Abstract
Aims
Currently, the ejection fraction [left ventricular ejection fraction (LVEF)] is the main criterion used for implanting implantable cardioverter defibrillators (ICDs) for primary prevention. However, many of ICD receivers would not have an event and do not have any gains from the device. Consequently, improving the discrimination strategies is needed. Here, we aimed at assessing the role of global longitudinal strain (GLS) for such purpose.
Methods and results
Seventy ischaemic or dilated cardiomyopathy cases characterized by LVEF ≤ 40% with a previously implanted ICD were enrolled. LVEF and GLS amounts were evaluated using 3D echocardiography. The occurrence of ventricular arrhythmias was checked by analysing the ICD history. Mean follow‐up period of patients was 1.8 ± 0.6 years. There was a significant difference in the amount of GLS in arrhythmic cases compared with non‐arrhythmic ones (−6.97 ± 3.06 vs −11.82 ± 4.25; P < 0.001). This difference was found in both ischaemic and dilated cardiomyopathy groups. A GLS below −10 cm/s could predict the occurrence of a ventricular event by 90% specificity and 72.2% sensitivity (area under the curve = 0.84, P < 0.001). While 27.39 (69.2%) patients with GLS below −10 cm/s had a ventricular event, only 3.31 (9.6%) of the patients with GLS above −10 had an event) P < 0.001). Those patients with a GLS ≥ 17 cm/s never experienced a ventricular arrhythmia.
Conclusions
Global longitudinal strain is a more accurate predictor of ventricular arrhythmias in patients with reduced LVEF. Whether it may help in selecting more appropriate patients for ICD implantation or not should be evaluated within a randomized trial in the future.
Keywords: Global longitudinal strain, Heart failure, Primary prevention, Implantable cardioverter defibrillator
Introduction
The main causes of mortality among those suffering from heart failure (HF) are sudden cardiac death (SCD) and pump failure. 1 Between these two, the unpredictable nature of SCD makes it less unpredictable, and as the treatment facilities are not available most of the times, it is more difficult to save the patient. 2 Indeed, according to Kandala et al., the rate of survival after out‐of‐hospital cardiac arrest has been reported to be only 10%. 1 In this regard, as shown in several studies, primary prevention using implantable cardioverter defibrillator (ICD) is significantly associated with the increase in the survival rate. 3 , 4 , 5 , 6 In fact, ICD implantation is the therapy of choice for ventricular tachyarrhythmia (VT) in ischaemic heart diseases, resulting in decreased left ventricular ejection fraction (LVEF) or non‐ischaemic dilated cardiomyopathies. 7 Decision for ICD implantation relies mainly on LVEF. According to the current guidelines, ICD implantation is indicated for the following groups: ischaemic cardiomyopathy with LVEF < 35% or 30% depending on New York Heart Association functional class and also for non‐ischaemic cardiomyopathy with LVEF of less than 35% and New York Heart Association functional class II or III. 8
However, its use comes along with some considerations. ICD therapy is a matter of cost‐effectiveness. Utilization and maintenance of this device through an individual's life span imposes financial burden on health care system and on the patient himself/herself. Useless implantation of ICD in MADIT‐II was shown to be correlated with the increased number of hospitalization. 9 Furthermore, a considerable number of patients who have implanted ICD, as the primary prevention, never receive therapeutic shocks. 3 Moreover, it had been demonstrated that in a group of patients, despite the existence of low LVEF, they remained at low risk of SCD occurrence. 4 , 10 Therefore, it seems that the current predictive assessment for ICD utilization is not quite distinctive and needs more effective risk stratification.
