Abstract
Aims
The aims of this paper were to investigate the analytical performance of the nine prognostic scales commonly used in heart failure (HF), in patients with dilated cardiomyopathy (DCM), and to develop a unique prognostic model tailored to DCM patients.
Methods and results
The hospital and outpatient records of 406 DCM patients were retrospectively analysed. The information on patient status was gathered after 48.2 ± 32.0 months. Tests were carried out to ascertain the prognostic accuracy in DCM using some of the most frequently applied HF prognostic scales (Barcelona Bio‐Heart Failure, Candesartan in Heart Failure‐Assessment of Reduction in Mortality and Morbidity, Studio della Streptochinasi nell'Infarto Miocardico‐Heart Failure, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure, Meta‐Analysis Global Group in Chronic Heart Failure, MUerte Subita en Insuficiencia Cardiaca, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure, Seattle Heart Failure Model) and one dedicated to DCM, that of Miura et al. At follow‐up, 70 DCM patients (17.2%) died. Most analysed scores substantially overestimated the mortality risk, especially in survivors. The prognostic accuracy of the scales were suboptimal, varying between 60% and 80%, with the best performance from Barcelona Bio‐Heart Failure and Seattle Heart Failure Model for 1–5 year mortality [areas under the receiver operating curve 0.792–0.890 (95% confidence interval 0.725–0.918) and 0.764–0.808 (95% confidence interval 0.682–0.934), respectively].Based on our accumulated data, a self‐developed DCM prognostic model was constructed. The model consists of age, gender, body mass index, symptoms duration, New York Heart Association class, diabetes mellitus, prior stroke, abnormal liver function, dyslipidaemia, left bundle branch block, left ventricle end‐diastolic diameter, ejection fraction, N terminal pro brain natriuretic peptide, haemoglobin, estimated glomerular filtration rate, and pharmacological and resynchronisation therapy. This newly created prognostic model outperformed the analysed HF scales.
Conclusions
An analysis of various HF prognostic models found them to be suboptimal for DCM patients. A self‐developed DCM prognostic model showed improved performance over the nine other models studied. However, further validation of the prognostic model in different DCM populations is required.
Keywords: Dilated cardiomyopathy, Mortality, Prognostic scale
Introduction
Modern medicine endeavours to estimate the clinical course and prognosis for various diseases. In the field of cardiology, many different types of prognostic scales have been developed for numerous clinical conditions, including acute and chronic heart failure (HF), myocardial infarction, atrial fibrillation (AF), and pulmonary embolism. Not only does the complexity of these scales differ, but also, more crucially, the accuracy of their scores. Due to the prevalence of HF, the ever increasing number of patients succumbing to the disease and its diverse clinical courses, risk stratification has been the subject of research for many years. Numerous prognostic scales have been developed based on large groundbreaking clinical trials evaluating thousands of patients. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 However, the HF population is highly heterogeneous, including as it does three types of HF categorized according to the ejection fraction (EF) and two types according to HF aetiology (ischaemic and non‐ischaemic). 9 Furthermore, HF prognostic scales were not validated for any particular type of HF, such as dilated cardiomyopathy (DCM).
It is important to note that the DCM population differs significantly from the rest of the HF population in terms of lower mortality rates, which can be partly explained by the patients' younger age and fewer comorbidities. 10 Therefore, scales constructed for the general HF population may not be suitable for patients with DCM, especially given the fact that these scales were created based on different patient populations, with dissimilar therapies, and before the introduction of angiotensin‐converting enzyme inhibitors (ACEIs), beta‐blockers (BBs), mineralocorticoid receptor antagonists (MRAs), and resynchronisation therapy (CRT). There has only been one previous analysis of the usefulness of HF prognostic scales in DCM; this, however, was conducted on a very selective population undergoing ventricular tachycardia (VT) ablation. 11
Another issue in relation to our current knowledge of DCM current mortality rates and prognosis in DCM population is that they are not well defined. The reason for this is that the available data were gathered quite a few years ago; the mortality rate findings also vary widely (from 6% to 43%). 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 Moreover, apart from the score created by Miura et al. based on a Japanese cohort from the 1990s, there is no prognostic model specific to mortality in DCM patients. 22 In addition, the Miura et al. score was created in different therapy settings and has not been validated on a European DCM population.
Therefore, in order to respond to this as yet unmet clinical need, two main objectives were formulated: (i) to analyse the accuracy of some common HF prognostic scales in our DCM cohort and (ii) to develop a unique prognostic model tailored to DCM patients in particular.
Methods
Study population and protocol
Between 2010 and 2018, we included 406 consecutive DCM patients with complete baseline and follow‐up data, who underwent detailed diagnostic work‐up, entailing clinical evaluation, a battery of laboratory tests [morphology, uric acid and creatinine levels, electrolytes, fasting glucose, lipid profile, liver enzymes, thyroid‐stimulating hormone, high‐sensitive troponin T, N terminal pro brain natriuretic peptide (NT‐proBNP), C‐reactive protein], electrocardiogram (ECG), echocardiography, and coronary catheterization or computed tomography coronary angiography. 9 , 29 , 30 In addition, some patients underwent magnetic resonance imaging, endomyocardial biopsy, and right‐heart catheterization. A similar number of patients were incorporated into the study each year (Supporting Information, Figure S1 ). DCM was diagnosed in accordance with the current European Society of Cardiology recommendations, based on (i) the presence of left ventricle (LV) dilation and impaired LV systolic function (EF < 45%) detected via echocardiogram and (ii) the exclusion of significant coronary artery disease (CAD; by coronary angiogram or computer tomography of coronary arteries), primary heart valve disease (by echocardiography and/or magnetic resonance), congenital heart disease (by echocardiography and/or magnetic resonance), and severe arterial hypertension. 29 , 30
The investigation conforms with the principles outlined in the Declaration of Helsinki. Prior to the study, the relevant institutional committees and the Jagiellonian University Ethical Committee approved the study, and all patients gave their written informed consent.
Endpoints
The endpoint was all‐cause mortality. Between April and September 2019, information on the status of the patients was collected through medical records and via telephone contact.
Prognostic scores analysis
Selecting from the most commonly applied prognostic scores used in HF, we chose those designed to estimate all‐cause mortality. We decided on the following (in alphabetic order): the Barcelona Bio‐Heart Failure risk calculator 2.0 (BCN Bio‐HF), Candesartan in Heart Failure‐Assessment of Reduction in Mortality and Morbidity (CHARM), Studio della Streptochinasi nell'Infarto Miocardico‐Heart Failure (GISSI‐HF), the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure, Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC), Miura et al. Score, MUerte Subita en Insuficiencia Cardiaca (MUSIC), Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure, and the Seattle Heart Failure Model (SHFM). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 22 Due to a lack of data on cardiopulmonary exercise tests, blood urea nitrogen levels, and apolipoproteins, the following scores were excluded from the study: Metabolic Exercise test data combined with Cardiac and Kidney Indexes, Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training, Heart Failure Survival Score, Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness, Acute Decompensated Heart Failure National Registry, Get With The Guidelines‐Heart Failure Registry, Enhanced Feedback for Effective Cardiac Treatment‐Heart Failure, and the Controlled rosuvastatin multinational study in heart failure.
All the variables needed to calculate the mortality risk with the selected scales were collected from patient hospital records and outpatient visits. In 163 out of 406 (40.1%) patients, available follow‐up data on troponin levels were missing, and in 296 patients (72.9%), information on uric acid levels were absent. In those cases of patients with missing data, in order to determine the mortality risk utilizing the BCN Bio‐HF calculator, we assigned a mean value of troponin (13 ng/L); for the SHFM model and GISSI‐HF score, we set uric acid levels at the mean value—7.63 mg/dL; for the MUSIC score, for the presence of elevated troponin level, 0.64 units were assigned to patients with missing data for troponin level (64% of the study population with available troponin level data had elevated troponin over normal values, at 14 ng/L). We calculated four models for the BCN Bio‐HF 2.0 calculator: (i) without troponin T and NT‐proBNP levels (M0), (ii) with NT‐proBNP level (M1), (iii) with troponin T level (M2), and (iv) with NT‐proBNP and troponin T levels (M3). 1 However, due to the highest accuracy of mortality prediction [i.e. highest area under the receiver operating curve (AUC)], only the fourth (M3) model was used in subsequent statistical analysis.
