Abstract
Aims
New‐onset atrial fibrillation (NOAF) complicating acute myocardial infarction (AMI) has been associated with poor survival, but the clinical implication of NOAF on heart failure (HF) is still not well characterized. We aimed to investigate the relationship between NOAF complicating AMI and HF hospitalization.
Methods and results
Adult AMI patients identified in the New‐Onset Atrial Fibrillation Complicating Acute Myocardial Infarction in Shanghai registry who, discharged alive, had complete echocardiography and follow‐up data from February 2014 to March 2018 were included. Patients were divided according to the presence of NOAF. The outcome measures were HF hospitalization and death during the observational period (until 10 April 2019). Cox proportional hazard models were performed in the whole population and propensity score‐matched (PSM) cohort to assess the adjusted hazard ratio (HR) and 95% confidence interval (CI). Overall, 2075 patients (mean age: 65.2 ± 12.3 years, 77.3% were men) with AMI were analysed, of whom 228 (11.0%) developed NOAF. Advanced age, admission HF (Killip II–IV), impaired renal function, decreased left ventricular ejection fraction, increased heart rate, and left atrial enlargement were independent predictors of NOAF. Over a median observational period of 2.7 years, the annual incidence rates of HF hospitalization were 18.4% and 2.8% for patients with NOAF and sinus rhythm, respectively. After adjustment for confounders, NOAF was significantly associated with HF hospitalization (HR: 3.14, 95% CI: 2.30–4.28, P < 0.001). Similar results were obtained when accounting for the competing risk of all‐cause death (subdistribution HR: 3.06, 95% CI: 2.18–4.30, P < 0.001) or from the PSM cohort (HR: 2.82, 95% CI: 1.99–4.00, P < 0.001). Patients with persistent NOAF (HR: 5.81, 95% CI: 3.59–9.41) were at significantly higher risk of HF hospitalization when compared with those with transient one (HR: 2.61, 95% CI: 1.84–3.70, P interaction = 0.008). Although post‐MI NOAF was significantly related to cardiovascular death (annual incidence rates for NOAF and sinus rhythm were 9.4% and 2.3%, respectively; HR: 1.97, 95% CI: 1.36–2.85, P < 0.001), such an association was attenuated when HF hospitalization (modelled as a time‐varying covariate) and antithrombotic treatment were adjusted (HR: 1.37, 95% CI: 0.92–2.02, P = 0.121).
Conclusions
In patients with AMI, NOAF is strongly associated with an increased long‐term risk of HF hospitalization. Our findings suggest that strengthened secondary prevention of HF should be considered in this high‐risk population.
Keywords: Acute myocardial infarction, Atrial fibrillation, Heart failure, Mortality, Risk factor
Introduction
Heart failure (HF) is a common finding after acute myocardial infarction (AMI) and has been associated with excess mortality and morbidity. 1 Owing to the effective utilization of invasive strategies and intensive pharmacotherapy, the incidence rate of HF after AMI has markedly decreased during the last decades, but the long‐term survival after HF complicating AMI remains poor. 2 , 3 , 4 Therefore, a better understanding of risk factors involving in the development of HF in long‐term AMI survivors will help identify individuals expected to benefit from the preventive measures.
New‐onset atrial fibrillation (NOAF) is a frequent complication during the acute phase of MI, and the incidence rate of which ranges from 5% to 21%. 5 Previous studies have shown that the presence of NOAF in the setting of AMI is independently associated with increased risks of death and ischemic stroke. 6 , 7 Given the fact that a transient NOAF episode is even related to poor outcome, NOAF during AMI should not be perceived as a benign event anymore. 8 AF and HF often coincide in clinical practice, and the reciprocal impact of both conditions on patients' prognosis has been validated in various settings. 9 , 10 However, until now, there remains a paucity of evidence regarding the association of NOAF with long‐term risk of HF in a contemporary AMI cohort.
Accordingly, in this analysis using data from the New‐Onset Atrial Fibrillation Complicating Acute Myocardial Infarction in Shanghai (NOAFCAMI‐SH) registry (ClinicalTrials.gov Identifier: NCT03533543), we aimed to investigate the relationship between NOAF and the long‐term risk of HF hospitalization, as well as the risk factors of NOAF during AMI hospitalization.
Methods
Data source
The NOAFCAMI‐SH registry was a retrospective hospital‐based AMI registry designed to investigate the association of NOAF‐related characteristics [including atrial fibrillation (AF) pattern, burden, duration, frequency, and with or without symptoms; details in Supporting Information, Table S1 ] with long‐term cardiovascular outcomes after AMI. The electronic medical records of 2698 patients who were over 18 years old and diagnosed with AMI at the coronary care unit of Shanghai Tenth People's Hospital between February 2014 and March 2018 had been reviewed for eligibility. AMI was diagnosed as an increase in serum troponin T (2 × upper limit of the normal range) associated with symptoms of ischemia and/or characteristic electrocardiogram (ECG) signs (ST‐segment–T wave changes, left bundle branch block, or presence of pathological Q waves). 11 After excluding patients whose clinical characteristics were not available (N = 157), those with a documented history of AF (N = 89), those with a rheumatic valvular disease (N = 4) or sick sinus syndrome (N = 2), those without heart rhythm data (N = 14), and those underwent emergent coronary artery bypass grafting surgery (N = 33), 2399 patients were included. Data within the NOAFCAMI‐SH registry included patient demographics, cardiovascular risk factors, medical history, laboratory tests, procedures, and medications.
