Abstract
Aims
Accelerometers are becoming increasingly commonplace for assessing physical activity; however, their use in patients with cardiovascular diseases is relatively substandard. We aimed to systematically review the methods used for collecting and processing accelerometer data in cardiology, using the example of heart failure, and to provide practical recommendations on how to improve objective physical activity assessment in patients with cardiovascular diseases by using accelerometers.
Methods and results
Four electronic databases were searched up to September 2019 for observational, interventional, and validation studies using accelerometers to assess physical activity in patients with heart failure. Study and population characteristics, details of accelerometry data collection and processing, and description of physical activity metrics were extracted from the eligible studies and synthesized. To assess the quality and completeness of accelerometer reporting, the studies were scored using 12 items on data collection and processing, such as the placement of accelerometer, days of data collected, and criteria for non‐wear of the accelerometer. In 60 eligible studies with 3500 patients (of those, 536 were heart failure with preserved ejection fraction patients), a wide variety of accelerometer brands (n = 27) and models (n = 46) were used, with Actigraph being the most frequent (n = 12), followed by Fitbit (n = 5). The accelerometer was usually worn on the hip (n = 32), and the most prevalent wear period was 7 days (n = 22). The median wear time required for a valid day was 600 min, and between two and five valid days was required for a patient to be included in the analysis. The most common measures of physical activity were steps (n = 20), activity counts (n = 15), and time spent in moderate‐to‐vigorous physical activity (n = 14). Only three studies validated accelerometers in a heart failure population, showing that their accuracy deteriorates at slower speeds. Studies failed to report between one and six (median 4) of the 12 scored items, with non‐wear time criteria and valid day definition being the most underreported items.
Conclusions
The use of accelerometers in cardiology lacks consistency and reporting on data collection, and processing methods need to be improved. Furthermore, calculating metrics based on raw acceleration and machine learning techniques is lacking, opening the opportunity for future exploration. Therefore, we encourage researchers and clinicians to improve the quality and transparency of data collection and processing by following our proposed practical recommendations for using accelerometers in patients with cardiovascular diseases, which are outlined in the article.
Keywords: Heart failure, Cut points, Steps, Physical activity, Counts, Raw acceleration
Introduction
Because physical activity (PA) plays a crucial role in preventing and managing cardiovascular diseases such as atherosclerosis, hypertension, and chronic heart failure (HF), 1 , 2 , 3 it is important that PA and sedentary behaviour can be properly assessed by researchers and clinicians alike. Traditionally, PA has been assessed using questionnaires, but recent evidence shows that questionnaires have numerous limitations, including recall bias, missing data, and less precision. 4 Therefore, interest in objectively assessing PA has proliferated, with accelerometers representing the most popular tool used in contemporary research. Despite this, several systematic reviews have recently reported that the quality of accelerometry‐based PA assessment is relatively poor in studies in patients with cardiovascular diseases. 5 , 6 , 7 As poor or inaccurate assessment of PA using accelerometry can deleteriously impact the advancement of PA research in cardiology and impede its translation into clinical practice, it is crucial that we understand how accelerometers have been used in cardiology to date.
To address this issue, we chose HF as an example of a cardiovascular disease, because of its increasing prevalence and economic burden, and because the benefits of PA for patients with HF are well established. 2 , 6 , 8 We conducted a systematic review of studies using accelerometers in HF patients with the aims (i) to explore the methods used for collecting and processing accelerometry data, (ii) to evaluate accelerometry‐based metrics used for the assessment of PA, (iii) to examine the quality of reporting on data collection and processing, (iv) to review the validity of accelerometers in this population, and (v) to provide practical recommendations for researchers and clinicians on how to objectively assess PA and sedentary behaviour in patients with cardiovascular diseases using accelerometers.
Methods
The present review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement, and the review protocol has been registered in the international prospective register of systematic reviews (PROSPERO: CRD42019125427).
Search strategy
The following electronic bibliographic databases were searched: PubMed, SPORTDiscus (via EBSCO), Scopus, and IEEE Xplore Digital Library. The search strategy combined terms for HF (e.g. ‘heart failure’ and ‘HFrEF’) AND terms to identify articles dealing with accelerometers (e.g. ‘accelerometer’ and ‘inertial sensor’). The search terms for individual databases are available as Data S1. The search was limited to papers published in English‐language academic journals.
Studies published from the inception of the databases up to September 2019 were sought. Additionally, the reference lists of recently published reviews 5 , 6 , 7 were hand searched for further studies. Titles and abstracts of the retrieved studies were screened to identify studies that potentially meet the eligibility criteria. The full texts of these potentially eligible studies were assessed for eligibility by two review authors (T. V. and C. C.); any disagreement was resolved through discussion with a third reviewer (M. B.).
Eligibility criteria
The eligibility criteria fall into four areas: study types, patient population, devices, and PA metrics.
-
(1)
Study types. A wide range of study types was eligible for the review, including observational and interventional studies, as well as validation and calibration studies (including lab‐based studies). Published study protocols and conference abstracts, whose results were not yet reported in a full paper, were also included to cover the latest advances in the use of accelerometers.
-
(2)
Patient population. Studies were eligible if they involved at least 10 adult HF patients; studies that involved mix of patients with various diagnoses were only included if they reported on the outcomes of HF patients separately. Furthermore, studies were excluded if they included patients with implanted left ventricular assist device or if they included patients on the basis of their self‐diagnosis of HF (e.g. papers analysing the UK Biobank database).
-
(3)
Devices. Studies employing all types of wearable devices containing accelerometers (capacitive, piezoresistive, and piezoelectric) were eligible. However, studies using pedometers with a mechanical pendulum were excluded. In addition, studies that used accelerometers embedded in implantable devices (e.g. in resynchronization devices) were excluded.
-
(4)
PA metrics. To be eligible, studies had to report on at least one PA or sedentary behaviour metric. Studies that used accelerometers only as a tool for patients' self‐monitoring without reporting on PA metrics were excluded. Similarly, feasibility, usability, and other studies that used accelerometers but did not report on PA metrics were excluded. Finally, studies that employed accelerometers for other purposes than assessing PA and sedentary behaviour (e.g. sleep analysis and seismocardiography) were also excluded.
Data extraction
Data were extracted by three of the reviewers (T. V., M. B., and C. C.), using an Excel spreadsheet. Extracted information included study and population characteristics, details of accelerometry data collection and processing, and description of PA metrics. When a paper referred to supplementary materials and previously published papers (e.g. study protocols and pilot studies) for details, theses were retrieved and extracted.
Quality of reporting
To assess the quality and completeness of accelerometer reporting, the papers were scored using 12 questions on accelerometer information and data collection and processing (Table 1 ) as proposed by Montoye et al. 9 Manuscripts were given a ‘+1’ score for adequately reported item and a ‘−1’ score for not adequately reported item. The number of ‘−1’ scores was summed, and each paper was, therefore, given a score of 0 to −12, with scores closer to 0 indicating more complete reporting.
TABLE 1.