For the purposes of calculating the mortality risk based on the BCN Bio‐HF and SHFM models, a dedicated IT software was designed, which entered data into the calculators available online and then inserted the results obtained regarding mortality risk into an Excel document; mortality risks assessed by other risk scores were calculated by formulas created in an Excel document.
Statistical analysis
All parameters are presented as mean ± standard deviation if continuous, or as counts and percentages in the case of categorical variables. All quantitative variables were tested for their normal distribution of data with the Shapiro–Wilk test. Comparisons of continuous parameters were conducted with t‐tests when normality was confirmed, or otherwise, with the Mann–Whitney test; the χ2 test was performed for the comparison of qualitative parameters. AUCs were calculated to assess the validity of prognostic models in their accuracy of prediction for 1 month and for 1, 2, 3, 4, and 5 year mortality. The Z test was used for comparing the AUC of each prognostic scale. Results were considered statistically significant when their P value was <0.05. The Statistica package, version 13.0 (StatSoft, TIBCO Software Inc.), was used for the statistical analysis.
The development of a prognostic model in dilated cardiomyopathy
All parameters from Table 1 were considered predictors in order to fit a prediction model for time to total death. In order to retain a sufficient number of subjects in the analysis, we only considered variables with missing data below 25%; therefore, troponin T and uric acid were not included in the model. For hospitalized patients, parameters incorporated into the model were obtained from hospital admission, unless otherwise indicated.
TABLE 1.
Baseline characteristics of study population divided according to outcome
| Non‐survivors (n = 70) | Survivors (n = 336) | P value | BCN Bio‐HF | CHARM | EMPHASIS | GISSI‐HF | MAGGIC | MUSIC | OPTIMIZE‐HF | SHFM | Miura et al. score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical characteristic | ||||||||||||
| Age (year) | 55.0 ± 14.4 | 53.3 ± 13.5 | 0.34 | X | X | X | X | X | X | X | X | |
| Male, n (%) | 60 (85.7%) | 269 (80.1%) | 0.29 | X | X | X | X | X | X | X | ||
| Symptoms duration (month) | 63.4 ± 83.5 | 35.4 ± 50.4 | 0.002 | X | X | |||||||
| Aetiology, n (%) | 0.69 | |||||||||||
| Inflammatory | 6 (8.6%) | 38 (11.3%) | ||||||||||
| Toxic | 11 (15.7%) | 51 (15.2%) | ||||||||||
| Tachyarrhythmic | 4 (5.7%) | 29 (8.6%) | ||||||||||
| Familial | 4 (5.7%) | 7 (2.1%) | ||||||||||
| Other | 3 (4.3%) | 13 (3.9%) | ||||||||||
| Unknown | 42 (60.0%) | 197 (58.6%) | ||||||||||
| Urgent HF hospitalization, n (%) | 17 (24.3%) | 45 (13.4%) | 0.02 | X | ||||||||
| CV prior hospitalization, n (%) | X | X | X | |||||||||
| 6 months | 41 (58.6%) | 184 (54.8%) | 0.56 | |||||||||
| 12 months | 42 (60.0%) | 194 (57.7%) | 0.73 | |||||||||
| >12 months | 36 (51.4%) | 101 (30.1%) | 0.0006 | |||||||||
| Blood pressure (mmHg) | X | X | X | X | X | X | ||||||
| Systolic | 115.3 ± 24.4 | 119.9 ± 17.7 | 0.01 | |||||||||
| Diastolic | 73.3 ± 11.9 | 76.2 ± 11.8 | 0.03 | |||||||||
| NYHA class | 2.94 ± 0.91 | 2.43 ± 0.86 | <0.0001 | X | X | X | X | X | X | |||
| Killip class | 1.27 ± 0.66 | 1.15 ± 0.45 | 0.05 | X | ||||||||
| Oedema, n (%) | 28 (40.0%) | 93 (27.8%) | 0.04 | X | ||||||||
| BMI (kg/m2) | 26.4 ± 5.3 | 27.8 ± 5.3 | 0.07 | X | X | X | X | X | ||||
| Comorbidities | ||||||||||||
| Current smoker, n (%) | 25 (35.7%) | 112 (33.4%) | 0.87 | X | X | |||||||
| Diabetes mellitus, n (%) | 21 (30.0%) | 68 (20.2%) | 0.07 | X | X | X | X | |||||
| COPD, n (%) | 4 (5.7%) | 23 (6.9%) | 0.73 | X | X | |||||||
| AF, n (%) | 25 (35.7%) | 103 (30.8%) | 0.42 | X | X | |||||||
| Prior stroke, n (%) | 11 (15.7%) | 13 (3.9%) | 0.0001 | |||||||||
| Dyslipidaemia, n (%) | 36 (51.4%) | 237 (70.8%) | 0.002 | |||||||||
| Abnormal liver function, n (%) | 13 (18.6%) | 40 (11.9%) | 0.13 | |||||||||
| ECG | ||||||||||||
| HR (bpm) | 82.6 ± 21.0 | 80.7 ± 20.2 | 0.48 | X | X | X | ||||||
| QRS (ms) | 113.2 ± 33.3 | 106.8 ± 28.0 | 0.31 | |||||||||
| Intraventricular delay, n (%) | 27 (38.6%) | 107 (31.9%) | 0.69 | X | X | X | ||||||
| LBBB, n (%) | 22 (31.4%) | 83 (24.7%) | 0.24 | |||||||||
| Ventricular extrasystole (per day) | 1847 ± 3,668 | 1876 ± 4,510 | 0.97 | X | ||||||||
| ns/sVT, n (%) | 20 (28.6%) | 65 (24.0%) | 0.046 | X | ||||||||
| Echocardiography | ||||||||||||
| EF (%) | 23.4 ± 9.4 | 26.6 ± 9.3 | 0.01 | X | X | X | X | X | X | X | X | |
| LVEDd (mm) | 68.5 ± 14.1 | 65.7 ± 9.4 | 0.04 | X | ||||||||
| RVd (mm) | 40.6 ± 10.2 | 37.4 ± 8.5 | 0.08 | |||||||||
| TAPSE (mm) | 17.9 ± 5.3 | 18.5 ± 6.3 | 0.59 | |||||||||
| LAd (mm) | 50.3 ± 11.7 | 46.8 ± 8.4 | 0.02 | X | ||||||||
| LAA (cm2) | 31.6 ± 10.2 | 29.0 ± 8.0 | 0.04 | |||||||||
| RAA (cm2) | 25.3 ± 9.4 | 22.7 ± 7.9 | 0.03 | |||||||||
| E wave (m/s) | 0.95 ± 0.37 | 0.83 ± 0.37 | 0.02 | |||||||||
| PASP (mmHg) | 39.6 ± 17.2 | 31.1 ± 17.5 | 0.0001 | |||||||||
| Mild or severe MR, n (%) | 40 (57.1%) | 152 (45.2%) | 0.07 | X | ||||||||
| Mild or severe TR, n (%) | 25 (35.7%) | 80 (23.8%) | 0.04 | |||||||||
| Laboratory results | ||||||||||||
| WBC (tys/uL) | 7.67 ± 2.04 | 7.95 ± 2.40 | 0.38 | |||||||||
| Lymphocytes (%) | 21.2 ± 7.8 | 24.7 ± 7.8 | 0.0008 | X | ||||||||
| Hb (g/dL) | 13.6 ± 1.5 | 14.4 ± 1.6 | 0.0003 | X | X | X | X | |||||
| Anaemia, n (%) | 18 (25.7%) | 43 (12.8%) | 0.006 | |||||||||
| Creatinine (umol/L) | 100.9 ± 46.6 | 92.6 ± 37.6 | 0.11 | X | X | |||||||
| eGFR < 60 mL/min, n (%) | 19 (27.2%) | 48 (14.3%) | 0.008 | X | X | X | X | |||||
| Urea acid (umol/L) | 480.1 ± 113.3 | 150.1 ± 135.4 | 0.32 | X | X | |||||||
