Abstract
Aims
In 2019, pulmonary vascular resistance (PVR) < 3WU was adopted to stratify patients at low risk in pulmonary hypertension due to left heart disease (PH‐LHD) as well those with isolated PH‐LHD. We sought to evaluate whether supervised machine learning with decision tree analysis, which provides more information than Cox Proportional analysis by forming a hierarchy of multiple covariates, confirms this risk stratification.
Methods and results
Two hundred two consecutive patients (mean age: 69 ± 11 years, female: 42%) with mean pulmonary artery pressure ≥ 20 mmHg and wedge pressure > 15 mmHg were recruited. Transpulmonary pressure gradient ⩾̸ 12 mmHg, PVR ⩾̸ 3WU, diastolic pressure gradient ⩾̸ 7 mmHg, pulmonary arterial capacitance < 1.1 mL/mmHg, tricuspid annular plane systolic excursion (TAPSE) < 16 mm, peak systolic tissue Doppler velocity < 10 cm/s, right ventricular end‐diastolic area ⩾̸ 25 cm2 were the seven categorical values entered into the model due to their prognostic significance in PH. We used the chi‐squared automatic interaction detection method to predict mortality. Each node and branch were compared using survival analysis at 6‐year follow‐up. Mean pulmonary artery pressure, wedge pressure, cardiac index, and PVR were 40.3 ± 10.0 mmHg, 22.3 ± 7.1 mmHg, 2.9 ± 0.8 L/min/m2, and 3.6 ± 2.1WU, respectively. Among the seven dichotomous, TAPSE was first selected following by PVR. Compared with patients with PVR < 3WU and TAPSE ⩾̸ 16 mm, patients with PVR ⩾̸ 3WU and TAPSE ⩾̸ 16 mm, or patients with PVR ⩾̸ 3WU and TAPSE<16 mm had significantly increased mortality, HR = 3.0, 95% CI = [1.4–6.4], P = 0.006 and HR = 3.3, 95% CI = [1.6–6.9], P = 0.002, respectively, while patients with PVR < 3WU and TAPSE < 16 mm exhibited the worst prognosis, HR = 7.2, 95% CI = [3.3–15.9], P = 0.0001.
Conclusions
Used for solving regression and classification problems, decision tree analysis confirms that PVR and TAPSE have to be analysed together in PH‐LHD and revealed the dangerous and contradictory prognostic significance of PVR < 3WU when TAPSE<16 mm.
Keywords: Pulmonary hypertension, Heart failure, Decision tree, Machine learning, Right ventricular function, Pulmonary vascular resistance
Introduction
The prognosis of pulmonary hypertension due to left heart disease (PH‐LHD) is mainly described by invasive means. The quest for an ideal predictor of outcome in PH‐LHD has led to conflicting results not only because of the underlying cardiac disease but mainly because patients were investigated at different time courses in their evolving disease, from ambulatory management to heart transplant candidates. A recent meta‐analysis including 10 articles and 2513 patients revealed that diastolic pressure gradient (DPG), pulmonary vascular resistance (PVR), and pulmonary arterial compliance (PAC) appeared to be correlated to survival. 1 Following this meta‐analysis, Vanderpool et al. 2 confirmed the prognostic significance of DPG, PVR, and transpulmonary pressure gradient (TPG) but not PAC. Although not corroborated to sufficient histological findings, PVR < 3WU and ≥ 3WU are believed to dichotomize patients with isolated PH‐LHD and patients with combined PH‐LHD on a prognostic basis, respectively. 3
Right heart catheterization remains the gold standard for monitoring pulmonary hypertension patients but tends to underestimate the remodelling of the right ventricle (RV), leaving echocardiography as the essential method for RV size and function assessment. 4 As emphasized in a recent publication, 5 the role of the RV function estimated by echocardiography is still emerging, with scarce observational studies in PH‐LHD. Recently, Guazzi et al. 6 concluded that a thorough assessment of RV function stratifies heart failure with preserved ejection fraction (HFpEF) patients at different levels of risk. Right ventricular dysfunction in HFpEF develops with increasing pulmonary pressure, and those patients with RV fractional area change <35% have a 2.4‐fold increased risk of death. 7
These observations emphasize the need for combining the right heart haemodynamic variables with a functional evaluation of the RV when trying to define the individual risk of patients with PH‐LHD. Thus, whether or not PVR < 3WU is associated with good prognosis and is further investigated in our work with the combination of right ventricular function parameters assessed by echocardiography. For modelling censored data, the standard Cox regression model has assumptions easily violated while classification tree analysis is a ‘decision‐tree’‐like classification model that provides parsimonious and transparent decision rules that are particularly appropriate in hierarchizing two or more prognostic factors simultaneously as reported in PH‐LHD. Decision tree, a supervised machine learning, maximizes predictive accuracy, derives exact P‐values via permutation tests, and evaluates model cross‐generalizability.