Questions assessing quality and completeness of accelerometer reporting
| Question | Number of assessed papers | Percentage of papers scoring ‘−1’ a |
|---|---|---|
| Brand of accelerometer used | 55 | 2 |
| Model of accelerometer used | 31 | 3 |
| Epoch length used | 55 | 60 |
| Placement of accelerometer (must indicate location and side of the body) | 55 | 64 |
| Number of participants enrolled at study start receiving accelerometers | 51 | 0 |
| How were accelerometers distributed | 55 | 0 |
| Days of data collected | 55 | 4 |
| Criteria for defining non‐wear of accelerometer | 51 | 80 |
| How many minutes of accelerometer data needed to be considered a valid day | 55 | 78 |
| Number of valid days of accelerometer data needed | 55 | 76 |
| What were physical activity outcome variables | 55 | 5 |
| Reported the number of people not meeting wear‐time criteria | 24 | 13 |
Score ‘−1’ is undesirable.
At several circumstances, some questions were not relevant to the study, and the items were not assessed. Firstly, when the manufacturer only had one model of a specific brand (e.g. GeneActiv and RT3), the question about the accelerometer model was not assessed (Question 1). Secondly, study protocols were not scored for the question about the number of participants enrolled at study start (Question 5). Thirdly, the question about compliance with wear‐time criteria was not assessed in studies that did not report any wear‐time criteria (Question 12). Finally, validation studies that were conducted in a laboratory setting, under direct observation of researchers, were not assessed at all, as most of the questions would be irrelevant.
Results
The database search yielded 1226 articles; of those, 139 full texts were retrieved, and 59 deemed eligible. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 Hand searching the reference lists of recent reviews identified one additional eligible paper. 68 Two of the papers used the same patient population but reported on two distinct studies; hence, they were both included. 27 , 28 In total, 60 papers were included in the review, as shown in PRISMA flow diagram (Figure 1 ). Their full list and the data extracted from these papers are available as Data S2 .
FIGURE 1.
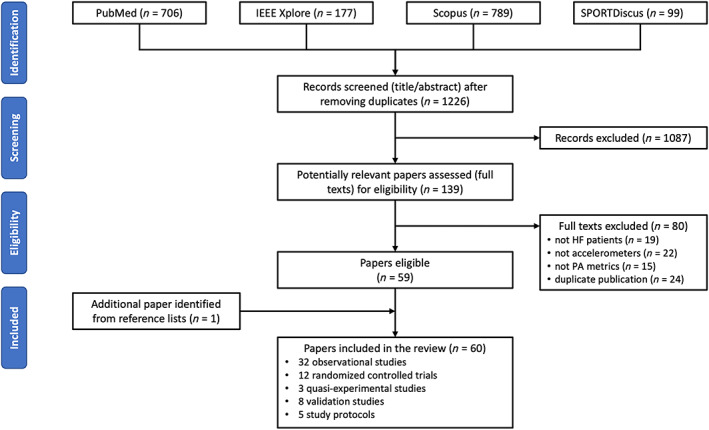
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram.
Study characteristics
Thirty‐two papers reported on observational studies, 10 , 11 , 12 , 13 , 17 , 18 , 20 , 21 , 23 , 24 , 25 , 28 , 33 , 36 , 37 , 39 , 40 , 41 , 43 , 44 , 46 , 47 , 50 , 52 , 53 , 56 , 60 , 61 , 64 , 65 , 66 12 on randomized controlled trials, 14 , 15 , 16 , 22 , 32 , 45 , 49 , 55 , 62 , 63 , 67 , 68 three on quasi‐experimental studies, 31 , 34 , 38 and eight on validation studies (of those, four were supervised lab‐based studies). 27 , 29 , 30 , 35 , 42 , 48 , 54 , 58 Furthermore, five published study protocols were included. 19 , 26 , 51 , 57 , 59
The vast majority (n = 52) of the papers were published in the past 10 years (since 2010), and almost a half (n = 26) were published between January 2017 and September 2019. The greatest number of studies originated in the USA (n = 23), followed by the UK (n = 8), Germany (n = 7), and Japan (n = 4).
Patient characteristics
Patient characteristics are reported from 54 studies: study protocols 19 , 26 , 51 , 57 , 59 are not included, and the two studies using the same population 27 , 28 are only included once. Altogether, 3500 HF patients participated in the reviewed studies; of those, 1088 in randomized controlled trials.
Twenty studies included only HF with reduced ejection fraction (HFrEF) patients, three studies included only HF with preserved ejection fraction (HFpEF) patients, and nine studies included both HFrEF and HFpEF patients (but only two of them reported their characteristics separately). In summary, these studies included 1813 HFrEF patients and 536 HFpEF patients. The remaining 22 studies did not specify the form of HF.
All but three studies reported participant sex; and 42 out of 51 studies recruited more men than women. Altogether, the studies included 1157 women and 2295 men. Thirty‐one studies indicated the proportion of patients according to their functional New York Heart Association (NYHA) class: 10% of NYHA I, 50% of NYHA II, 38% of NYHA III, and 2% of NYHA IV patients were recruited. The descriptive statistics of other reported patient characteristics, together with the number of reporting papers, are summarized in Table 2 .
TABLE 2.
Patient characteristics
| Minimum | Quartile 1 | Median | Quartile 3 | Maximum | Number of reporting papers (out of 54) | |
|---|---|---|---|---|---|---|
| Sample size (n) | 10 | 24 | 50 | 91 | 243 | 54 |
| Age (years) | 46 | 60 | 66 | 70 | 81 | 53 |
| BMI (kg/m2) | 22 | 28 | 29 | 31 | 37 | 35 |
| LVEF (%) a | 23 | 26 | 27 | 33 | 38 | 17 |
| 6MWD (m) | 243 | 306 | 386 | 413 | 510 | 22 |
| PeakVO2 (mL/min/kg) | 10 | 14 | 15 | 19 | 21 | 12 |
| BNP (ng/L) | 148 | 164 | 190 | 263 | 379 | 7 |
| NT‐proBNP (ng/L) | 230 | 737 | 2027 | 2593 | 3727 | 6 |
LVEF, left ventricular ejection fraction; 6MWD, 6‐min walk distance.
HFrEF patients only.
Data collection
In total, 27 accelerometer brands and 46 models were used in the reviewed studies. In addition, four studies used custom‐made accelerometers, and two studies did not specify the brand. The most frequently used brand was Actigraph (n = 12); followed by Fitbit and RT3 (both n = 5); Actiwatch, Aipermon, Omron, and SenseWear (all n = 4); and GeneActiv (n = 3).
The most common placement of the accelerometer was at the hip/waist/belt (n = 32), followed by wrist (n = 17), thigh (n = 7), chest (n = 7), and ankle (n = 3). In 11 cases, the placement site was not reported. The accelerometers were equally placed on the right/dominant (n = 17) or left/non‐dominant (n = 16) side. In two cases, the accelerometers were alternated between left and right sides. The side was not reported in 42 cases. Note that the number of placement cases is higher than number of studies, as some studies used multiple accelerometers at different sites and/or sides.
