Abstract
Aims
This study aimed to describe baseline characteristics of patients with atrial fibrillation (AF) at risk of stroke with and without history of heart failure (HF) and report 2‐year outcomes in the dabigatran‐treated subset of a prospective, global, observational study (GLORIA‐AF).
Methods and results
Newly diagnosed patients with AF and CHA2DS2‐VASc score ≥ 1 were consecutively enrolled. Baseline characteristics were assessed by the presence or absence of HF diagnosis at enrolment. Incidence rates for outcomes in dabigatran‐treated patients were estimated with and without standardization by stroke (excluding HF component) and bleeding risk scores. A total of 15 308 eligible patients were enrolled, including 15 154 with known HF status; of these, 3679 (24.0%) had been diagnosed with HF, 11 475 (75.0%) had not. Among 4873 dabigatran‐treated patients, 1169 (24.0%) had HF, and 3658 (75.1%) did not; the risk of stroke was high (CHA2DS2‐VASc score ≥ 2) for 94.3% of patients with HF and 85.8% without, while 6.0% and 7.0%, respectively, had a high bleeding risk (HAS‐BLED ≥ 3). Incidence rates of all‐cause death in dabigatran‐treated patients with and without HF, standardized for CHA2DS2‐VASc and HAS‐BLED scores, were 4.76 vs. 1.80 per 100 patient years (py), with roughly comparable rates of stroke (0.82 vs. 0.60 per 100 py) and major bleeding (1.20 vs. 0.92 per 100 py).
Conclusions
Patients with AF and history of HF may have greater disease burden at AF diagnosis and increased mortality rates vs. patients without HF. Stroke and major bleeding rates were roughly comparable between groups confirming the long‐term safety and effectiveness of dabigatran in patients with HF.
Keywords: Anticoagulation, Atrial fibrillation, Dabigatran, Heart failure, Major bleed, Stroke
Introduction
The relationship between atrial fibrillation (AF) and heart failure (HF) is not yet fully understood, and further information is needed to optimize management strategies. Overall, AF is more prevalent in patients with vs. without HF, 1 and HF is more prevalent in patients with vs. without AF. 2 A recent analysis from the Framingham Heart Study identified AF in over half of the patients with new‐onset HF and HF in over one third of patients with new‐onset AF. 3 In addition, registry‐based data from patients hospitalized for HF showed that approximately 40% had a history of AF, up to 50% had AF at baseline, and almost 20% experienced new‐onset AF during hospitalization. 4 Several clinical characteristics of patients with AF have been identified as potential independent predictors of HF, 2 and the two conditions share many risk factors and pathophysiological mechanisms. 5 , 6 However, patients with AF and HF have increased mortality compared with patients with AF but without HF, 2 , 3 , 6 , 7 , 8 and both conditions predispose patients to an increased risk of thromboembolic events. 2 , 9 , 10 According to guidelines from the European Society of Cardiology (ESC), the only therapy with proven prognostic value for reducing risk of thromboembolic events is anticoagulation, and appropriate oral anticoagulants should be prescribed in all patients with AF at risk of stroke, regardless of the presence of HF. 11
The ESC HF Long‐Term Registry, a prospective, multicentre, observational study of almost 15 000 patients with HF, was published recently. 12 Mean age was 66 ± 13 years, and 67% of the patients were male. The prevalence of AF was generally age dependent in both men and women and reached 50% in patients with HF aged > 80 years. 12
Four pivotal, phase 3, clinical trials (ARISTOTLE, RE‐LY, ROCKET AF, and ENGAGE AF) have shown the benefit of non‐vitamin K oral anticoagulants (NOACs) on outcomes compared with warfarin in patients with AF. 13 , 14 , 15 , 16 For example, in the RE‐LY trial, dabigatran etexilate (dabigatran) 110 mg twice daily showed similar rates of stroke or systemic embolism (SSE) and reduced bleeding rates compared with warfarin, while dabigatran 150 mg twice daily showed reduced rates of SSE but similar bleeding rates. 13 Moreover, the relevant benefits of dabigatran over warfarin were consistent in patients with and without HF, as well as in those with reduced (≤ 40%) or preserved (> 40%) left ventricular ejection fraction (LVEF). 17 A meta‐analysis of the outcomes of patients with AF and HF, using published data from these four pivotal phase 3 trials, 17 , 18 , 19 , 20 also showed no interactions in the efficacy and safety of NOACs between patients with AF both with and without HF. 7 Further data are needed from prospective, observational studies to help optimize effective management strategies and to evaluate real‐world safety and effectiveness in routine clinical care.
GLORIA™‐AF is a large, international, observational registry programme that enrolled patients with newly diagnosed AF at risk for stroke 21 in 44 countries from five geographical regions (Europe, North America, Asia, Latin America, and Africa and the Middle East). 21
Data from Phase II of GLORIA‐AF, which began early after approval of dabigatran in a given country, have been used to compare the baseline characteristics and outcome events at 2 years (safety and effectiveness) in the subset of dabigatran‐treated patients with AF, with or without HF at baseline. We also describe the overall baseline clinical characteristics of all patients newly diagnosed with AF, with or without HF, regardless of anticoagulation treatment.
Methods
The study design has been described previously. 21 In brief, consecutively enrolled adult patients with newly diagnosed (within 3 months before the baseline visit; 4.5 months in Latin America) AF at risk of stroke (CHA2DS2‐VASc scores ≥ 1, based on the presence of congestive HF, hypertension, age ≥ 75 years [doubled], diabetes, stroke/transient ischaemic attack/systemic embolism [doubled], vascular disease, age 65–74 years, and sex category [female patients]) were included. Patients were excluded if they had mechanical heart valves, prior vitamin K antagonist (VKA) therapy for > 60 days, or AF with a generally reversible cause. Patients were recruited from different outpatient settings, including university hospitals, community hospitals, specialist offices, and general practice offices. 21 Phase II of GLORIA‐AF, which enrolled patients between November 2011 and December 2014, collected baseline data of all included patients with AF and monitored the outcomes of those prescribed dabigatran through 2 years of follow‐up.