Methods
Our study complies with the Declaration of Helsinki, and the locally appointed ethics committee has approved the research protocol and that informed consent has been obtained from the subjects.
Population
We constituted a prospective cohort from two pulmonary hypertension referral centres. All symptomatic patients with a maximum trans‐tricuspid regurgitation peak velocity > 2.8 m/s revealed by echocardiography were invited to undergo right heart catheterization. Pressures were recorded as published elsewhere. 8 No vasoreactivity testing was performed. Inclusion criteria were patients ≥18 years old classified as having post‐capillary PH based on invasive mean pulmonary artery (PA) pressure (mPAP) ⩾̸ 20 mmHg and PA wedge pressure (WP) > 15 mmHg from the new suggested definition. 3 Exclusion criteria were post‐capillary PH associated with surgically treatable valvular heart disease, infiltrative or genetic cardiomyopathy, and pericardial disease. Patients at risk for independent precapillary PH following a meticulous workout, including high‐resolution computed tomography, contrast‐enhanced computed tomography, ventilation/perfusion lung scan, pulmonary function tests, arterial blood gases, targeted biology parameters, and those receiving or candidate for pulmonary vasodilation therapy, were excluded.
Risk stratification, irrespective of PH classification, included collection of disease progression, syncope, WHO functional class, 6 min walk distance, right heart failure signs, N terminal pro brain natriuretic peptide plasma level, and haemodynamics were evaluated at each hospital visit. Further investigations comprised complete echocardiography focused on left ventricular size and function, right atrial surface, pericardial effusion, right ventricular size and function including the RV 2D strain, peak systolic tissue Doppler velocity, and tricuspid annular plane systolic excursion (TAPSE) were measured. 9 Heart rate and cardiac rhythm were determined from surface 12‐lead electrocardiogram.
All clinical, biological, ultrasound, and haemodynamic parameters were measured and collected by investigators blinded to patient outcomes, as were ultrasound measurements that were performed blindly as to cardiac catheterization results.
Machine learning and decision tree
Decision tree algorithms belongs to the family of supervised learning algorithms that can be used for solving regression and classification problems. A decision tree is a tree where each node represents a feature (attribute), each link (branch) represents a decision (rule), and each leaf represents an outcome. The goal of using a decision tree is to create a model to predict the mortality from our cohort. Because it is preferred for feature values to be categorical, all continuous variables were disregarded before entering the decision tree algorithm. Patients were dichotomized from published predictors of mortality in PH‐LHD, including TPG ⩾̸ 12 mmHg, 10 PVR ⩾̸ 3WU 11 , 12 and DPG ⩾̸ 7 mmHg, 10 , 12 PAC < 1.1 mL/mmHg, 13 TAPSE<16 mm, peak systolic tissue Doppler velocity < 10 cm/s, 14 and right ventricular end‐diastolic area ⩾̸ 25 cm2. 9 Less investigated and validated in PH‐LHD, cardiac index < 2.5 L/min/m2, right atrial pressure ⩾̸ 8 mmHg, and right atrial area ⩾̸ 18 cm2 were also explored. 15 Isolated and combined PH‐LHD were classified using recent publications from the Sixth World Symposium on Pulmonary Hypertension in 2018. 2 Records were distributed recursively based on attribute values. The order for placing attributes as root or internal node of the tree was performed using the chi‐squared automatic interaction detection's method that uses chi‐square statistics. 16
Additional statistics
Clinical, biological, electrocardiographic, echocardiographic, and haemodynamic characteristics were compared using the χ2 test for categorical variables, and ANOVA (or Kruskal‑Wallis test, as appropriate) for continuous variables. Patients were followed up at 6 years (mean follow‐up time was 2.6 ± 1.7 years). The primary endpoint was either cardiac or non‐cardiac death following baseline catheterization. Six‐year survival according to the phenogroup was assessed using a Cox proportional regression model. Statistics were performed with R software (v 3.4.3), using bilateral tests, with P < 0.05 considered statistically significant.
Results
Baseline characteristics
Overall, 202 consecutive patients (mean age: 69 ± 11 years) were prospectively included in this study (Table 1). There were 85 (42%) female patients, 20% patients with coronary artery disease, and 49% in atrial fibrillation. Systemic hypertension and diabetes were the two most common comorbidities ejection fraction (EF) averaged 57 ± 11%, with 175 (87%), 14 (7%), and 13 (6%) patients classified as having preserved EF (≥50%), mid‐range EF (between 40–50%), and reduced EF (<40%), respectively. Overall, the echocardiographic tricuspid regurgitation peak velocity was 3.5 ± 0.6 m/s.
TABLE 1.