The wear protocol was either full 24 h (n = 23) or limited to waking hours (n = 16). In four validation studies, the accelerometer was worn only for the purpose of a lab‐based testing, and 17 studies did not report the wear protocol at all. The wear period ranged from 1 day to 1 year (median: 7 days, inter‐quartile range: 4–9 days), and a 7‐day wear period was the most frequent (n = 22). Three studies did not report the wear period at all.
Data processing
The length of epochs to which the accelerometer signal was aggregated was indicated in 23 studies. The epoch length varied from 1 s to 15 min, with 60 s being the most common epoch length (n = 13).
The method for detecting non‐wear time was described in 10 studies; these included the algorithm of Troiano 2007 (n = 2), another algorithm based on vector magnitude units (n = 2), a proprietary algorithm by Apple (n = 1), participants' daily logs (n = 3), and impedance (n = 1) or heart rate (n = 1) sensors combined with the accelerometer in the form of a multi‐sensor device. Specifically, of the 12 studies using an Actigraph, only three reported on the algorithm used to detect non‐wear time.
The valid day definition, that is the minimum wear time required for a day to be included in the analysis, was indicated in 12 studies; it ranged from 480 to 1200 min (median: 600 min). Among studies using an Actigraph, only four reported the valid day definition.
The minimum number of valid days required for a patient to be included in the analysis was reported by 13 studies. Of those, there were nine studies with a 7 day wear protocol, and these required 2 (n = 2), 3 (n = 3), 4 (n = 2), or 5 (n = 2) valid days. Of the 13 studies that reported the minimum number of valid days, only seven indicated the valid day definition, and only three described how non‐wear time was detected.
Physical activity metrics
The daily number of steps was the most often reported measure of PA (n = 20), followed by arbitrary activity ‘counts’ (n = 15), time spent in PA intensity levels (n = 14), energy expenditure (n = 8), and time spent in various body postures and activities (n = 5).
Of the 14 studies that reported on the time spent in different intensities of PA, seven employed an Actigraph accelerometer, and of these, four used Freedson 1998 cut points (in one case combined with Matthews 2005 cut points), one used Troiano 2008 cut points, and one did not state what cut points they used to distinguish between the PA levels. Three studies employed a SenseWear system, which uses multiple sensors (including accelerometers) to calculate intensity levels on the basis of the proprietary algorithm. One study employing a GeneActiv accelerometer used a raw acceleration cut point (>100 mg) to calculate time spent in moderate‐to‐vigorous physical activity (MVPA).
Other measures included performance during the most active period of the day (e.g. most active 30 min), measures of variance (e.g. skewness and kurtosis) calculated from the most active periods, time accumulated in intensity bins of raw acceleration, and measures of rest–activity patterns.
Quality of reporting
The quality and completeness of reporting were assessed in 55 studies (four lab‐based validation studies 27 , 29 , 30 , 54 and a study protocol 19 with a negligible accelerometer role were not assessed). Studies were scored on 9 to 12 items (mean: 10.9 items). The percentage of ‘−1’ scores for each of the 12 items is depicted in Table 1 .
No study achieved the highest score (0), and only three studies achieved a score of −1. 23 , 40 , 58 The median score was −4 (range −1 to −6). Scores of individual studies can be found in Data S3.
Validation studies
Eight validation studies were included in our review. Three studies used wearable accelerometers to examine concurrent validity of PA data from accelerometers embedded in cardiac implantable devices in free living. 35 , 42 , 48 Two studies investigated the validity of accelerometers to measure patients' performance during a 6‐min walk test. 27 , 30
Three studies assessed the validity of a wearable accelerometer to quantify PA during a structured supervised protocol. 29 , 54 , 58 In one study, the patients were asked to perform several everyday activities (e.g. reading, dressing, and walking), and the output of a custom‐made accelerometer was compared with visual analysis of simultaneously made video recordings. 54 The two other studies assessed step accuracy of accelerometers by visual observation on the treadmill and during free walking at various speeds. They both found that at speeds slower than 3.0–3.6 km/h, the accuracy of the devices substantially deteriorates. 29 , 58
In addition, one of these studies evaluated the free‐living validity of consumer‐level activity monitors as measures of step count, with an Actigraph wGT3X‐BT used as the criterion. The study evaluated six devices (Fitbit Charge 2, Garmin vívofit and vívofit 3, Withings Go, Omron HJ‐322U‐E, SmartLAB walk+) and concluded that most of them perform to a reasonable enough standard to be useful tools that clinicians can use to motivate HF patients to walk more. 58
Discussion
This systematic review is the first study to provide a comprehensive overview of the use of accelerometers in the assessment of PA and sedentary behaviour in HF patients. Despite the fact that accelerometers are becoming more commonplace in the assessment of PA in HF patients, until the current review, there was no consensus to provide much needed guidance to clinicians and exercise scientists wishing to employ accelerometers in this population.
In this review, we assessed 60 studies that used a wide variety of accelerometer brands and models, with Actigraph being the most frequent (n = 12). The accelerometer was usually worn on the hip (n = 32), and the most prevalent wear period was 7 days (n = 22). The most common measures of PA were steps (n = 20), activity counts (n = 15), and time spent in MVPA (n = 14). Only two studies used raw accelerometry data. 16 , 32 No study used machine learning or other emerging analytical techniques. 69
Considering the quality of reporting, studies failed to report between one and six (median 4) of the 12 items that were suggested as important for replication and correct interpretation of results of accelerometer studies. 9 The most underreported items included the criteria for defining non‐wear time, minimum number of minutes needed to be considered a valid day, and number of valid days needed for a patient to be included in the analysis.
Quality of reporting
Two systematic reviews have recently evaluated the quality and completeness of reporting on data collection and processing in general populations 9 and cancer survivors. 70 When comparing our results with the aforementioned reviews, caution must be taken, as the scoring has not been sufficiently standardized among the studies.