Study population
All NOAFCAMI‐SH registry participants were included in the present analysis, except those who died during hospitalization or did not have transthoracic echocardiographic data within 7 days after AMI admission (N = 252), or those lost to follow‐up (N = 72). A total of 2075 patients were included in the final analysis. The study was performed following the Declaration of Helsinki, and the ethics committee of the Shanghai Tenth People's Hospital approved the study protocol (SHSY‐IEC‐KY‐4.1/18‐199/01). Informed consent was waived as information was extracted anonymously and participants were assigned to a code number.
New‐onset atrial fibrillation ascertainment
Atrial fibrillation was diagnosed in accordance with the consensus guidelines as the absence of P waves with irregular RR intervals lasting at least 30 s. 12 NOAF was defined as patients with no prior history of AF who developed the first documented AF episode. Continuous electronic monitoring (IntelliVue MP40, Philips, Netherlands) was started immediately after admission and continued throughout the hospitalization. All participants' electronic monitoring data were reviewed to adjudicate the occurrence of NOAF. Patients were divided into two groups according to the presence of NOAF.
Data collection
A detailed review of the electronic medical records was performed to collect patients' demographic characteristics, cardiovascular risk factors, baseline comorbidities, biological, echocardiography, and angiography data, as well as medications. Blood samples were obtained after 12 h of fasting and were examined in a central laboratory. Left atrial diameter, left ventricular end‐diastolic diameter, left ventricular end‐systolic diameter, and left ventricular ejection fraction (LVEF) were measured according to standardized criteria. 13 Definitions of essential clinical characteristics were described in Table S2 .
Outcome measures
The primary outcome was HF hospitalization, defined as a minimum of an overnight hospital stay of a participant who presented with symptoms and signs of HF or received intravenous diuretics. The secondary outcomes included cardiovascular and all‐cause death. All deaths without a clear non‐cardiovascular cause would be classified as cardiovascular deaths. Each patient was followed from the index AMI discharge to the date of HF hospitalization, death, or the end of the observational period (as of the censoring date of 10 April 2019), whichever came first. Clinical outcomes that occurred during the observational period were evaluated either by telephone interview or by a comprehensive review of the patient's inpatient and outpatient records.
Statistical analysis
Baseline characteristics were compared between patients with sinus rhythm (SR) and NOAF during index AMI hospitalization. Statistical significance for categorical variables was tested using the χ2 or Fisher's exact test and the analysis of Student's t‐test or Mann–Whitney U test for continuous variables, as appropriate. Independent predictors of in‐hospital NOAF were identified using a logistic regression model (details in Supporting Information).
Incidence rates were calculated using the total number of HF hospitalization and death during the observational period divided by person‐years at risk. Kaplan–Meier method was used to calculate the cumulative incidence of HF hospitalization and death stratified by rhythm status, and the difference was compared using the log‐rank test. Cox proportional hazard regression analysis was used to investigate the association of post‐MI NOAF and HF hospitalization and death. Baseline covariates incorporated in the multivariable models were selected on the basis of clinical plausibility and previous literature 14 including age, sex, current smoking, comorbidity [hypertension, diabetes, hyperlipidaemia, chronic kidney disease (CKD), HF, MI, and stroke/transient ischemic attack (TIA), initial presentation (Killip class on arrival > I, heart rate, systolic blood pressure) in‐hospital percutaneous coronary intervention (PCI) with stent, LVEF, and medications at discharge (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, statin, β‐blocker, and diuretic). No missing data existed in the abovementioned covariates. The assumption of proportional hazards was validated by a visual examination of the log (minus log) curves. In addition, we performed an exploratory analysis to demonstrate the association of NOAF pattern with HF hospitalization; patients with NOAF who discharged with SR were considered as with transient NOAF, while those remained with AF at discharge were considered as with persistent NOAF. 8
Propensity score matching analysis
We used a propensity score matching (PSM) method to balance important patient characteristics between groups. A logistic regression model was used to calculate the propensity score that represented each participants' probability of developing NOAF during the index AMI hospitalization. The covariates introduced in the logistic regression model were selected based on the measured baseline characteristics, including age, sex, smoking status, comorbidity (hypertension, diabetes, hyperlipidaemia, CKD, HF, MI, PCI, stroke/TIA), initial presentation [ST‐segment elevation myocardial infarction (STEMI), Killip class, heart rate, systolic blood pressure, and out‐of‐hospital cardiac arrest], and LVEF. A 1:2 (exposed to unexposed) nearest‐neighbour matching without replacement algorithm was performed using a caliper of 0.05 to ensure adequate matches.
Subgroup and sensitivity analysis
The relationship between NOAF and HF hospitalization was evaluated in several subgroups: age (< or ≥ 65 years), sex, hypertension, diabetes, Killip class (II–IV vs. I), estimated glomerular filtration rate (eGFR; < or ≥ 60 mL/min/1.73m2), MI category (STEMI vs. NSTEMI), and the use of PCI with stent.
Several sensitivity analyses were also performed. First, we further adjusted for LDL‐c, HDL‐c, peak TnT, and peak NT‐proBNP (N terminal pro brain natriuretic peptide). Second, we repeated the analysis after excluding all patients with a history of HF or developed acute HF during the index AMI hospitalization. Third, we repeated the analysis after excluding patients with an LVEF level of ≤40%. Fourth, HF hospitalization that occurred within the first month after discharge was censored. Fifth, a competing risk analysis using the method of Fine and Gray was conducted to treat all‐cause death as a competing event for the outcomes of interest. 15 Besides, we further adjusted the HF hospitalization during the observational period (modelled as a time‐varying covariate) as well as the use of dual‐antiplatelet therapy (DAPT) and oral anticoagulation at discharge to evaluate the association of post‐MI NOAF with long‐term mortality. All tests were two sided at the 0.05 significance level. All analyses were performed using Stata 14.0 (StataCorp, College Station, Texas).