Demographic, invasive, and echocardiographic data
| Variables | All patients (n = 202) |
Low PVR High TAPSE (n = 72) |
High PVR High TAPSE (n = 52) |
High PVR Low TAPSE (n = 57) |
Low PVR Low TAPSE (n = 21) |
P‐value |
|---|---|---|---|---|---|---|
| Age (years) | 69 ± 11 | 68 ± 10 | 69 ± 12 | 71 ± 11 | 68 ± 12 | 0.35 |
| Female | 42% | 44% | 31% | 40% | 67% | 0.42 |
| NYHA III or IV | 57% | 49% | 62% | 69% | 62% | 0.23 |
| Comorbidities | ||||||
| BMI (kg/m2) | 31.0 ± 7.2 | 32.2 ± 7.7 | 32.4 ± 7.1 | 29.3 ± 6.4 | 28.2 ± 7.2 | 0.33 |
| Systemic hypertension (%) | 70% | 69% | 71% | 75% | 62% | 0.69 |
| Diabetes (%) | 44% | 47% | 48% | 42% | 29% | 0.43 |
| Coronary artery disease (%) | 20% | 18% | 19% | 28% | 14% | 0.44 |
| Kidney disease 3, 4, 5 (%) | 49% | 43% | 48% | 51% | 71% | 0.18 |
| 6MWD (m) | 325 ± 125 | 354 ± 132 | 302 ± 133 | 289 ± 108 | 325 ± 125 | 0.009 |
| Blood pressure | ||||||
| Systolic pressure (mmHg) | 141 ± 26 | 144 ± 25 | 145 ± 28 | 140 ± 25 | 127 ± 23 | 0.54 |
| Diastolic pressure (mmHg) | 74 ± 13 | 74 ± 14 | 77 ± 12 | 74 ± 12 | 70 ± 12 | 0.48 |
| EKG | ||||||
| Heart rate (beats/min) | 76 ± 18 | 74 ± 15 | 80 ± 21 | 75 ± 18 | 77 ± 18 | 0.37 |
| Atrial fibrillation | 49% | 38% | 38% | 67% | 67% | 0.001 |
| Biology | ||||||
| NT‐pro BNP (pg/mL) | 2828 ± 3620 | 2041 ± 1730 | 2730 ± 1769 | 2859 ± 2143 | 4738 ± 8521 | 0.001 |
| Sodium (mmol/L) | 138 ± 4 | 138 ± 4 | 138 ± 6 | 139 ± 4 | 136 ± 4 | 0.21 |
| eGFR (mL/min/1.73 m2) | 62.0 ± 22.0 | 66.4 ± 21.1 | 63.6 ± 21.4 | 57.8 ± 22.0 | 54.2 ± 23.3 | 0.046 |
| Hb (g/dL) | 12.5 ± 1.8 | 12.5 ± 1.8 | 12.5 ± 1.6 | 12.4 ± 1.9 | 12.2 ± 2.1 | 0.66 |
| Right heart catheterization | ||||||
| Mean RAP (mmHg) | 13.8 ± 6.2 | 12.5 ± 5.5 | 13.7 ± 6.9 | 14.1 ± 6.1 | 17.9 ± 6.4 | 0.18 |
| Systolic PAP (mmHg) | 63.6 ± 18.1 | 53.5 ± 12.6 | 72.5 ± 14.9 | 72.9 ± 19.7 | 51.6 ± 9.7 | 0.005 |
| Diastolic PAP (mmHg) | 26.2 ± 7.7 | 22.6 ± 5.8 | 28.8 ± 8.7 | 29.0 ± 7.5 | 24.5 ± 5.7 | 0.025 |
| Mean PAP (mmHg) | 40.3 ± 10.0 | 34.2 ± 6.9 | 45.5 ± 9.3 | 45.0 ± 10.1 | 35.8 ± 6.9 | 0.004 |
| PWP (mmHg) | 22.3 ± 5.1 | 22.1 ± 5.3 | 22.2 ± 5.2 | 21.7 ± 4.6 | 24.6 ± 5.4 | 0.89 |
| CO (L/min) | 5.5 ± 1.7 | 6.5 ± 1.8 | 5.5 ± 1.4 | 4.4 ± 1.2 | 5.4 ± 1.8 | 0.0001 |
| Cardiac index (L/min/m2) | 2.9 ± 0.8 | 3.4 ± 0.8 | 2.8 ± 0.6 | 2.4 ± 0.6 | 2.9 ± 0.8 | 0.0001 |
| TPG (mmHg) | 18.0 ± 8.5 | 12.1 ± 4.5 | 23.3 ± 7.0 | 23.3 ± 8.0 | 11.1 ± 5.4 | 0.0001 |