Eligible patients for the present analysis of baseline characteristics were those with a baseline visit in Phase II of GLORIA‐AF and whose status with respect to the presence or absence of HF had been recorded. HF was defined as New York Heart Association (NYHA) classes II–IV or ejection fraction ≤ 40%. Baseline medical history, concomitant medications, and antithrombotic treatments prescribed were recorded, and outcome events were collected at planned visit intervals at 3, 6, and 12 months and 2 years after the baseline visit.
GLORIA‐AF was performed in accordance with the provisions of the Declaration of Helsinki, 22 and the protocol and procedures were approved by the European Medicines Agency, as well as relevant institutional review boards and ethics committees where required. All patients provided written informed consent before entering the registry.
Outcome measures
The subset of patients receiving dabigatran (defined as taking at least one dose of dabigatran after enrolment) was followed for up to 2 years to assess outcomes. Outcome measures were the incidence of stroke, myocardial infarction (MI), major bleed, all‐cause death and vascular death, and a composite of SSE, MI, life‐threatening bleed, and vascular death. Occurrence of outcome measures was evaluated according to the presence or absence of HF at baseline.
Statistical analysis
Baseline characteristics were analysed descriptively in patients with AF both with and without HF. To compare the clinical demography of patients with and without HF, we report the standardized difference between groups. 23 We considered variables with standardized differences < 10% to be similar between the groups. Patients who were prescribed dabigatran but did not take at least one dose (n = 14) were excluded from the outcome analysis. Incidence rates and 95% confidence intervals (CIs) of outcomes were evaluated according to the presence or absence of HF. In addition to crude estimates, to eliminate the confounding effect of risk score components (except for HF) accounting for differences in outcomes between patients with and without HF, the incidence rates were standardized by stroke [modified CHA2DS2‐VASc score (excluding prior congestive HF as a component) < 3, 3, and >3] and bleeding risk [HAS‐BLED score < 2 or ≥ 2 (excluding labile international normalized ratios)]. The HAS‐BLED score is based on the presence of hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, elderly (> 65 years), and drugs/alcohol concomitantly. The strata for standardization were (i) CHA2DS2‐VASc < 3 and HAS‐BLED < 2; (ii) CHA2DS2‐VASc = 3 and HAS‐BLED < 2; (iii) CHA2DS2‐VASc > 3 and HAS‐BLED < 2; (iv) CHA2DS2‐VASc < 3 and HAS‐BLED ≥ 2; (v) CHA2DS2‐VASc = 3 and HAS‐BLED ≥ 2; and (vi) CHA2DS2‐VASc > 3 and HAS‐BLED ≥ 2.
Standardization involved averages of the stratum‐specific incidence rates, weighted in each stratum by the total patient years (py) that fell in that stratum. For missing data on HAS‐BLED scores [1804 (11.8%) dabigatran‐treated patients overall; 114 (9.8%) with HF and 379 (10.4%) without HF] and HF [46 (0.9%) dabigatran‐treated patients], we used multiple imputation with chained equations 24 based upon approximately 50 baseline variables including demographics and medical history. The CIs of the standardized incidence rates were constructed using the bootstrap method. 25 All analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics and treatment patterns of all patients
A total of 15 308 patients with AF from Phase II of GLORIA‐AF were eligible for analysis in the full baseline set. HF status was missing for 154 (1.0%) patients. Baseline characteristics according to HF status were evaluated for the remaining 15 154 patients; of these, 3679 (24.3%) were diagnosed with HF, while 11 475 (75.7%) were not. Most patients with HF [n = 1861 (50.6%)] were classified in NYHA functional class II; 910 (24.7%) were NYHA class III, 354 (9.6%) were NYHA class I, and 168 (4.6%) were NYHA class IV. For 386 patients (10.5%), NYHA class was unknown. Just over one third (n = 1405; 38.2%) of patients with HF had LVEF ≤ 40%.
Baseline clinical characteristics and cardiovascular and antithrombotic medications of all patients with and without prior HF are as follows: the proportion of patients aged ≥ 75 years with or without HF was similar (40.7% vs. 38.6%). Compared with patients without HF, those with HF were more often male (60.9% vs. 52.3%) and had higher rates of prior MI (18.8% vs. 7.8%) and coronary artery disease (31.6% vs. 16.7%), as well as greater proportions of symptomatic (39.5% vs. 24.6%), persistent (43.9% vs. 33.0%), and permanent AF (15.2% vs. 9.7%). Patients with HF were at a higher risk of stroke [mean (standard deviation; SD) CHA2DS2‐VASc score 3.9 (1.6) vs. 3.0 (1.4)], while the risk of bleeding was comparable [mean (SD) HAS‐BLED score 1.4 (0.9) vs. 1.4 (0.9)]. However, it should be considered that HF is part of the CHA2DS2‐VASc score, which may explain the observed difference in stroke risk. The use of antiarrhythmic and antihypertensive medications was higher among patients with HF than those without.
The most frequently prescribed antiarrhythmic medications in patients with vs. without HF were amiodarone (17.8% vs. 10.8%) and digoxin/digitoxin (21.9% vs. 7.9%), while β‐blockers were the most frequently prescribed antihypertensive (70.7% vs. 57.3%) medication. Overall anticoagulant treatment patterns at baseline were similar between patients with and without HF (Figure 1 ), with VKAs and NOACs the most frequently prescribed (35.1% vs. 31.5% for VKAs and 46.6% vs. 48.4% for NOACs). Of the NOACs, dabigatran was prescribed in 31.8% vs. 31.9% of patients with and without HF; rivaroxaban in 10.5% vs. 11.8%, and apixaban in 4.3% vs. 4.7%.
Figure 1.