Results
Patient characteristics
The flow diagram of patient inclusion was illustrated in Figure 1 . Of 2075 analysed patients, 228 (11.0%) developed NOAF during hospitalization. Baseline characteristics were summarized in Table 1 . The mean age was 65.2 ± 12.3 years, and 77.3% were men. Patients with NOAF were older, less likely to be female and current smokers, more likely to have a history of hypertension, CKD, HF, and stroke/TIA, were more likely to present with a higher Global Registry of Acute Coronary Events risk score compared with those with SR. In addition, patients with NOAF had higher levels of serum creatinine and peak NT‐proBNP, while lower total and LDL cholesterol and LVEF values when compared with those with SR. Out of 228 patients with NOAF, SR was restored during hospitalization in 188 (82.5%) patients of whom only one had undergone direct current cardioversion. Amiodarone (146/228) was the most commonly applied antiarrhythmic agent for cardioversion, as well as maintaining SR. NOAF individuals were more likely to be prescribed with amiodarone, diuretic, oral anticoagulants, and aldosterone antagonist, but less likely to receive DAPT and β‐blocker at discharge compared with SR (Table 2 ). Baseline characteristics stratified by NOAF pattern were shown in Table S3 .
Figure 1.
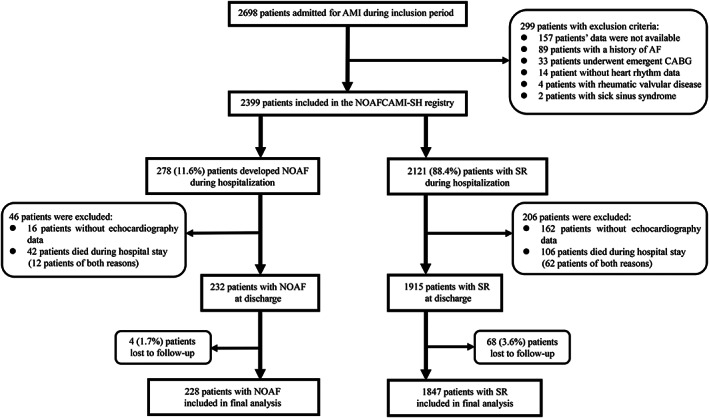
Flow diagram depicting the study population. AF, atrial fibrillation; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; NOAF, new‐onset atrial fibrillation; NOAFCAMI‐SH, New‐Onset Atrial Fibrillation Complicating Acute Myocardial Infarction in Shanghai; SR, sinus rhythm.
Table 1.
Baseline characteristics
| Characteristics | Overall (n = 2075) | Sinus rhythm (n = 1847) | NOAF (n = 228) | P value |
|---|---|---|---|---|
| Age (years) | 65.2 ± 12.3 | 64.1 ± 12.1 | 74.1 ± 10.6 | <0.001 |
| Male, n (%) | 1605 (77.3) | 1451 (78.6) | 154 (67.5) | <0.001 |
| BMI (kg/m2) | 24.6 ± 3.3 | 24.6 ± 3.3 | 24.3 ± 3.4 | 0.282 |
| Smoking status | <0.001 | |||
| Never | 946 (45.6) | 817 (44.2) | 129 (56.6) | |
| Former | 202 (9.7) | 171 (9.3) | 31 (13.6) | |
| Current | 927 (44.7) | 859 (46.5) | 68 (29.8) | |
| Comorbidity | ||||
| Hypertension | 1307 (63.0) | 1149 (62.2) | 158 (69.3) | 0.036 |
| Diabetes | 763 (36.8) | 675 (36.5) | 88 (38.6) | 0.545 |
| Hyperlipidaemia | 547 (26.4) | 497 (26.9) | 50 (21.9) | 0.107 |
| Chronic kidney disease | 174 (8.4) | 147 (8.0) | 27 (11.8) | 0.046 |
| History of heart failure | 109 (5.3) | 79 (4.3) | 30 (13.2) | <0.001 |
| Prior MI | 135 (6.5) | 117 (6.3) | 18 (7.9) | 0.367 |
| Prior PCI | 183 (8.8) | 155 (8.4) | 28 (12.3) | 0.051 |
| Prior TIA | 235 (11.3) | 196 (10.6) | 39 (17.1) | 0.004 |
| Initial presentation | ||||
| Out‐of‐hospital cardiac arrest | 44 (2.1) | 35 (1.9) | 9 (4.0) | 0.042 |
| STEMI | 1262 (60.8) | 1122 (60.7) | 140 (61.4) | 0.848 |
| Anterior location | 654 (51.8) | 585 (52.1) | 69 (49.3) | 0.429 |
| Inferior location | 552 (43.7) | 485 (43.2) | 67 (47.9) | |
| Other | 56 (4.4) | 52 (4.6) | 4 (2.9) | |
| Heart rate (bpm) | 79.7 ± 16.7 | 79.1 ± 15.6 | 84.7 ± 23.5 | <0.001 |
| SBP (mmHg) | 138.1 ± 24.2 | 138.2 ± 23.8 | 137.1 ± 26.9 | 0.563 |
| Killip class >I | 288 (13.9) | 221 (12.0) | 67 (29.4) | <0.001 |
| GRACE risk score | 119.5 ± 27.3 | 116.6 ± 25.9 | 142.7 ± 27.0 | <0.001 |
BMI, body mass index; GRACE, Global Registry of Acute Coronary Events; MI, myocardial infarction; NOAF, new‐onset atrial fibrillation; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST‐segment elevation myocardial infarction; TIA, transient ischemic attack.
Values are presented as number (percentage) or mean ± SD, as appropriate.
Table 2.