| Na (mmol/L) | 139.3 ± 3.7 | 140.3 ± 3.1 | 0.07 | X | X | X | X | |||||
| K (mmol/L) | 4.53 ± 0.48 | 4.57 ± 0.45 | 0.53 | |||||||||
| Fasting glucose (mg/dL) | 6.3 ± 0.3 | 6.17 ± 1.7 | 0.56 | |||||||||
| Total cholesterol (mmol/L) | 4.18 ± 1.26 | 4.69 ± 1.12 | 0.0005 | X | ||||||||
| Cholesterol LDL (mmol/L) | 2.66 ± 1.00 | 3.00 ± 0.97 | 0.003 | |||||||||
| TSH (uIU/mL) | 2.19 ± 1.66 | 2.35 ± 2.61 | 0.69 | |||||||||
| hsTnT (ng/mL) | 0.016 ± 0.037 | 0.012 ± 0.071 | <0.0001 | X | X | |||||||
| CRP (mg/L) | 10.4 ± 15.3 | 7.3 ± 16.0 | 0.0007 | |||||||||
| log10 of NT‐proBNP | 3.5 ± 0.2 | 3.1 ± 0.8 | <0.0001 | X | X | |||||||
| Therapy | ||||||||||||
| ACEI/ARB/ARNI, n (%) | 59 (84.3%) | 311 (92.6%) | 0.03 | X | X | X | ||||||
| BB, n (%) | 66 (94.3%) | 327 (97.6%) | 0.62 | X | X | X | ||||||
| MRA, n (%) | 64 (91.4%) | 292 (87.2%) | 0.32 | X | ||||||||
| Furosemide (mg/day) | 70.1 ± 87.4 | 40.5 ± 55.1 | 0.007 | X | X | |||||||
| Torsemide (mg/day) | 9.1 ± 18.9 | 5.8 ± 19.7 | 0.20 | X | X | |||||||
| Other diuretics, n (%) | 3 (4.3%) | 15 (4.5%) | 0.94 | X | ||||||||
| Anticoagulants, n (%) | 35 (50.0%) | 134 (39.9%) | 0.09 | |||||||||
| Digoxin, n (%) | 27 (38.6%) | 74 (22.2%) | 0.004 | |||||||||
| Ivabradine, n (%) | 3 (4.3%) | 32 (9.6%) | 0.15 | |||||||||
| Amiodarone, n (%) | 13 (18.6%) | 40 (11.9%) | 0.13 | |||||||||
| Statins, n (%) | 18 (25.7%) | 145 (43.3%) | 0.06 | X | X | |||||||
| Insulin, n (%) | 4 (5.7%) | 7 (2.1%) | 0.09 | |||||||||
| Allopurinol, n (%) | 9 (12.9%) | 32 (9.6%) | 0.40 | X | ||||||||
| ICD, n (%) | 10 (14.3%) | 29 (8.7%) | 0.15 | X | ||||||||
| CRT/CRT‐D, n (%) | 5 (7.1%) | 8 (2.4%) | 0.04 | X | ||||||||
ACEI, angiotensin‐converting‐enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; BB‐beta‐blocker; BCN Bio‐HF, Barcelona Bio‐Heart Failure; BMI, body mass index; CHARM, Candesartan in Heart Failure‐Assessment of Reduction in Mortality and Morbidity; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; CRT‐P/CRT‐D, cardiac resynchronization therapy‐pacemaker/defibrillator; CV, cardiovascular; eGFR, estimated glomerular filtration rate; EF, ejection fraction; EMPHASIS‐HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; GISSI‐HF, Studio della Streptochinasi nell'Infarto Miocardico‐Heart Failure; Hb, haemoglobin; HF, heart failure; HR, heart rate; hs‐TnT, high‐sensitive troponin T; ICD, implantable cardioverter‐defibrillator; K, potassium; LAd, left atria dimeter; LAA/RAA, left/right atrium area; LBBB, left bundle branch block; LDL, low‐density lipoprotein; LVEDd, left ventricle end‐diastolic diameter; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; MUSIC, MUerte Subita en Insuficiencia Cardiaca; Na, sodium; ns/sVT, non‐sustained/sustained ventricular tachycardia; NYHA, New York Heart Association; OPTIMIZE‐HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure; PASP, pulmonary arterial systolic pressure; RVd, right ventricle basal diameter; SHFM, Seattle Heart Failure Model. TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TSH, thyroid‐stimulating hormone; WBC, white blood cells.
The parameters included into analysed prognostic scores are presented in the Table 1 (the cross reflects the use of the parameter in the model). All parameters are presented as mean ± standard deviation if continuous or counts and percentages in the case of categorical variables.
All variables with P < 0.1 for the univariable analysis were considered candidate predictors and were included in a prediction model for time to total death (Appendix). An accelerated failure time model was adopted for the DCM patients' data, and internal validation was applied. To avoid overfitting, the model was trained and tested on two separate data sets, randomly split into a testing and a validating sample, encompassing 69% and 31% of patients (280 and 126 patients, respectively). The size of the testing and validation set was chosen to ensure an appropriate number of cases (e.g. deaths in the first year) in a test set. The application of the 80–20 rule (325 patients in the testing set) resulted in a testing set including no such cases, so the testing set was reduced in size. A sensitivity analysis on the testing set size was conducted to make sure that the arbitrary choice of 280 patients for inclusion did not affect results (280 and 126 patients, respectively). Subsequently, the model's performance was compared with the BCN Bio‐HF model (Supporting Information, Table S1 ).
The model training included (i) choosing a distribution for the accelerated failure time model from Weibull, exponential, log‐normal, and log‐logistic; (ii) selecting variables with the backward stepwise method; and (iii) choosing an appropriate transformation (log, second and third power, binning) for continuous variables. Given the good discrimination yielded from the tested model in both the derivation and validation samples, the data sets were collapsed and a final Weibull parametric model was constructed based on the entire cohort of 406 patients. The performance metric used in the model training stage was the mean of AUCs obtained for 1, 2, 3, 4, and 5 year mortality prediction. R software (version 3.6.1) was used for model building.
Results
Baseline characteristics
During a follow‐up of mean 48.2 ± 32.0 months, 70 (17.2%) of the patients died: sixty‐three patients due to cardiovascular causes, six patients due to neoplasms (three patients—lung cancer, one patient—pancreatic cancer, one patient—mesothelioma, and one patient—throat cancer), and one patient owing to complications following laparotomy (Figure 1 ). In terms of procedures undergone, seven patients received left ventricular assist device implantations and thirteen patients HTX; one patient received both procedures.
Figure 1.
