Abstract
Aims
To explore the effects of dapagliflozin on congestion through CardioMEMS (Abbott Inc., Atlanta, USA) and Cordella™ pulmonary artery Sensor (Endotronix Inc., Lisle, Il, USA) devices, which are implantable systems that provide real‐time remote monitoring of pulmonary artery pressure (PAP).
Methods and results
Single‐centre open label observational pilot trial, to investigate the short‐term effects of dapagliflozin in consecutive heart failure and reduced ejection fraction patients with elevated PAP between October and December 2019, previously implanted with CardioMEMS or Cordella™ Sensor. Changes in PAP were evaluated with an area under the curve methodology to estimate the total sum increase or decrease in pressures (mmHg/day) for 7 days before and after starting dapagliflozin relative to the first day of each period. Nine patients (72 ± 10 years, N‐terminal pro b‐type natriuretic peptide 1027 ± 510 pg/mL, estimated glomerular filtration rate 45 ± 15 mL/kg/m2, left ventricular ejection fraction 35 ± 10%), all on optimal guideline‐directed therapy was included. The mean PAP was reduced from 42 ± 9.16 to 38 ± 9.95 mmHg with dapagliflozin therapy (P < 0.05). The average area under the curve for the week leading to dapagliflozin therapy remained unchanged compared to the drop observed for the week after therapy (P < 0.05). Interestingly, the drop in PAP occurred within the first 2 days of dapagliflozin and remained stable for the week following the start of the therapy.
Conclusions
This is the first study to demonstrate a direct effect of dapagliflozin on achieving effective hemodynamic decongestion, providing further mechanistic data regarding the potential mechanisms of sodium‐glucose co‐transporter‐2 inhibitor benefits on heart failure.
Keywords: Dapagliflozin, Heart failure, MEMS, Pulmonary artery pressure
CardioMEMS (Abbott Inc., Atlanta, USA) and Cordella™ pulmonary artery sensor (Endotronix Inc., Lisle, Il, USA) devices are implantable systems that provide real‐time remote monitoring of pulmonary artery pressure (PAP). Incorporation of a PA pressure‐guided treatment algorithm with the goal of maintaining this pressure within a therapeutic range by adjusting medications, in response to PAP trends, was more effective in reducing heart failure (HF) hospitalizations than management of patient clinical signs or symptoms alone. 1 , 2 Currently, mostly alterations in diuretics and vasodilators are used to achieve the desired PAP. 2 Sodium‐glucose co‐transporter‐2 inhibitors (SGLT2i) are recommended to be used in type 2 diabetes mellitus patients with either established cardiovascular disease or at high cardiovascular risk in order to prevent or delay the onset of and hospitalizations for HF. 3 Recently, the SGLT2i dapagliflozin also demonstrated to reduce the risk of cardiovascular death or worsening HF, in patients with HF and reduced ejection fraction (HFrEF), including those without diabetes mellitus. 4 Multiple pleiotropic mechanisms beyond glycaemic control might account for the HF benefits of SLGT2i. 5 No previous study has explored the effects of SGLT2i effects on congestion directly.
In this single‐centre open label observational pilot trial, we wanted to investigate the short‐term effects of dapagliflozin on congestion in HFrEF patients with diabetes mellitus, previously implanted with CardioMEMS or Cordella Sensor. Patients were instructed to perform daily transmission of their PAP. Data from the remote monitoring Merlin.net and Sirona.com database were used to examine PAP trends. The study was carried out in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional ethics committee, and all patients provided written informed consent. Changes in PAP were evaluated with an area under the curve (AUC) methodology to estimate the total sum increase or decrease in pressures (mmHg/day) for 7 days before and after starting dapagliflozin relative to the first day of each period. Importantly, no concomitant changes in any other therapy were allowed during the study. This method accomplishes a quantification of the frequency and duration of time that a patient spends at a pressure lower than their baseline pressure. The area between the PAP timelines and baseline values was computed for each patient by simple numeric integration. The AUC results is presented as mean ± 2 SE, and P value compared both areas under the curve which were computed by the t‐test with equal variance. Twenty patients were implanted with such a device. After exclusion of four patients with HFpEF, three patients with normal PAP, two patients already receiving dapagliflozin, and two other with severe renal impairment, the final study population consisted of nine patients with HFrEF and elevated PAP (mean PAP > 25 mmHg) between October and December 2019. They were seen back in the outpatient clinic 1 week later, and at that moment further, medical changes were allowed whenever indicated by their treating physician.