In‐hospital examination and medications at discharge
| Overall (n = 2075) | Sinus rhythm (n = 1847) | NOAF (n = 228) | P value | |
|---|---|---|---|---|
| Laboratory indices | ||||
| Total cholesterol (mmol/L) | 4.38 (3.76–5.07) | 4.42 (3.79–5.10) | 4.18 (3.53–4.94) | 0.002 |
| LDL‐c (mmol/L) | 2.64 (2.12–3.24) | 2.66 (2.15–3.25) | 2.44 (1.89–3.08) | 0.002 |
| HDL‐c (mmol/L) | 0.99 (0.83–1.17) | 0.99 (0.84–1.17) | 1.02 (0.83–1.23) | 0.234 |
| Serum creatinine (mg/dL) | 0.88 (0.75–1.05) | 0.88 (0.75–1.03) | 0.96 (0.82–1.27) | <0.001 |
| eGFR (mL/min/1.73 m2) | 86.0 (67.7–97.3) | 88.0 (70.4–98.3) | 69.8 (48.6–84.4) | <0.001 |
| Peak TnT (ng/mL) | 3.02 (0.80–8.06) | 2.91 (0.79–7.76) | 3.86 (0.76–10.0) | 0.051 |
| Log peak NT‐proBNP (pg/mL) | 3.18 (2.86–3.53) | 3.14 (2.83–3.47) | 3.64 (3.34–3.98) | <0.001 |
| Angiographic data | ||||
| In‐hospital PCI with stent | 1750 (84.3) | 1577 (85.4) | 173 (75.9) | <0.001 |
| Infarct‐related artery a | 0.132 | |||
| Left anterior descending | 618 (51.5) | 561 (52.5) | 57 (43.5) | |
| Right coronary artery | 451 (37.6) | 392 (36.7) | 59 (45.0) | |
| Left circumflex | 130 (10.8) | 115 (10.8) | 15 (11.5) | |
| Left main disease | 89 (4.7) | 68 (4.0) | 21 (10.3) | <0.001 |
| Echocardiographic data | ||||
| Left atrial diameter (mm) | 38.1 ± 4.6 | 37.9 ± 4.5 | 40.2 ± 5.0 | <0.001 |
| LVEDD (mm) | 45.6 ± 4.8 | 45.5 ± 4.6 | 46.1 ± 5.8 | 0.115 |
| LVESD (mm) | 31.0 ± 5.5 | 30.8 ± 5.4 | 32.1 ± 6.5 | <0.001 |
| LVEF (%) | 50.4 ± 10.6 | 50.9 ± 10.3 | 46.5 ± 11.6 | <0.001 |
| Medications at discharge | ||||
| Aspirin | 1908 (92.0) | 1708 (92.5) | 200 (87.7) | 0.013 |
| P2Y12 receptor inhibitor | 1994 (96.1) | 1782 (96.5) | 212 (93.0) | 0.010 |
| DAPT | 1843 (88.8) | 1655 (89.6) | 188 (82.5) | 0.001 |
| Oral anticoagulation | 12 (0.6) | 2 (0.1) | 10 (4.4) | <0.001 |
| ACEI/ARB | 1227 (59.1) | 1104 (59.8) | 123 (53.9) | 0.091 |
| β‐blocker | 1502 (72.4) | 1367 (74.0) | 135 (59.2) | <0.001 |
| Aldosterone antagonist | 379 (18.3) | 325 (17.6) | 54 (23.7) | 0.009 |
| Statin | 1989 (95.9) | 1775 (96.1) | 214 (93.9) | 0.109 |
| Diuretic | 303 (14.6) | 236 (12.8) | 67 (29.4) | <0.001 |
| Amiodarone | 54 (2.6) | 24 (1.3) | 30 (13.2) | <0.001 |
ACEI/ARB, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; DAPT, dual‐antiplatelet therapy; eGFR, estimated glomerular filtration rate; HDL‐c, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐c, low‐density lipoprotein cholesterol; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; NOAF, new‐onset atrial fibrillation; NT‐proBNP, N terminal pro brain natriuretic peptide; PCI, percutaneous coronary intervention.
Values are presented as number (percentage), mean ± SD, or median (IQR), as appropriate.
For patients with STEMI undergoing angiography.
Predictors of new‐onset atrial fibrillation development
Findings from a multivariable logistic model identifying predictors of NOAF were shown in Table 3. Every 10 year increase in age was the strongest predictor of the occurrence of NOAF [odds ratio: 1.88, 95% confidence interval (CI): 1.60–2.19]. Other significant predictors (in decreasing order) included admission Killip > I, impaired kidney function (eGFR < 60 mL/min/1.73m2), decreased LVEF value, elevated admission heart rate, and left atrial enlargement.
Table 3.
Independent predictors of post‐MI NOAF
| Variables | Multivariable‐adjusted risk | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Age (+10 years) | 1.88 | 1.60–2.19 | <0.001 |
| Admission Killip > I | 1.68 | 1.14–2.48 | 0.008 |
| eGFR<60 mL/min/1.73m2 | 1.60 | 1.10–2.32 | 0.014 |
| LVEF<40% | 1.59 | 1.10–2.31 | 0.015 |
| Admission heart rate (+10 bpm) | 1.19 | 1.09–1.30 | <0.001 |
| Left atrial diameter (+1 mm) | 1.07 | 1.03–1.11 | <0.001 |
Discrimination: C index: 0.77 (95% CI: 0.74–0.81); calibration: H–L test P value: 0.869.
CI, confidence interval; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NOAF, new‐onset atrial fibrillation; OR, odds ratio.