Despite the non‐standardized scoring procedures, many observations can be made. Notably, most studies in our review failed to report the details of data processing. Specifically, 80% of the studies failed to report criteria for defining non‐wear, as compared with 69% in general populations 9 and 49% in cancer survivors. 70 Furthermore, 78% of our studies did not report how many minutes of accelerometer data were needed to be considered as a valid day, and 76% failed to mention the number of valid days needed for the inclusion of a patient in the analysis. These percentages are substantially worse than those reported in general populations (50% and 52%, respectively) 9 and cancer survivors (38% and 43%, respectively). 70 We are reluctant to use this anecdotal evidence to claim that the quality of reporting in studies with HF patients is inferior to that in general populations or in cancer survivors. However, we are confident in asserting that reporting on data collection and especially processing methods needs to be markedly improved, as it is crucial for replicating and comparing future accelerometry‐based studies. Thus, clinicians and researchers are strongly encouraged to report accelerometry studies in accordance with the checklist developed by Montoye et al. 9 and presented in Table 1 , or other best practices. 71
Physical activity metrics
Although the majority of the studies were published in the past 10 years, most of them included traditional PA metrics such as steps, activity counts, and time spent in arbitrary PA intensities. When these metrics were first introduced, they simply reflected the capabilities of the technologies available at that time. Whilst these traditional metrics greatly contributed to the advancement of PA research, in particular the objectification of PA, they have several limitations that need to be considered. 72
Even though we excluded studies that used pendulum‐based pedometers, step count was the most frequently used measure of PA, reported in 33% of the studies. Because step count is intuitively understood by researchers, clinicians, and patients, it is useful for self‐monitoring purposes and as a basis for simple practical recommendations. However, it only accounts for locomotion (walking and running) and does not cover the whole spectrum of PA (swimming and cycling). Furthermore, the step‐counting algorithms provided by various manufacturers are all proprietary and use different thresholds of acceleration to detect a step, which limits the comparability among studies using different devices. 73 Despite numerous validation studies showing that most commercially available accelerometers are more or less equal in general populations with normal gait, 74 , 75 other studies have reported that step‐counting accuracy deteriorates in patients with chronic conditions, particularly during slower gait speeds. 58 , 76 As such, steps can still remain a useful self‐monitoring metric for patients but should be avoided by researchers striving for high accuracy and reproducibility of their findings.
Along with step counts, activity counts were the second most common metric, reported in 25% of the studies. The common method to obtain activity counts includes using a proprietary integration algorithm to calculate the area under the curve of the accelerometer signal for a given time window. 77 If steps were criticized for the proprietary character of step‐counting algorithms, activity counts are not only proprietary but also arbitrary in that they do not describe any specific construct of PA. Consequently, studies using different devices cannot be directly compared, and the use of activity counts should be discouraged. Albeit in normal populations, Rowlands et al. have robustly demonstrated the utility of assessing the raw acceleration signal to gain inference into PA, where such an approach is free from user‐selected arbitrary thresholds. 78
Time spent in PA intensity levels, particularly in MVPA, was reported in 23% of the studies. Time spent in MVPA is a standard measure of PA, which is related to the most current PA guidelines that recommend achieving at least 150 min of MVPA weekly. 1 To calculate the time spent in MVPA, accelerometer output needs to be calibrated, usually against indirect calorimetry, to establish cut points distinguishing between various intensity levels. 79 However, different populations (e.g. children vs. adults vs. elderly) have drastically different cut points, whilst different placements (e.g. hip vs. wrist) and number of accelerometer axes (vertical axis vs. sum of all three axes) also produce different cut points. 80 , 81 Hence, for each specific situation, there needs to be a calibration study producing specific cut points. Once this prerequisite is met, time spent in MVPA can be directly compared among studies with different accelerometers, in theory. In reality, wearing the same accelerometer model concurrently at various placements produces strikingly varying results (4–66 min of MVPA per day) even though the appropriate cut points are applied. 81 Furthermore, using cut points that were derived from normal healthy populations to calculate time spent in MVPA in patients with reduced cardiorespiratory fitness [e.g. HF, chronic obstructive pulmonary disease (COPD), and obese] is inherently flawed. Unfortunately, to the best of our knowledge, no cut points specific for HF patients have been devised.
Recently, it has been suggested that raw acceleration (Euclidean Norm minus 1 g, ENMO), rather than activity counts, could and should be used to develop PA intensity levels cut points. 82 Even though this approach does not solve the above‐mentioned calibration issue, it enables us to compare studies that use different accelerometers, provided they produce raw acceleration output (Actigraph, GeneActiv, and Axivity). 74 Unfortunately, this approach has been used only by one of the reviewed studies. 16
Regardless whether cut points are expressed in activity counts or raw acceleration, using time spent in PA intensity levels has other disadvantages. 72 For example, many HF patients are likely to fail to obtain any activity above the MVPA cut point; consequently, a large number of study participants score 0 min, as was the case in one of the reviewed studies where median minutes of MVPA were zero. 65 Rowlands et al. recently suggested several new metrics on the basis of raw acceleration, such as the level of acceleration above which a person's most active 30 min are accumulated (M30), 72 or intensity gradient, which can be used in combination with average acceleration to fully describe PA profile. 83 A similar approach has been employed by one of the reviewed studies that calculated time accumulated in narrow intensity bins (0–20–40–60–80–100 mg) of raw acceleration. 32
Practical recommendations
-
(1)
Limit the use of steps only to the studies that focus on walking, for example, when the aim of the intervention is to increase daily number of steps, or when participants are instructed to self‐monitor their steps. 57 In addition, researchers and clinicians must be cognizant of the inaccuracy of most devices at slow gait speeds. 58
-
(2)
Use time spent in PA intensity levels with caution, especially in patients with reduced cardiorespiratory fitness. Consider developing cut points on the basis of raw acceleration, specific for the population of interest or individually tailored cut points. 79 , 84
-
(3)
Whenever possible, use accelerometers that provide raw acceleration output. 78 Also, consider using recently developed metrics such as average acceleration, intensity gradient, or acceleration above which the most active 30 min is accumulated. 72 , 83 These can be easily calculated using a GGIR package in R. 85
-
(4)
When choosing the accelerometer brand and model, any device featuring triaxial accelerometry and providing raw acceleration data is suitable. There are currently three major, research‐grade brands on the market that comply with these requirements: Actigraph, Axivity, and GeneActiv. On the other hand, additional compliant devices are likely available. 74
-
(5)
With regard to the data collection and processing, there are no validation/calibration studies or studies comparing different protocols specifically in cardiovascular patients; therefore, we can only mirror the recommendations that were devised for general populations 86 and other chronic conditions, such as COPD. 87 As such, these recommendations need to be considered as merely a starting point for the specific choices that will always reflect the study aims and the previously selected PA metrics.
Wear time
Most researchers agree on 24 h wear for seven continuous days. As opposed to waking‐hours‐only protocols, 24 h data cover all physical behaviours (including sleep) and enable the analysis of their interplay using techniques such as compositional analysis or isotemporal substitution. However, when a study only focuses on MVPA or daily step count, there is no need for 24 h wear, and waking‐hours‐only protocols should suffice.