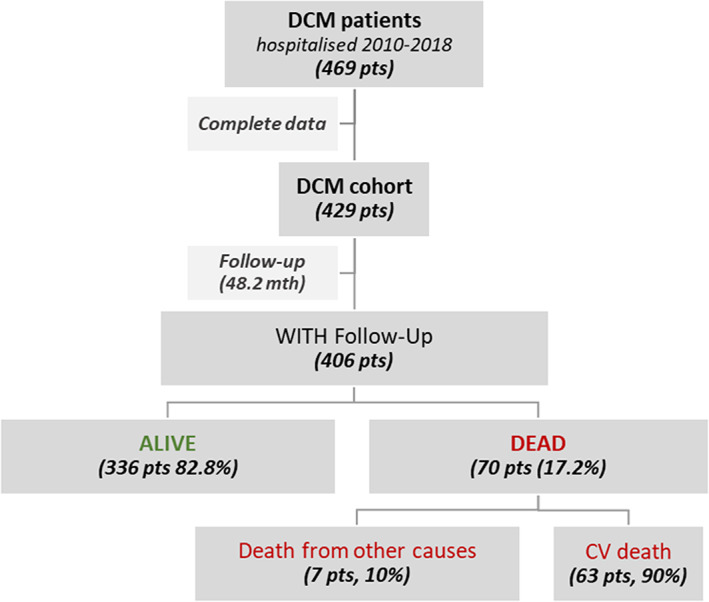
Patient flowchart with the outcome. DCM, dilated cardiomyopathy.
Patients who died differed from surviving patients in terms of clinical, vital, echocardiographic, laboratory parameters, and therapies implemented (Table 1 ). Indeed, surviving and non‐surviving DCM patients were differentiated by mortality risk calculated by most of the analysed prognostic scores (Table 2 ). However, most HF scales substantially overestimated mortality risk for surviving patients.
TABLE 2.
Comparison of calculated mortality risk for non‐survivors and survivors dilated cardiomyopathy patients
| Non‐survivors (n = 70) | Survivors (n = 336) | P value | |
|---|---|---|---|
| Follow‐up time (month) | 32.1 ± 26.3 | 51.6 ± 32.1 | <0.0001 |
| In‐hospital mortality | |||
| Outcome of study population, n (%) | 4 (1.0%) | 402 (99%) | |
| Mortality risk by OPTIMIZE‐HF (%) | 2.95 ± 2.26 | 2.24 ± 1.52 | 0.01 |
| 1‐year mortality | |||
| Outcome of study population, n (%) | 19 (5.5%) | 345 (94.5%) | |
| Mortality risk by MAGGIC (%) | 13.91 ± 8.15 | 10.21 ± 5.76 | <0.0001 |
| Mortality risk by SHFM (%) | 10.82 ± 16.3 | 5.48 ± 6.14 | <0.001 |
| Mortality risk by BCN Bio‐HF (%) | 15.32 ± 15.95 | 9.9 ± 10.51 | <0.0001 |
| 2‐year mortality | |||
| Outcome of study population, n (%) | 32 (11.4%) | 281 (88.6%) | |
| Mortality risk by CHARM (%) | 32.49 ± 24.5 | 25.38 ± 19.63 | 0.002 |
| Mortality risk by GISSI‐HF (%) | 14.05 ± 10.97 | 11.68 ± 8.77 | 0.02 |
| Mortality risk by SHFM (%) | 15.23 ± 16.36 | 9.93 ± 10.22 | <0.001 |
| Mortality risk by BCN Bio‐HF (%) | 26 ± 22.18 | 18.96 ± 16.8 | <0.0001 |
| Mortality risk by EMPHASIS (%) | 5.07 ± 1.94 | 4.37 ± 1.88 | <0.0001 |
| 3‐year mortality | |||
| Outcome of study population, n (%) | 42 (17.8%) | 236 (82.2%) | |
| Mortality risk by MAGGIC (%) | 35.5 ± 25.15 | 27.08 ± 20.6 | <0.0001 |
| Mortality risk by BCN Bio‐HF (%) | 27.15 ± 13.1 | 23.8 ± 12.31 | 0.009 |
| 44‐month mortality | |||
| Outcome of study population, n (%) | 49 (23.3%) | 210 (76.7%) | |
| Mortality risk by MUSIC (%) | 45.61 ± 22.04 | 27.9 ± 16.83 | <0.0001 |
| 4‐year mortality | |||
| Outcome of study population, n (%) | 52 (26.8%) | 194 (73.2%) | |
| Mortality risk by GISSI‐HF (%) | 24.32 ± 15.32 | 22.94 ± 14.98 | 0.36 |
| Mortality risk by BCN Bio‐HF (%) | 43.59 ± 26.61 | 33.93 ± 22.41 | 0.0001 |
| 5‐year mortality | |||
| Outcome of study population, n (%) | 59 (39.9%) | 148 (60.1%) | |
| Mortality risk by SHFM (%) | 70.31 ± 23.55 | 43.25 ± 24.53 | <0.001 |
| Mortality risk by BCN Bio‐HF (%) | 42.61 ± 26.02 | 23.06 ± 17.07 | <0.0001 |
| Mortality risk by Miura et al. score (%) | 24.21 ± 11.87 | 19.43 ± 10.36 | 0.001 |
BCN Bio‐HF, Barcelona Bio‐Heart Failure; CHARM, Candesartan in Heart Failure‐Assessment of Reduction in Mortality and Morbidity; EMPHASIS‐HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; GISSI‐HF, Studio della Streptochinasi nell'Infarto Miocardico‐Heart Failure; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure, MUSIC, MUerte Subita en Insuficiencia Cardiaca; OPTIMIZE‐HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure; SHFM, Seattle Heart Failure Model.
Follow‐up time was presented as mean ± SD. The mortality in study population was presented as n (%). Predicted mortality was calculated based on scores presented as mean ± standard deviation.
Diagnostic ability of heart failure prognostic scales
All HF scales analysed in the study produced a reasonably good performance in terms of their prognostic ability in DCM patients (all AUCs > 0.6), with the highest accuracy yielded by the BCN Bio‐HF and SHFM calculators for 1–5 year mortality (Figure 2 ).
Figure 2.
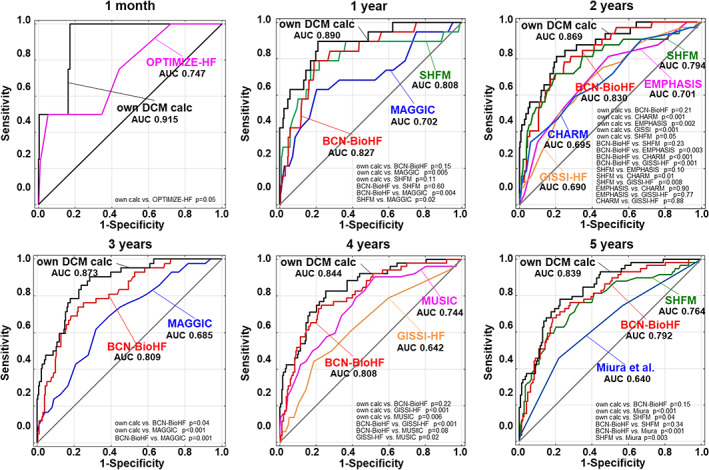
Receiver operating characteristic curves for prognosis accuracy of all scores for 1 month and 1, 2, 3, 4, and 5 year mortality with a comparison of all the models. AUC, area under the receiver operating curve; BCN Bio‐HF, Barcelona Bio‐Heart Failure; CHARM, Candesartan in Heart Failure‐Assessment of Reduction in Mortality and Morbidity; DCM, dilated cardiomyopathy; EMPHASIS‐HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; GISSI‐HF, Studio della Streptochinasi nell'Infarto Miocardico‐Heart Failure; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure, MUSIC, MUerte Subita en Insuficiencia Cardiaca; OPTIMIZE‐HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure; SHFM, Seattle Heart Failure Model.
Prognostic model construction
After an initial evaluation of the variables presented in Table 1 and the Appendix, a prognostic model was finally created. Table 3 provides the multivariable regression model. Our calculator exceeded the accuracy of all analysed scales for the prognosis of 1 month and 1–5 years mortality risk, with AUC of 0.915 [95% confidence interval (CI) 0.832–0.999], 0.89 (95%CI 0.812–0.968), 0.869 (95%CI 0.805–0.934), 0.873 (95%CI 0.820 0.926), 0.844 (95%CI 0.785–0.902), and 0.839 (95%CI 0.781–0.896), relatively (Figure 2 ).