New‐onset atrial fibrillation complicating acute myocardial infarction and heart failure hospitalization
During a median observational period of 2.7 years (interquartile range: 1.6–3.9), a total of 72 (3.5%) patients lost to follow‐up and were excluded from this analysis. Overall, 205 patients (9.9%) experienced HF hospitalization, with an annual incidence rate of 18.4% and 2.8% for patients with NOAF and SR, respectively (Figure 2 A ). After adjustment for confounding factors, NOAF remained a predictor of HF hospitalization [hazard ratio (HR): 3.14, 95% CI: 2.30–4.28, P < 0.001] (Table 4 ). Results were similar when accounting for the competing risk of all‐cause death [subdistribution HR (sHR): 3.06, 95% CI: 2.18–4.30, P < 0.001]. In our exploratory analyses, the risk of HF hospitalization was significantly higher in patients with persistent NOAF (HR: 5.81, 95% CI: 3.59–9.41) compared with that in those with transient NOAF (HR: 2.61, 95% CI: 1.84–3.70, P interaction = 0.008).
Figure 2.
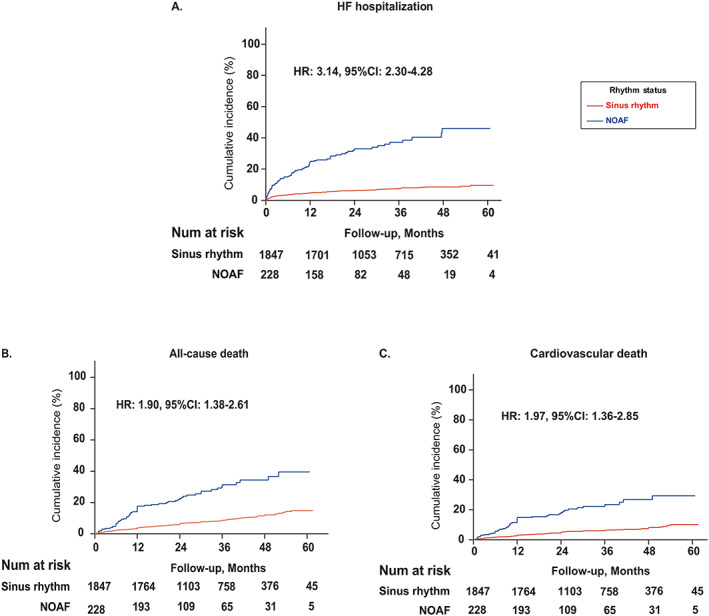
Kaplan–Meier curves illustrating the cumulative incidence of (A) heart failure (HF) hospitalization, (B) all‐cause death, and (C) cardiovascular death according to rhythm status. CI, confidence interval; HR, hazard ratio; NOAF, new‐onset atrial fibrillation.
Table 4.
Long‐term clinical outcomes
| NOAF development during hospitalization | Multivariable adjusted risk a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical outcome | Absent | Present | Unadjusted risk | ||||||||
| Events/Patients | %/Year (95% CI) | Events/Patients | %/Year (95% CI) | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||||
| HF hospitalization | 130/1847 | 2.8 (2.3–3.3) | 75/228 | 18.4 (14.6–23.0) | 5.81 (4.36–7.73) | <0.001 | 3.14 (2.30–4.28) | <0.001 | |||
| All‐cause death | 158/1847 | 3.2 (2.8–3.8) | 62/228 | 12.3 (9.6–15.8) | 3.76 (2.80–5.05) | <0.001 | 1.90 (1.38–2.61) | <0.001 | |||
| Cardiovascular death | 112/1847 | 2.3 (1.9–2.7) | 47/228 | 9.4 (7.0–12.5) | 3.95 (2.81–5.55) | <0.001 | 1.97 (1.36–2.85) | <0.001 | |||
CI, confidence interval; HF, heart failure; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; OR, odds ratio.
Adjusted for age, sex, current smoking, comorbidity (hypertension, diabetes, hyperlipidaemia, chronic kidney disease, HF, myocardial infarction, stroke/transient ischemic attack), initial presentation (Killip on arrival > I, heart rate, systolic blood pressure), in‐hospital percutaneous coronary intervention with stent, LVEF, and the medications at discharge (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, statin, β blocker, diuretic).
New‐onset atrial fibrillation complicating acute myocardial infarction and mortality
A total of 220 patients (10.6%) died during the observational period, of whom 159 were cardiovascular deaths. The annual incidence rates of all‐cause mortality in patients with SR and NOAF were 3.2% and 12.3%, respectively, and of cardiovascular mortality were 2.3% and 9.4%, respectively (Figure 2 B,C ). Following multivariable adjustment, NOAF was independently associated with the risk of cardiovascular (HR: 1.97, 95% CI: 1.36–2.85, P < 0.001) and all‐cause death (HR: 1.90, 95%CI: 1.38–2.61, P < 0.001) (Table 4 ). Results were similar when performing competing risk analysis for cardiovascular death with non‐cardiovascular death as a competing factor (sHR: 1.98, 95% CI: 1.36–2.88, P < 0.001). When we further adjusted for the HF hospitalization (modelled as a time‐varying covariate) as well as the use of DAPT and oral anticoagulation at discharge, we found that NOAF was not an independent risk factor of cardiovascular death (HR: 1.37, 95% CI: 0.92–2.02, P = 0.121), and its association with all‐cause death was attenuated (HR: 1.43, 95% CI: 1.02–2.00, P = 0.037). In contrast, post‐discharge HF hospitalization was determined as the strongest predictor for both cardiovascular death (HR: 7.23, 95% CI: 5.01–10.43, P < 0.001) and all‐cause death (HR: 5.21, 95% CI: 3.78–7.18, P < 0.001).
