Abstract
Aims
Atrial fibrillation (AF) and heart failure (HF) are the most common cardiac diseases and often coexist leading to increased mortality and morbidity compared with AF patients without HF. As shown previously, AF ablation using radio frequency (RF) in HF patients leads to a reduction of AF burden, an increase of left ventricular ejection fraction (LVEF) and consequently to reduced hospitalization and mortality. Previous AF ablation studies on HF patients have been liberal about additional targets beyond pulmonary vein isolation (PVI). Thus, the aim of this study was to assess systematically the impact of a straightforward PVI‐only strategy on LVEF, NYHA functional class, and cardiovascular hospitalization rate in HF patients.
Methods and results
Out of 414 consecutive patients undergoing PVI, only with the cryoballoon 113 patients with reduced LVEF [mean: 38.4 ± 10.8%, reduced ejection fraction (rEF) group] and 301 patients with normal LVEF (>55%) at baseline were identified [normal ejection fraction (nEF) group]. Remarkably, even though freedom from arrhythmia recurrence after 1 year was significantly lower in the rEF group (64.9%) compared with the nEF group (71.2%, P = 0.036), mean LVEF improved from 38.4 ± 10.8% to 52.5 ± 17.2% (P < 0.001) after cryoballoon ablation in the rEF group. Accordingly, HF‐related symptoms as well as hospitalization rate declined significantly in the rEF group during follow‐up compared with baseline.
Conclusions
The results of the present study suggest that catheter ablation restricted to a straightforward PVI‐only strategy using the cryoballoon leads to improved left ventricular ejection fraction as well as improvement of NYHA functional class and increased freedom from cardiovascular rehospitalization.
Keywords: Atrial fibrillation, Heart failure, Cryoballoon, Left ventricular systolic function, Hospitalization, Pulmonary vein isolation
Introduction
Mortality and morbidity are significantly increased in atrial fibrillation (AF) patients with reduced left ventricular ejection fraction (LVEF) compared with AF patients with normal LVEF. 1 , 2 As shown in the CASTLE‐AF trial, AF ablation by radio frequency (RF) current in heart failure (HF) patients leads to a significant reduction of AF burden, an increase of LVEF and consequently to a reduction of HF‐related hospitalization and mortality. As in similar previous trials in the CASTLE‐AF trial ablation was not limited to PVI, 3 , 4 , 5 instead additional ablation targets such as left atrial lines and ablation of complex fractionated atrial electrograms (CFAE) were left to the operator's discretion. 6 The impact of PVI only on LVEF has not been analysed systematically in HF patients so far.
Cryoballoon pulmonary vein isolation (PVI) has emerged as an effective therapy to treat symptomatic AF. 7 , 8 , 9 As shown in the FIRE and ICE trial, advantages of cryoballoon PVI include significantly shorter procedure duration, reduction in all‐cause and cardiovascular (CV) rehospitalizations, and reduction in repeat ablations and electrical cardioversions compared with RF catheter ablation. 10 Nevertheless, cryoballoon PVI does not routinely enable ablation of extrapulmonary vein targets potentially limiting effectiveness of this approach in specific patient population such as patients with persistent AF or HF patients in which atrial substrate is considered to play a more pronounced role in AF pathogenesis compared with paroxysmal AF patients without HF. 11 , 12
Current guidelines clearly recommend considering AF ablation in HF patients; however, no explicit recommendations regarding the optimal ablational strategy is provided, because evidence for this issue, in particular for the cryoballoon, is sparse. 13 , 14 , 15 Thus, the aim of our study was to systematically assess procedural parameters as well as clinical outcome, especially left ventricular systolic function, HF‐related symptoms, recurrence rate, and rate of annual CV hospitalization, in HF patients undergoing PVI with the cryoballoon.
Methods
Study population
Four hundred fourteen consecutive patients, which successfully underwent first‐time PVI for the treatment of symptomatic paroxysmal or persistent AF at Ulm University Electrophysiology Center between January 2013 and November 2017, were included retrospectively into our study (Figure 1 ). All the 414 patients had a clinical follow‐up >90 days, and an LVEF assessment before and after index procedure. Left ventricular systolic function was determined by echocardiography, cardiac MRI, or cardiac ventriculography. LVEF was routinely quantified by 2D transthoracic echocardiography or cardiac MRI. Patients with reduced LVEF <55% at baseline were assigned to the reduced ejection fraction (rEF) group, whereas patients with unaffected LVEF (≥55%) were assigned to the normal ejection fraction (nEF) group. Patients of the rEF group were stratified in three subgroups according to their LVEF (mild LVEF reduction: 45–54%; moderate LVEF reduction: 35–44%; and severe LVEF reduction: <35%). Patients with LVEF improvement during clinical follow‐up in the context of specific HF therapy, in particular myocardial revascularization, intensified HF medication (defined as increase in dosage to the therapeutic level or initiation of a new HF drug) according to the current guidelines and/or CRT implantation were excluded from our study, 1 because in these patients, it is impossible to attribute LVEF improvement to either AF ablation or specific HF therapy. The study complies with the Declaration of Helsinki and was approved by the local ethics committee. All patients gave written informed consent prior ablation.
Figure 1.