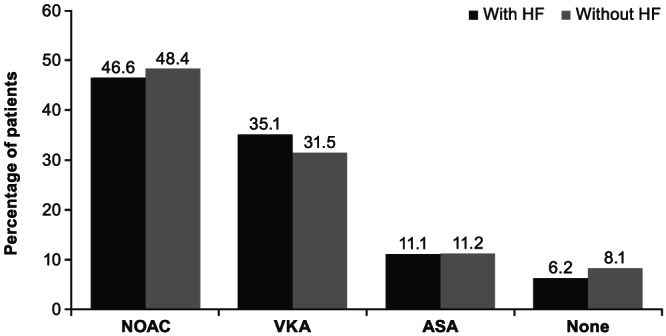
Overall anticoagulant treatment patterns in all patients with and without prior HF at baseline. Four patients without prior HF received other anticoagulant medication such as a combination of OACs. Thirty‐six patients (1.0%) with prior HF and 86 patients (0.7%) without prior HF received antiplatelet treatment other than ASA. ASA, acetylsalicylic acid; HF, heart failure; NOAC, non‐vitamin K oral anticoagulants; OAC, oral anticoagulant; VKA, vitamin K antagonist.
Baseline characteristics and treatment patterns of dabigatran‐treated patients
A total of 4873 patients were treated with dabigatran; prior HF disease status was unknown for 46 (0.9%) patients and 1169 (24.0%) and 3658 (75.1%) patients with or without prior HF, respectively. Most patients with HF were NYHA functional class II [n = 675 (57.7%)]; 289 (24.7%) were NYHA class III, 112 (9.6%) were NYHA class I, and 23 (2.0%) were NYHA class IV. NYHA class was unknown for 70 (6.0%) patients. One third [n = 394 (33.7%)] of the patients with HF had LVEF ≤ 40%.
Most patients treated with dabigatran were managed by cardiologists (87.5%), followed by neurologists (4.0%), general practitioners/primary care physicians/gerontologists (3.4%), or others (5.1%). Dabigatran‐treated patients were followed in community hospitals (34.6%), specialists' offices (30.0%), university hospitals (23.6%), general practitioners/primary care sites (6.4%), outpatient or anticoagulation clinics (4.1%), or other health care facilities (1.3%). Baseline characteristics of dabigatran‐treated patients with and without HF are presented in Table 1 . The proportion of dabigatran‐treated patients aged ≥ 75 years with or without HF was similar (38.2% vs. 36.2%). Similar to the overall population, dabigatran‐treated patients with vs. without HF were more often male (60.7% vs. 54.0%), had higher rates of prior MI (15.2% vs. 6.6%) and coronary artery disease (29.8% vs. 15.5%), as well as greater proportions of symptomatic (39.6% vs. 25.7%), persistent (43.2% vs. 32.6%), and permanent (18.1% vs. 11.2%) AF. Dabigatran‐treated patients with HF were also at a higher risk of stroke [mean (SD) CHA2DS2‐VASc score 3.8 (1.5) vs. 3.0 (1.4) without HF], while the risk of bleeding was comparable [mean (SD) HAS‐BLED score 1.2 (0.8) vs. 1.3 (0.9) without HF]. In both patient groups, slightly more patients received dabigatran 150 mg than 110 mg twice daily (50.1% compared with 47.3% with HF and 56.2% compared with 41.6% without HF). The most frequently prescribed antiarrhythmics were amiodarone (22.2% with HF and 14.8% without HF) and digoxin/digitoxin (19.9% and 7.2%). The most frequently prescribed antihypertensive was still β‐blockers (70.9% and 60.2%). In addition to their use as an antihypertensive, β‐blockers are used to control heart rhythm and increase cardiac action, which may account for this observation.
Table 1.
Baseline demographic and clinical characteristics of 4827 phase 2 dabigatran‐treated patients with recorded presence or absence of prior HF
| Characteristic | AF with prior HF (n = 1169) | AF without prior HF (n = 3658) | Standardized difference a |
|---|---|---|---|
| Age | −0.03 | ||
| Mean (SD), years | 69.9 (11.0) | 70.3 (10.2) | |
| < 75 years, n (%) | 723 (61.8) | 2333 (63.8) | −0.04 |
| ≥ 75 years, n (%) | 446 (38.2) | 1325 (36.2) | 0.04 |
| Sex, n (%) | |||
| Male | 709 (60.7) | 1974 (54.0) | 0.14 |
| Female | 460 (39.3) | 1684 (46.0) | −0.14 |
| Ethnicity, n (%) b | |||
| White | 870 (74.4) | 2470 (67.5) | 0.15 |
| Asian | 72 (6.2) | 342 (9.3) | −0.12 |
| Arab/Middle East | 81 (6.9) | 221 (6.0) | 0.04 |
| Black/African American | 11 (0.9) | 28 (0.8) | 0.02 |
| Other | 52 (4.4) | 113 (3.1) | 0.07 |
| Body mass index, n (%) c | 0.05 | ||
| < 30 kg/m2 | 735 (62.9) | 2364 (64.6) | −0.04 |
| ≥ 30 kg/m2 | 428 (36.6) | 1253 (34.3) | 0.05 |
| Medical status, n (%) | |||
| Coronary artery disease | 348 (29.8) | 567 (15.5) | 0.35 |
| Hyperlipidaemia | 464 (39.7) | 1538 (42.0) | −0.05 |
| Prior myocardial infarction | 178 (15.2) | 240 (6.6) | 0.28 |
| Prior stroke | 94 (8.0) | 480 (13.1) | −0.17 |
| Prior stroke or TIA | 118 (10.1) | 639 (17.5) | −0.22 |
| History of hypertension | 898 (76.8) | 2844 (77.7) | −0.02 |
| Prior bleeding | 57 (4.9) | 188 (5.1) | −0.01 |
| Diabetes mellitus | 279 (23.9) | 819 (22.4) | 0.04 |
| Abnormal kidney function d | 10 (0.9) | 8 (0.2) | 0.09 |
| CHA2DS2‐VASc score | 0.53 | ||
| Mean (SD) | 3.8 (1.5) | 3.0 (1.4) | |
| High risk (score ≥ 2), n (%) | 1102 (94.3) | 3138 (85.8) | 0.29 |
| Moderate risk (score = 1), n (%) | 67 (5.7) | 520 (14.2) | −0.29 |
| HAS‐BLED score e | −0.08 | ||
| Mean (SD) | 1.2 (0.8) | 1.3 (0.9) | |
| High (score ≥ 3), n (%) | 70 (6.0) | 255 (7.0) | −0.04 |
| Low (score < 3), n (%) | 985 (84.3) | 3024 (82.7) | 0.04 |
| AF category, n (%) | |||
| Asymptomatic | 212 (18.1) | 1186 (32.4) | −0.33 |