Propensity score matching analyses
The PSM cohorts were well balanced (Table S4 ). In the matched cohorts, the annual incidence rate of HF hospitalization was 6.2% for SR and 18.4% for NOAF (HR:2.82, 95% CI:1.99–4.00, P < 0.001); of all‐cause death was 7.8% for SR and 12.2% for NOAF (HR: 1.73, 95% CI: 1.23–2.45, P = 0.002); of cardiovascular death was 5.8% for SR and 9.2% for NOAF (HR: 1.91, 95% CI: 1.27–2.87, P = 0.002) (Figure 3 A–C ).
Figure 3.
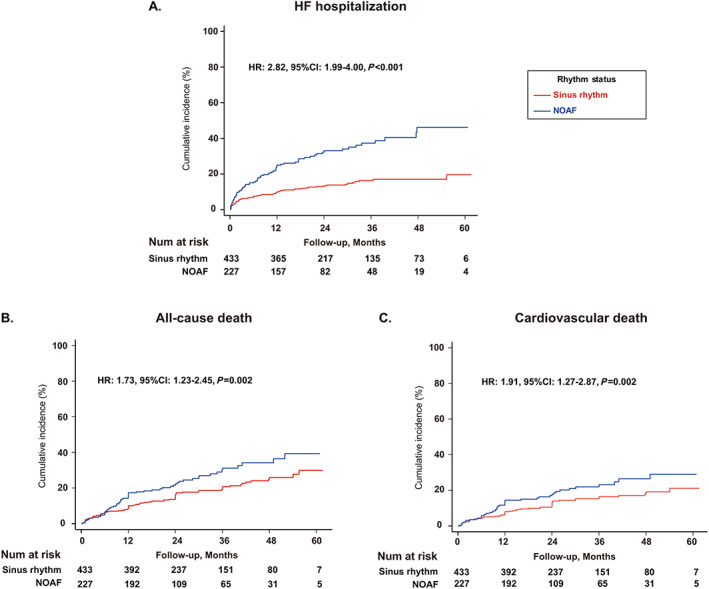
Long‐term risk of (A) heart failure (HF) hospitalization, (B) all‐cause death, (C) cardiovascular death in the propensity score‐matched cohorts. CI, confidence interval; HR, hazard ratio; NOAF, new‐onset atrial fibrillation.
Subgroup and sensitivity analyses
As illustrated in Figure 4 , consistent results were observed across all subgroups compared with that in the primary analysis. As for sensitivity analysis, adjusted HR for HF hospitalization was similar after further adjustment for biological data (LDL‐c, HDL‐c, peak TnT, and peak NT‐proBNP; HR: 3.04, 95% CI: 2.18–4.23, P < 0.001). Comparable results were also observed after excluding patients who had a history of HF or developed acute HF during AMI hospitalization (HR: 2.05, 95% CI: 1.30–3.21, P = 0.002), or after excluding those who had an LVEF value of ≤40% (HR: 4.21, 95% CI: 2.65–6.68, P < 0.001). When censoring the HF hospitalization events that occurred within the first month after discharge, the association between NOAF and HF hospitalization remained significant (HR: 3.18, 95% CI: 2.25–4.49, P < 0.001).
Figure 4.
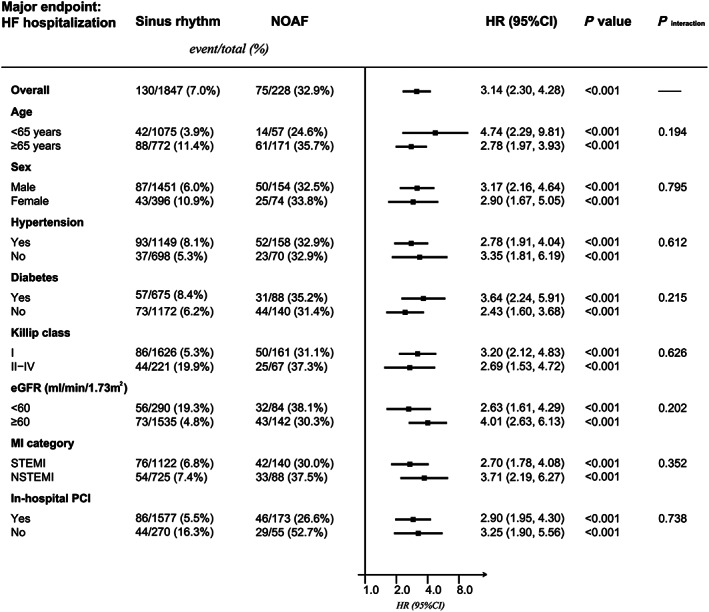
Subgroup analyses evaluating the association between post‐myocardial infarction (MI) new‐onset atrial fibrillation (NOAF) and heart failure (HF) hospitalization. CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
Discussion
In this analysis, we found that the presence of NOAF during AMI was significantly associated with a nearly three‐fold increased long‐term risk of HF hospitalization. The consistent results obtained from the PSM cohorts as well as accounting for the competing risk of death further strengthen our findings. The risk of HF hospitalization was more pronounced in patients with persistent AF compared with those with transient AF, which indicated a dose–response relationship. Although patients with NOAF had a higher risk of death compared with those with SR, such an association was attenuated when accounting for the impact of HF hospitalization.
