furthermore, DD affects female patients in their reproductive age. Timely and correct diagnosis is therefore also critical for genetic counselling in their families.
There are several studies that used molecular genetic testing and evaluated the frequency of DD in specifically selected patient cohorts. Again, male DD patients predominated among probands identified by most of these studies.
Prevalence of DD was suggested to be ~1–3% among patients with HCM. 18 , 19 The values are higher among similarly affected paediatric patients (4–6%) 20 or patients with end‐stage HCM (7.7%). 21 DD is, nonetheless, most frequent (~33%) among patients presenting with both HCM and preexcitation. 22 When tested among patients, who underwent endomyocardial biopsy because of suspected cardiac storage disease, the prevalence of DD reached 8% after exclusion of patients with amyloidosis. 23 Interestingly, a large scale study that combined oligonucleotide hybridization‐based and dideoxy‐based DNA sequencing techniques of selected genes including LAMP2 did not identify any DD individual among 558 HCM patients. 24
Almost all reported LAMP2 mutations (106–108 in December 2019 by the authors' review of literature) putatively result in the absence of the protein. LAMP2 missense mutations are very rare in DD patients. 6 Residual LAMP2 was unambiguously documented for very few variants (e.g. a leaky splice‐site mutation 25 or a mutation in the isoform specific exon 9B 26 ). Given this mutation spectrum, LAMP2 protein testing identifies uniform LAMP2 deficiency in cells and tissues of male DD patients (Figure 1C ).
XCI triggers mosaic expression of the wild‐type and mutant LAMP2 alleles in tissues of female DD patients (Figure 1B,C ). 10 , 12 , 13 , 14 Setting thresholds for skewing or extreme skewing of XCI is arbitrary. Importantly, ~98% of females have WBC XCI ratios within the >5:95/<95:5 range. 15 A large‐scale survey evaluating XCI ratios in DD female patients has never been reported; however, a systematic extreme skewing beyond the aforementioned range is unlikely in these patients based on the dataset presented in this manuscript. The only patient in our cohort (#4), who had extremely skewed WBC XCI ratios resulting in fractions of LAMP2def granulocytes (and monocytes) <5%, had a complex Xq24 re‐arrangement that deleted not only the entire LAMP2 but also several C‐terminal exons of the neighbouring CUL4B gene. 14 Although clinically asymptomatic, female patients heterozygous for mutations in the latter gene have extremely skewed WBC XCI ratios as a result of selective pressure, most likely tissue‐specific, against the CUL4B deficient cellular clones. 14
Critical for interpretation of the sensitivity and specificity values of the LAMP2 FC assay in WBCs, all six female DD patients (#1–3 and #5–7) with mutations impacting solely the LAMP2 gene had unambiguously detectable LAM2def populations larger than 5%. Identifying patient #4 among the individuals with LAMP2def fractions <5% supports the robustness of the method. Rather than expression‐related, we attribute the LAMP2def populations <5% in the other four patients with no LAMP2 molecular abnormality to sample collection/processing‐induced errors.
Clinical implications
Prognostic stratification of patients with non‐ischemic heart failure is difficult. It is particularly problematic in patients with recent‐onset DCM, because clinical outcomes in these patients may range from full recovery to sudden death or progressive heart failure. 27 Clarification of a monogenic aetiology (DD in this case) may considerably improve the risk assessment and facilitate family counselling. Although no specific therapy is currently available, further development of efficient diagnostic algorithms for DD is substantiated by the currently ongoing LAMP2B gene‐therapy trial in male patients. 28
It is not easy to establish the diagnosis of DD in male patients. However, identification of female patients is even more complicated. 6 As we show in this study, hypertrophic cardiomyopathy and/or the presence of delta waves may suggest DD. Documenting these clinical clues, however, does not have the sufficient sensitivity and specificity to effectively support the diagnosis in female patients. A study by Boucek et al. 3 evaluated 18 DD female patients and reported pre‐excitation in 27% of them. Brambatti et al. 6 identified pre‐excitation in 32% of 56 DD female patients included into their review of previously published DD reports. The slightly higher fraction of patients with delta waves (43%), that we observed in our study, potentially reflects a combined effect of the relatively limited number of evaluated DD female patients and stringent electrocardiogram assessment criteria.