Patients (mean age 72 ± 10 years) received optimal guideline‐directed medical and device therapy (100% on beta blockers, 88% on ACEI/ARB/ARNI, 100% on magnetic resonance angiograms, 100% on loop diuretics with mean daily dose bumetanide 3 ± 1.6 mg, 44% with implantable cardioverter‐defibrillator, 22% with cardiac resynchronization therapy). Baseline mean N‐terminal pro b‐type natriuretic peptide was 1027 ± 510 pg/mL, estimated glomerular filtration rate 45 ± 15 mL/kg/m2, left ventricular ejection fraction 35 ± 10%, and left ventricular diastolic volume 215 ± 82 mL. The mean PAP was measured and averaged during the week prior to dapagliflozin therapy and after (starting 24 h after treatment). It was reduced from 42 ± 9.16 to 38 ± 9.95 mmHg with dapagliflozin therapy (P < 0.05). As illustrated in Figure 1, the average AUC for the week leading to dapagliflozin therapy remained unchanged compared to the drop observed for the week after therapy (P < 0.05). Interestingly, the drop in PAP occurred within the first 2 days of dapagliflozin and remained stable for the week following the start of the therapy. There also was a significant decrease in body weight after 1 week (92.4 ± 21 vs. 90.8 ± 22 kg, P 0.01).
Figure 1.
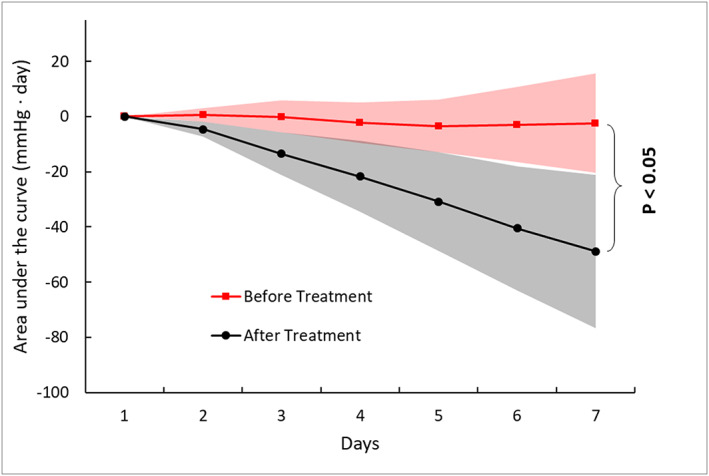
Area under the curve (AUC) trends for mean pulmonary artery pressure before and after dapagliflozin.
As illustrated by this pivotal small observational study, dapagliflozin effectively reduced PAP in HFrEF patients with elevated PAP, already within days of initiation which was sustained during at least 1 week. Historically, alterations in diuretics and vasodilator therapy are used to “target” remote PAP, which has proven to result in lower rates of decompensation and all‐cause mortality. 1 , 2 Importantly, we are the first to demonstrate that lowering of PAP also occurs with solely adding dapagliflozin. Of note, the decongestive effects were observed in patients already receiving guideline‐directed therapy for HFrEF. The mechanisms beyond glycaemic control behind the HF benefits of SGLT2i remain largely unknown. 4 , 5 However, as the reduction in congestion is seen very early after the initiation of dapagliflozin, diuretic/natriuretic and even vasodilatory effects of dapagliflozin are probably involved. 5 Though the patient number is small, AUC analysis is a robust method to assess treatment effects and relies on comparing baseline PAP and the expected alterations in PAP after adjustment of medical management in patients with initially high filling pressures. 1 , 6 Additionally, when the time element of pressure analysis was removed from the AUC calculation in the present study, the magnitude of absolute pressure change was up to 5 mmHg, which is consistent with clinical benefit seen in other haemodynamic monitoring clinical trials. 6 , 7 , 8
In conclusion, this is the first study to demonstrate a direct effect of dapagliflozin on achieving effective haemodynamic decongestion, hereby, providing further mechanistic data regarding the potential mechanisms of SGLT2i benefits on HF.
Mullens, W. , Martens, P. , Forouzan, O. , Dauw, J. , Vercammen, J. , Luwel, E. , Ceyssens, W. , Kockaerts, V. , Ameloot, K. , and Dupont, M. (2020) Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Heart Failure, 7: 2071–2073. 10.1002/ehf2.12850.
References
- 1. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 2. Constanzo M, Stevenson LW, Adamson PB, Deasi AS, Heywood JT, Bourge RC, Bauman J, Abraham WT. Interventions linked to decreased heart failure hospitalizations during ambulatory pulmonary artery pressure monitoring. JACC Heart Fail 2016; 4: 333–344. [DOI] [PubMed] [Google Scholar]
- 3. Seferovic P, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D‐HT and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 5. Verbrugge FH, Martens P, Mullens W. SGLT‐2 inhibitors in heart failure: implications for the kidneys. Curr Heart Fail Rep 2017; 14: 331–337. [DOI] [PubMed] [Google Scholar]
- 6. Heywood JT, Jermyn R, Shavelle D, Abraham WT, Bhimaraj A, Bhatt K, Sheikh F, Eichorn E, Lamba S, Bharmi R, Agarwal R, Kumar C, Stevenson LW. Impact of practice‐based management of pulmonary artery intracardiac pressures pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation 2017; 135: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 7. Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM Jr, Magalski A, Zile MR, Smith AL, Smart FW, O'Shaughnessy MA, Jes‐sup ML, Sparks B, Naftel DL, Stevenson LW, COMPASS‐HF Study Group . Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS‐HF study. J Am Coll Cardiol 2008; 51: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 8. Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT, Bourge RC, Warner Stevenson L. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail 2017; 10: e003594. [DOI] [PubMed] [Google Scholar]