| Minimally symptomatic | 494 (42.3) | 1532 (41.9) | 0.01 |
| Symptomatic | 463 (39.6) | 940 (25.7) | 0.30 |
| AF type, n (%) | |||
| Paroxysmal | 452 (38.7) | 2054 (56.2) | −0.36 |
| Persistent | 505 (43.2) | 1193 (32.6) | 0.22 |
| Permanent | 212 (18.1) | 411 (11.2) | 0.20 |
| Dabigatran dose, n (%) | |||
| 75 mg | 23 (2.0) | 64 (1.7) | 0.02 |
| 110 mg | 553 (47.3) | 1520 (41.6) | 0.12 |
| 150 mg | 586 (50.1) | 2055 (56.2) | −0.12 |
| Other | 7 (0.6) | 19 (0.5) | 0.01 |
| Additional antiplatelet therapy | 185 (15.8) | 429 (11.7) | 0.12 |
| Medications, n (%) | |||
| Antiarrhythmics: any | 531 (45.4) | 1278 (34.9) | 0.22 |
| Amiodarone | 259 (22.2) | 541 (14.8) | 0.19 |
| Dronedarone | 3 (0.3) | 37 (1.0) | −0.10 |
| Verapamil | 15 (1.3) | 54 (1.5) | −0.02 |
| Quinidine | 0 (0.0) | 1 (0.0) | NE |
| Digoxin/Digitoxin | 233 (19.9) | 264 (7.2) | 0.38 |
| Mexiletine | 0 (0.0) | 1 (0.0) | NE |
| Propafenone | 25 (2.1) | 140 (3.8) | −0.10 |
| Flecainide | 4 (0.3) | 173 (4.7) | −0.28 |
| Diltiazem | 48 (4.1) | 188 (5.1) | −0.05 |
| Other | 90 (7.7) f | 144 (3.9) g | 0.16 |
| Other medications: any | 1112 (95.1) | 3297 (90.1) | 0.19 |
| ARBs | 298 (25.5) | 1058 (28.9) | −0.08 |
| ACE inhibitors | 537 (45.9) | 1073 (29.3) | 0.35 |
| α‐blockers or other vasodilators | 87 (7.4) | 263 (7.2) | 0.01 |
| β‐blockers | 829 (70.9) | 2202 (60.2) | 0.23 |
| Diuretics | 778 (66.6) | 1144 (31.3) | 0.75 |
| Other antihypertensive agents | 185 (15.8) | 770 (21.0) | −0.14 |
| Statins | 536 (45.9) | 1593 (43.5) | 0.05 |
| Other anti‐inflammatory therapy | 24 (2.1) f | 68 (1.9) g | 0.01 |
AF, atrial fibrillation; ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CHA2DS2‐VASc, congestive HF, hypertension, age ≥ 75 years (doubled), diabetes, stroke/TIA/systemic embolism (doubled), vascular disease, age 65–75 years, and sex category (female); CHF, congestive HF; HAS‐BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, elderly (> 65 years), drugs/alcohol concomitantly; HF, heart failure; NE, not evaluated; SD, standard deviation; TIA, transient ischaemic attack.
Around 46 (0.9%) dabigatran patients had unknown or missing history of prior CHF.
Standardized differences ≥ 10% (or ≥ 0.1) indicate a difference between the compared groups.
Data missing for 83 (7.1%) patients with HF and for 484 (13.2%) patients without HF. “Other” race category includes American Indian/American Native/Alaskan Native, African, and Other.
Data missing for 6 (0.5%) patients with HF and for 41 (1.1%) patients without HF.
Abnormal kidney function is defined as the presence of chronic dialysis or renal transplantation or serum creatinine ≥ 200 μmol/L.
Data missing for 114 (9.8%) patients with HF and for 379 (10.4%) patients without HF.
Percentages based on the total number of eligible patients with prior HF.
Percentages based on the total number of eligible patients with no prior HF.
Two‐year follow‐up of dabigatran‐treated patients
Mean therapy duration for the index dabigatran period was 18.0 ± 9.4 months. The crude and standardized incidence rates (per 100 py) of the outcome events for dabigatran‐treated patients with and without prior HF are shown in Table 2 . The standardized incidence rates were comparable between the groups with and without HF for stroke (0.82 [95% CI: 0.41, 1.30] vs. 0.60 [0.40, 0.80] per 100 py, respectively), MI (0.87 [0.45, 1.37] vs. 0.40 [0.24, 0.58] per 100 py), and major bleed (1.20 [0.71, 1.77] vs. 0.92 [0.67, 1.18] per 100 py). AF patients with HF had higher standardized incidence rates than those without HF for all‐cause death (4.76 [3.74, 5.86] vs. 1.80 [1.46, 2.16] per 100 py) and vascular death (1.92 [1.28, 2.62] vs. 0.53 [0.35, 0.74] per 100 py), as well as for the composite outcome of SSE, MI, life‐threatening bleed, and vascular death (3.37 [2.53, 4.33] vs. 1.70 [1.37, 2.06] per 100 py, respectively).
Table 2.
Clinical outcomes in phase 2 patients treated with dabigatran by recorded presence or absence of prior HF
| Outcome | Standardized a incidence rates per 100 py (95% CI) | |
|---|---|---|
| With prior HF | Without prior HF | |
| Stroke | 0.82 (0.41, 1.30) | 0.60 (0.40, 0.80) |
| Major bleed | 1.20 (0.71, 1.77) | 0.92 (0.67, 1.18) |
| MI | 0.87 (0.45, 1.37) | 0.40 (0.24, 0.58) |
| All‐cause death | 4.76 (3.74, 5.86) | 1.80 (1.46, 2.16) |
| Vascular death | 1.92 (1.28, 2.62) | 0.53 (0.35, 0.74) |
| Composite outcome of SSE, MI, life‐threatening bleed, and vascular death | 3.37 (2.53, 4.33) | 1.70 (1.37, 2.06) |
CHA2DS2‐VASc, congestive HF, hypertension, age ≥ 75 years (doubled), diabetes, stroke/TIA/systemic embolism (doubled), vascular disease, age 65–75 years, and sex category (female); CHF, congestive HF; CI, confidence interval; HAS‐BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, elderly (> 65 years), drugs/alcohol concomitantly; HF, heart failure; MI, myocardial infarction; py, patient‐years; SSE, stroke or systemic embolism; TIA, transient ischaemic attack.