Laboratory diagnostic implications
Massively parallel sequencing technologies increased the efficiency of molecular genetic DD diagnostics. 17 However, pathogenicity validation/prediction of many of the variants identified by these methods is often inconclusive and certain LAMP2 mutation types (e.g. copy number variants) can be easily missed. 13 Contrary to these high‐throughput genomic analytical techniques, the outlined FC method is a selective protein‐level expression test. Unlike to analyses of WBCs homogenates by western blotting, 8 the fraction of LAMP2def cells is directly quantified by the FC method. The FC test identifies uniform LAMP2 deficiency in X‐hemizygous male patients as well as mosaic LAMP2 deficiency in X‐heterozygous female DD patients (Figure 1C ). In specific pedigree situations, the test may also be modified to allow identification of individuals with extremely small LAMP2def populations (<1%) resulting from somatic mosaicism. 11 , 12
Sample collection is minimally invasive, the FC test uses commercially available reagents and the turnover time is short (<24 h). Flow cytometers are a standard laboratory equipment and the overall cost of the test is low. Most importantly, the test is easy to set‐up—Standard Operating Protocol is part of the supporting information (pages 7–9) for those interested in using the methodology in their local diagnostic practice.
Given the high prevalence of DD among young female patients with non‐ischemic cardiomyopathy, that we identified in this study, we suggest screening of similarly affected patients by flow cytometry since this technique allows direct assessment of the (XCI‐driven) mosaic expression of the mutant LAMP2 allele. Additionally, the information about the presence or absence of LAMP2def cells may facilitate molecular genetic testing and expedite the correct diagnostic classification.
Study limitations
Patients with AHF constitute ~5% of the patients with heart failure. 29 AHF is also rare among female patients aged 20–40 years (e.g. 6–23 cases per one million people in a study by authors from Northeast France 30 ). Genetic cardiomyopathies, myocarditis, and peripartum cardiomyopathy are the most common causes of heart failure in young female patients. Contrary to infectious and pregnancy‐related insults, genetic defects almost exclusively result in irreversible cardiac damage. The high prevalence (12%) of DD seen in our cohort likely reflects a combination of the listed reasons—that is, small number of young female AHF patients in the general population and possible proportional increase of patients with genetic causes of cardiomyopathy in the tested population.
Patients included in our study were gathered from two centres that participate in the nation‐wide heart transplantation program and jointly serve the entire population of the Czech Republic (~10 million). The studied population of female patients with non‐ischemic heart failure younger than 40 years is not composed of patients who were followed long‐term to reach the inclusion criteria but rather is a snapshot group of young female patients recruited within 24 months, who lived with a heart transplant, ventricular assist device or were undergoing a pre‐transplant assessment.
Lastly, almost all of currently known LAMP2 pathogenic variants result in the absence of the protein. The set‐up of the LAMP2 FC assay allows identification of LAMP2 deficient cells. Given the spectrum of LAMP2 variants identified in the Czech DD patient cohort, samples of patients with residual LAMP2 were not tested.
Conclusions
Danon disease is an underdiagnosed cause of AHF in young female patients. LAMP2 flow cytometry in peripheral WBCs can be used as an effective screening method to facilitate the timely diagnosis, treatment, and family counselling in these patients.
Conflict of interest
None declared.
Supporting information
Figure S1. Family pedigrees and LAMP2 mutations.
Figure S2. ECG curves in female DD patients.
Acknowledgements
This study was supported by the research grants of the Ministry of Health of the Czech Republic (MZ 15‐27682A, NV19‐08‐00122) and Charles University in Prague (Grant/Award SVV 260367, UNCE 204064, and PROGRES Q26/LF1). It was institutionally funded by the project 00023001 of the Ministry of Health of the Czech Republic (IKEM, Prague, Czech Republic).
Gurka, J. , Piherova, L. , Majer, F. , Chaloupka, A. , Zakova, D. , Pelak, O. , Krebsova, A. , Peichl, P. , Krejci, J. , Freiberger, T. , Melenovsky, V. , Kautzner, J. , Kalina, T. , Sikora, J. , and Kubanek, M. (2020) Danon disease is an underdiagnosed cause of advanced heart failure in young female patients: a LAMP2 flow cytometric study. ESC Heart Failure, 7: 2534–2543. 10.1002/ehf2.12823.
Jiri Gurka and Lenka Piherova contributed equally to the work.