| TPG ≥ 12 (%) | 74% | 49% | 98% | 98% | 38% | 0.0001 |
| PVR (Wood units) | 3.6 ± 2.1 | 1.8 ± 0.6 | 4.3 ± 1.3 | 5.8 ± 2.3 | 2.1 ± 0.6 | 0.0001 |
| PVR ≥ 3WU (%) | 54% | 0% | 100% | 100% | 0% | — |
| DPG (mmHg) | 3.9 ± 6.6 | 0.5 ± 4.7 | 6.7 ± 8.8 | 7.3 ± 6.1 | 0.0 ± 4.4 | 0.0001 |
| DPG ≥ 7 (%) | 29% | 11% | 42% | 49% | 5% | 0.0001 |
| PAC (mmHg/mL) | 0.37 ± 0.16 | 0.37 ± 0.16 | 0.66 ± 0.25 | 0.80 ± 0.57 | 0.39 ± 0.12 | 0.0001 |
| PAC < 1.1 mmHg/mL (%) | 96% | 100% | 96% | 89% | 100% | 0.016 |
| Echocardiography | ||||||
| LVEF (%) | 57 ± 11 | 60 ± 7 | 57 ± 11 | 56 ± 11 | 53 ± 13 | 0.020 |
| LVDd (cm) | 49.6 ± 9.9 | 51.3 ± 8.9 | 47.9 ± 10.8 | 47.5 ± 9.9 | 53.5 ± 9.2 | 0.654 |
| LVDs (cm) | 37.8 ± 11.3 | 37.0 ± 8.0 | 37.4 ± 15.3 | 36.7 ± 11.3 | 41.1 ± 12.4 | 0.024 |
| LA volume index (mL/m2) | 54.6 ± 24.6 | 51.7 ± 21.2 | 46.3 ± 26.7 | 61.6 ± 22.7 | 66.0 ± 27.4 | 0.001 |
| LV mass index (g/m2) | 90 ± 36 | 95 ± 41 | 82 ± 30 | 87 ± 33 | 103 ± 33 | 0.059 |
| E/A | 2.6 ± 1.3 | 2.4 ± 1.5 | 3.0 ± 1.4 | 2.7 ± 0.8 | 2.4 ± 1.3 | 0.820 |
| E/e′ | 12.9 ± 5.7 | 12.4 ± 5.4 | 12.4 ± 5.4 | 13.6 ± 5.6 | 13.7 ± 7.8 | 0.567 |
| e′ | 9.4 ± 3.0 | 9.7 ± 3.0 | 9.2 ± 2.9 | 9.6 ± 3.3 | 8.6 ± 2.8 | 0.464 |
| Tricuspid regurgitation (grade) | 1.3 ± 0.9 | 1.3 ± 0.7 | 1.5 ± 1.0 | 1.3 ± 0.8 | 1.7 ± 1.1 | 0,101 |
| RV end‐systolic area (cm2) | 23. 2 ± 7.3 | 22.0 ± 7.6 | 22.3 ± 6.6 | 23.9 ± 7.5 | 27.2 ± 5.9 | 0.0001 |
| RV end‐systolic area ≥ 25 cm2 (%) | 36% | 32% | 27% | 39% | 67% | 0.011 |
| TAPSE (mm) | 17.9 ± 6.3 | 22.3 ± 5.2 | 20.9 ± 3.8 | 11.8 ± 2.4 | 12.0 ± 3.2 | 0.0001 |
| TAPSE≥16 mm (%) | 61% | 100% | 100% | 0% | 0% | — |
| RV peak systolic TDI (cm/s) | 10.2 ± 3.3 | 12.5 ± 2.8 | 11.2 ± 2.9 | 7.8 ± 1.9 | 7.5 ± 2.1 | 0.0001 |
| RV peak systolic TDI ≥ 10 cm/s (%) | 56% | 92% | 71% | 19% | 11% | 0.0001 |
| RV fractional shortening (%) | 34.1 ± 11.5 | 37.5 ± 11.9 | 34.2 ± 10.7 | 31.9 ± 10.9 | 28.4 ± 10.1 | 0.023 |
| RV strain (%) | 15.0 ± 4.3 | 16.6 ± 4.3 | 16.7 ± 4.5 | 13.8 ± 3.6 | 11.7 ± 3.1 | 0.003 |
| Right atrial atrium area (cm2) | 24.8 ± 7.5 | 23.2 ± 7.6 | 23.2 ± 6.9 | 25.6 ± 6.0 | 30.1 ± 9.0 | 0.003 |
Mean PAP averaged 40.3 ± 10.0 mmHg, PA wedge pressure 22.3 ± 7.1 mmHg, and cardiac index 2.9 ± 0.8 L/min/m2. The calculated PVR was 3.6 ± 2.1WU, while DPG averaged 1.17 ± 5.72 mmHg. Based on the new definition, (2) 54% of patients displayed a combined postcapillary PH form defined by PVR ≥3WU, leaving 47% patients with isolated postcapillary PH.