For standardized incidence rates, missing data were imputed using the multiple imputation approach (based on the entire eligible population), including congestive HF.
Standardized by stroke [CHA2DS2‐VASc (excluding prior CHF as a component) < 3, 3, and > 3] and bleeding risk [HAS‐BLED < 2 or ≥ 2 (excluding labile international normalized ratios)], with HF and HAS‐BLED missing values imputed. Only events occurring while the patient was on the first treatment regimen were considered; in case of recurrent events for one patient, only the first event was considered.
The unadjusted cumulative probability of the composite outcome of SSE, MI, life‐threatening bleed, and vascular death in dabigatran‐treated patients with and without prior HF for 2 years of follow‐up were evaluated. The Kaplan–Meier curves suggest that the probability of this composite endpoint was higher in AF patients with HF than in those without HF over the 2‐year follow‐up period. Of note, the unadjusted cumulative probability of stroke or major bleeding was comparable in dabigatran‐treated patients over the 2 years of follow‐up (Figure 2 ).
Figure 2.
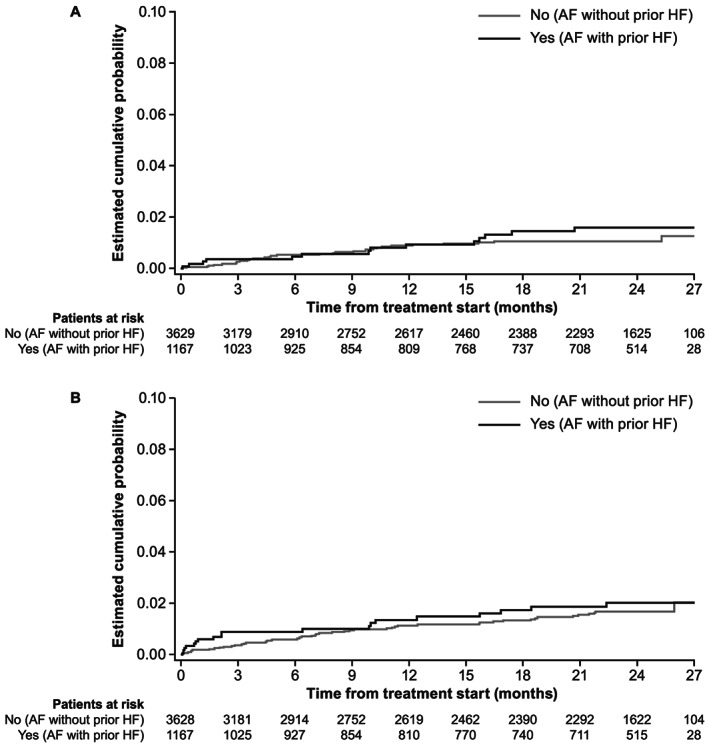
Kaplan–Meier curves for the cumulative probability, in dabigatran‐treated patients by HF status, of stroke (A) and major bleeding (B). AF, atrial fibrillation; HF, heart failure.
Discussion
AF and HF are associated with substantial morbidity and mortality and are associated with a poor prognosis. 26 In the Fushimi AF registry, persistent AF was associated with a higher incidence of the composite of cardiac death and HF compared with paroxysmal AF. 27 In the Framingham Heart Study, 1470 participants developed either new AF or HF between the years 1948 and 1995. Among these participants, a total of 383 individuals (26%) developed both AF and HF. 8 In our study, baseline characteristics across all patients in Phase II of GLORIA‐AF revealed that the incidence of HF in patients with AF (24.3%) was similar to that previously reported. Furthermore, patients with HF were more often male patients, had higher rates of prior MI and coronary artery disease, and greater proportions had symptomatic, persistent, and permanent AF compared with those with no HF.
Similar to our findings, the EURObservational Research Programme pilot survey on patients with AF (EORP‐AF) reported that patients with AF (electrocardiogram‐documented diagnosis of AF confirmed in the year before enrolment) and HF were more likely to have symptomatic and permanent AF and coronary artery disease (including MI), as well as a higher risk of stroke (CHA2DS2‐VASc score ≥ 2) and bleeding risk (HAS‐BLED ≥ 3). 28 However, there are differences between GLORIA‐AF and EORP‐AF that may be reflected in the discrete patient populations and perhaps variations in the timing of the AF diagnosis in each trial (< 3 months before the baseline visit in GLORIA‐AF and < 12 months before enrolment in EORP‐AF). Importantly, to be included in GLORIA‐AF, patients had to be at risk for stroke as defined by a CHA2DS2‐VASc score of at least 1. This shifts the demographic and clinical characteristics of the group without HF, who were expected to show higher rates of other stroke risk factors compared with the general population of AF patients with comorbid HF. This may explain why, in GLORIA‐AF, there was little difference in mean age between the HF and no HF groups and little difference in diabetes mellitus, while prior stroke and transient ischaemic attack were higher in patients without HF. When compared with EORP‐AF, where AF patients were included irrespective of stroke risk, patients with AF and HF were older than those without HF and had higher rates of both diabetes and prior stroke.
Within the group of patients with HF in GLORIA‐AF, which should not have been affected by the inclusion criteria, half were classified as having NYHA class II HF and just over one‐third had reduced LVEF. In EORP‐AF, almost half the patients were classified as having European Heart Rhythm Association class III, and over half had reduced LVEF. 28 There were also differences in anticoagulation treatment between GLORIA‐AF and EORP‐AF, with additional antiplatelet therapy given to < 16% of patients who received dabigatran in GLORIA‐AF compared with > 34% of patients in EORP‐AF. 28 Moreover, in GLORIA‐AF, no patients reported the use of other antithrombotic medications such as heparin or fondaparinux, while in EORP‐AF, 38.3% of the patients with HF and 48.4% of those without HF reported the use of other antithrombotic medications. 28 Consistent with previously reported regional differences in anticoagulation treatment for stroke prevention in AF from the GLORIA‐AF study, 29 we also observed regional differences in the prescription of anticoagulation treatment between HF groups (data not shown).