In fact, LVEF is not an accurate method of risk stratification, and more novel echocardiographic indices such as the global longitudinal strain (GLS) would help to predict the outcome more accurately. 11 , 12 Speckle‐tracking echocardiography (STE) has been reported as a non‐Doppler‐oriented and angle‐independent evaluation technique of the LVEF. Measuring the length of myocardial segments is performed by tracking the displacement of myocardial speckles via an algorithm. 13 As measuring the STE is done directly, variety in ventricular loading, compliance of myocardium, and afterload properties have less influence on it compared with LVEF. 14 Strain is the most common evaluation unit in STE, which is the alteration in the myocardial fibre length at end‐systole than its primary length at end‐diastole and is reported as percentage. There are longitudinal, radial, and circumferential strains. By using STE, GLS serves as a novel technique that could provide detection and quantification of fine LV disturbances in terms of systolic function. 15
This study is the first study designed in order to assess the value of GLS beside LVEF as a benchmark for ICD implantation. In this regard, the rate of VT/ventricular fibrillations (VFs) was assessed in patients with ischaemic heart disease or dilated cardiomyopathy (DCM) who received ICD. Also, GLS was measured in these patients as well. The correlation between the probability of VT/VFs incidence, as a marker for the efficiency of implanted ICD and GLS amount, was investigated.
Methods
Study population
Patients with HF (ischaemic or DCM) who had undergone ICD implantation for primary prevention of ventricular arrhythmias and referred for ICD analyses to the Faghihi clinic of arrhythmia, ICD, and Permanent Pace Maker (PMM) were evaluated for enrolment. Inclusion criteria were presence of ejection fraction (EF) ≤ 35% and an implanted ICD. Patients who needed Cardiac re‐synchronization Therapy Defibrillator (CRT‐D) implantation were excluded from study. Patients with the following characteristics were excluded from the study: absence of normal sinus rhythm, less than 1 year duration of ICD implantation, a history of myocardial infarction (MI) through the past 3 months, coronary artery bypass graft surgery for 3 months, existence of congenital heart defect, valvular disorder, or serious liver/kidney dysfunction.
Ischaemic cardiomyopathy was regarded as more than one stenosis of one of the main coronary arteries (left anterior descending artery, left circumflex artery, or right coronary artery) diagnosed with coronary angiography or a history of old MI. DCM was documented by the absence of obstructive coronary disease. Demographic information of patients was collected through a questionnaire as well.
The study protocol was in accordance with the Helsinki declaration as well as the Iranian National Committee for Ethics in Biomedical Research. Also, it was approved by the research ethics committee of Shiraz University of Medical Sciences (reference number: IR.SUMS.REC.700/112). The written informed consent was obtained from the subjects followed by approval of the research ethics committee.
Echocardiographic study
All echocardiographic studies were done by expert subspecialty echo man before ICD implantations and at the end of follow‐up period. Imaging was done in the left lateral decubitus position via the general electric E9 conventional echocardiography machine. The echocardiograms were assessed via a specified reader. LVEF was determined from the apical four‐chamber and two‐chamber views by a 3D echocardiographic probe and using automated techniques.
Speckle‐tracking echocardiography was performed using the same machine; analysis of myocardial speckles shift in each spot was done and traced frame to frame. Longitudinal strain was evaluated by automated functional imaging. The GLS peak was determined as a mean of the peak longitudinal strain in three image planes (apical two‐chamber and four‐chamber as well as long‐axis visions).
Implantable cardioverter defibrillator evaluation
Implanted ICD was evaluated through interrogation of each device with its compatible analyser. Events leading to therapy were assessed to see if treatments were appropriate or inappropriate. Only appropriate treatments for VT or VF were considered as an acceptable endpoint.
Statistical analysis
Shapiro–Wilks test was used for assessing normal distribution of data. Data were introduced as mean ± standard deviation. Student's t‐test or Mann–Whitney was applied for measuring the differences between variables. Receiver operating characteristic (ROC) curve was analysed for assessing the specificity as well as sensitivity of each variable to predict VT/VF occurrence. Data were assessed by SPSS 17 (SPSS, Chicago, Illinois) and MedCalc 15.8 (MedCalc Software bvba, Ostend, Belgium). P values smaller than 0.05 were regarded significant.
Results
Seventy HF patients were enrolled in our study: 37 cases were categorized as ischaemic and 33 cases were in the DCM group (Table 1 ). Follow‐up duration period after ICD implantation was 1.8 ± 0.6 years (1 to 3 years).