In this study, increasing age, admission HF, impaired kidney function, decreased LVEF level, elevated admission heart rate, and left atrial enlargement were identified as independent predictors of NOAF during AMI. Advanced age is a well‐established risk factor of AF. 16 Left atrial enlargement that reflects the adverse structural remodelling in the left atrium has also proven to be a predictor of AF after AMI. 17 Because left ventricular (LV) dysfunction and impaired haemodynamics have been recognized as potential mechanisms leading to the occurrence of NOAF, it was not surprising to find that patients with admission HF, decreased LVEF, or elevated admission heart rate (a surrogate of LV dysfunction) were at higher risk of AF compared with those with normal cardiac function. 16 In an analysis of the Atherosclerosis Risk in Communities study, 18 the incidence rates of AF in patients with an eGFR of 30 to 59 mL/min/1.73m2 and 15 to 30 mL/min/1.73m2 were nearly 1.6‐fold and 3.2‐fold higher compared with that in patients with an optimal eGFR (≥90 mL/min/1.73m2), respectively, which supported the predictive value of impaired kidney function for AF occurrence. Taken together, our results suggested that extensive electrical monitoring should be considered in patients with renal or cardiac dysfunction after AMI for underlying NOAF detection.
The prognostic impact of NOAF in patients with AMI has been extensively studied. In a previous meta‐analysis, Jabre et al. demonstrated that post‐MI NOAF was associated with a 37% greater all‐cause mortality compared with the SR, but the majority of studies included in their analysis were conducted in the thrombolysis era. 6 Therefore, the clinical implication of post‐MI NOAF remains to be validated in contemporary cohorts. Our results showed that post‐MI NOAF was significantly associated with 90% higher all‐cause and 97% higher cardiovascular mortality after multivariable adjustment. However, when accounting for the impact of HF hospitalization, NOAF was no longer an independent risk factor of cardiovascular death. Similarly, in Asanin et al., after adjustment for baseline characteristics together with in‐hospital HF, NOAF was not associated with long‐term mortality (adjusted relative risk: 1.14, 95% CI: 0.72–1.79). 19 As a result, our analysis showed that the association between NOAF during AMI and long‐term mortality might be HF‐dependent, which underscored the adverse impact of HF after AMI.
The finding that NOAF developing during the acute phase of MI was associated with an increased risk of HF adds to a growing body of published studies highlighting the prognostic importance of post‐MI HF. In an analysis of the Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO‐Kyoto) AMI registry, the onset of HF hospitalization within 1 year after STEMI was independently associated with a 64% increased risk of all‐cause death. 4 In a large cohort of 199 851 patients with AMI, Desta et al. reported nearly two‐fold higher mortality in patients with HF compared with those without HF. 2 Finally, in a nationwide analysis of 47 673 patients without a history of HF who were discharged alive after AMI, post‐discharge HF during 1 year follow‐up accounted for 5.4% of AMI patients but contributed 24.5% and 29.1% of all‐cause and cardiovascular death, respectively. After multivariable analysis, post‐discharge HF was identified as a strong predictor of cardiovascular (HR: 7.93, 95% CI: 6.84–9.19) and all‐cause mortality (HR: 5.98, 95% CI: 5.39–6.64). 20 In line with previous studies, we also demonstrated that HF hospitalization was an independent predictor of mortality after AMI. In this regard, our results were of paramount clinical importance because the identification of NOAF as a strong predictor of long‐term HF after AMI would give clinicians a new way to recognize high‐risk patients.
Interestingly, in our exploratory analysis, we uncovered a dose–response relationship as patients with persistent NOAF were challenged by a higher risk of HF hospitalization compared with those with transient NOAF. The explicit reasons for such a divergence in NOAF pattern are still elusive, and we postulated that the progression of AF burden might be one of the causes. In a post‐hoc analysis of ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial) trial, Wong et al. precisely quantified the duration of subclinical AF and demonstrated that the progression of subclinical AF duration from 6 min–24 h to >24 h (or overt AF) was associated with increased risk of HF hospitalization (HR: 4.58, 95% CI: 1.64–12.8). 21 As there were still no data available to address the impact of NOAF pattern on HF after AMI, our study filled this knowledge gap in the literature and indicated that patients with persistent NOAF could benefit more from the preventive strategies compared with those with transient one.
Limitations
This was a single‐centre retrospective study conducted in a hospital‐based setting. Because of the observational design of our study, the observed effects were limited to associations rather than causality given potential unmeasured confounders. Second, although patients with a history of AF had been excluded according to their medical records, some patients who had an undiagnosed AF before admission might be misclassified into the NOAF group. Third, despite the usefulness of ECG parameters (e.g. P‐wave duration, axis, etc.) in predicting AF, 22 we could not evaluate their influences on the occurrence of post‐MI NOAF because the NOAFCAMI‐SH registry specifically focused on the prognostic implication of AF‐related characteristics (Table S1 ) and had not included individuals' ECG data. Similarly, because of the lack of information on angiotensin receptor‐neprilysin inhibitor, we could not examine its effect on clinical outcomes.
Conclusions
Our findings indicate that NOAF during AMI hospitalization is independently associated with increased long‐term risk of HF hospitalization. Patients with persistent NOAF are at higher risk of HF hospitalization compared with those with transient NOAF. As a result, NOAF complicating AMI can be a suitable preventive and therapeutic target of post‐MI HF.
Author contributions
JCL and YDW contributed to the conception and design of this work; JCL, SLX, HQL, ZQL, MMG, BXL, BBS, and XMQ contributed to the acquisition, analysis, and interpretation of data for this work. JCL drafted the manuscript. All authors had critically revised and gave the final approval of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81270193) and the Natural Science Foundation of Shanghai (grant number 18ZR1429700).
Conflict of Interest
None declared.
Supporting information
Table S1. Definitions of NOAF‐related characteristics in the NOAFCAMI‐SH registry
Table S2. Definitions of baseline clinical characteristics
Table S3. Baseline characteristics of included patients stratified by the pattern of NOAF
Table S4. Baseline characteristics of patients with sinus rhythm and NOAF before and after propensity‐score matching
Supplemental references
Luo, J. , Xu, S. , Li, H. , Li, Z. , Liu, B. , Qin, X. , Gong, M. , Shi, B. , and Wei, Y. (2020) Long‐term impact of new‐onset atrial fibrillation complicating acute myocardial infarction on heart failure. ESC Heart Failure, 7: 2762–2772. 10.1002/ehf2.12872.