TABLE 3.
Multivariable Weibull prognostic model for death from any cause
| Parameters | Acceleration factors (AF) [95%CI] | P value | Coefficient for mortality prediction analysis |
|---|---|---|---|
| Gender (female) | 1.56 [0.70–3.45] | 0.28 | +0.442 |
| Age (years) | 1.01 [0.99–1.04] | 0.44 | +0.01 |
| BMI (kg/m2) | 1.03 [0.96–1.10] | 0.41 | +0.028 |
| Symptoms duration (months) | 0.99 [0.99–1.00] | 0.03 | −0.004 |
| NYHA II | 0.57 [0.18–1.78] | 0.33 | −0.562 |
| NYHA III | 1.05 [0.33–3.37] | 0.94 | +0.046 |
| NYHA IV | 0.25 [0.07–0.84] | 0.03 | −1.398 |
| DM | 0.42 [0.21–0.85] | 0.02 | −0.868 |
| Prior stroke | 0.39 [0.17–0.90] | 0.03 | −0.945 |
| Abnormal liver function | 0.55 [0.28–1.07] | 0.08 | −0.601 |
| Dyslipidaemia | 1.75 [0.96–3.19] | 0.07 | +0.559 |
| LBBB | 0.44 [0.23–0.84] | 0.01 | −0.816 |
| LVEDd (mm) | a | 0.04 | c |
| EF (%) | 0.96 [0.93–1.00] | 0.07 | −0.037 |
| log10NT‐proBNP | 0.35 [0.18–0.67] | 0.001 | −1.054 |
| eGFR (mL/min) | b | 0.76 | c |
| Hb (g/dl) | 1.32 [1.09–1.60] | 0.004 | +0.289 |
| BB | 0.39 [0.12–1.31] | 0.13 | −0.934 |
| ACEi/ARB/ARNI | 3.67 [1.67–8.06] | 0.001 | +1.299 |
| MRA | 0.38 [0.14–1.07] | 0.07 | −0.959 |
| Digoxin | 0.55 [0.30–1.00] | 0.05 | −0.598 |
| Furosemide daily doses (mg/day) | 0.99 [0.99–1.00] | 0.32 | −0.02 |
| CRT‐P/CRT‐D | 0.32 [0.10–1.02] | 0.05 | −1.131 |
ACEi, angiotensin‐converting‐enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor‐neprilysin inhibitor; BB, beta‐blocker; BMI, body mass index; CRT‐P/CRT‐D, cardiac resynchronization therapy‐pacemaker/defibrillator; DM, diabetes mellitus; EF, ejection fraction; GFR, globular filtration rate; Hb, haemoglobin; LBBB, left bundle brunch block; LVEDd, left ventricle end‐diastolic diameter; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association class.
Model P value < 0.001. The formula for assessment of individual mortality risk is presented in the Results section. For acceleration factor computation used in the formula, it is necessary to calculate the sum of the products of coefficients and the value of all factors included in the table.
1.17 [1.01–1.35] for LVEDd + 0.999 [0.998–0.999] for LVEDd2.
1.016 [0.919–1.123] for GFR + 2.425 [<0.001–6.5105] for GFR2 and 0.165 [0.002–17.2] for GFR3.
For calculating the relevance of LVEDd and eGFR, the following calculation should be included: +0.154*LVEDd −0.001*LVEDd2 +0.016*eGFR +0.886*(eGFR/100)2 –1.8*(eGFR/100)3.
Individual mortality risk calculator
To compute the mortality risk, an algorithm was derived from the model. To calculate the probability of developing an event at a specific time, the particular combination of covariates should be used along with corresponding beta‐coefficients of the Weibull model:
where
AF are acceleration factors and t is the time expressed in months.
AF = +0.442*gender +0.01*age +0.028*BMI −0.004*(symptoms duration) −0.562*(NYHA II) +0.046*(NYHA III) −1.398*(NYHA IV) −0.868*DM −0.945*(prior stroke) −0.601*(abnormal liver function) +0.559*dyslipidaemia −0.816*LBBB +0.154*LVEDd −0.001*LVEDd2 –0.037*EF −1.054*log10(NT‐proBNP) +0.016*GFR +0.886*(GFR/100)2 –1.8*(GFR/100)3 + 0.289*Hb −0.934*BB +1.299(ACEI/ARB/ARNI) −0.959*MRA −0.598*digoxin −0.02(furosemide daily dosage) −1.131*(CRT‐P/CRT‐D).
To calculate mortality risk, age is expressed in years, symptoms duration in months, BMI as kg/m2, LVEDd in mm, EF in percent, GFR in mL/min/1.73 m2, Hb in g/dL, and furosemide in mg/day.
Gender is 1 if female, 0 if male.
NYHA II/III/IV, DM, prior stroke, abnormal liver function, dyslipidaemia, LBBB, BB, ACEI/ARB/ARNI, MRA, digoxin, CRT‐D/CRT‐P = 1 if present or used, 0 otherwise.
Discussion
The main findings of the study can be summarized as follows. First, during the 48.2 ± 32.0 months of observation, 17% out of 406 DCM patients died. Briefly, the prognostic HF scales under study were suboptimal as their accuracy varied between 60% and 80%. Based on the accumulated data, a self‐developed DCM prognostic model was created. External validation of the newly created model showed the highest accuracy in comparison with the other prognostic scales under study.
Overview of prognostic studies in dilated cardiomyopathy
We identified 17 studies analysing mortality in DCM since the 1980s up to the present. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 The reported mortality rate varied from 6% (reported by Marume et al.) to 43% (from Sobrino‐Márquez et al.). Overall, the studies differ fundamentally, especially in terms of the dates (from 1978 to 2017), the duration of observation (from 1 to over 9 years), the number of recruited patients (from 87 to 881—on average, 350 patients—while one Japanese study looked at 1554 patients). However, in most, the mortality rate reported after approximately 5 years was 15–25%, which is in line with our observations.
The largest study, analysing 5 year mortality in 1554 DCM patients, was conducted on the basis of a nationwide survey of cardiomyopathies conducted by the Japanese Research Committee on Idiopathic Cardiomyopathies. 22 However, the study was conducted 15 years ago before the introduction of most modern HF therapies; for instance, ACEI, BB, and electrotherapy were not in use. Moreover, the Japanese study also included patients with EF over 40% and 50% (encompassing 34% of the study population).
The enormous changes in HF therapy over the last decades may well have led to effects in DCM mortality rates, and therefore, more recent studies on the subject are required. 27 There has been only one study focused on DCM mortality to date conducted by Miura et al. 12 However, they found a surprisingly low death risk (6.2%) after a 4 year observation, which is not consistent with our results, nor with the subanalysis of the PARADIGM‐HF study (16.8% patients had died after 27 months). 31
Comparison of heart failure prognostic scales in dilated cardiomyopathy
We examined the eight most popular HF prognostic scales as part of our study. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 22 Typically reported mortality in unselected HF patients varies between 24% and 40% after approximately 3 years of observation, which seems to be higher than in DCM. The scales differed appreciably in terms of the general derivation cohorts and, consequently, in parameters included in the calculation of the scores (Supporting Information, Table S2 ). From the scales we looked at, only four included HF aetiology, while patients with non‐ischaemic HF covered about half of the derivation cohorts (DCM about 10–16%). Moreover, the analysed scores differed significantly in terms of the size of the study population, follow‐up time, and observation period; the derivation cohorts also differed, in terms of age, sex, New York Heart Association (NYHA) class, EF, and type of HF (five scales included both acute and chronic HF patients, and six scales covered HF with reduced and preserved EF). In addition, the overall score designs vary significantly with regard to the number of factors involved (from 5 to 22), the type of parameters incorporated, and the method for calculating mortality risk. It is also worth noting that most of these studies did not incorporate NT‐proBNP and troponin levels, nor even pharmacotherapy, all of which are known prognostic factors in HF and DCM.