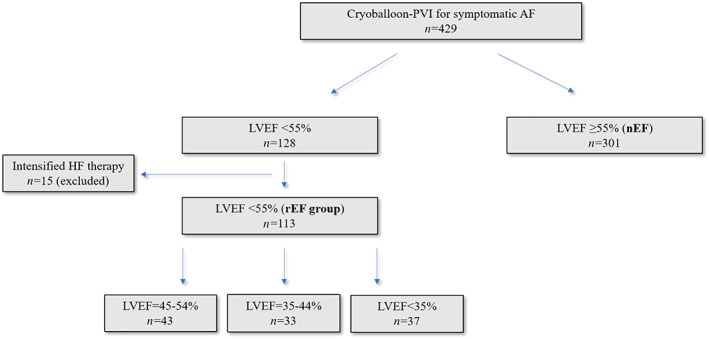
Flowchart showing distribution of study patients with and without reduced left ventricular ejection fraction (rEF group). Included patients (n = 414) had a clinical follow‐up >90 days and LVEF assessment before and after cryoballoon PVI. Patients with modified heart failure therapy after index procedure, namely, revascularization, cardiac resynchronization, or intensified drug therapy were excluded from our study (n = 15).
Aim of the study
The aim of the study was to compare LVEF, NYHA‐classification, and CV hospitalization rate before cryoballoon PVI and during clinical follow‐up in patients with reduced LVEF as well as to compare procedural parameters, EHRA score and atrial arrhythmia recurrence 12 months after the index procedure off class I or class III antiarrhythmic drugs (AAD) between patients with reduced LVEF and patients with normal LVEF.
Preprocedural management and cryoballoon ablation procedure
Preprocedural management was performed as described before. 9 Briefly, left atrial thrombus was ruled out by transesophageal echocardiography in all patients prior to PVI. The cryoballoon procedure was guided by fluoroscopy only, no additional pre‐procedural or intra‐procedural imaging such as CT, MRI, or intracardiac echocardiography was applied. Vitamin K antagonists (VKAs) were administered uninterruptedly to a target INR of 2.0–2.5 at the time of the procedure. Patients treated with non‐VKA oral anticoagulants (NOACs) were advised to hold their anticoagulant 24 h prior to the ablation procedure.
The cryoballoon ablation procedure was performed under deep sedation. A 10‐polar diagnostic catheter was placed in the coronary sinus (cs). The cryoballoon was advanced to the LA via a 12F steerable sheeth (Flexcath Advance, Medtronic, Minneapolis, MN) after single transseptal puncture and inflated at the pulmonary vein (PV) ostia. Instead of a guidewire, a spiral mapping catheter (20 mm Achieve or 25 mm Achieve Advance, Medtronic, Minneapolis, MN) was advanced through the balloon inner lumen and positioned in the PV at the closest achievable proximity to the cryoballoon in order to record real‐time PV potentials during PV isolation. PV occlusion was documented by injection of contrast medium. During PV isolation, the potentials from the PV were recorded. The time to isolation (TTI) was defined as the time of the last recording of a PV potential before sustained isolation.
In the first 178 patients, freeze cycles of 240 s were administered at each PV until acute PVI could be documented by entrance block and exit block. A single 240 s bonus freeze was applied at each PV thereafter. In the last 236 patients, freeze duration was modified depending on the observed TTI: In case of a TTI <30 s, the total freeze cycle duration was decreased to 120 s, and no bonus freeze was applied. If TTI was between 30 and 60 s, a single 180 s freeze cycle was applied. If TTI was >60 s, a 180 s freeze cycle followed by a 180 s bonus freeze was applied. If no TTI could be documented, but isolation was achieved, a single 180 s freeze cycle was applied. PV isolation was reassessed at the end of the procedure by documentation of entrance block and exit block. Oesophageal temperature was monitored by a transnasally introduced temperature probe (Sensitherm; St. Jude Medical Inc, St Paul, MN or Circa; Circa Scientific Inc., Englewood, CO) in all patients. Cryoablation was prematurely terminated if luminal oesophageal temperature (LET) decreased below 15°C in the first 218 patients. According to the guidelines, LET cut‐off value was increased to 20°C in the last 196 patients. Phrenic nerve function was monitored by phrenic nerve stimulation via the diagnostic catheter placed in the superior vena cava and palpation of the right‐sided diaphragm and with additional recording of compound motor action potentials (CMAP) of the right sided diaphragm by surface ECG. 16 , 17 A decrease in CMAP potentials or weakening of palpable diaphragm contractions led to immediate termination of the freeze cycle.
Postprocedural management and clinical follow‐up
Echocardiography was performed in every patient immediately after the procedure and before hospital discharge to rule out pericardial tamponade or pericardial effusion. Oral anticoagulation was resumed on the day of the ablation procedure. Patients were scheduled for outpatient clinic visits including clinical assessment, echocardiography, 12‐lead rest‐ECG, and 7‐day‐Holter‐monitoring at 1, 3, and 6 months after the procedure and thereafter every 6 months. For LVEF reevaluation we analysed the last available LVEF reassessment after index procedure. Individual LVEF improvement was defined as 20% relative LVEF augmentation during follow‐up compared with baseline. CV hospitalization 12 months before and after index procedure was defined as any unplanned hospital admission because of the following international classification of diagnoses (ICD‐10): I10–I15 hypertensive diseases; I20–I25 ischaemic heart diseases; I26–I28 pulmonary heart disease and diseases of pulmonary circulation; I30–I52 other forms of heart disease; I60–I69 cerebrovascular diseases; I70–I79 diseases of arteries, arterioles, and capillaries; I80–I89 diseases of veins, lymphatic vessels, and lymph nodes, not elsewhere classified; I95–I99 other and unspecified disorders of the circulatory system; R55 syncope and collapse.