TABLE 1.
Demographic data
| Age (years) | 54.5 ± 18.2 |
| Ischaemic cardiomyopathy (%) | 52.8 |
| Dilated cardiomyopathy (%) | 47.2 |
| Female (%) | 51.4 |
| LBBB (%) | 17.1 |
| Diabetes mellitus (%) | 31.4 |
| Hypertension (%) | 71.4 |
| Medications | |
| Beta‐blockers (%) | 100 |
| Antiarrhythmic medications (%) | 0 |
| ACE inhibitors (%) | 62.8 |
| Angiotensin receptor blockers (%) | 31.2 |
| Aldosterone antagonists (%) | 62.8 |
| Aspirin (%) | 52.8 |
| Statins (%) | 52.8 |
ACE, angiotensin‐converting enzyme; LBBB, left bundle branch block.
Comparison of LVEF and GLS showed no difference between the patients with ischaemic versus DCM origin. However, both values were significantly lower in patients with a history of ventricular arrhythmias (Table 2 ). There was a significant but moderate correlation between GLS values and EF (r = −0.425, P < 0.001).
TABLE 2.
The comparison of LVEF and GLS in ischaemic CMP with DCM and in patients with VT/VF with those without VT/VF
| Number | LVEF | P value | GLS | P value | |
|---|---|---|---|---|---|
| Ischaemic CMP | 37 | 28.64 ± 7.33 | 0.489 | −9.84 ± 5.45 | 0.846 |
| DCM | 33 | 29.87 ± 7.46 | −9.63 ± 3.11 | ||
| VT/VF | 30 | 26.56 ± 6.62 | 0.008 | −6.97 ± 3.06 | <0.001 |
| No VT/VF | 40 | 31.22 ± 7.33 | −11.82 ± 4.25 |
CMP, Cardiomyopathy; DCM, dilated cardiomyopathy; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; VT/VF, ventricular tachycardia/fibrillation.
About 43.2% of the patients of ischaemic and 42.4% of those with a DCM origin had a VT/VF, which showed no statistical difference. Among the patients who suffered from VT/VF, LVEF was similar in those of ischaemic and DCM origin (P = 0.97), while GLS was significantly lower (the absolute number is higher) in those from ischaemic origin (−12.6 vs −10.8 cm/s, difference = 1.78, P = 0.009; Table 3 ). In those without VT/VF, none of the values was different.
TABLE 3.
The comparison of GLS and LVEF amount in patients with and without VT/VF incidence categorized based on ischaemic versus DCM origin
| Ischaemic | DCM | |||
|---|---|---|---|---|
| VT/VF | No VT/VF | VT/VF | No VT/VF | |
| Number | 16 | 21 | 14 | 19 |
| GLS (mean ± SD) | −6.13 ± 3.46 | −12.66 ± 5.02 | −7.92 ± 2.29 | −10.88 ± 3.7 |
| P value | <0.001 | 0.005 | ||
| LVEF (mean ± SD) | 25.93 ± 6.37 | 30.71 ± 7.47 | 27.28 ± 7.07 | 31.78 ± 7.33 |
| P value | 0.048 | 0.087 | ||
DCM, dilated cardiomyopathy; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; SD, standard deviation; VT/VF, ventricular tachycardia/fibrillation.
Receiver operating characteristic curve analyses showed that a GLS more than −10 cm/s (absolute values less than 10) could predict the occurrence of a ventricular event by 90% specificity and 72.2% sensitivity [area under the curve (AUC) = 0.84, P < 0.001]. However, an LVEF below 32% could predict the occurrence of VT/VF with just 76.7% specificity and 55% sensitivity (AUC = 0.68). Comparison of ROC curves showed significantly higher power of GLS for predicting VT/VF, as compared with LVEF (AUC = 0.84 vs 0.68, difference = 0.15, P = 0.02; Figure 1 ). While 27 out of 39 (69.2%) patients with GLS below −10 cm/s had a ventricular event, only 3.31 (9.6%) of patients with GLS above −10 had an event (P < 0.001). Those patients with a GLS equal or below −17 cm/s (absolute values above 17; 7% of study population) never experienced a ventricular arrhythmia (positive predictive value = 100%). However, no LVEF cut‐off showed a positive predictive value of 100%.