Baseline characteristics in dabigatran‐treated patients with AF were similar to the overall population included in this analysis, irrespective of anticoagulant therapy; patients with HF showed a greater disease burden compared with those without HF (e.g. fewer had asymptomatic AF and more had persistent or permanent AF). Despite this higher disease burden, the standardized incidence rates of stroke and major bleeding in dabigatran‐treated patients with AF were similar in those with or without HF. In the randomized, phase 3 RE‐LY trial, HF was not meaningfully associated with the occurrence of SSE (hazard ratio with vs. without HF 1.08; 95% CI: 0.89, 1.31), or major bleeding (hazard ratio 0.96; 95% CI: 0.83, 1.10). 17 Moreover, for patients treated with either dabigatran 110 or 150 mg twice daily, the rates of SSE and major bleeding events were unaffected by NYHA class or ≤ 40% LVEF compared with warfarin. 17 It is important to note that in the pivotal RE‐LY study, two‐thirds of the patients had pre‐existing AF, and they were older and had more comorbidities than those in GLORIA‐AF. 13 In addition, HF remained a powerful independent predictor of vascular death in RE‐LY 17 ; therefore, it is unsurprising that our analysis also observed higher rates of mortality in patients with AF with vs. without HF.
In the HF Long‐Term Registry of the ESC, AF was progressively more common with an increase in LVEF and was associated with signs and symptoms of HF regardless of LVEF subtype. Compared with sinus rhythm, AF was associated with worse long‐term cardiovascular outcomes across the LVEF subtypes. 12
Regional differences were observed in the proportion of patients with AF who were diagnosed with comorbid HF, with the highest proportion in the Middle East/South Africa and the lowest in North America. Therefore, the low incidence of ACE inhibitors or ARBs and the high incidence of digitalis could represent local strategies in different parts of the world and how patients were treated. The observed differences in patient characteristics could result from differences in other patient characteristics (e.g. regional differences in age could explain differences in the prevalence or treatment of HF) that were not explored in this analysis.
Regarding outcomes, this subgroup analysis of the 2‐year outcomes of dabigatran in Phase II of GLORIA‐AF 30 is the first prospective analysis to evaluate the effect of HF on outcomes over 2 years of follow‐up when dabigatran is used according to local labels, providing additional insights into newly diagnosed patients with AF who have a diagnosis of comorbid HF. The roughly similar rates of stroke and major bleeding with dabigatran use between patients with and without HF provide important data from clinical practice settings on the long‐term safety and effectiveness of dabigatran in HF patients. These patients were more likely to have existing coronary artery disease, a history of MI, and be at a higher risk of stroke. In addition, the burden of AF was greater in patients with comorbid HF, as they had symptomatic and persistent AF more frequently compared with those without HF, suggesting that HF in patients with AF represents a more burdensome form of AF. As expected, the use of antihypertensive, anti‐HF, and antiarrhythmic medications was higher among patients with HF. It is therefore unsurprising that higher standardized rates of mortality and MI were observed in dabigatran‐treated patients with AF with vs. without HF.
Clinical practice data regarding outcomes with dabigatran in patients with HF were previously only available based on retrospective analyses (e.g. database or health claims analyses). In an analysis of elderly Medicare beneficiaries with AF, propensity score‐matched incidence rates of ischaemic stroke and major bleeds for dabigatran‐treated patients were 1.13 and 4.27 per 100 py, respectively. 31 An analysis of two health insurance databases reported propensity score‐matched incidence rates in dabigatran‐treated patients of 0.77 and 4.42 per 100 py for ischaemic stroke and major bleeds, respectively. 32 Additionally, in a study of dabigatran‐treated patients in the US Department of Defence claims database, propensity score‐matched incidence rates of ischaemic stroke and major bleeds were 0.92 and 3.08 per 100 py, respectively. 33 Our data presented here from a prospective, observational study design, in general, support these findings, despite differences in study type and patient populations. 31 , 32 , 33
There are limitations to the study. First, due to the limited number of events observed, comparisons of outcomes of patients with and without HF can only be adjusted for a limited number of potential confounders. We focused on stroke and bleeding risk scores because they are key predictors of outcomes and aggregate multiple other variables (such as age and hypertension). Residual confounding remains possible. Second, the follow‐up included only dabigatran‐treated patients in Phase II of the GLORIA‐AF study. Consequently, no direct comparisons can be made with other anticoagulants. However, data can be put into perspective by using results from other non‐interventional and interventional studies. Indirect comparisons with other studies are limited by the presence of differences in setting (e.g. country), patient baseline characteristics, and study design. Importantly, in the ongoing Phase III GLORIA‐AF global registry programme, follow‐up data will be collected from VKA‐treated patients, allowing for comparison of safety and effectiveness. 21 In this study, HF was diagnosed at baseline based on functional classes II–IV or ejection fraction < 40%, and follow‐up was performed according to the initial diagnosis. Therefore, there was potential for patients with HF at baseline and an ejection fraction of ≥ 40% to be misclassified. Finally, this study compared patients with a CHA2DS2‐VASc score ≥ 1 with and without HF. As HF is a component of CHA2DS2‐VASc, patients with HF may have a score of ≤ 9 while those without HF can only have a score of ≤ 8; thus, differences in CHA2DS2‐VASc scores cannot be interpreted. Differences in the prevalence of risk components for patients without HF could be distorted as patients with HF alone (CHA2DS2‐VASc = 1) could be included in GLORIA‐AF, but patients without HF need to present ≥ 1 additional stroke risk factor. Given that for patients with HF, the proportion of patients with no additional stroke risk factors is limited (6.6%), the analysis is mostly about patients with HF and an additional risk factor (CHA2DS2‐VASc ≥ 2) and patients without HF and an additional risk factor (CHA2DS2‐VASc ≥ 1); as a consequence, the impact on the observed proportions of stroke risk factors should be limited. For the same reason, the standardized incidence rates were standardized by a modified CHA2DS2‐VASc score that excludes HF.