We identified only one study that compared the accuracy of the most popular HF prognostic scores in DCM. 11 However, the analysis of Muser et al. was conducted on a smaller (282 patients) and highly select group of DCM patients undergoing VT ablation. The authors reported similar survival rates of 15% over 4 years and found that SHFM yielded the highest prognostic accuracy.
In our study, the best performance (highest AUC and no significant lack of fit) in predicting 1, 2, 3, 4, and 5 year mortality was from both BCN Bio‐HF and SHFM, probably due to their advanced statistical methods (model linearity, continuous variables), the large number of parameters incorporated, including those typically associated with HF [NYHA, EF, laboratory parameters, and therapy (pharmacotherapy and CRT)], and factors also germane to DCM. The utility of most of the analysed scales showed moderate accuracy (most showing an AUC of 0.65–0.75) due to the overestimation of the mortality risk in the majority of patients.
The most probable explanation for the overestimation of the risk of death is the significantly higher mortality rate in HF derivation cohorts than in the DCM population (Supporting Information, Table S2 ). Without doubt, age is one the strongest parameters affecting mortality risk. Typically, general HF patients are 15 to 20 years older than DCM patients (in our study, the mean age was 54 years in comparison with the mean age of HF cohorts i.e. 65–73 years, a data point that was used for previously created prognostic models). Additional factors profoundly impacting HF prognosis are the type and number of comorbidities. Recent studies show that elderly HF patients have typically three (and more in most cases) clinically relevant and outcome‐affecting comorbidities. Younger DCM populations are significantly less burdened with comorbidities, which can also be seen in Supporting Information, Table S2 . HF aetiology is another significant influence on prognoses. HF due to CAD constitutes the majority of HF populations in various HF randomized controlled trials and registries, while numerous mechanisms causing HF development, such as acute ischaemic injury (as in the case of myocardial infarction), prolonged suboptimal myocardial perfusion, inflammation, or fibrosis, impact on the poor prognosis in HF patients due to CAD. Moreover, in the presentation of most patients with HF, for instance, as a result of CAD, primary valvular heart diseases or congenital heart diseases, there are many exacerbations that significantly worsen their prognosis. In contrast, DCM patients are rather unique in that a substantial number of them (between 30 to 50%) undergo left ventricular reverse remodelling (LVRR), which critically improves prognosis. Obviously, morphological and functional cardiac improvement may be seen in HF patients of other aetiologies; however, this is much rarer and more unpredictable in comparison with DCM. Thus, the phenomenon of LVRR must be taken into account when studying prognoses in DCM patients. Moreover, certain methods of treatment have a positive effect on a patient's prognosis, especially in light of LVRR. It has been shown that the novel pharmacotherapy angiotensin receptor‐neprilysin inhibitor (ARNI) significantly improves prognosis via the improvement of LV function, regardless of the HF aetiology. 31 Moreover, the patient response to therapy also determines outcome; for example, DCM patients respond better to CRT than ischaemic HF patients. Therefore, the mortality rate reported in the previous studies is also probably higher due to a lack of optimal novel HF therapy; in the derived cohorts, BBs were used in about 62% of cases and MRA in 28% of study populations.
Furthermore, we have clearly shown that simpler HF risk models, that is, those that are simpler in terms of the number of parameters used and the statistical methodology employed (such as MUSIC, CHARM), showed very poor accuracy for DCM patients. On the other hand, sophisticated risk models, incorporating much larger numbers of parameters (including continuous variables) and relying on linear statistical models, such as BCN Bio‐HF and SHFM, showed much better accuracy (70–80%) for our DCM population.
To the best of our knowledge, there has been only one prognostic scale dedicated to DCM patients to date, that performed by Miura et al. 9 However, its prognostic accuracy was not satisfactory (predicting correctly the outcome for only about 60% of patients) probably due to the small number of factors included—just five parameters [sex, age, NYHA, left ventricular end‐diastolic diameter (LVEDd) indexed on body surface area, EF]—and the simple method of calculation used, that is, numerical values attributed to the parameters under analysis.
The development of a mortality calculator tailored for dilated cardomyopathy
Many factors influence the mortality rate in DCM; among these are symptom severity, functional capacity, LV remodelling, the degree of impairment in left and right ventricular contractility, LV enlargement, QRS duration, the presence of late post‐contrast enhancement in magnetic resonance imaging, left bundle brunch block (LBBB) or VT, NYHA, and HF therapy. 12 , 16 , 21 , 22 , 24 , 26 , 32 However, their exact impact on death in a multivariable model is unclear because most of the studies identifying these factors focussed on a single specific parameter rather than investigating multivariable prognostic factors. The largest study on mortality in DCM, from Miura et al., performed a regression analysis, but their mortality rate differs significantly from the other studies mentioned above. As far as we know, ours is the first exploration of independent prognostic factors in such a large cohort of DCM patients. To the best of our knowledge, we are the first to address LVEDd as an independent mortality predictor. Moreover, we found symptom severity and duration, diabetes mellitus, LBBB, prior stroke, haemoglobin, NT‐proBNP, and angiotensin blocker usage to be independent prognostic factors. In contrast to the most recent discoveries of Cannata et al. with regard to a lower mortality rate in women, in our own analysis, gender was not found to be related to the outcome in multivariate prognostic analyses, a finding which is in accordance with the BCN Bio‐HF and SHFM studies. 33 , 34 , 35 Intriguingly, EF was not found to be a significant independent prognostic factor, which is in line with the BCN Bio‐HF study, but not with the latest prognostic analysis in HF, that is, PARADIGM‐HF (Simpson et al. reported a 7% decrease in mortality rate for every 5% increase of EF). 36 Similarly, BB usage was not a crucial factor (probably due to the high rate of use of this treatment in both survivors and non‐survivors). As in the BCN Bio‐HF, we did not find other HF therapies in the HF cohort to have any significant impact on outcomes in DCM (via multivariate analysis).
In light of the results of the scales comparison for HF, our own DCM prognostic calculator incorporated numerous variables and used model linearity for the calculation of mortality risk rather than creating a simple numerical score. Having carried out a detailed analysis, a calculator consisting of 21 parameters (including clinical, ECG, echocardiographic, and laboratory parameters as well as applied therapy) emerged as the one with the highest accuracy for mortality risk prediction.
We report here that our own DCM model outperformed all the other HF models under study, including BCN Bio‐HF and SHFM. Although most of the incorporated parameters in BCN Bio‐HF and SHFM and in our model are similar, there are also major differences that may well be responsible for the upgraded performance of our model. Cardiac morphology (e.g. LVEDd) is important both in the clinical course and prognosis in DCM (e.g. LVRR). Therefore, uniquely, we inserted LVEDd into our model, whereas no such parameter exists in other models, including BCN Bio‐HF and SHFM. Furthermore, the duration of HF symptoms, which roughly reflects the onset of HF, also seems to be a highly relevant parameter; however, apart from CHARM and MAGGIC scores (that in any performed less well than our model), this parameter was not included in other models. In contrast to other models, we included the highest number of disease‐modifying therapies (e.g. BBs, ACEI/ARB/ARNI, MRA, CRT‐D) and other therapies (digoxin, furosemide daily doses).