Any documented sustained atrial arrhythmia on 12‐lead rest ECG or any atrial arrhythmia [AF and atrial tachycardia (AT)] of ≥30 s on Holter ECG was counted as AT/AF recurrence. Initiation of class I or class III AAD or failure to hold them after the 3 months blanking period was also counted as arrhythmia recurrence.
Statistical analysis
Significance of differences of numeric values between the two groups was calculated by t‐test if normal distribution with equal variance was given. Normal distribution was determined by Shapiro–Wilk test and equal variance by Brown–Forsythe test. Numeric variables that were not normally distributed were analysed by Mann–Whitney rank sum test. Categorical variables were analysed by χ 2 test or Fisher's exact test. For identification of independent predictors of LVEF improvement, we analysed baseline characteristics (age, gender, and co‐morbidities) as well as echocardiographic parameters [LVEF, left ventricular systolic diameter (LVsD) and left ventricular diastolic diameter (LVdD), tricuspid annular plane systolic excursion (TAPSE), and left atrial diameter (LAD)] by univariate logistic regression. Parameters with P ≤ 0.2 were further tested for independency by multivariate logistic regression. Survival analysis was performed by Kaplan–Meier and log‐rank test. A P‐value <0.05 was considered significant. Statistical assessment was performed with Excel (Version 2016, Microsoft Inc., Redmond, WA) or XLStat software (V 2016.02.28430, Addinsoft, New York, NY).
Results
Characteristics of the study population at baseline
Between January 2013 and November 2017, in 429 AF patients PVI, using the cryoballoon was performed at Ulm University Medical Center. Of these 429 patients, 15 AF patients with reduced LVEF and intensified HF therapy, including modified drug therapy, myocardial revascularization, or cardiac resynchronization, were excluded from our study. Thus, study cohort consists of a total of 414 individuals with a clinical follow‐up and LVEF assessment before and after index procedure; 301/414 (72.7%) had normal LVEF (nEF group), whereas 113/414 patients (27.3%) presented with reduced LVEF <55% (rEF group, Figure 1 ). Baseline characteristics for both groups are shown in Table 1 . Data on HF medication at baseline and at final assessment for the rEF patients are presented in Table S1 . As expected, patients in the rEF group are characterized by significantly more co‐morbidities, higher CHA2DS2Vasc‐Score, larger LA size, history of myocardial infarction, and higher proportion of male patients reflecting higher prevalence of reduced LVEF in men. (Table 1 ).
Table 1.
Baseline characteristics
| Baseline characteristics | rEF group | nEF group | P‐value |
|---|---|---|---|
| Patients [n (%)] | 113 (27.3%) | 301 (72.7%) | |
| Gender, male [n (%)] | 73 (64.6%) | 150 (49,8%) | 0.007 |
| Age [years ± SD (median)] | 67.6 ± 10.2 (69.0) | 65.9 ± 10.6 (68.0) | 0.149 |
| BMI [kg/m2 ± SD (median)] | 27.8 ± 5.0 (27.5) | 29.2 ± 5.7 (28.1) | 0.032 |
| CHA2DS2Vasc [mean ± SD (median)] | 3.6 ± 1.6 (4.0) | 2.6 ± 1.5 (3.0) | <0.001 |
| Persistent AF [n (%)] | 62 (54.9%) | 75 (24.9%) | <0.001 |
| Left atrial diameter [mm ± SD (median)] | 46.3 ± 11.0 (48.0) | 42.7 ± 10.7 (44.0) | <0.001 |
| LVEF [mean ± SD (median)] | 38.4 ± 10.8 (39.0) | 67.1 ± 7.6 (67.0) | <0.001 |
| LVEF [n (%)] | |||
| ≥55% | 0 (0.0%) | 301 (100.0%) | |
| 45–54% | 43 (38.1%) | ||
| 35–44% | 33 (29.2%) | ||
| <35% | 37 (32.7%) | ||
| Non‐ischemic cardiomyopathy | 55 (48.7%) | ||
| Coronary artery disease [n (%)] | 58 (51.3%) | 100 (33.2%) | 0.001 |
| Myocardial infarction [n (%)] | 17 (15.0%) | 23 (7.6%) | 0.023 |
| Hypertension [n (%)] | 99 (87.6%) | 235 (78.1%) | 0.029 |
| Diabetes mellitus [n (%)] | 35 (31.0%) | 42 (14.0%) | <0.001 |
| Hyperlipidaemia [n (%)] | 73 (64.6%) | 181 (60.3%) | 0.427 |
| Obstructive sleep apnoea [n (%)] | 12 (10.6%) | 28 (9.3%) | 0.686 |
Procedural data
In 414 included patients, we identified a total of 1639 PV with 452 PV in the rEF group and 1187 PV in the nEF group. PVI was successful in 1639/1639 (100%) PV without additional RF ablation to achieve complete PV isolation. Interestingly, relevant procedural parameters such as procedure duration, fluoroscopy time, number of freezes per patient, or complication rate were statistically not different. Also, after stratification of the rEF group according to the grade of LVEF reduction, procedural parameters did not differ in comparison with the nEF group (Table 2 ). Similarly, mean TTI, PV isolation with the first freeze, or incidence of prematurely aborted freezes were comparable for both study groups.
Table 2.