Conclusions
In this analysis of GLORIA‐AF Phase II, patients with AF with comorbid HF were found to have a greater disease burden. In the 2‐year follow‐up of those taking dabigatran, increased rates of mortality were observed compared with patients without comorbid HF. The incidence rates of stroke and major bleeding were roughly similar between the patient groups with and without HF, showing that the long‐term safety and effectiveness of dabigatran in patients with AF extends to those with comorbid HF.
The increased mortality in patients with AF with comorbid HF highlights the clinical importance of managing this growing patient population to reduce outcomes and burden of disease.
Conflict of interest
S.J.D. has received consultancy fees for serving as a steering committee member for Boehringer Ingelheim. He also holds research grants from Abbott (St Jude Medical). M.V.H. has received honoraria for research grants, consultation, and presentations from Actelion, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, GlaxoSmithKline, and Pfizer. H‐C.D. has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from Abbott, Allergan, AstraZeneca, Bayer Vital, Boehringer Ingelheim, Bristol‐Myers Squibb, CoAxia, Corimmun, Covidien, Daiichi Sankyo Pharma, D‐Pharm, Fresenius, GlaxoSmithKline, Janssen‐Cilag, Johnson & Johnson, Knoll, Lilly, Medtronic, Merck Sharp & Dohme Corp., MindFrame, Neurobiological Technologies, Novartis, Novo Nordisk, Paion, Parke‐Davis, Pfizer, Sanofi Aventis, Schering‐Plough, Servier, Solvay, St Jude Medical, Syngis, Talecris, Thrombogenics, WebMD Global, Wyeth, and Yamanouchi. Financial support for research projects was provided by AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Lundbeck, Novartis, Janssen‐Cilag, Sanofi Aventis, Syngis, and Talecris. The Department of Neurology at the University Duisburg‐Essen received research grants from the German Research Council, German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz‐Nixdorf Foundation. He has no ownership interest and does not own stocks in any pharmaceutical company. J.H. has received consulting fees/honoraria or research support from Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo Pharma, Janssen Pharmaceuticals, Johnson & Johnson, Medtronic, Pfizer, and Sanofi Aventis. K.J.R. is an employee of RTI Health Solutions, an independent, non‐profit research organization that does work for government agencies and pharmaceutical companies. C‐S.M. has received consultancy fees/honoraria from Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Bristol‐Myers Squibb, Johnson & Johnson, and Pfizer. E.C‐V. has received consultancy fees from Boehringer Ingelheim. K.Z. and C.T. are employees of Boehringer Ingelheim International GmbH, Ingelheim, Germany. L.R.F. was an employee of Boehringer Ingelheim International GmbH at the time of manuscript writing and is now employed by Sanofi‐Aventis. S.L. is an employee of Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA. M.P. is an employee of Boehringer Ingelheim, Burlington, ON, Canada. G.Y.H.L. has served as a consultant for Bayer/Janssen, Bristol‐Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi Sankyo; and as a speaker for Bayer, Bristol‐Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi Sankyo. No fees are directly received personally. J.B‐K reports no conflict of interest.
Funding
This study was supported by Boehringer Ingelheim.
Information for submission
Article classification(s)
620. Drug therapy/100. Antithrombotic drugs
925. Heart Failure Programmes
1090. Incidence
1170. Ischaemia
1355. Morbidity
1370. Mortality
1650. Pharmacotherapy
1980. Rhythm disturbance/105. Atrial fibrillation
2120. Thromboembolism
2223. Ejection fraction
Acknowledgements
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Parexel during the preparation of this article. The authors would like to thank all the patients who participated in this study.
Dubner, S. J. , Teutsch, C. , Huisman, M. V. , Diener, H.‐C. , Halperin, J. , Rothman, K. J. , Ma, C.‐S. , Chuquiure‐Valenzuela, E. , Bergler‐Klein, J. , Zint, K. , Riou França, L. , Lu, S. , Paquette, M. , and Lip, G. Y. H. (2020) Characteristics and 2‐year outcomes of dabigatran treatment in patients with heart failure and atrial fibrillation: GLORIA‐AF. ESC Heart Failure, 7: 2679–2689. 10.1002/ehf2.12857.