References
- 1. Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, Sue CM, Yamamoto A, Murakami N, Shanske S, Byrne E, Bonilla E, Nonaka I, DiMauro S, Hirano M. Primary LAMP‐2 deficiency causes X‐linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 2000; 406: 906–910. [DOI] [PubMed] [Google Scholar]
- 2. Rowland TJ, Sweet ME, Mestroni L, Taylor MRG. Danon disease—dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J Cell Sci 2016; 129: 2135–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boucek D, Jirikowic J, Taylor M. Natural history of Danon disease. Genet Med 2011; 13: 563–568. [DOI] [PubMed] [Google Scholar]
- 4. D'Souza RS, Levandowski C, Slavov D, Graw SL, Allen LA, Adler E, Mestroni L, Taylor MR. Danon disease: clinical features, evaluation, and management. Circ Heart Fail 2014; 7: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu H, Luo J, Yu H, Rattner A, Mo A, Wang Y, Smallwood PM, Erlanger B, Wheelan SJ, Nathans J. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron 2014; 81: 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brambatti M, Caspi O, Maolo A, Koshi E, Greenberg B, Taylor MRG, Adler ED. Danon disease: gender differences in presentation and outcomes. Int J Cardiol 2019; 286: 92–98. [DOI] [PubMed] [Google Scholar]
- 7. Miani D, Taylor M, Mestroni L, D'Aurizio F, Finato N, Fanin M, Brigido S, Proclemer A. Sudden death associated with Danon disease in women. Am J Cardiol 2012; 109: 406–411. [DOI] [PubMed] [Google Scholar]
- 8. Fanin M, Nascimbeni AC, Fulizio L, Spinazzi M, Melacini P, Angelini C. Generalized lysosome‐associated membrane protein‐2 defect explains multisystem clinical involvement and allows leukocyte diagnostic screening in Danon disease. Am J Pathol 2006; 168: 1309–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regelsberger G, Höftberger R, Pickl WF, Zlabinger GJ, Körmöczi U, Salzer‐Muhar U, Luckner D, Bodamer OA, Mayr JA, Muss WH, Budka H, Bernheimer H. Danon disease: case report and detection of new mutation. J Inherit Metab Dis 2009; 32: S115–S122. [DOI] [PubMed] [Google Scholar]
- 10. Majer F, Vlaskova H, Krol L, Kalina T, Kubanek M, Stolnaya L, Dvorakova L, Elleder M, Sikora J. Danon disease: a focus on processing of the novel LAMP2 mutation and comments on the beneficial use of peripheral white blood cells in the diagnosis of LAMP2 deficiency. Gene 2012; 498: 183–195. [DOI] [PubMed] [Google Scholar]
- 11. Majer F, Pelak O, Kalina T, Vlaskova H, Dvorakova L, Honzik T, Palecek T, Kuchynka P, Masek M, Zeman J, Elleder M, Sikora J. Mosaic tissue distribution of the tandem duplication of LAMP2 exons 4 and 5 demonstrates the limits of Danon disease cellular and molecular diagnostics. J Inherit Metab Dis 2014; 37: 117–124. [DOI] [PubMed] [Google Scholar]
- 12. Sikora J, Majer F, Kalina T. LAMP2 flow cytometry in peripheral white blood cells is an established method that facilitates identification of heterozygous Danon disease female patients and mosaic mutation carriers. J Cardiol 2015; 66: 88–89. [DOI] [PubMed] [Google Scholar]
- 13. Majer F, Piherova L, Reboun M, Stara V, Pelak O, Norambuena P, Stranecky V, Krebsova A, Vlaskova H, Dvorakova L, Kmoch S, Kalina T, Kubanek M, Sikora J. LAMP2 exon‐copy number variations in Danon disease heterozygote female probands: infrequent or underdetected? Am J Med Genet A 2018; 176: 2430–2434. [DOI] [PubMed] [Google Scholar]
- 14. Majer F, Kousal B, Dusek P, Piherova L, Reboun M, Mihalova R, Gurka J, Krebsova A, Vlaskova H, Dvorakova L, Krihova J, Liskova P, Kmoch S, Kalina T, Kubanek M, Sikora J. Alu‐mediated contiguous Xq24 deletion encompassing CUL4B, LAMP2, ATP1B4, TMEM255A, and ZBTB33 genes causes Danon disease in a female patient. Am J Med Genet A 2020; 182: 219–223. [DOI] [PubMed] [Google Scholar]
- 15. Amos‐Landgraf JM, Cottle A, Plenge RM, Friez M, Schwartz CE, Longshore J, Willard HF. X chromosome‐inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet 2006; 79: 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalia S, Adelman K, Bale S, Chung WK, Eng C, Evans JP, Herman GE, Hufnagel SB, Klein TE, Korf BR, McKelvey KD. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med 2017; 19: 249–255. [DOI] [PubMed] [Google Scholar]