Placement
There is no consensus regarding device placement. Wrist‐worn accelerometers seem to have a slight edge, mostly because they achieve the best compliance with 24 h wear 88 and because the most widespread open‐source tool for analysing raw data (GGIR package in R) was originally developed for wrist‐worn data. 85 However, the traditional hip/waist placement is still preferred by many researchers, as it seems to have better validity for some physical behaviours. 82 , 89 When the study focus is on sedentary behaviour, thigh placement is currently considered the gold standard. 90 In populations with decreased functional capacity, ankle placement is also gaining popularity as it performs well even with slow gait where other placements usually fail. 79 , 91 In a review by Clark et al., the authors advocated that there is no ‘one site fits all’ approach to the selection of accelerometer site or analytical technique, and that study design and focus should always inform the most suitable location of attachment, and be driven by the type of activity being characterized. 92
Processing methods
The first step in data processing that requires some decision making is the detection of non‐wear time. The algorithms used for non‐wear detection are usually embedded in the software used for accelerometer data analysis. For example, the open‐source GGIR package estimates non‐wear time on the basis of the standard deviation and the value range of the raw data from each accelerometer axis. 85 Actilife, the proprietary software developed by the manufacturer of Actigraph devices, enables a choice between several algorithms on the basis of Actigraph‐specific activity counts. In the absence of an algorithm specific for HF patients, we tentatively recommend using the Choi algorithm, which is considered to be the best performing in older adults. 86 Following non‐wear detection, a decision of how many minutes of wear time is required for a day to be valid needs to be made; the general consensus is 600 min (10 h). 86 Alternatively, the GGIR package replaces the non‐wear sections by imputed values calculated as an average of the same time point on all other days of the measurement. 85 Finally, for a patient to be included in the analysis, at least four valid days, including at least one weekend day, is usually required. 86
Conclusions
In our systematic review of 60 studies using accelerometers in HF patients, we demonstrated large heterogeneity and a lack of consensus in the methods used for accelerometer data collection, handling, and processing. Furthermore, we found that despite well‐founded limitations, traditional metrics such as steps, activity counts, and time spent in PA intensity levels still prevail, revealing an opportunistic gap in the use of metrics based on raw acceleration and machine learning techniques. Finally, we encourage researchers and clinicians to improve the quality and transparency of reporting on data collection and processing by following our proposed practical recommendations for the use of accelerometers in patients with cardiovascular diseases.
Conflict of interest
None declared.
Funding
This work was supported by Czech Health Research Council of the Ministry of Health of the Czech Republic (grant number NV18‐09‐00146).
Supporting information
Data S1. Supporting Information
Data S2. Supporting Information
Data S3. Supporting Information
Vetrovsky, T. , Clark, C. C. T. , Bisi, M. C. , Siranec, M. , Linhart, A. , Tufano, J. J. , Duncan, M. J. , and Belohlavek, J. (2020) Advances in accelerometry for cardiovascular patients: a systematic review with practical recommendations. ESC Heart Failure, 7: 2021–2031. 10.1002/ehf2.12781.
References
- 1. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 2018; 320: 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W. Associations of leisure‐time physical activity with cardiovascular mortality: a systematic review and meta‐analysis of 44 prospective cohort studies. Eur J Prev Cardiol 2018; 25: 1864–1872. [DOI] [PubMed] [Google Scholar]
- 3. Vetrovsky T, Cupka J, Dudek M, Kuthanova B, Vetrovska K, Capek V, Bunc V. A pedometer‐based walking intervention with and without email counseling in general practice: a pilot randomized controlled trial. BMC Public Health 2018; 18: 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Limb ES, Ahmad S, Cook DG, Kerry SM, Ekelund U, Whincup PH, Victor CR, Iliffe S, Ussher M, Fox‐Rushby J, Furness C, Ibison J, DeWilde S, Harris T. Measuring change in trials of physical activity interventions: a comparison of self‐report questionnaire and accelerometry within the PACE‐UP trial. Int J Behav Nutr Phys Act 2019; 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dibben GO, Dalal HM, Taylor RS, Doherty P, Tang LH, Hillsdon M. Cardiac rehabilitation and physical activity: systematic review and meta‐analysis. Heart 2018; 104: 1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan MKH, Wong JKL, Bakrania K, Abdullahi Y, Harling L, Casula R, Rowlands AV, Athanasiou T, Jarral OA. Can activity monitors predict outcomes in patients with heart failure? A systematic review. Eur Heart J Qual Care Clin Outcomes 2019; 5: 11–21. [DOI] [PubMed] [Google Scholar]
- 7. Shoemaker MJ, Tresh T, Hart J, Wood T. Objective improvement in daily physical activity in heart failure remains elusive. Cardiopulm Phys Ther J 2018; 29: 63–80. [Google Scholar]
- 8. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise‐based cardiac rehabilitation for adults with heart failure. Cochrane Heart Group, ed. Cochrane Database Syst Rev 2019; 8: 860–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montoye AHK, Moore RW, Bowles HR, Korycinski R, Pfeiffer KA. Reporting accelerometer methods in physical activity intervention studies: a systematic review and recommendations for authors. Br J Sports Med 2018; 52: 1507–1516. [DOI] [PubMed] [Google Scholar]
- 10. Alosco ML, Spitznagel MB, Miller L, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Waechter D, Hughes J, Rosneck J, Gunstad J. Depression is associated with reduced physical activity in persons with heart failure. Health Psychol 2012; 31: 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andreae C, Årestedt K, Evangelista L, Strömberg A. The relationship between physical activity and appetite in patients with heart failure: a prospective observational study. Eur J Cardiovasc Nurs 2019; 18: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atalla A, Carlisle TW, Simonds AK, Cowie MR, Morrell MJ. Sleepiness and activity in heart failure patients with reduced ejection fraction and central sleep‐disordered breathing. Sleep Med 2017; 34: 217–223. [DOI] [PubMed] [Google Scholar]