References
- 1. Davis RC, Hobbs FD, Kenkre JE, Roalfe AK, Iles R, Lip GY, Davies MK. Prevalence of atrial fibrillation in the general population and in high‐risk groups: the ECHOES study. Europace 2012; 14: 1553–1559. [DOI] [PubMed] [Google Scholar]
- 2. Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, Piccini JP, Fonarow GC, ORBIT‐AF Investigators and Patients . Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail 2017; 5: 44–52. [DOI] [PubMed] [Google Scholar]
- 3. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016; 133: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 5. Ferreira JP, Santos M. Heart failure and atrial fibrillation: from basic science to clinical practice. Int J Mol Sci 2015; 16: 3133–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lip GY, Heinzel FR, Gaita F, Juanatey JR, Le Heuzey JY, Potpara T, Svendsen JH, Vos MA, Anker SD, Coats AJ, Haverkamp W, Manolis AS, Chung MK, Sanders P, Pieske B, Gorenek B, Lane D, Boriani G, Linde C, Hindricks G, Tsutsui H, Homma S, Brownstein S, Nielsen JC, Lainscak M, Crespo‐Leiro M, Piepoli M, Seferovic P, Savelieva I. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace 2016; 18: 12–36. [DOI] [PubMed] [Google Scholar]
- 7. Savarese G, Giugliano RP, Rosano GM, McMurray J, Magnani G, Filippatos G, Dellegrottaglie S, Lund LH, Trimarco B, Perrone‐Filardi P. Efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation and heart failure: a meta‐analysis. JACC Heart Fail 2016; 4: 870–880. [DOI] [PubMed] [Google Scholar]
- 8. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003; 107: 2920–2925. [DOI] [PubMed] [Google Scholar]
- 9. Agarwal M, Apostolakis S, Lane DA, Lip GY. The impact of heart failure and left ventricular dysfunction in predicting stroke, thromboembolism, and mortality in atrial fibrillation patients: a systematic review. Clin Ther 2014; 36: 1135–1144. [DOI] [PubMed] [Google Scholar]
- 10. Thihalolipavan S, Morin DP. Atrial fibrillation and heart failure: update 2015. Prog Cardiovasc Dis 2015; 58: 126–135. [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18: 1609–1678. [DOI] [PubMed] [Google Scholar]
- 12. Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo‐Leiro MG, Coats AJS, Anker SD, Filippatos G, Seferovic PM, Maggioni AP, De Mora Martin M, Polonski L, Silva‐Cardoso J, Amir O, ESC‐HFA HF Long‐Term Registry Investigators . Prognostic implications of atrial fibrillation in heart failure with reduced, mid‐range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long‐Term Registry. Eur Heart J 2018; 39: 4277–4284. [DOI] [PubMed] [Google Scholar]
- 13. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 14. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 15. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 16. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira J, Ezekowitz MD, Connolly SJ, Brueckmann M, Fraessdorf M, Reilly PA, Yusuf S, Wallentin L, RE‐LY Investigators . Dabigatran compared with warfarin in patients with atrial fibrillation and symptomatic heart failure: a subgroup analysis of the RE‐LY trial. Eur J Heart Fail 2013; 15: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 18. Magnani G, Giugliano RP, Ruff CT, Murphy SA, Nordio F, Metra M, Moccetti T, Mitrovic V, Shi M, Mercuri M, Antman EM, Braunwald E. Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF‐TIMI 48. Eur J Heart Fail 2016; 18: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 19. McMurray JJ, Ezekowitz JA, Lewis BS, Gersh BJ, van Diepen S, Amerena J, Bartunek J, Commerford P, Oh BH, Harjola VP, Al‐Khatib SM, Hanna M, Alexander JH, Lopes RD, Wojdyla DM, Wallentin L, Granger CB, ARISTOTLE Committees and Investigators . Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail 2013; 6: 451–460. [DOI] [PubMed] [Google Scholar]
- 20. van Diepen S, Hellkamp AS, Patel MR, Becker RC, Breithardt G, Hacke W, Halperin JL, Hankey GJ, Nessel CC, Singer DE, Berkowitz SD, Califf RM, Fox KA, Mahaffey KW. Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: insights from ROCKET AF. Circ Heart Fail 2013; 6: 740–747. [DOI] [PubMed] [Google Scholar]
- 21. Huisman MV, Lip GY, Diener HC, Dubner SJ, Halperin JL, Ma CS, Rothman KJ, Teutsch C, Zint K, Ackermann D, Clemens A, Bartels DB. Design and rationale of global registry on long‐term oral antithrombotic treatment in patients with atrial fibrillation: a global registry program on long‐term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J 2014; 167: 329–334. [DOI] [PubMed] [Google Scholar]
- 22. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 23. Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. Am Stat 1986; 40: 249–251. [Google Scholar]
- 24. Berglund P, Heeringa S. Multiple Imputation of Missing Data Using SAS. Cary, North Carolina, USA: SAS Institute Inc.; 2014. [Google Scholar]
- 25. Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med 2018; 37: 2252–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu CY, Wang CY, Li JY, Ma J, Li ZQ. Relationship between atrial fibrillation and heart failure. Eur Rev Med Pharmacol Sci 2016; 20: 4593–4600. [PubMed] [Google Scholar]
- 27. An Y, Ogawa H, Esato M, Ishii M, Iguchi M, Masunaga N, Aono Y, Ikeda S, Doi K, Tsuji H, Wada H, Hasegawa K, Abe M, Lip GYH, Akao M, Fushimi AFRI. Age‐dependent prognostic impact of paroxysmal versus sustained atrial fibrillation on the incidence of cardiac death and heart failure hospitalization (the Fushimi AF Registry). Am J Cardiol 2019; 124: 1420–1429. [DOI] [PubMed] [Google Scholar]
- 28. Lip GY, Laroche C, Popescu MI, Rasmussen LH, Vitali‐Serdoz L, Dan GA, Kalarus Z, Crijns HJ, Oliveira MM, Tavazzi L, Maggioni AP, Boriani G. Heart failure in patients with atrial fibrillation in Europe: a report from the EURObservational Research Programme Pilot survey on atrial fibrillation. Eur J Heart Fail 2015; 17: 570–582. [DOI] [PubMed] [Google Scholar]
- 29. Mazurek M, Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, Halperin JL, Ma CS, Zint K, Elsaesser A, Lu S, Lip GYH, Gloria‐AF Investigators . Regional differences in antithrombotic treatment for atrial fibrillation: insights from the GLORIA‐AF phase II registry. Thromb Haemost 2017; 117: 2376–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazurek M, Teutsch C, Diener HC, Dubner SJ, Halperin JL, Ma CS, Rothman KJ, Paquette M, Zint K, Franca LR, Lu S, Bartels DB, Huisman MV, Lip GYH, GLORIA‐AF Investigators . Safety and effectiveness of dabigatran at 2 years: Final outcomes from Phase II of the GLORIA‐AF registry program. Am Heart J 2019; 218: 123–127. [DOI] [PubMed] [Google Scholar]
- 31. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015; 131: 157–164. [DOI] [PubMed] [Google Scholar]
- 32. Seeger JD, Bykov K, Bartels DB, Huybrechts K, Zint K, Schneeweiss S. Safety and effectiveness of dabigatran and warfarin in routine care of patients with atrial fibrillation. Thromb Haemost 2015; 114: 1277–1289. [DOI] [PubMed] [Google Scholar]
- 33. Villines TC, Schnee J, Fraeman K, Siu K, Reynolds MW, Collins J, Schwartzman E. A comparison of the safety and effectiveness of dabigatran and warfarin in non‐valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost 2015; 114: 1290–1298. [DOI] [PubMed] [Google Scholar]