Decision tree algorithm
The 10 parameters utilized for the decision tree were primarily tested using traditional statistics. We began with a univariate Cox proportional hazard model that retained DPG (P = 0.03), right ventricular end‐diastolic area (P = 0.003), right ventricular peak systolic tissue Doppler velocity (P = 0.0001), and TAPSE (P = 0.0001). Then, all variables which were significant in the univariate model at the 5% level entered the multivariate Cox proportional hazard analysis, keeping right ventricular end‐diastolic area (P = 0.013) and right ventricular peak systolic tissue Doppler velocity (P = 0.027).
A decision tree is an elegant multivariate technique that enables us to examine multiple complex relationships very quickly and simultaneously. Figure 1 illustrates the descriptive decision tree. We were interested in whether or not death was determined by seven dichotomous variables during follow‐up in PH‐LHD patients. Only two variables entered into the model while the other five variables were excluded. The most important information that was considered first was the TAPSE (P = 0001). If TAPSE was <16 mm, patients were more likely to die irrespective of PVR including those patients with PVR < 3WU, whereas if TAPSE was ⩾̸16 mm, patients were more likely to survive. However, patients with TAPSE ⩾̸ 16 mm and PVR < 3WU had better prognosis compared with those with PVR ⩾̸ 3WU (P = 0.006). In our study, we had 66.7% accuracy in predicting survival using this algorithm (P = 0.03). Cardiac index <2.5 L/min/m2, right atrial pressure > 8 mmHg, and right atrial area > 18 cm2 were also investigated in the decision tree, but they did not provide additional information.
FIGURE 1.
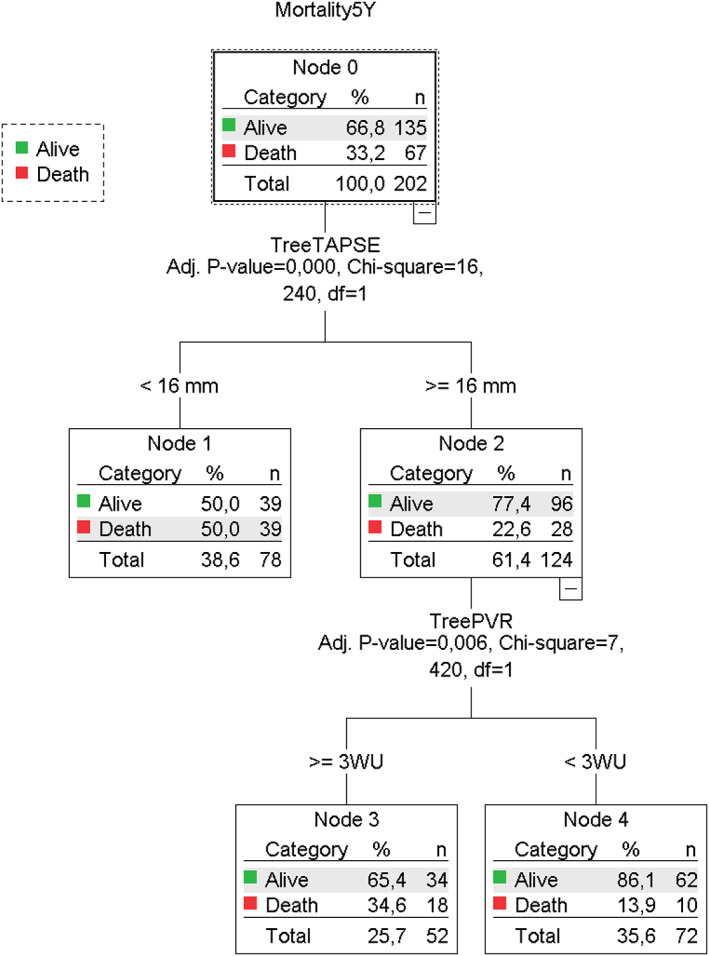
Decision tree solving regression and classification of prognosis from seven dichotomous variables. In our decision tree, each node represents a feature (attribute), each link (branch) represents a decision (rule), and each leaf represents an outcome. TAPSE was the first variable, followed by PVR in the model to predict the mortality. The other five variables were excluded. In PH‐LHD, the prognostic significance of PVR < 3WU must be immediately interpreted from TAPSE.
Survival
In order to translate the decision tree into clinical relevance, we analysed survival at 6‐year follow‐up. Included in a prospective cohort, we had only two patients lost to follow‐up. Overall, 67 patients (33%) died. Compared with patients with PVR < 3WU and TAPSE ⩾̸ 16 mm, patients with PVR < 3WU and TAPSE < 16 mm exhibiting the worst prognosis, HR = 7.2, 95% CI = [3.3–15.9], P = 0.0001, while patients with PVR ⩾̸ 3WU and TAPSE ⩾̸ 16 mm or patients with PVR ⩾̸ 3WU and TAPSE < 16 mm had significantly increased mortality, HR = 3.0, 95% CI = [1.4–6.4], P = 0.006, and HR = 3.3, 95% CI = [1.6–6.9], P = 0.002, respectively. Figure 2 is the Kaplan–Meier curves displaying the estimated survival probability for four different groups of patients with PH‐LHD.