- 13. Baril J‐F, Bromberg S, Moayedi Y, Taati B, Manlhiot C, Ross HJ, Cafazzo J. Use of free‐living step count monitoring for heart failure functional classification: validation study. JMIR Cardio 2019; 3: e12122–e12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, LeWinter MM, Mann DL, Margulies KB, Smith AL, Tang WHW, Whellan DJ, Chen HH, Davila‐Roman VG, McNulty S, Desvigne‐Nickens P, Hernandez AF, Braunwald E, Redfield MM, for the National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network . Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction. JAMA 2018; 320: 1764–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cowie A, Thow MK, Granat MH, Mitchell SL. A comparison of home and hospital‐based exercise training in heart failure: immediate and long‐term effects upon physical activity level. Eur J Cardiovasc Prev Rehabil 2011; 18: 158–166. [DOI] [PubMed] [Google Scholar]
- 16. Dalal HM, Taylor RS, Jolly K, Davis RC, Doherty P, Miles J, Van Lingen R, Warren FC, Green C, Wingham J, Greaves C, Sadler S, Hillsdon M, Abraham C, Britten N, Frost J, Singh S, Hayward C, Eyre V, Paul K, Lang CC, Smith K. The effects and costs of home‐based rehabilitation for heart failure with reduced ejection fraction: the REACH‐HF multicentre randomized controlled trial. Eur J Prev Cardiol 2018; 26: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deka P, Pozehl B, Williams MA, Norman JF, Khazanchi D, Pathak D. MOVE‐HF: an internet‐based pilot study to improve adherence to exercise in patients with heart failure. Eur J Cardiovasc Nurs 2019; 18: 122–131. [DOI] [PubMed] [Google Scholar]
- 18. Dontje ML, van der Wal MHL, Stolk RP, Brügemann J, Jaarsma T, Wijtvliet PEPJ, van der Schans CP, de Greef MHG. Daily physical activity in stable heart failure patients. J Cardiovasc Nurs 2014; 29: 218–226. [DOI] [PubMed] [Google Scholar]
- 19. Edelmann F, Bobenko A, Gelbrich G, Hasenfuss G, Herrmann‐Lingen C, Duvinage A, Schwarz S, Mende M, Prettin C, Trippel T, Lindhorst R, Morris D, Pieske‐Kraigher E, Nolte K, Düngen H‐D, Wachter R, Halle M, Pieske B. Exercise training in Diastolic Heart Failure (Ex‐DHF): rationale and design of a multicentre, prospective, randomized, controlled, parallel group trial. Eur J Heart Fail 2017; 19: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 20. Floegel TA, Dickinson JM, DerAnanian C, McCarthy M, Hooker SP, Buman MP. Association of posture and ambulation with function 30 days after hospital discharge in older adults with heart failure. J Card Fail 2018; 24: 126–130. [DOI] [PubMed] [Google Scholar]
- 21. Gad SA, Martin S, Kimber S, Williams R, Gulamhusein S, Lockwood E, Haennel RG. Impact of cardiac resynchronization therapy on daily physical activity in heart failure patients. J Cardiopulm Rehabil Prev 2018; 38: E1–E4. [DOI] [PubMed] [Google Scholar]
- 22. Gottlieb SS, Fisher ML, Freudenberger R, Robinson S, Zietowski G, Alves L, Krichten C, Vaitkevicus P, McCarter R. Effects of exercise training on peak performance and quality of life in congestive heart failure patients. J Card Fail 1999; 5: 188–194. [DOI] [PubMed] [Google Scholar]
- 23. Howell J, Strong BM, Weisenberg J, Kakade A, Gao Q, Cuddihy P, Delisle S, Kachnowski S, Maurer MS. Maximum daily 6 minutes of activity: an index of functional capacity derived from actigraphy and its application to older adults with heart failure. J Am Geriatr Soc 2010; 58: 931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howie‐Esquivel J, Zaharias E. Using novel technology to determine mobility among hospitalized heart failure patients: a pilot study. Cardiol Res 2013; 4: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Izawa KP, Watanabe S, Oka K, Hiraki K, Morio Y, Kasahara Y, Osada N, Omiya K, Shimizu H. Association between mental health and physical activity in patients with chronic heart failure. Disabil Rehabil 2013; 36: 250–254. [DOI] [PubMed] [Google Scholar]
- 26. Jaarsma T, Klompstra L, Ben Gal T, Boyne J, Vellone E, Bäck M, Dickstein K, Fridlund B, Hoes A, Piepoli MF, Chialà O, Mårtensson J, Strömberg A. Increasing exercise capacity and quality of life of patients with heart failure through Wii gaming: the rationale, design and methodology of the HF‐Wii study; a multicentre randomized controlled trial. Eur J Heart Fail 2015; 17: 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jehn M, Schmidt‐Trucksäess A, Schuster T, Hanssen H, Weis M, Halle M, Koehler F. Accelerometer‐based quantification of 6‐minute walk test performance in patients with chronic heart failure: applicability in telemedicine. J Card Fail 2009; 15: 334–340. [DOI] [PubMed] [Google Scholar]
- 28. Jehn M, Schmidt‐Trucksäss A, Schuster T, Weis M, Hanssen H, Halle M, Koehler F. Daily walking performance as an independent predictor of advanced heart failure: prediction of exercise capacity in chronic heart failure. Am Heart J 2009; 157: 292–298. [DOI] [PubMed] [Google Scholar]
- 29. Jehn M, Schmidt‐Trucksäss A, Schuster T, Hanssen H, Halle M, Köhler F. Pedometer accuracy in patients with chronic heart failure. Int J Sports Med 2010; 31: 186–191. [DOI] [PubMed] [Google Scholar]
- 30. Jehn M, Prescher S, Koehler K, von Haehling S, Winkler S, Deckwart O, Honold M, Sechtem U, Baumann G, Halle M, Anker SD, Koehler F. Tele‐accelerometry as a novel technique for assessing functional status in patients with heart failure: feasibility, reliability and patient safety. Int J Cardiol 2013; 168: 4723–4728. [DOI] [PubMed] [Google Scholar]
- 31. Klompstra L, Jaarsma T, Strömberg A. Exergaming to increase the exercise capacity and daily physical activity in heart failure patients: a pilot study. BMC Geriatr 2014; 14: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lang CC, Smith K, Wingham J, Eyre V, Greaves CJ, Warren FC, Green C, Jolly K, Davis RC, Doherty PJ, Miles J, Britten N, Abraham C, Van Lingen R, Singh SJ, Paul K, Hillsdon M, Sadler S, Hayward C, Dalal HM, Taylor RS, REACH‐HF investigators . A randomised controlled trial of a facilitated home‐based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH‐HFpEF Pilot Study. BMJ Open 2018; 8: e019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liebzeit D, Phelan C, Moon C, Brown R, Bratzke L. Rest–activity patterns in older adults with heart failure and healthy older adults. J Aging Phys Act 2017; 25: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCarthy MM, Dickson VV, Katz SD, Chyun DA. An exercise counseling intervention in minority adults with heart failure. Rehabil Nurs 2017; 42: 146–156. [DOI] [PubMed] [Google Scholar]
- 35. Melczer C, Melczer L, Goják I, Oláh A, Ács P. A comparative analysis between external accelerometer and internal accelerometer's physical activity data from implanted resynchronization devices in patients with heart failure. Eur J Integr Med 2016; 8: 18–22. [Google Scholar]
- 36. Melin M, Hagerman I, Gonon A, Gustafsson T, Rullman E. Variability in physical activity assessed with accelerometer is an independent predictor of mortality in CHF patients. PLoS ONE 2016; 11: e0153036–e0153013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miyahara S, Fujimoto N, Dohi K, Sugiura E, Moriwaki K, Omori T, Takeuchi T, Kumagai N, Nakamori S, Yamada N, Ito M. Postdischarge light‐intensity physical activity predicts rehospitalization of older Japanese patients with heart failure. J Cardiopulm Rehabil Prev 2018; 38: 182–186. [DOI] [PubMed] [Google Scholar]