Procedural data
| Procedural data | rEF group | nEF group | P‐value |
|---|---|---|---|
| Procedure duration[mean ± SD (median) (min)] | 98.3 ± 31.6 (95.0) | 103.7 ± 37.0 (100.0) | 0.358 |
| LVEF reduction: mild | 93.9 ± 30.9 (91.0) | 0.155 | |
| moderate | 102.4 ± 33.5 (102.0) | 0.998 | |
| severe | 99.7 ± 30.8 (98.0) | 0.819 | |
| Fluoroscopy time [mean ± SD (median) (min)] | 20.6 ± 9.7 (19.0) | 21.2 ± 10.2 (18.3) | 0.883 |
| LVEF reduction: mild | 19.6 ± 7.2 (19.1) | 0.717 | |
| moderate | 22.0 ± 10.9 (21.4) | 0.526 | |
| severe | 20.7 ± 11.0 (17.4) | 0.622 | |
| No. of Ablations per patient [mean ± SD (median)] | 7.0 ± 2.3 (7.0) | 7.2 ± 2.3(8.0) | 0.248 |
| LVEF reduction: mild | 7.0 ± 2.1 (7.0) | 0.517 | |
| moderate | 7.3 ± 2.4 (7.0) | 0.904 | |
| severe | 6.7 ± 2.4 (6.0) | 0.157 | |
| Isolation with 1. Freeze [n/total n (%)] | 371/452 (82.1%) | 959/1187 (80.8%) | 0.551 |
| LVEF reduction: mild | 145/173 (83.8%) | 0.342 | |
| moderate | 104/132 (78.8%) | 0.581 | |
| severe | 122/147 (83.0%) | 0.521 | |
| Mean TTI [mean ± SD (median) (sec.)]: | |||
| LSPV | 51.0 ± 23.1 (48.0) | 49.0 ± 25.3 (42.0) | 0.183 |
| LIPV | 40.5 ± 21.6 (35.0) | 38.3 ± 22.0 (32.0) | 0.259 |
| LCPV | none | 42.0 ± 20.5 (41.5) | |
| RSPV | 42.8 ± 20.6 (36.0) | 39.4 ± 22.5 (34.0) | 0.103 |
| RIPV | 47.6 ± 26.3 (44.0) | 46.2 ± 25.7 (39.0) | 0.552 |
| RMPV | 32.5 ± 12.0 (32.5) | none | |
| Aborted freezes [n/total n (%)] | 63/784 (8.0%) | 179/2157 (8.3%) | 0.819 |
| Complications [n (%)]: | 10/113 (9.7%) | 24/301 (7.8%) | 0.772 |
| Pers. Phrenicus nerve palsy | 3 (2.7%) | 3 (1.0%) | 0.209 |
| Pericardial effusion | 0 (0%) | 4 (1.3%) | 0.578 |
| Vascular access | 3 (2.7%) | 14 (4.7%) | 0.362 |
| Stroke | 0 (0%) | 1 (0.3%) | 1.000 |
| Bleeding | 4 (3.5%) | 2 (0.7%) | 0.050 |
Clinical outcome
The mean duration of the clinical follow‐up in the rEF group was 1.6 ± 1.2 years (median: 1.2 years) and 1.8 ± 1.2 (median: 1.6; P = 0.052) in the nEF group after index procedure. 5/113 patients (4.4%) in the rEF group and 1/301 (0.3%) patients in the nEF group died during follow‐up. Data on heart rate, blood pressure, and HF medication before and after ablation can be found in Table S2 .
Left ventricular ejection fraction
Even though patients with extended HF therapy after cryoballoon PVI were excluded from our study, mean LVEF in the rEF group significantly increased from 38.4 ± 10.8% before cryoballoon PVI to 52.5 ± 17.2% (P < 0.001, Figure 2 A ) after index procedure. Thus, absolute LVEF improvement in the rEF group was 14.1% during follow‐up. Out of 113 patients in the rEF group, a total of 57 (50.4%) patients experienced complete LVEF reconstitution (Figure 2 B ). Subgroup analysis revealed that 34/43 (79.1%, P < 0.001) patients with initially mild LVEF reduction and 15/33 (45.5%; P < 0.001) patients with initially moderate LVEF reduction experienced complete reconstitution after ablation. Also, in patients with severely reduced LVEF, 8/37 (21.6%; P = 0.005) patients presented with normal LVEF during further follow‐up.
Figure 2.
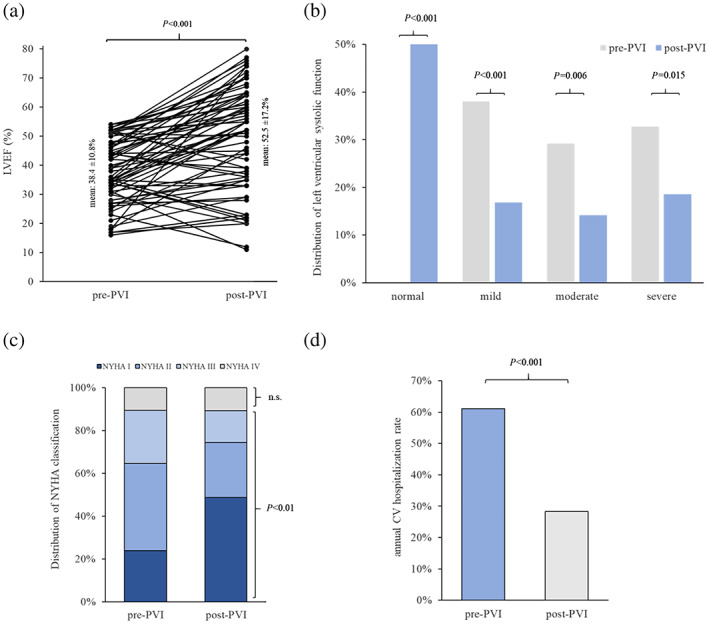
(A) Ladder plot of cardiac systolic pump function comparing quantitative LVEF before and after index procedure. Mean LVEF increased from 38.4 ± 10.8% to 52.5 ± 17.2% after cryoballoon PVI. (B) Distribution of patients with reduced LVEF before and after index procedure. In every subgroup, prevalence of systolic dysfunction is significantly decreased compared with baseline. (C) Significantly more patients had heart failure related symptoms classified as NYHA I after PVI than before AF ablation. Distribution of patients with NYHA II and III decreased significantly after cryoballoon PVI. (D) 12 months after procedure 28.2% patients were hospitalized because of cardiovascular reasons, whereas annual CV hospitalization rate was 61.1% before ablation (P < 0.01).