FIGURE 1.
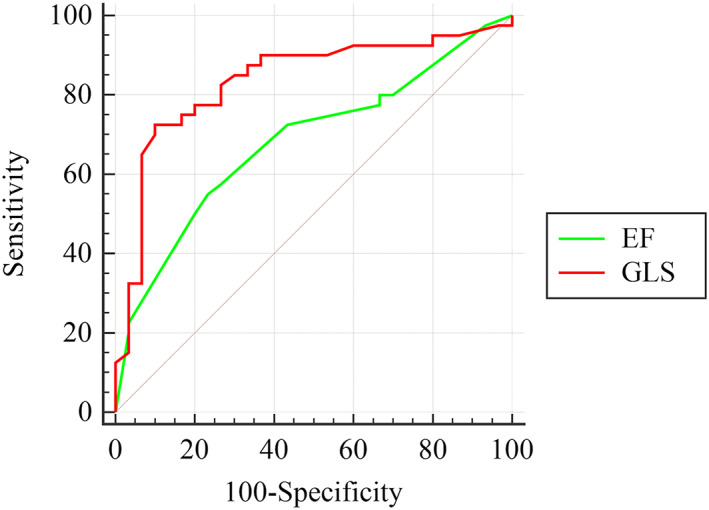
Receiver operating characteristic curve analysis. Comparison of the accuracy of GLS versus LVEF in predicting VT/VF occurrence. GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; VT/VF, ventricular tachycardia.
In patients with ischaemic cardiomyopathy, a GLS more than −7.2 cm/s (absolute values less than 7.2) could predict the occurrence of a ventricular event by 90.5% specificity and 75% sensitivity (AUC = 0.85, P < 0.001). In patients with DCM, a GLS more than −10.1 cm/s (absolute values less than 10.1) could predict the occurrence of a ventricular event by 92.9% specificity and 68.4 sensitivity (AUC = 0.8, P < 0.001).
Discussion
This research aimed at investigating the GLS potential as an indicator for ICD implantation for primary prevention among the cases suffering from HF. In this regard, GLS was measured in ICD‐implanted HF patients and the rate of VT/VFs incidence was evaluated. The occurrence of tachyarrhythmia events translates into the appropriate ICD prophylactic therapy for the subject. The results showed that GLS acted more precisely than LVEF alone in identification of patients who would really face VT/VFs in the future because of ischaemic heart disease/DCM.
In spite of a moderate relationship between GLS and LVEF, GLS was a much better prognostic indicator in our study as well as in other studies. In our study, GLS could predict the occurrence of VT/VF with a very high accuracy, while LVEF was not capable of such discrimination. Consistent with the results of the current study, Haugaa et al. reported that in post‐MI patients, GLS was severely reduced in those who experienced a VT/VF episode as compared with those who did not (−14.8 ± 4.7% compared with −18.2 ± 3.7%, P = 0.001). They found mechanical dispersion as well as global strain as markers for arrhythmias among the cases with non‐ST segment elevation MI (P < 0.05) and among those with LVEFs of more than 35% (P < 0.05), while LVEF was not (P = 0.33). 16 In the study of Banasik et al., patients with electrical events demonstrated greater mechanical dispersion as compared with patients without electrical events (99.14 ± 33.60 vs 72.98 ± 19.70). 17 In another study, Mignot and colleagues showed that in HF cases, among the echocardiographic factors including LVEF, GLS showed the highest prognostic accuracy to predict the major cardiac events. 18 In agreement, Perry and colleagues studied 1014 patients and ROC analysis determined the optimal cut‐off for GLS as ≥−15%. Also, only GLS was independently predictive of Major adverse cardiac events (MACE). 19 Similarly, Guerra et al. showed that impaired GLS is associated with an increased risk of ventricular arrhythmias and appropriate ICD therapies in a consecutive ‘real‐world’, unselected population of remotely monitored patients with structural heart disease. 20 In a large study by Hwang and collegues on 4312 patients, GLS was the best information used to develop a mortality risk prediction model for patients with acute HF syndrome. 21 Similarly, in Park and colleagues' study, GLS (not LVEF) was an independent predictor of death among the cases suffering from HF with reduced EF, HF with mid‐range EF, and HF with preserved EF. 12 In a study by Abtahi et al., it was shown that GLS was a more appropriate predictor of iron cardiac overload among thalassemia cases, while LVEF was not capable of such detection. 13 It is also utilized to evaluate LV remodelling, which may occur following an acute MI. 22