An example of a calculation for individualized risk
Based on the model presented here, the individual mortality risk of a DCM patient can be calculated using the formula from the Results section. For example, the 5 year mortality risk of a 35‐year‐old male patient with DCM diagnosed 22 months ago in NYHA class II, with a normal body mass index (24 kg/m2), dyslipidaemia but with no other concomitant disease (e.g. diabetes mellitus, prior stroke, abnormal liver function), with QRS 90 ms in ECG, LVEDd 72 mm and EF 25% via echocardiography, Hb 14.2 g/dL, glomerular filtration rate (GFR) 75 mL/min/1.73 m2, and NT‐proBNP 1756 pg/mL in laboratory tests, on adequate HF therapy (BB, ARNI, MRA; without digoxin and CRT), requiring 80 mg of furosemide per day is 4.3% In contrast, a 60‐year‐old woman diagnosed with DCM 5 years (60 months) ago with NYHA class III, obesity (body mass index 33 kg/m2), diabetes mellitus and dyslipidemia (without prior stroke and abnormal liver function), with LBBB, LVEDd 65 mm and EF 35%, Hb 12 g/dL, GFR 40 mL/min/1.73m2, and NT‐proBNP 500 pg/mL, on adequate HF therapy (BB, ARNI, MRA, digoxin; without CRT), requiring 160 mg of furosemide per day, has a 5‐year mortality risk of 24.4%
Study limitations
Although the size of the current study population is relatively large, we clearly acknowledge that the validity of any survival analyses may be considerably diminished for the purpose of investigating mortality in DCM, as this is a single‐centre retrospective analysis. Although only a few patients were excluded due to a lack of data or follow‐up information, there were deficiencies in the data for troponin and uric acid levels. However, this lack of data did not in any way influence the results of the analysis of the prognostic scales. Moreover, these parameters (troponin and uric acid levels) were not included in our own prognostic model. However, we included nine HF prognostic scales in our analysis, and we excluded several commonly used ones, such as Metabolic Exercise test data combined with Cardiac and Kidney Indexes, Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness, Enhanced Feedback for Effective Cardiac Treatment‐Heart Failure, and Heart Failure Survival Score scores, due to the lack of data. The prognostic calculator was created based on an unambiguous endpoint—overall mortality—and is a multiparameter model. It is the first model of such complexity tailored to DCM patients. As with previous studies on prognostic HF scales, which included both stable and unstable patients, our study population represents a mixture of stable DCM (85%) and urgently admitted patients due to HF worsening (15%).
Conclusions
The mortality rates calculated by common HF prognostic scales (BCN Bio‐HF, CHARM, GISSI‐HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure, MAGGIC, the score from Miura et al., MUSIC, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) vary in their results in terms of DCM survivals and non‐survivals; however, their performance in prognosis prediction seems to be limited for patients with DCM. A newly developed prognostic model tailored to DCM patients outperformed all of the scales under analysis (including BCN‐BioHF and SHFM) derived from heterogeneous HF populations.
Conflict of interest
None declared.
Funding
This work was supported by the Department of Scientific Research and Structural Funds of Medical College, Jagiellonian University (grant SAP N41/DBS/000130).
Author contributions
The contributions of the authors were as follows: Ewa Dziewięcka for data curation, formal analysis, investigation, methodology, software, visualization, and writing of the original draft; Matylda Gliniak, Mateusz Winiarczyk, and Arman Karapetyan for data curation; Aleksandra Karabinowska and Sylwia Wiśniowska‐Śmiałek for data curation and investigation; Marcin Dziewięcki for software and formal analysis; Piotr Podolec for validation and methodology; and Paweł Rubiś for conceptualization, investigation, methodology, supervision, validation, and writing, reviewing, and editing.
Supporting information
Figure S1. Study population according to hospitalization date.
Table S1. External validation of our own prognostic model compared with the BCN Bio‐HF calculator. The size of testing set was in line with the 80–20 rule to eliminate errors associated with an arbitrary choice of size for the test group.
Table S2. Comparison of derivation studies from analysed prognostic scales and their models.
APPENDIX 1.
The parameters considered to be predictors were defined as follows: weight and echocardiographic parameters were obtained from hospital admission in stable patients or after stabilization in the case of urgent hospitalization; the presence of concomitant disease was established based on patients' medical documentation, pharmacotherapy in use, and the results obtained during hospitalization; the presence of abnormal liver function was defined as chronic hepatic disease (e.g. cirrhosis) or biochemical evidence of significant hepatic derangement (bilirubin twice the normal upper limit, in association with aspartate or alanine aminotransferase/alkaline phosphatase three times the normal upper limit, labile INR without antithrombotic therapy); and pharmacological therapy was assessed at hospital discharge.
Dziewięcka, E. , Gliniak, M. , Winiarczyk, M. , Karapetyan, A. , Wiśniowska‐Śmiałek, S. , Karabinowska, A. , Dziewięcki, M. , Podolec, P. , and Rubiś, P. (2020) Mortality risk in dilated cardiomyopathy: the accuracy of heart failure prognostic models and dilated cardiomyopathy‐tailored prognostic model. ESC Heart Failure, 7: 2455–2467. 10.1002/ehf2.12809.
References
- 1. Lupón J, Simpson J, McMurray JJV, de Antonio M, Vila J, Subirana I, Barallat J, Moliner P, Domingo M, Zamora E, Bayes‐Genis A. Barcelona Bio‐HF Calculator Version 2.0: incorporation of angiotensin II receptor blocker neprilysin inhibitor (ARNI) and risk for heart failure hospitalization. Eur J Heart Fail 2018; 20: 938–940. [DOI] [PubMed] [Google Scholar]
- 2. Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJV, Swedberg KB, Östergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006; 27: 65–75. [DOI] [PubMed] [Google Scholar]
- 3. Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 4. Collier TJ, Pocock SJ, McMurray JJV, Zannad F, Krum H, Van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. The impact of eplerenone at different levels of risk in patients with systolic heart failure and mild symptoms: insight from a novel risk score for prognosis derived from the EMPHASIS‐HF trial. Eur Heart J 2013; 34: 2823–2829. [DOI] [PubMed] [Google Scholar]
- 5. Barlera S, Tavazzi L, Franzosi MG, Marchioli R, Raimondi E, Masson S, Urso R, Lucci D, Nicolosi GL, Maggioni AP, Tognoni G. Predictors of mortality in 6975 patients with chronic heart failure in the Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto Miocardico‐Heart Failure trial. Proposal for a nomogram. Circ Heart Fail 2013; 6: 31–39. [DOI] [PubMed] [Google Scholar]
- 6. Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Predictors of in‐hospital mortality in patients hospitalized for heart failure. Insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE‐HF). J Am Coll Cardiol 2008; 52: 347–356. [DOI] [PubMed] [Google Scholar]