As shown in Figure 1 B , severe LVEF reduction was observed in 37/113 (32.7%) patients before index procedure compared with 21/113 (18.5%) patients after ablation (P = 0.015). Similarly, prevalence of patients with moderate LVEF reduction was significantly lower after PVI with the cryoballoon [16/113 (14.2%)] compared with the incidence of moderate LVEF reduction at baseline [33/113 (29.2%); P = 0.006]. Also, mild LVEF reduction was diagnosed less frequently after PVI than at baseline [baseline: 43/113 (38.1%) vs. follow‐up: 19/113 (16.8%); P < 0.001].
To identify independent predictors for LVEF improvement, we performed univariate as well as multivariate logistic regression analysis of relevant baseline characteristics and echocardiographic parameters (Table 3 ). Interestingly, we found that pulmonary hypertension (PH) measured via 2D echocardiography and/or via cardiac catheterization at baseline is the only independent and strong predictor for LVEF non‐improvement with an odds ratio of 0.15 (95% CI: 0.041–0.540, P = 0.004) in PH patients. Remarkably, severity of LVEF reduction did not have an influence on LVEF improvement after ablation rendered by univariate regression analysis (P = 0.672).
Table 3.
Predictors of LVEF non‐improvment
| Logistic regression | Univariate | Multivariate | ||
|---|---|---|---|---|
| Variable | OR (95% CI) | P‐value | OR (95% CI) | P‐value |
| Left atrial diameter | 0.95 (0.90–1.01) | 0.09 | 1.01 (0.92–1.11) | 0.77 |
| Age | 0.99 (0.95–1.03) | 0.52 | ||
| CHA2DS2‐VASc‐Score | 0.91 (0.72–1.14) | 0.40 | ||
| Sex | 0.91 (0.42–1.98 | 0.82 | ||
| Persistent AF | 1.44 (0.68–3.04) | 0.34 | ||
| Hypertension | 0.42 (0.13–1.45 | 0.17 | 0.55 (0.11–2.89) | 0.48 |
| Diabetes | 1.69 (0.75–3.83) | 0.20 | 0.49 (0.16–1.50) | 0.21 |
| LVEF | 0.99 (0.95–1.03) | 0.67 | ||
| Non‐ischemic cardiomyopathy | 0.94 (0.43–2.03) | 0.87 | ||
| Mitral regurgitation | 0.87 (0.58–1.30) | 0.49 | ||
| LVdD | 1.00 (0.95–1.04) | 0.83 | ||
| LVsD | 0.99 (0.95–1.03) | 0.55 | ||
| Recurrence of AF/AT | 0.58 (0.27–1.26) | 0.17 | 0.93 (0.29–2.98) | 0.90 |
| TAPSE | 1.02 (0.93–1.12) | 0.66 | ||
| Pulmonary Hypertension | 0.29 (0.11–0.77) | 0.01 | 0.15 (0.04–0.54) | <0.001 |
NYHA classification and cardiovascular hospitalization
To study the impact of cryoballoon PVI on clinical symptoms in patients with abnormal LVEF, we compared NYHA classification of the rEF group before and after index procedure. We found that 86/113 (76.1%) patients in the rEF group had HF‐related symptoms that were classified as NYHA II or higher (Figure 2 C ); 28/113 (24.8%) patients were classified as NYHA III and 12/113 (10.6%) as NYHA IV before index procedure. By contrast, after cryoballoon PVI only 58/113 (51.3%) patients had symptoms classified as NYHA II or higher (P < 0.001). Also, significantly fewer patients presented with symptoms classified as NYHA III [17/113 (15.0%, P < 0.001)]. Proportion of patients suffering from HF symptoms according to class IV did not differ before and after index procedure (pre‐PVI: 10.6% vs. post‐PVI: 10.6%, P = 1.0).
In order to evaluate whether reduction of HF‐related symptoms in the rEF group translates into decreased CV hospitalizations, we compared the annual CV hospitalization rate before and after ablation (Figure 2 D ). Remarkably, in the first year after procedure, 32/113 (28.2%) patients experienced unplanned hospital readmission. In contrast, unplanned CV hospitalization rate was 61.1% (69/113 patients) 12 months before ablation. Hence, CV hospitalization rate was reduced by 53.8% (P < 0.001) in HF patients with AF in the first year after cryoballoon PVI.
Arrhythmia recurrence and EHRA symptom score
After a blanking period of 3 months following ablation, 51/113 (45.1%) patients of the rEF group experienced atrial arrhythmia recurrence compared with 107/301 (35.5%) patients of the nEF group. In the rEF group, 17/51 (33.3%) patients with AF recurrence underwent a second AF ablation procedure. In 15/17 (88.2%) patients with AT/AF recurrence reconnected PV were re‐isolated. In 2/17 (11.8%) patients, all PV were still isolated. In only two patients of the rEF group CFAE as well as introduction of a roof line were performed.