Limitations of LVEF are related to several points. LVEF results from endocardium geometric deformation. 23 To obtain a precise evaluation, proper echocardiographic views characterized by an approved quality are essential. Oh et al. in their laboratory evaluation of the baseline echocardiographic investigations in the STICH trial on cases suffering from ischaemic cardiomyopathy with an LVEF of less than 35% indicated that 18% of the cases showed an EF < 35%. In addition, evaluation of LVEF in 73% of the subjects was done by the Simpson technique, due to the low imaging quality, which indicates that LVEF had a low reproducibility as well as feasibility. 24 LVEF is associated with the intrinsic restriction, as it is not representative of the myocardial contractility. Among those with definite regional wall motion disorders due to MI, LVEF is possibly normal owing to the compensatory overcontractions in other regions of the heart, but these cases evidently had elevated neurohumoral activation with an adverse effect on clinical results. 25 These findings can explain the inconsistent and controversial relationship between LVEF and clinical results. Moreover, measurement of LVEF has shown to be associated with changes in geometry and is strongly dependent on the operators' experience. On the contrary, by STE, myocardial changes are directly measured. Longitudinal contraction of the myocardium is defined by GLS. Validity of GLS accuracy was documented against magnetic resonance imaging technique. Indeed, measuring GLS has some merits in terms of operator independency, reproducibility especially with respect to EF, and integration to standard echocardiography. 26 Consequently, Karlsen and colleagues showed that GLS was a better reproducible measure of left ventricular function compared with ejection fraction without echocardiographic course. 27
Nowadays, candidates for ICD therapy, as the primary prevention, are selected based on LVEF. However, it seems that this strategy is not sensitive enough to truly differentiate the patients in need from the others. For instance, an SCD death study showed that only one‐fifth of the subjects had LVEF < 35%. It means that 80% of them who faced SCD are ruled out from the therapy with ICD. 28 Otherwise, there are people with low LVEF (<35%) who are simultaneously at low risk of SCD occurrence. 10 Therefore, it seems that making decision based solely on LVEF should be revised or improved by utilizing other approaches. The present research was the first to show that GLS was a more reliable predictor of VT/VF in both ischaemic and DCM subject as compared with LVEF. This may help in better discrimination of patients in true need of ICD implantation. In some other studies, the value of longitudinal strain in predicting arrhythmic events in MI patients was highlighted. 16 , 29 Meanwhile, LVEF suffers from a low sensitivity in detecting the risk for VT/VF. 30 , 31
This study faced some limitations. First of all, the number of patients enrolled was low and all the patients were from one centre. Second, the nature of study was prospective, while for reaching a definitive recommendation, a large randomized trial is needed.
Conclusions
Our study showed that in both ischaemic and DCM HF patients with ICD implantation, in using GLS beside LVEF, the accuracy in prediction of ventricular tachycardia would be more than LVEF criteria alone. Our results also showed that more than 88.8% of patients who had GLS amount less than −10.0 received Direct Current (DC) shock for defibrillator therapy. This may help to select more appropriate patients for ICD therapy, which should be evaluated within a randomized trial in future.
Conflict of interest
None declared.