- 38. Mohri M, Motohama R, Sato N. Home‐based cardiac rehabilitation decreases red cell distribution width in chronic heart failure. Acta Cardiol 2017; 68: 615–619. [DOI] [PubMed] [Google Scholar]
- 39. Nguyen HQ, Steele BG, Dougherty CM, Burr RL. Physical activity patterns of patients with cardiopulmonary illnesses. Arch Phys Med Rehabil 2012; 93: 2360–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Donnell J, Velardo C, Shah SA, Khorshidi GS, Salvi D, Rahimi K, Tarassenko L. Physical activity and sleep analysis of heart failure patients using multi‐sensor patches. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2018. 10.1109/EMBC.2018.8513594 [DOI] [PubMed]
- 41. Pozehl BJ, McGuire R, Duncan K, Hertzog M, Deka P, Norman J, Artinian NT, Saval MA, Keteyian SJ. accelerometer‐measured daily activity levels and related factors in patients with heart failure. J Cardiovasc Nurs 2018; 33: 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pressler A, Danner M, Esefeld K, Haller B, Scherr J, Schömig A, Halle M, Kolb C. Validity of cardiac implantable electronic devices in assessing daily physical activity. Int J Cardiol 2013; 168: 1127–1130. [DOI] [PubMed] [Google Scholar]
- 43. Redeker NS, Hilkert R. Sleep and quality of life in stable heart failure. J Card Fail 2005; 11: 700–704. [DOI] [PubMed] [Google Scholar]
- 44. Redeker NS, Muench U, Zucker MJ, Walsleben J, Gilbert M, Freudenberger R, Chen M, Campbell D, Blank L, Berkowitz R, Adams L, Rapoport DM. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep 2010; 33: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WHW, McNulty SE, Velazquez EJ, Shah MR, Braunwald E. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373: 2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shen H, Zhao J, Zhou X, Li J, Wan Q, Huang J, Li H, Wu L, Yang S, Wang P. Impaired chronotropic response to physical activities in heart failure patients. BMC Cardiovasc Disord 2017; 17: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six‐minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J 2013; 24: 21–29. [PMC free article] [PubMed] [Google Scholar]
- 48. Shoemaker MJ, Cartwright K, Hanson K, Serba D, Dickinson MG, Kowalk A. Concurrent validity of daily activity data from Medtronic ICD/CRT devices and the Actigraph GT3X triaxial accelerometer. Cardiopulm Phys Ther J 2017; 28: 3–11. [Google Scholar]
- 49. Smagula SF, Freedland KE, Steinmeyer BC, Wallace MJ, Carney RM, Rich MW. Moderators of response to cognitive behavior therapy for major depression in patients with heart failure. Psychosom Med 2019; 81: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stocker TJ, Scheck F, Orban M, Braun D, Hertell H, Lackermair K, Deseive S, Mehr M, Orban M, Karam N, Nabauer M, Massberg S, Hausleiter J. Physical activity tracking in correlation to conventional heart failure monitoring assessing improvements after transcatheter mitral and tricuspid valve repair. Eur J Heart Fail 2019; 144: 524–523. [DOI] [PubMed] [Google Scholar]
- 51. Suchy C, Massen L, Rognmo O, Van Craenenbroeck EM, Beckers P, Kraigher‐Krainer E, Linke A, Adams V, Wisløff U, Pieske B, Halle M. Optimising exercise training in prevention and treatment of diastolic heart failure (OptimEx‐CLIN): rationale and design of a prospective, randomised, controlled trial. Eur J Prev Cardiol 2014; 21: 18–25. [DOI] [PubMed] [Google Scholar]
- 52. Tai M‐K, Meininger JC, Frazier LQ, Chan W. Ambulatory blood pressure and physical activity in heart failure. Biol Res Nurs 2010; 11: 269–279. [DOI] [PubMed] [Google Scholar]
- 53. Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan DW, Ades PA. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol 2010; 143: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van den Berg‐Emons HJ, Bussmann JB, Balk AH, Stam HJ. Validity of ambulatory accelerometry to quantify physical activity in heart failure. Scand J Rehabil Med 2000; 32: 187–192. [DOI] [PubMed] [Google Scholar]
- 55. van den Berg‐Emons R, Balk A, Bussmann H, Stam H. Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure? Eur J Heart Fail 2005; 6: 95–100. [DOI] [PubMed] [Google Scholar]
- 56. Velikic G, Modayil J, Thomsen M, Bocko M, Pentland A. Predicting the near‐future impact of daily activities on heart rate for at‐risk populations. 2011 IEEE 13th International Conference on e‐Health Networking, Applications and Services 2011.
- 57. Vetrovsky T, Siranec M, Parenica J, Griva M, Stastny J, Precek J, Pelouch R, Bunc V, Linhart A, Belohlavek J. Effect of a 6‐month pedometer‐based walking intervention on functional capacity in patients with chronic heart failure with reduced (HFrEF) and with preserved (HFpEF) ejection fraction: study protocol for two multicenter randomized controlled trials. J Transl Med 2017; 15: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vetrovsky T, Siranec M, Marencaková J, Tufano JJ, Capek V, Bunc V, Belohlavek J. Validity of six consumer‐level activity monitors for measuring steps in patients with chronic heart failure. PLoS ONE 2019; 14: e0222569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wagner J, Knaier R, Infanger D, Arbeev K, Briel M, Dieterle T, Hanssen H, Faude O, Roth R, Hinrichs T, Schmidt‐Trucksäss A. Functional aging in health and heart failure: the COmPLETE Study. BMC Cardiovasc Disord 2019; 19: 180–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Waring T, Gross K, Soucier R, ZuWallack R. Measured physical activity and 30‐day rehospitalization in heart failure patients. J Cardiopulm Rehabil Prev 2017; 37: 124–129. [DOI] [PubMed] [Google Scholar]
- 61. Werhahn SM, Dathe H, Rottmann T, Franke T, Vahdat D, Hasenfuss G, Seidler T. Designing meaningful outcome parameters using mobile technology: a new mobile application for telemonitoring of patients with heart failure. ESC Heart Failure 2019; 6: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Witham MD, Gray JM, Argo IS, Johnston DW, Struthers AD, McMurdo MET. Effect of a seated exercise program to improve physical function and health status in frail patients ≥70 years of age with heart failure. Am J Cardiol 2005; 95: 1120–1124. [DOI] [PubMed] [Google Scholar]
- 63. Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo MET. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure. Circ Heart Fail 2010; 3: 195–201. [DOI] [PubMed] [Google Scholar]
- 64. Yamazaki T, Asanoi H, Ueno H, Yamada K, Takagawa J, Kameyama T, Hirai T, Nozawa T, Inoue H. Circadian dynamics of heart rate and physical activity in patients with heart failure. Clin Exp Hypertens 2009; 27: 241–249. [PubMed] [Google Scholar]
- 65. Yates BC, Pozehl B, Kupzyk K, Epstein CM, Deka P. Are heart failure and coronary artery bypass surgery patients meeting physical activity guidelines? Rehabil Nurs 2017; 42: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yavari M, Haykowsky MJF, Savu A, Kaul P, Dyck JRB, Haennel RG, Alberta HEART Investigators . Volume and patterns of physical activity across the health and heart failure continuum. Can J Cardiol 2017; 33: 1465–1471. [DOI] [PubMed] [Google Scholar]