The Kaplan–Meier estimated freedom from atrial arrhythmia 12 months after the procedure off AAD was 64.9% in the rEF group and 71.2% in the nEF group; 2 years after ablation the Kaplan–Meier estimated freedom from AT/AF recurrence rate was 45.1 in the rEF group and 60.8% in the nEF group (log‐rank P = 0.036, Figure 3 ). Despite significant higher AT/AF recurrence rate in the rEF group, mean EHRA score of 1.5 ± 0.8 was statistically not different compared with 1.5 ± 0.8 in the nEF group (P = 0.285).
Figure 3.
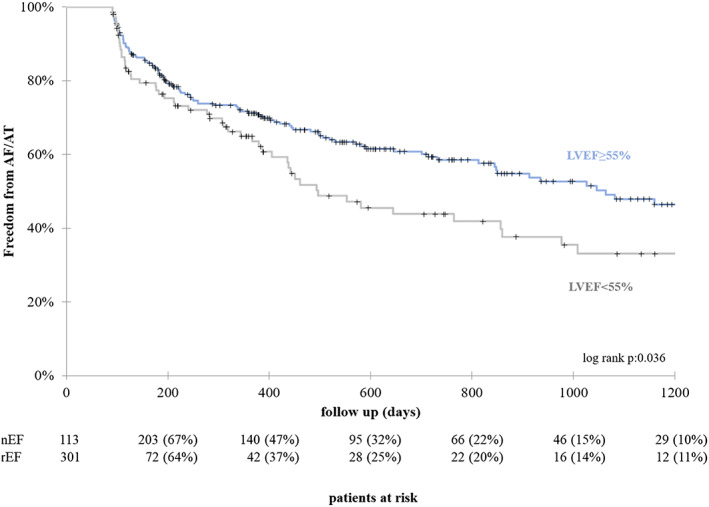
Kaplan–Meier survival curve: 1 year after index procedure freedom from AT/AF off ADD is 64.9% in the rEF group compared with 71.2% in the nEF group. Freedom from AT/AF after 2 years is 42.9% in the rEF group compared with 60.8% in the nEF group (P = 0.036).
Discussion
Several studies have demonstrated a clinical as well as a prognostic benefit of an ablation procedure in HF patients with concomitant AF. 18 , 19 However, in these studies, AF ablation was performed by RF current and besides PVI additional ablation targets such as left atrial lines and ablation of CFAE were applied. 6
In contrast, we here assessed for the first‐time systematically the impact of a PVI‐only strategy with the cryoballoon on procedural parameters and on clinical outcome in patients with abnormal systolic function. 20 , 21 , 22 Also, contrary to previous trials, we included patients with “mid range systolic” dysfunction in our study as recommended by the current guidelines for the management of HF patients. According to these guidelines, physician and clinical scientists are encouraged to study patients with mild systolic dysfunction more in detail with the aim to identify underlying characteristics, pathophysiology, and treatment strategies of this special population. 1 This is especially interesting considering that in a subgroup analysis of the CASTLE‐AF trial patients with LVEF ≥25% profited from ablation, while patients with LVEF <25% did not. Because study design limited inclusion to patients with an LVEF <35%, patients with mid range systolic dysfunction have not been assessed.
Interestingly, we found that in the rEF group, essential procedural parameters of the cryoballoon ablation procedure including acute PV isolation, procedure duration, fluoroscopy time, and complication rate did not differ compared with patients without structural heart disease and less co‐morbidities, indicating that cryoballoon PVI procedure is feasible even in patients with severely reduced LVEF. With regard to outcome parameters, we found a significant LVEF improvement in the entire rEF group as well as in the respective subgroups stratified by LVEF reduction during follow‐up. Hence, in more than half of the patients in the rEF group and even in more than one third of patients with initially severe systolic dysfunction LVEF normalized after ablation. The mechanism how elimination of AF or at least reduction of AF burden leads to improvement or even normalization of LVEF can only be speculated on. We find a decrease in resting heart rate at follow‐up compared with baseline in the rEF patients despite reduction of beta‐blocker usage. Whether this decrease in resting heart rate alone or restoration of sinus rhythm with reinstallation of atrioventricular synchrony and atrial contraction mediated increased ventricular filling or molecular atrial and ventricular remodelling is responsible for LVEF improvement cannot be deducted from our data. Nevertheless, the extend of LVEF improvement in our study is similar to that observed in previous studies. 6 , 18
On the search for an independent predictor for LVEF development after ablation, we analysed patient‐specific baseline characteristics such as LAD, age, sex, LVEF, mitral regurgitation, diabetes, type of AF, arrhythmia recurrence, and PH. Remarkably, we found that only in PH patients probability of LVEF improvement during follow‐up was significantly reduced.
Congruously, several HF studies have demonstrated that PH is a direct consequence of left ventricular systolic dysfunction and is an ominous clinical condition leading to tremendously increased morbidity and mortality. 23 , 24 Often, secondary PH is diagnosed in patients with extremely severe or‐long‐lasting cardiac systolic dysfunction. Hence, PH can also be considered as a reliable hemodynamic risk marker to estimate severity as well as reversibility of systolic HF.
Interestingly, we found in our study that severely reduced LVEF without PH is not associated with lower probability of LVEF recovery. One might speculate that in contrast to HFrEF patients without PH HFrEF patients with PH can be considered as “endstage” HF patients in our study population with a complete loss of compensatory mechanisms to avoid hemodynamic congestion of the blood flow from the left ventricle to the pulmonary vessels. According to our results HF patients should be considered for AF ablation rather in an early stage of HF than to allow progression of systolic dysfunction and development of secondary PH.