Funding
This study was supported by grants numbers 95‐01‐01‐11829 and 97‐01‐21‐17707 from vice chancellor of research of Shiraz University of Medical Sciences.
Acknowledgements
The authors would like to thank Shiraz University of Medical Sciences, Shiraz, Iran, and also Center for Development of Clinical Research of Nemazee Hospital and Dr Nasrin Shokrpour for editorial assistance.
Nikoo, M. H. , Naeemi, R. , Moaref, A. , and Attar, A. (2020) Global longitudinal strain for prediction of ventricular arrhythmia in patients with heart failure. ESC Heart Failure, 7: 2956–2961. 10.1002/ehf2.12910.
All authors declare that all material to this submission is original.
References
- 1. Kandala J, Oommen C, Kern KB. Sudden cardiac death. Br Med Bull 2017; 122: 5–15. [DOI] [PubMed] [Google Scholar]
- 2. McMurray JV, Pfeffer MA. Heart Fail Lancet [Internet] 2005; 365: 1877–1889. [DOI] [PubMed] [Google Scholar]
- 3. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty S, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225–237. [DOI] [PubMed] [Google Scholar]
- 4. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 2004: 2140–2150. [DOI] [PubMed] [Google Scholar]
- 5. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ. MADIT‐I and MADIT‐II. J Cardiovasc Electrophysiol 2003; 14: S96–S98. [DOI] [PubMed] [Google Scholar]
- 7. Satake H, Fukuda K, Sakata Y, Miyata S, Nakano M, Kondo M, Hasebe Y, Segawa M, Shimokawa H, behalf of the CHART‐2 Investigators . Current status of primary prevention of sudden cardiac death with implantable cardioverter defibrillator in patients with chronic heart failure. Circ J 2015; 79: 381–390. [DOI] [PubMed] [Google Scholar]
- 8. Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, Taylor AJ. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter‐defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol 2011; 57: 821–828. [DOI] [PubMed] [Google Scholar]
- 9. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335: 1933–1940. [DOI] [PubMed] [Google Scholar]
- 10. Al‐Khatib SM, Sanders GD, Bigger JT, Buxton AE, Califf RM, Carlson M, Curtis A, Curtis J, Fain E, Gersh BJ, Gold MR, Haghighi‐Mood A, Hammill SC, Healey J, Hlatky M, Hohnloser S, Kim RJ, Lee K, Mark D, Mianulli M, Mitchell B, Prystowsky EN, Smith J, Steinhaus D, Zareba W. Preventing tomorrow's sudden cardiac death today: part I: current data on risk stratification for sudden cardiac death. Am Heart J 2007; 153: 941–950. [DOI] [PubMed] [Google Scholar]
- 11. Haugaa KH, Dejgaard LA. Global longitudinal strain: ready for clinical use and guideline implementation. J Am Coll Cardiol 2018; 71: 1958–1959. [DOI] [PubMed] [Google Scholar]
- 12. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 2018; 71: 1947–1957. [DOI] [PubMed] [Google Scholar]
- 13. Abtahi F, Abdi A, Jamshidi S, Karimi M, Babaei‐Beigi MA, Attar A. Global longitudinal strain as an indicator of cardiac iron overload in thalassemia patients. Cardiovasc Ultrasound 2019; 17: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zamirian M, Afsharizadeh F, Moaref A, Abtahi F, Amirmoezi F, Attar A. Reduced myocardial reserve in cirrhotic patients: an evaluation by dobutamine stress speckle tracking and tissue Doppler imaging (TDI) echocardiography. J Cardiovasc Thorac Res 2019; 11: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Attar A, Sayadi M, Jannati M. Effect of intensive blood pressure lowering on cardiovascular outcomes based on cardiovascular risk: a secondary analysis of the SPRINT trial. Eur J Prev Cardiol 2019; 26: 238–245. [DOI] [PubMed] [Google Scholar]
- 16. Haugaa KH, Grenne BL, Eek CH, Ersbøll M, Valeur N, Svendsen JH, Florian A, Sjøli B, Brunvand H, Køber L, Voigt JU. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 2013; 6: 841–850. [DOI] [PubMed] [Google Scholar]