FIGURE 2.
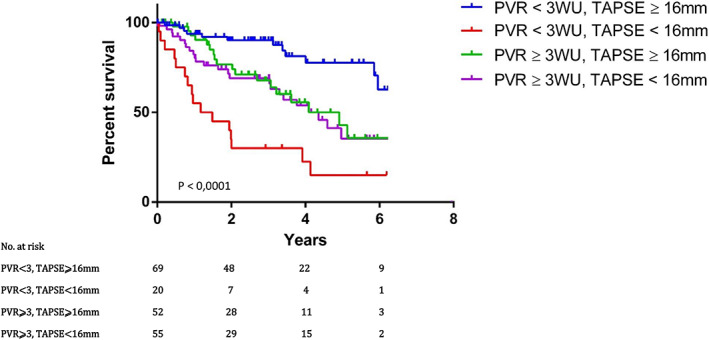
Kaplan–Meier curves displaying the estimated survival probability for four different groups of patients with PH‐LHD. Patients were classified according to PVR and TAPSE. A visual inspection suggests that survival seems to be more favourable for patients who had TAPSE ⩾̸ 16 mm and PVR < 3WU. Those with PVR < 3WU and TAPSE < 16 mm had the worst prognosis. The log‐rank test indicates a significant difference between the survival curves.
Discussion
To the best of our knowledge, this is the first study looking at predictors of mortality with simultaneous comparison of invasive and echocardiographic parameters in PH‐LHD from original supervised machine learning based on a decision tree algorithm. In PH‐LHD, PVR is of prognostic significance only if interpreted from TAPSE in a form of hierarchy.
Decision tree algorithm in pulmonary hypertension due to left heart disease
A common approach to assessing endpoints in registries with time‐to‐event outcomes is to estimate propensity scores, compute weights using logistic regression, test for covariate balance, and then estimate endpoints using Cox regression. When complex relationships between covariates are not found using conventional regression‐based analysis, machine‐learning alternative‐classification tree analysis is much more appropriate. 17 The decision tree algorithm is based on conditional probabilities like mortality in our work. The overall advantages of the decision tree are that it is faster to perform, easy to interpret, robust on small datasets, and simple to understand. When compared with Cox regression, the advantages of decision trees are explicit maximization of predictive accuracy, parsimony, statistical robustness, and transparency and above all classification that is particularly appropriate to hierarchizing two or more prognostic factors as reported in PH‐LHD. 18
Our decision tree is relatively robust and easy to understand because there are only two decisions. The flow starts at the root node with the entire PH‐LHD population and mortality as a dependent variable. TAPSE was the first relevant feature to split the root node into one terminal node, also called leaf node when TAPSE < 16 mm and one decision node if TAPSE ≥ 16 mm. This decision node splits into two terminal/leaf sub‐nodes based on PVR. In other words, TAPSE < 16 mm is of prognostic significance for both PVR < 3WU and ≥3WU (P = 0.0001), but TAPSE ≥ 16 mm is associated with a worse prognosis only if PVR ≥ 3WU (P = 0.006).
Tricuspid annular plane systolic excursion in pulmonary hypertension due to left heart disease
Tricuspid annular plane systolic excursion (TAPSE) is a measurement of the lateral tricuspid valve annulus, used as a surrogate for the RV systolic function. In PH, TAPSE has shown the best correlation with techniques estimating RV EF, such as magnetic resonance imaging. 9 While TAPSE has been investigated in pulmonary arterial hypertension, 19 no study has assessed the prognostic implications of TAPSE in invasively determined PH‐LHD. In heart failure with preserved EF, nosologically closed to PH‐LHD, the meaning of deteriorated TAPSE could be extrapolated to postcapillary PH. Causes of low TAPSE in HF‐PEF are not only PH but also atrial fibrillation, cardiac dyssynchrony, and end‐stage left ventricular diastolic dysfunction, suggesting a role for chronic pressure overload in contributing to right ventricular systolic dysfunction. 20 , 21 Therefore, TAPSE decline in PH‐LHD should reflect more of a systemic disease with frequent comorbidities than a pure haemodynamic disorder indirectly supported by the weak correlation between TAPSE and cardiac output in our work (y = 1.4x + 10.2, r = 0.39, P < 0.0001).