It is well known that, co‐morbidities such as diabetes and arterial hypertension are clearly associated with worsening of HF during patients' further clinical course. 1 Indeed, these co‐morbidities, show a trend towards LVEF non‐improvement in our univariate analysis. However, they are not identified as independent predictors in our multivariate analysis. Interestingly, recurrence of AT/AF, persistent AF, and LA enlargement also showed a trend in the univariate regression model, but they were not independent predictors of LVEF non‐improvement. It is widely accepted, that persistent AF patients and LA enlargement are associated with higher arrhythmia recurrence rates, 25 which might explain why they are not independent predictors in the multivariate regression analysis. In addition, this is perfectly in line with the results of the CASTLE‐AF trial, where only reduction in AF burden, but not recurrence any AT/AF episode correlated with reduction in HF hospitalization and mortality. Unfortunately, in contrast to the CASTLE‐AF trial, which required that patients had an ICD implanted, we do not have data on the AF burden in our patients.
The effect of the severity of LVEF reduction on HF improvement by AF ablation has been controversial. In the recent AMICA trial, HF patients did not benefit from AF ablation. 26 However, only patients with persistent or long‐standing persistent AF were included. In the CASTLE‐AF trial, AF ablation in HF patient results in a benefit with respect to the combined primary endpoint of HF hospitalization and mortality; however, subgroup analysis only identified a beneficial effect on AF patients with LVEF ≥25%. 6 In our study, we did not identify LVEF as a risk factor for LVEF non‐improvement.
According to normalization of systolic function, also HF‐related symptoms improved significantly after ablation in comparison with initial NYHA classification in the rEF group. Moreover, CV hospitalization rate after index procedure was reduced by more than 50% in the rEF group indicating that increased LVEF translates into a clinical benefit in our study.
As expected from the baseline characteristics, arrhythmia recurrence rate in the HF group was significantly higher compared with patients without structural heart disease. However, as revealed by logistic regression analysis, arrhythmia recurrence was not associated with reduced probability of LVEF improvement. As shown in the CASTLE‐AF trial favourable clinical outcome based on reduction of AF burden rather than on freedom from arrhythmia recurrence. Although, AF burden was not monitored continuously in our study, one might speculate that moderately higher recurrence rate in the rEF group did not translate into an excessive augmentation of the duration, in which patients were in AF during follow‐up. In accordance to this, CV hospitalization rate in the rEF group 12 months after index procedure as well as AF related symptoms measured by EHRA symptom scale during follow‐up were significantly reduced compared with baseline. Hence, we conclude that higher AF recurrence rate in the rEF group does not translate in reduced quality of life or even recurrence of symptoms as severe requiring hospitalization or might be counterbalanced by LVEF improvement and reduction of HF‐related symptoms after cryoballoon PVI.
Limitation
With the aim to alleviate the selection bias in our retrospective study and to avoid overestimation of the impact of the ablation on NYHA classification, patients with LVEF improvement and intensified HF drug medication, CRT implantation, or myocardial revascularization were excluded. However, this non‐randomized study is of retrospective nature and data were collected only from one EP centre limiting scientific quality. Furthermore, AF burden was not investigated continuously limiting the evidence of a potential link between AF duration and LVEF.
Conclusions
We here show, that PVI only with the cryoballoon in HF patients with AF, that are suitable for catheter ablation, significantly improves LVEF as well as NYHA functional and leads to significant reduction in CV hospitalization rate. Moreover, procedural parameters as well complication rates are comparable with patients with normal LVEF.
Conflict of interest
T. Dahme received speaker's honoraria and consulting fees from Medtronic. A. Pott received speaker's honoraria from Medtronic and is invited fellow of the Boston Scientific EP training program. The other authors have no conflicts to report.
Supporting information
Table S1. Heart failure medical therapy at baseline and follow‐up.
Table S2. Heart failure variables at baseline and follow‐up.