- 7. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006; 113: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 8. Vazquez R, Bayes‐Genis A, Cygankiewicz I, Pascual‐Figal D, Grigorian‐Shamagian L, Pavon R, Gonzalez‐Juanatey JR, Cubero JM, Pastor L, Ordonez‐Llanos J, Cinca J, De Luna AB. The MUSIC Risk score: a simple method for predicting mortality in ambulatory patients with chronic heart failure. Eur Heart J 2009; 30: 1088–1096. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 10. Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, Di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, Di Lenarda A. Prevalence and prognostic impact of non‐cardiac co‐morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community‐based study. Eur J Heart Fail 2018; 20: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 11. Muser D, Liang JJ, Castro SA, Lanera C, Enriquez A, Kuo L, Magnani S, Birati EY, Lin D, Schaller R, Supple G, Zado E, Garcia FC, Nazarian S, Dixit S, Frankel D, Callans DJ, Marchlinski FE, Santangeli P. Performance of prognostic heart failure models in patients with nonischemic cardiomyopathy undergoing ventricular tachycardia ablation. JACC Clin Electrophysiol 2019; 5: 801–813. [DOI] [PubMed] [Google Scholar]
- 12. Marume K, Noguchi T, Tateishi E, Morita Y, Kamakura T, Ishibashi K, Noda T, Miura H, Nishimura K, Nakai M, Yamada N, Tsujita K, Anzai T, Kusano K, Ogawa H, Yasuda S. Mortality and sudden cardiac death risk stratification using the noninvasive combination of wide QRS duration and late gadolinium enhancement in idiopathic dilated cardiomyopathy. Circ Arrhythm Electrophysiol 2018; 11: e006233. [DOI] [PubMed] [Google Scholar]
- 13. Sobrino‐Márquez JM, Grande‐Trillo A, Cantero‐Pérez EM, Rangel‐Sousa D, Lage‐Galle E, Adsuar‐Gómez A. Prognostic value of blood panel parameters in patients with dilated cardiomyopathy and advanced heart failure. Transplant Proc 2018; 50: 650–652. [DOI] [PubMed] [Google Scholar]
- 14. Gulati A, Ismail TF, Jabbour A, Ismail NA, Morarji K, Ali A, Raza S, Khwaja J, Brown TDH, Liodakis E, Baksi AJ, Shakur R, Guha K, Roughton M, Wage R, Cook SA, Alpendurada F, Assomull RG, Mohiaddin RH, Cowie MR, Pennell DJ, Prasad SK. Clinical utility and prognostic value of left atrial volume assessment by cardiovascular magnetic resonance in non‐ischaemic dilated cardiomyopathy. Eur J Heart Fail 2013; 15: 660–670. [DOI] [PubMed] [Google Scholar]
- 15. Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA, Raza S, Khwaja J, Brown TDH, Morarji K, Liodakis E, Roughton M, Wage R, Pakrashi TC, Sharma R, Carpenter J‐P, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013; 128: 1623–1633. [DOI] [PubMed] [Google Scholar]
- 16. Riad A, Weitmann K, Herda LR, Empen K, Gross S, Nauck M, Dörr M, Klingel K, Kandolf R, Hoffmann W, Felix SB. Initial white blood cell count is an independent risk factor for survival in patients with dilated cardiomyopathy. Int J Cardiol 2013; 168: 1207–1213. [DOI] [PubMed] [Google Scholar]
- 17. Ertaş G, Kozdaǧ G, Emre E, Vural A, Akbulut T, Ural D, Göktekin Ö. Renal function has an effect on cardiovascular mortality in patients with dilated cardiomyopathy. J Cardiovasc Med 2012; 13: 554–558. [DOI] [PubMed] [Google Scholar]
- 18. Sha J, Zhang S, Tang M, Chen K, Zhao X, Wang F. Fragmented QRS is sssociated with all‐cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol 2011; 16: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aleksova A, Merlo M, Zecchin M, Sabbadini G, Barbati G, Vitrella G, Di Lenarda A, Sinagra G. Impact of atrial fibrillation on outcome of patients with idiopathic dilated cardiomyopathy: data from the Heart Muscle Disease Registry of Trieste. Clin Med Res 2010; 8: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vischer A, Osswald S, Sticherling C, Schaer B. Outcome of patients with dilated cardiomyopathy in a contemporary Swiss population. Acta Cardiol 2009; 64: 347–350. [DOI] [PubMed] [Google Scholar]
- 21. Venner C, Selton‐Suty C, Huttin O, Erpelding M‐L, Aliot E, Juillière Y. Right ventricular dysfunction in patients with idiopathic dilated cardiomyopathy: prognostic value and predictive factors. Arch Cardiovasc Dis 2016; 109: 231–241. [DOI] [PubMed] [Google Scholar]
- 22. Miura K, Matsumori A, Nasermoaddeli A, Soyama Y, Morikawa Y, Sakurai M, Kitabatake A, Nagai M, Inaba Y, Nakagawa H. Prognosis and prognostic factors in patients with idiopathic dilated cardiomyopathy in Japan. Results From a Nationwide Study. Circ J 2008; 72: 343–348. [DOI] [PubMed] [Google Scholar]
- 23. Chen R, Lu A, Wang J, Ma X, Zhao L, Wu W, Du Z, Fei H, Lin Q, Yu Z, Liu H. Using machine learning to predict one‐year cardiovascular events in patients with severe dilated cardiomyopathy. Eur J Radiol 2019; 117: 178–183. [DOI] [PubMed] [Google Scholar]
- 24. Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol 2011; 57: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 25. Halliday BP, Gulati A, Ali A, Newsome S, Lota A, Tayal U, Vassiliou VS, Arzanauskaite M, Izgi C, Krishnathasan K, Singhal A, Chiew K, Gregson J, Frenneaux MP, Cook SA, Pennell DJ, Collins P, Cleland JGF, Prasad SK. Sex‐ and age‐based differences in the natural history and outcome of dilated cardiomyopathy. Eur J Heart Fail 2018; 20: 1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karatolios K, Holzendorf V, Richter A, Schieffer B, Pankuweit S, Competence Network Heart Failure Germany . Long‐term outcome and predictors of outcome in patients with non‐ischemic dilated cardiomyopathy. Int J Cardiol 2016; 220: 608–612. [DOI] [PubMed] [Google Scholar]
- 27. Merlo M, Pivetta A, Pinamonti B, Stolfo D, Zecchin M, Barbati G, Di Lenarda A, Sinagra G. Long‐term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. Eur J Heart Fail 2014; 16: 317–324. [DOI] [PubMed] [Google Scholar]
- 28. Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TDH, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013; 309: 896–908. [DOI] [PubMed] [Google Scholar]
- 29. Rubiś P. The diagnostic work‐up of genetic and inflammatory dilated cardiomyopathy. E‐J Cardiol Pract 2015; 13: 19. [Google Scholar]
- 30. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 31. Balmforth C, Simpson J, Shen L, Jhund PS, Lefkowitz M, Rizkala AR, Rouleau JL, Shi V, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM‐HF. JACC Hear Fail 2019; 7: 457–465. [DOI] [PubMed] [Google Scholar]
- 32. Sinagra G, Iorio A, Merlo M, Cannatà A, Stolfo D, Zambon E, Di Nora C, Paolillo S, Barbati G, Berton E, Carriere C, Magrì D, Cattadori G, Confalonieri M, Di Lenarda A, Agostoni P. Prognostic value of cardiopulmonary exercise testing in idiopathic dilated cardiomyopathy. Int J Cardiol 2016; 223: 596–603. [DOI] [PubMed] [Google Scholar]
- 33. Cannatà A, Fabris E, Merlo M, Artico J, Gentile P, Pio Loco C, Ballaben A, Ramani F, Barbati G, Sinagra G. Sex differences in the long‐term prognosis of dilated cardiomyopathy. Can J Cardiol 2020; 36: 37–44. [DOI] [PubMed] [Google Scholar]
- 34. Lupón J, De Antonio M, Vila J, Peñafiel J, Galán A, Zamora E, Urrutia A, Bayes‐Genis A. Development of a novel heart failure risk tool: the Barcelona Bio‐Heart Failure risk calculator (BCN bio‐HF calculator). PLoS ONE 2014; 9: e85466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JGF, Carson PE, Maggioni AP, Mann DL, Pitt B, Poole‐Wilson PA, Levy WC. Prediction of mode of death in heart failure: The Seattle Heart Failure Model. Circulation 2007; 116: 392–398. [DOI] [PubMed] [Google Scholar]
- 36. Simpson J, Jhund PS, Lund LH, Padmanabhan S, Claggett BL, Shen L, Petrie MC, Abraham WT, Desai AS, Dickstein K, Køber L, Packer M, Rouleau JL, Mueller‐Velten G, Solomon SD, Swedberg K, Zile MR, McMurray JJV. Prognostic models derived in PARADIGM‐HF and validated in ATMOSPHERE and the Swedish Heart Failure Registry to predict mortality and morbidity in chronic heart failure. JAMA Cardiol 2020; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study population according to hospitalization date.
Table S1. External validation of our own prognostic model compared with the BCN Bio‐HF calculator. The size of testing set was in line with the 80–20 rule to eliminate errors associated with an arbitrary choice of size for the test group.
Table S2. Comparison of derivation studies from analysed prognostic scales and their models.