In our work, the decision tree primarily assigned TAPSE to outcomes before PVR in PH‐LHD, which corroborated the univariate Cox proportional analysis (P = 0.0001). To our knowledge, there are no studies addressing the prognostic significance of TAPSE in invasively characterized PH‐LHD. Mohammed et al. 22 reported comparable results by echocardiography in which survival varied across PASP tertiles among patients in the highest, middle, or lowest TAPSE tertiles, later confirmed by Huston et al. 23 in HF‐PEF. Not only an isolated index of RV systolic function, TAPSE is apparently a systemic marker of the whole heart including loading conditions, rhythm, valvular, pericardial and left heart disease, and many more if we consider comorbidities. Regardless heart disease involvement, TAPSE is altered in diabetes, systemic sclerosis, and chronic obstructive pulmonary disease. Therefore, it is suspected that the mortality is not only driven by the right ventricular dysfunction in response to pulmonary hypertension but also by all the comorbidities, TAPSE being only an overall indicator of this pathophysiological state.
Like any disease with heterogeneous phenotypes and numerous comorbidities, the fact that TAPSE appeared stronger than PVR in PH‐LHD was expected. Indeed, TAPSE is a broader marker of the severity and prognosis of cardiac and non‐cardiac diseases, including HFpEF without PH along with PH‐LHD.
Pulmonary vascular resistance in pulmonary hypertension due to left heart disease and the prognostic biphasic response of pulmonary vascular resistance < 3WU
Our work emphasizes the need to combine right heart haemodynamic variables with a functional evaluation of the RV, that is, TAPSE, when trying to define the individual risk of patients with PH‐LHD. PVR was the second decision node in our decision tree model, chronologically right after TAPSE, meaning the role of haemodynamics is key. As published, the presence of PVR ⩾̸ 3WU has two meanings in PH. First, it suggests the presence of significant pulmonary vascular disease in all forms of precapillary PH and a combined phenotype in PH‐LHD, and second, 24 it has been shown that elevated PVR ⩾̸ 3WU is associated with a poor survival rate in PH‐LHD. 3
Our paper seriously challenges the proceedings on the prognostic significance of PVR < 3WU in PH‐LHD. 3 All PH‐LHD patients with TAPSE below 16 mm at baseline regardless PVR< or ⩾̸3WU are at a high‐risk of death. Patients with PVR < 3WU and TAPSE<16 mm have a 7.2‐fold increase in mortality because TAPSE<16 mm is individually associated with comorbidities, clinical and echocardiographic evidence of more advanced HF, and is predictive of poorer outcomes. This may lead to controversies as to whether PVR would significantly, 12 , 25 slightly 11 or would not 26 predict outcome in patients with PH‐LHD.
The prognostic biphasic response of PVR < 3WU is pathophysiologically explained by the nature of its calculation, with a crucial impact of TPG on results. The transpulmonary pressure is the gradient between mean pulmonary arterial pressure and left atrial pressure, that is, PA wedge pressure, and is normally ≤12 mmHg. At early stage, the TPG is normal and becomes pseudo‐normalized at end‐stage of the disease in response to RV systolic dysfunction thus pulmonary pressure declines while left atrial pressure keeps increasing independently. Therefore, normal TPG has the dual meaning of right ventricular systolic to left ventricular diastolic coupling or uncoupling highlighting the importance to parallel simultaneously PA wedge pressure and right systolic ventricular not on cardiac output but from echo indices in a prognostic perspective. It is of note that this biphasic response of TPG is applicable to only PH‐LHD, not to precapillary PH in which TGP is likely to progressively increase in the evolving course of the disease.
The meaning of PVR ⩾̸ 3WU driven by elevated TPG is more complex to understand. PVR ⩾̸ 3WU is nowadays correlated to pulmonary vascular remodelling. In fact, there are limited data in the literature supporting this haemodynamic/histological parallel, and Gerges et al. 10 reported only 38 morphometric analyses of pulmonary vascular remodelling out of 2351 patients enrolled in this study. PVR ⩾̸ 3WU may have other explanations, such as the Anrep effect combined with the Bowditch effect. 27 For example, patients with PVR ⩾̸ 3WU and TAPSE ⩾̸ 16 mm demonstrated normal RV function by echocardiography, that is, 2D strain, FAC, and tissue Doppler velocity with higher heart rate, supporting the Bowditch response.
Conclusions
Used for solving regression and classification problems, decision tree analysis indicates that among seven prognostic factors, both TAPSE and PVR have to be analysed simultaneously and altogether for mortality assessment in PH‐LHD. Therefore, in future research, PVR < 3WU should be interpreted primarily based on right ventricular function by echocardiography whether TAPSE is ⩾̸16 mm or not.
Conflict of interest
None declared.
Raitière, O. , Berthelot, E. , Fauvel, C. , Guignant, P. , Si Belkacem, N. , Sitbon, O. , and Bauer, F. (2020) The dangerous and contradictory prognostic significance of PVR<3WU when TAPSE<16mm in postcapillary pulmonary hypertension. ESC Heart Failure, 7: 2398–2405. 10.1002/ehf2.12785.