References
- 1. Steg PG, Dabbous OH, Feldman LJ, Cohen‐Solal A, Aumont MC, Lopez‐Sendon J, Budaj A, Goldberg RJ, Klein W, Anderson FA Jr. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004; 109: 494–499. [DOI] [PubMed] [Google Scholar]
- 2. Desta L, Jernberg T, Lofman I, Hofman‐Bang C, Hagerman I, Spaak J, Persson H. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail 2015; 3: 234–242. [DOI] [PubMed] [Google Scholar]
- 3. Hung J, Teng TH, Finn J, Knuiman M, Briffa T, Stewart S, Sanfilippo FM, Ridout S, Hobbs M. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: a population‐based study of 20,812 patients with first acute myocardial infarction in Western Australia. J Am Heart Assoc 2013; 2: e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taniguchi T, Shiomi H, Morimoto T, Watanabe H, Ono K, Shizuta S, Kato T, Saito N, Kaji S, Ando K, Kadota K, Furukawa Y, Nakagawa Y, Horie M, Kimura T. Incidence and prognostic impact of heart failure hospitalization during follow‐up after primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction. Am J Cardiol 2017; 119: 1729–1739. [DOI] [PubMed] [Google Scholar]
- 5. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J 2009; 30: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 6. Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta‐analysis. Circulation 2011; 123: 1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo J, Li H, Qin X, Liu B, Zhao J, Maihe G, Li Z, Wei Y. Increased risk of ischemic stroke associated with new‐onset atrial fibrillation complicating acute coronary syndrome: a systematic review and meta‐analysis. Int J Cardiol 2018; 265: 125–131. [DOI] [PubMed] [Google Scholar]
- 8. Batra G, Svennblad B, Held C, Jernberg T, Johanson P, Wallentin L, Oldgren J. All types of atrial fibrillation in the setting of myocardial infarction are associated with impaired outcome. Heart 2016; 102: 926–933. [DOI] [PubMed] [Google Scholar]
- 9. Lip GY, Laroche C, Popescu MI, Rasmussen LH, Vitali‐Serdoz L, Dan GA, Kalarus Z, Crijns HJ, Oliveira MM, Tavazzi L, Maggioni AP, Boriani G. Heart failure in patients with atrial fibrillation in Europe: a report from the EURObservational Research Programme Pilot survey on Atrial Fibrillation. Eur J Heart Fail 2015; 17: 570–582. [DOI] [PubMed] [Google Scholar]
- 10. Nieuwlaat R, Eurlings LW, Cleland JG, Cobbe SM, Vardas PE, Capucci A, Lopez‐Sendon JL, Meeder JG, Pinto YM, Crijns HJ. Atrial fibrillation and heart failure in cardiology practice: reciprocal impact and combined management from the perspective of atrial fibrillation: results of the Euro Heart Survey on atrial fibrillation. J Am Coll Cardiol 2009; 53: 1690–1698. [DOI] [PubMed] [Google Scholar]
- 11. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Eur Heart J 2012; 33: 2551–2567. [DOI] [PubMed] [Google Scholar]
- 12. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 14. Biering‐Sorensen T, Querejeta Roca G, Hegde SM, Shah AM, Claggett B, Mosley TH Jr, Butler KR Jr, Solomon SD. Left ventricular ejection time is an independent predictor of incident heart failure in a community‐based cohort. Eur J Heart Fail 2018; 20: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 16. Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO‐I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol 1997; 30: 406–413. [DOI] [PubMed] [Google Scholar]
- 17. Parkash R, Green MS, Kerr CR, Connolly SJ, Klein GJ, Sheldon R, Talajic M, Dorian P, Humphries KH. The association of left atrial size and occurrence of atrial fibrillation: a prospective cohort study from the Canadian Registry of Atrial Fibrillation. Am Heart J 2004; 148: 649–654. [DOI] [PubMed] [Google Scholar]
- 18. Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123: 2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asanin M, Perunicic J, Mrdovic I, Matic M, Vujisic‐Tesic B, Arandjelovic A, Vasiljevic Z, Ostojic M. Prognostic significance of new atrial fibrillation and its relation to heart failure following acute myocardial infarction. Eur J Heart Fail 2005; 7: 671–676. [DOI] [PubMed] [Google Scholar]
- 20. Sulo G, Igland J, Nygard O, Vollset SE, Ebbing M, Poulter N, Egeland GM, Cerqueira C, Jorgensen T, Tell GS. Prognostic impact of in‐hospital and postdischarge heart failure in patients with acute myocardial infarction: a nationwide analysis using data from the Cardiovascular Disease in Norway (CVDNOR) Project. J Am Heart Assoc 2017; 6: e005277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang J, Gold MR, Hohnloser SH, Lau CP, Capucci A, Botto G, Gronefeld G, Israel CW, Connolly SJ, Healey JS. Progression of device‐detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol 2018; 71: 2603–2611. [DOI] [PubMed] [Google Scholar]
- 22. Maheshwari A, Norby FL, Soliman EZ, Koene R, Rooney M, O'Neal WT, Alonso A, Chen LY. Refining prediction of atrial fibrillation risk in the general population with analysis of P‐wave axis (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2017; 120: 1980–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions of NOAF‐related characteristics in the NOAFCAMI‐SH registry
Table S2. Definitions of baseline clinical characteristics
Table S3. Baseline characteristics of included patients stratified by the pattern of NOAF
Table S4. Baseline characteristics of patients with sinus rhythm and NOAF before and after propensity‐score matching
Supplemental references