Pott, A. , Jäck, S. , Schweizer, C. , Baumhardt, M. , Stephan, T. , Rattka, M. , Weinmann, K. , Bothner, C. , Scharnbeck, D. , Keßler, M. , Rottbauer, W. , and Dahme, T. (2020) Atrial fibrillation ablation in heart failure patients: improved systolic function after cryoballoon pulmonary vein isolation. ESC Heart Failure, 7: 2258–2267. 10.1002/ehf2.12735.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Sartipy U, Dahlstrom U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid‐range, and reduced ejection fraction. JACC Heart Fail 2017; 5: 565–574. [DOI] [PubMed] [Google Scholar]
- 3. Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquié JL, Scavée C, Bordachar P. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med 2004; 351: 2373–2383. [DOI] [PubMed] [Google Scholar]
- 4. Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, Potenza D. Pulmonary‐vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med 2008; 359: 1778–1785. [DOI] [PubMed] [Google Scholar]
- 5. Smer A, Salih M, Darrat YH, Saadi A, Guddeti R, Mahfood Haddad T, Kabach A, Ayan M, Saurav A, Abuissa H, Elayi CS. Meta‐analysis of randomized controlled trials on atrial fibrillation ablation in patients with heart failure with reduced ejection fraction. Clin Cardiol 2018; 41: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D, CASTLE‐AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018; 378: 417–427. [DOI] [PubMed] [Google Scholar]
- 7. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KJ, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque J. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374: 2235–2245. [DOI] [PubMed] [Google Scholar]
- 8. Cardoso R, Mendirichaga R, Fernandes G, Healy C, Lambrakos LK, Viles‐Gonzalez JF, Goldberger JJ, Mitrani RD. Cryoballoon versus radiofrequency catheter ablation in atrial fibrillation: a meta‐analysis. J Cardiovasc Electrophysiol 2016; 27: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 9. Pott A, Kraft, C , Stephan, T , Petscher, K Rottbauer, W , Dahme, T ,. Time‐to‐Isolation guided titration of freeze duration in 3rd generation short‐tip cryoballoon pulmonary vein isolation—comparable clinical outcome and shorter procedure duration. Int J Cardiol 2017; 255: 80–84. [DOI] [PubMed] [Google Scholar]
- 10. Chun KRJ, Brugada J, Elvan A, Gellér L, Busch M, Barrera A, Schilling RJ, Reynolds MR, Hokanson RB, Holbrook R, Brown B, Schlüter M, Kuck KH, for the FIRE AND ICE Investigators . The impact of cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation on healthcare utilization and costs: an economic analysis from the FIRE AND ICE trial. J Am Heart Assoc 2017; 6: e006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jalife J. Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol 2014; 29: 20–27. [DOI] [PubMed] [Google Scholar]
- 12. Hatem S. Biology of the substrate of atrial fibrillation. Biol Aujourdhui 2012; 206: 5–9. [DOI] [PubMed] [Google Scholar]
- 13. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, de Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18: 1609–1678. [DOI] [PubMed] [Google Scholar]
- 14. Pruszkowska P, Lenarczyk R, Gumprecht J, Jedrzejczyk‐Patej E, Mazurek M, Kowalski O, Sokal A, Podolecki T, Morawski S, Streb W, Mitręga K, Kalarus Z. Cryoballoon ablation of atrial fibrillation in patients with advanced systolic heart failure and cardiac implantable electronic devices. Kardiol Pol 2018; 76: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 15. Guhl EN, Smith B, Lehmann H, Adelstein E, Bhonsale A, Kancharla K, Voigt A, Wang NC, Saba S, Jain SK. Improvement in ejection fraction after cryoballoon pulmonary vein isolation for atrial fibrillation in individuals with systolic dysfunction. J Interv Card Electrophysiol 2018; 54: 225–229. [DOI] [PubMed] [Google Scholar]
- 16. Lakhani M, Saiful F, Parikh V, Goyal N, Bekheit S, Kowalski M. Recordings of diaphragmatic electromyograms during cryoballoon ablation for atrial fibrillation accurately predict phrenic nerve injury. Heart Rhythm 2014; 11: 369–374. [DOI] [PubMed] [Google Scholar]
- 17. Mondesert B, Andrade JG, Khairy P, Guerra PG, Shohoudi A, Dyrda K, Macle L, Rivard L, Thibault B, Talajic M, Roy D. Clinical experience with a novel electromyographic approach to preventing phrenic nerve injury during cryoballoon ablation in atrial fibrillation. Circ Arrhythm Electrophysiol 2014; 7: 605–611. [DOI] [PubMed] [Google Scholar]
- 18. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, Goromonzi F, Sawhney V, Duncan E, Page SP, Ullah W, Unsworth B, Mayet J, Dhinoja M, Earley MJ, Sporton S, Schilling RJ. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014; 7: 31–38. [DOI] [PubMed] [Google Scholar]
- 19. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016; 133: 1637–1644. [DOI] [PubMed] [Google Scholar]
- 20. Elgendy AY, Mahmoud AN, Khan MS, Sheikh MR, Mojadidi MK, Omer M, Elgendy IY, Bavry AA, Ellenbogen KA, Miles WM, McKillop M. Meta‐analysis comparing catheter‐guided ablation versus conventional medical therapy for patients with atrial fibrillation and heart failure with reduced ejection fraction. Am J Cardiol 2018; 122: 806–813. [DOI] [PubMed] [Google Scholar]
- 21. Briceno DF, Markman TM, Lupercio F, Romero J, Liang JJ, Villablanca PA, Birati EY, Garcia FC, Di Biase L, Natale A, Marchlinski FE. Catheter ablation versus conventional treatment of atrial fibrillation in patients with heart failure with reduced ejection fraction: a systematic review and meta‐analysis of randomized controlled trials. J Interv Card Electrophysiol 2018; 53: 19–29. [DOI] [PubMed] [Google Scholar]
- 22. Kheiri B, Osman M, Abdalla A, Haykal T, Ahmed S, Bachuwa G, Hassan M, Bhatt DL. Catheter ablation of atrial fibrillation with heart failure: an updated meta‐analysis of randomized trials. Int J Cardiol 2018; 269: 170–173. [DOI] [PubMed] [Google Scholar]
- 23. Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs JS, Martinez F. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013; 62: D100–D108. [DOI] [PubMed] [Google Scholar]
- 24. Farber HW, Gibbs S. Under pressure: pulmonary hypertension associated with left heart disease. Eur Respir Rev 2015; 24: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim YG, Choi JI, Boo KY, Oh SK, Park HS, Lee KN, Shim J, Kim JS, Park SW, Park SM, Shim WJ. Clinical and echocardiographic risk factors predict late recurrence after radiofrequency catheter ablation of atrial fibrillation. Sci Rep 2019; 9: 1–9 6890–019–43283‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schlüter M, Tilz RR, Piorkowski C, Gellér L, Kleemann T, Hindricks G. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol 2019; 12: e007731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Heart failure medical therapy at baseline and follow‐up.
Table S2. Heart failure variables at baseline and follow‐up.