- 67. Young L, Hertzog M, Barnason S. Effects of a home‐based activation intervention on self‐management adherence and readmission in rural heart failure patients: the PATCH randomized controlled trial. BMC Cardiovasc Disord 2016; 16: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Witham MD, Fulton RL, Greig CA, Johnston DW, Lang CC, van der Pol M, Boyers D, Struthers AD, McMurdo MET. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure. Circ Heart Fail 2012; 5: 209–216. [DOI] [PubMed] [Google Scholar]
- 69. Clark CCT, Barnes CM, Stratton G, McNarry MA, Mackintosh KA, Summers HD. A review of emerging analytical techniques for objective physical activity measurement in humans. Sports Med 2017; 47: 439–447. [DOI] [PubMed] [Google Scholar]
- 70. Peddle‐McIntyre CJ, Cavalheri V, Boyle T, McVeigh JA, Jeffery E, Lynch BM, Vallance JK. A review of accelerometer‐based activity monitoring in cancer survivorship research. Med Sci Sports Exerc 2018; 50: 1790–1801. [DOI] [PubMed] [Google Scholar]
- 71. Matthews CE, Hagströmer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population‐based research. Med Sci Sports Exerc 2012; 44: S68–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rowlands AV, Sherar LB, Fairclough SJ, Yates T, Edwardson CL, Harrington DM, Davies MJ, Munir F, Khunti K, Stiles VH. A data‐driven, meaningful, easy to interpret, standardised accelerometer outcome variable for global surveillance. J Sci Med Sport 2019; 22: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 73. John D, Morton A, Arguello D, Lyden K, Bassett D. ‘What is a step?’ Differences in how a step is detected among three popular activity monitors that have impacted physical activity research. Sensors (Basel) 2018; 18: 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rowlands AV, Plekhanova T, Yates T, Mirkes EM, Davies M, Khunti K, Edwardson CL. Providing a basis for harmonization of accelerometer‐assessed physical activity outcomes across epidemiological datasets. J Meas Phys Behav 2019; 2: 131–142. [Google Scholar]
- 75. Šimůnek A, Dygrýn J, Gába A, Jakubec L, Stelzer J, Chmelík F. Validity of Garmin vívofit and Polar Loop for measuring daily step counts in free‐living conditions in adults. Acta Gymnica 2016; 46: 129–135. [Google Scholar]
- 76. Fokkema T, Kooiman TJM, Krijnen WP, van der Schans CP, De Groot M. Reliability and validity of ten consumer activity trackers depend on walking speed. Med Sci Sports Exerc 2017; 49: 793–800. [DOI] [PubMed] [Google Scholar]
- 77. Chen KY, Bassett DR Jr. The technology of accelerometry‐based activity monitors: current and future. Med Sci Sports Exerc 2005; 37: S490–S500. [DOI] [PubMed] [Google Scholar]
- 78. Rowlands AV, Dawkins NP, Maylor B, Edwardson CL, Fairclough SJ, Davies MJ, Harrington DM, Khunti K, Yates T. Enhancing the value of accelerometer‐assessed physical activity: meaningful visual comparisons of data‐driven translational accelerometer metrics. Sports Med Open 2019; 5: 47–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Duncan MJ, Rowlands A, Lawson C, Wright SL, Hill M, Morris M, Eyre E, Tallis J. Using accelerometery to classify physical activity intensity in older adults: what is the optimal wear‐site? Eur J Sport Sc 2019. 10.1080/17461391.2019.1694078 [DOI] [PubMed] [Google Scholar]
- 80. Migueles JH, Cadenas‐Sanchez C, Rowlands AV, Henriksson P, Shiroma EJ, Acosta FM, Rodriguez Ayllon M, Esteban Cornejo I, Plaza‐Florido A, Gil‐Cosano JJ, Ekelund U, van Hees VT, Ortega FB. Comparability of accelerometer signal aggregation metrics across placements and dominant wrist cut points for the assessment of physical activity in adults. Sci Rep 2019; 9: 18235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Migueles JH, Cadenas‐Sanchez C, Tudor‐Locke C, Löf M, Esteban Cornejo I, Molina Garcia P, Mora‐Gonzalez J, Rodriguez Ayllon M, Garcia Marmol E, Ekelund U, Ortega FB. Comparability of published cut‐points for the assessment of physical activity: implications for data harmonization. Scand J Med Sci Sports 2019; 29: 566–574. [DOI] [PubMed] [Google Scholar]
- 82. Hildebrand M, van Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist‐ and hip‐worn monitors. Med Sci Sports Exerc 2014; 46: 1816–1824. [DOI] [PubMed] [Google Scholar]
- 83. Rowlands AV, Edwardson CL, Davies MJ, Khunti K, Harrington DM, Yates T. Beyond cut points. Med Sci Sports Exerc 2018; 50: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 84. Gil‐Rey E, Maldonado‐Martín S, Gorostiaga EM. Individualized accelerometer activity cut‐points for the measurement of relative physical activity intensity levels. Res Q Exerc Sport 2019; 90: 327–335. [DOI] [PubMed] [Google Scholar]
- 85. Migueles JH, Rowlands AV, Huber F, Sabia S, van Hees VT. GGIR: a research community–driven open source R package for generating physical activity and sleep outcomes from multi‐day raw accelerometer data. J Meas Phys Behav 2019; 2: 188–196. [Google Scholar]
- 86. Migueles JH, Cadenas‐Sanchez C, Ekelund U, Nyström CD, Mora‐Gonzalez J, Löf M, Labayen I, Ruiz JR, Ortega FB. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med 2017; 47: 1821–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Byrom B, Rowe DA. Measuring free‐living physical activity in COPD patients: deriving methodology standards for clinical trials through a review of research studies. Contemp Clin Trials 2016; 47: 172–184. [DOI] [PubMed] [Google Scholar]
- 88. Doherty A, Jackson D, Hammerla N, Plötz T, Olivier P, Granat MH, White T, van Hees VT, Trenell MI, Owen CG, Preece SJ, Gillions R, Sheard S, Peakman T, Brage S, Wareham NJ. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS ONE 2017; 12: e0169649–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Marcotte RT, Petrucci GJ Jr, Cox MF, Freedson PS, Staudenmayer JW, Sirard JR. Estimating sedentary time from a hip‐ and wrist‐worn accelerometer. Med Sci Sports Exerc 2019; 52: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Crowley P, Skotte J, Stamatakis E, Hamer M, Aadahl M, Stevens ML, Rangul V, Mork PJ, Holtermann A. Comparison of physical behavior estimates from three different thigh‐worn accelerometers brands: a proof‐of‐concept for the Prospective Physical Activity, Sitting, and Sleep consortium (ProPASS). Int J Behav Nutr Phys Act 2019; 16: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Treacy D, Hassett L, Schurr K, Chagpar S, Paul SS, Sherrington C. Validity of different activity monitors to count steps in an inpatient rehabilitation setting. Phys Ther 2017; 97: 581–588. [DOI] [PubMed] [Google Scholar]
- 92. Clark CCT, Nobre GC, Fernandes JFT, Moran J, Drury B, Mannini A, Gronek P, Podstawski R. Physical activity characterization: does one site fit all? Physiol Meas 2018; 39: 09TR02–TR29. [DOI] [PubMed] [Google Scholar]
- 93. da Silva VZM , Lima AC, Vargas FT, Cahalin LP, Arena R, Cipriano G. Association between physical activity measurements and key parameters of cardiopulmonary exercise testing in patients with heart failure. J Card Fail 2013; 19: 635–640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data S2. Supporting Information
Data S3. Supporting Information