- 17. Fu L, Luo S, Cai S, Hong W, Guo Y, Wu J, Liu T, Zhao C, Li F, Huang H, Huang M, Wang J. Identification of LAMP2 mutations in early‐onset Danon disease with hypertrophic cardiomyopathy by targeted next‐generation sequencing. Am J Cardiol 2016; 118: 888–894. [DOI] [PubMed] [Google Scholar]
- 18. Charron P, Villard E, Sébillon P, Laforêt P, Maisonobe T, Duboscq‐Bidot L, Romero N, Drouin‐Garraud V, Frébourg T, Richard P, Eymard B, Komajda M. Danon's disease as a cause of hypertrophic cardiomyopathy: a systematic survey. Heart 2004; 90: 842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arad M, Maron BJ, Gorham JM, Johnson WH Jr, Saul JP, Perez‐Atayde AR, Spirito P, Wright GB, Kanter RJ, Seidman CE, Seidman JG. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med 2005; 352: 362–372. [DOI] [PubMed] [Google Scholar]
- 20. Yang Z, McMahon CJ, Smith LR, Bersola J, Adesina AM, Breinholt JP, Kearney DL, Dreyer WJ, Denfield SW, Price JF, Grenier M, Kertesz NJ, Clunie SK, Fernbach SD, Southern JF, Berger S, Towbin JA, Bowles KR, Bowles NE. Danon disease as an underrecognized cause of hypertrophic cardiomyopathy in children. Circulation 2005; 112: 1612–1617. [DOI] [PubMed] [Google Scholar]
- 21. Garcia‐Pavia P, Vázquez ME, Segovia J, Salas C, Avellana P, Gómez‐Bueno M, Vilches C, Gallardo ME, Garesse R, Molano J, Bornstein B, Alonso‐Pulpon L. Genetic basis of end‐stage hypertrophic cardiomyopathy. Eur J Heart Fail 2011; 13: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Chen X, Wang F, Liang Y, Deng H, Liao H, Zhang Q, Zhang B, Zhan X, Fang X, Shehata M, Wang X, Xue Y, Wu S. Prevalence and clinical characteristics of Danon disease among patients with left ventricular hypertrophy and concomitant electrocardiographic preexcitation. Mol Genet Genomic Med 2019; 7: e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng Z, Cui Q, Tian Z, Xie H, Chen L, Fang L, Zhu K, Fang Q. Danon disease as a cause of concentric left ventricular hypertrophy in patients who underwent endomyocardial biopsy. Eur Heart J 2012; 33: 649–656. [DOI] [PubMed] [Google Scholar]
- 24. Li Q, Gruner C, Chan RH, Care M, Siminovitch K, Williams L, Woo A, Rakowski H. Genotype‐positive status in patients with hypertrophic cardiomyopathy is associated with higher rates of heart failure events. Circ Cardiovasc Genet 2014; 7: 416–422. [DOI] [PubMed] [Google Scholar]
- 25. Cetin H, Wöhrer A, Rittelmeyer I, Gencik M, Zulehner G, Zimprich F, Ströbel T, Zimprich A. The c.65‐2A>G splice site mutation is associated with a mild phenotype in Danon disease due to the transcription of normal LAMP2 mRNA. Clin Genet 2016; 90: 366–371. [DOI] [PubMed] [Google Scholar]
- 26. van der Kooi AJ, van Langen IM, Aronica E, van Doorn PA, Wokke JHJ, Brusse E, Langerhorst CT, Bergin P, Dekker LRC, dit Deprez RH, de Visser M. Extension of the clinical spectrum of Danon disease. Neurology Apr 2008; 70: 1358–1359. [DOI] [PubMed] [Google Scholar]
- 27. Givertz MM, Mann DL. Epidemiology and natural history of recovery of left ventricular function in recent onset dilated cardiomyopathies. Curr Heart Fail Rep 2013; 10: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ClinicalTrials.gov [Internet] . Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03882437, Gene therapy for male patients with Danon disease using RP‐A501; AAV9.LAMP2B; 2019. 20. https://clinicaltrials.gov/ct2/show/study/NCT03882437 (12 January 2020).
- 29. Costanzo MR, Mills RM, Wynne J. Characteristics of "Stage D" heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am Heart J 2008; 155: 339–347. [DOI] [PubMed] [Google Scholar]
- 30. Zannad F, Briancon S, Juilliere Y, Mertes PM, Villemot JP, Alla F, Virion JM. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL Study. Epidemiologie de l'Insuffisance Cardiaque Avancee en Lorraine. J Am Coll Cardiol 1999; 33: 734–742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Family pedigrees and LAMP2 mutations.
Figure S2. ECG curves in female DD patients.