- 17. Banasik G, Segiet O, Elwart M, Szulik M, Lenarczyk R, Kalarus Z, Kukulski T. LV mechanical dispersion as a predictor of ventricular arrhythmia in patients with advanced systolic heart failure: a pilot study. Herz 2016; 41: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, Monzy S, Roudaut R, Habib G, Lafitte S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr 2010; 23: 1019–1024. [DOI] [PubMed] [Google Scholar]
- 19. Perry R, Patil S, Horsfall M, Marx C, Chew D, Joseph M, Ganesan A, McGavigan A, Nucifora G, Selvanayagam J. Global longitudinal strain and mechanical dispersion improves risk stratification of malignant ventricular arrhythmias and major adverse cardiac events over ejection fraction alone. J Am Coll Cardiol 2018; 71: A1663. [Google Scholar]
- 20. Guerra F, Malagoli A, Contadini D, Baiocco E, Menditto A, Bonelli P, Rossi L, Sticozzi C, Zanni A, Cai J, Maitra P, Villani GQ, Capucci A. Global longitudinal strain as a predictor of first and subsequent arrhythmic events in remotely monitored ICD patients with structural heart disease. JACC Cardiovasc Imaging 2020; 13: 1–9. [DOI] [PubMed] [Google Scholar]
- 21. Hwang IC, Cho GY, Choi HM, Yoon YE, Park JJ, Park JB, Park JH, Lee SP, Kim HK, Kim YJ, Sohn DW. Derivation and validation of a mortality risk prediction model using global longitudinal strain in patients with acute heart failure. Eur Heart J Cardiovasc Imaging 2019. 10.1093/ehjci/jez300 [DOI] [PubMed] [Google Scholar]
- 22. Gjesdal O, Hopp E, Vartdal T, Lunde K, Helle‐Valle T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Global longitudinal strain measured by two‐dimensional speckle tracking echocardiography is closely related to myocardial infarct size in chronic ischaemic heart disease. Clin Sci (Lond) 2007; 113: 287–296. [DOI] [PubMed] [Google Scholar]
- 23. Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T, Remme EW. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol 2017; 70: 942–954. [DOI] [PubMed] [Google Scholar]
- 24. Oh JK, Pellikka PA, Panza JA, Biernat J, Attisano T, Manahan BG, Wiste HJ, Lin G, Lee K, Miller FA Jr, Stevens S, Sopko G, She L, Velazquez EJ, STICH Trial Investigators . Core lab analysis of baseline echocardiographic studies in the STICH trial and recommendation for use of echocardiography in future clinical trials. J Am Soc Echocardiogr 2012; 25: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaney C, Waeber B, Turini G, Margalith D, Brunner HR, Perret C. Renin and the complications of acute myocardial infarction. Chest 1984; 86: 40–43. [DOI] [PubMed] [Google Scholar]
- 26. Ypenburg C, Roes SD, Bleeker GB, Kaandorp TAM, de Roos A, Schalij MJ, van der Wall EE, Bax JJ. Effect of total scar burden on contrast‐enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am J Cardiol 2007; 99: 657–660. [DOI] [PubMed] [Google Scholar]
- 27. Karlsen S, Dahlslett T, Grenne B, Sjøli B, Smiseth O, Edvardsen T, Brunvand H. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc Ultrasound 2019; 17: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Bijl P, Delgado V, Bax JJ. Sudden cardiac death: the role of imaging. Int J Cardiol 2017; 237: 15–18. [DOI] [PubMed] [Google Scholar]
- 29. Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C, Søgaard P, Køber L. Prediction of all‐cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013; 61: 2365–2373. [DOI] [PubMed] [Google Scholar]
- 30. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 31. Passman R, Kadish A. Sudden death prevention with implantable devices. Circulation 2007; 116: 561–571. [DOI] [PubMed] [Google Scholar]