References
- 1. Caravita S, Dewachter C, Soranna D, D'Araujo SC, Khaldi A, Zambon A, Parati G, Bondue A, Vachiery JL. Haemodynamics to predict outcome in pulmonary hypertension due to left heart disease: a meta‐analysis. Eur Respir J 2018; 51: 1702427. [DOI] [PubMed] [Google Scholar]
- 2. Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol 2018; 3: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vachiery JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, Coghlan G, Chazova I, De Marco T. Pulmonary hypertension due to left heart disease. Eur Respir J 2019; 53: 1801897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato T, Tsujino I, Ohira H, Oyama‐Manabe N, Yamada A, Ito YM, Goto C, Watanabe T, Sakaue S, Nishimura M. Validation study on the accuracy of echocardiographic measurements of right ventricular systolic function in pulmonary hypertension. J Am Soc Echocard: off publ Am Soc Echocard 2012; 25: 280–286. [DOI] [PubMed] [Google Scholar]
- 5. Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019; 53: 1801900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guazzi M, Dixon D, Labate V, Beussink‐Nelson L, Bandera F, Cuttica MJ, Shah SJ. Rv contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. J Am Coll Cardiol Img 2017; 10: 1211–1221. [DOI] [PubMed] [Google Scholar]
- 7. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenkranz S, Preston IR. Right heart catheterisation: best practice and pitfalls in pulmonary hypertension. Eur Resp Rev: off j Eur Resp Soc 2015; 24: 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the american society of echocardiography endorsed by the european association of echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocard: off publ Am Soc Echocard 2010; 23: 685–713 quiz 786–688. [DOI] [PubMed] [Google Scholar]
- 10. Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out‐of‐proportion” pulmonary hypertension. Chest 2013; 143: 758–766. [DOI] [PubMed] [Google Scholar]
- 11. Tampakakis E, Leary PJ, Selby VN, De Marco T, Cappola TP, Felker GM, Russell SD, Kasper EK, Tedford RJ. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart failure 2015; 3: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palazzini M, Dardi F, Manes A, Bacchi Reggiani ML, Gotti E, Rinaldi A, Albini A, Monti E, Galie N. Pulmonary hypertension due to left heart disease: analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. Eur J Heart Fail 2018; 20: 248–255. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart failure 2015; 3: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darahim K. Right ventricular systolic echocardiographic parameters in chronic systolic heart failure and prognosis. Egypt Heart J 2013; 66: 317–325. [Google Scholar]
- 15. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 16. Kaas GV. An exploratory technique for investigating large quantities of categorical data. Appl Stat 1980; 29: 119–127. [Google Scholar]
- 17. Linden A, Yarnold PR. Estimating causal effects for survival (time‐to‐event) outcomes by combining classification tree analysis and propensity score weighting. J Eval Clin Pract 2018; 24: 380–387. [DOI] [PubMed] [Google Scholar]
- 18. Linden A, Yarnold PR. Modeling time‐to‐event (survival) data using classification tree analysis. J Eval Clin Pract 2017; 23: 1299–1308. [DOI] [PubMed] [Google Scholar]
- 19. Ameloot K, Palmers PJ, Vande Bruaene A, Gerits A, Budts W, Voigt JU, Delcroix M. Clinical value of echocardiographic Doppler‐derived right ventricular dp/dt in patients with pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging 2014; 15: 1411–1419. [DOI] [PubMed] [Google Scholar]
- 20. Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH. Right ventricular dysfunction in left‐sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 2017; 19: 1664–1671. [DOI] [PubMed] [Google Scholar]
- 21. Gorter TM, van Veldhuisen DJ, Voors AA, Hummel YM, Lam CSP, Berger RMF, van Melle JP, Hoendermis ES. Right ventricular‐vascular coupling in heart failure with preserved ejection fraction and pre‐ vs. post‐capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2018; 19: 425–432. [DOI] [PubMed] [Google Scholar]
- 22. Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community‐based study. Circulation 2014; 130: 2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huston JH, Maron BA, French J, Huang S, Thayer T, Farber‐Eger EH, Wells QS, Choudhary G, Hemnes AR, Brittain EL. Association of mild echocardiographic pulmonary hypertension with mortality and right ventricular function. JAMA Cardiol 2019; 4: 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dragu R, Hardak E, Ohanyan A, Adir Y, Aronson D. Prognostic value and diagnostic properties of the diastolic pulmonary pressure gradient in patients with pulmonary hypertension and left heart disease. Int J Cardiol 2019; 290: 138–143. [DOI] [PubMed] [Google Scholar]
- 26. Yamabe S, Dohi Y, Fujisaki S, Higashi A, Kinoshita H, Sada Y, Hidaka T, Kurisu S, Yamamoto H, Kihara Y. Prognostic factors for survival in pulmonary hypertension due to left heart disease. Circ J: off j Japan Circ Soc 2016; 80: 243–249. [DOI] [PubMed] [Google Scholar]
- 27. von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol 1912; 45: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]


