ABSTRACT
Triclocarban (TCC) is a widely used antimicrobial ingredient in consumer products and is a ubiquitous contaminant in the environment. In 2016, the FDA removed TCC from over-the-counter handwashing products, but this compound is still approved for use in many other personal care products. A better understanding of its impact on human health could lead to significant impact for public health and regulatory policies. Here we show that exposure to low-dose TCC exaggerated the severity of colitis and exacerbated the development of colitis-associated colon tumorigenesis, via gut microbiota-dependent mechanisms. Exposure to TCC increased dextran sodium sulfate (DSS)- and interleukin 10 (IL-10) knockout-induced colitis, and exaggerated azoxymethane (AOM)/DSS-induced colon tumorigenesis in mice. Regarding the mechanisms, TCC exposure reduced the diversity and altered the composition of gut microbiota and failed to promote DSS-induced colitis in mice lacking the microbiota, supporting that the presence of the microbiota is critical for the pro-colitis effects of TCC. Together, these results support TCC could be a novel risk factor for colitis and colitis-associated colon cancer, and further regulatory policies on this compound could be needed.
KEYWORDS: Gut microbiota, inflammatory bowel disease, colon cancer, environmental chemical, triclocarban
Introduction
Inflammatory bowel disease (IBD), including Ulcerative colitis that is characterized by chronic inflammation in the colon and rectum and Crohn’s disease that involves inflammation in the small intestine, severely impacts patient quality of life; symptoms include abdominal pain, vomiting, diarrhea, and rectal bleeding. In addition, the IBD patients have increased risks of developing colon cancer.1,2 The incidences of IBD have risen dramatically in recent decades:3 in 2015, ~1.3% of the US adults (3 million) were estimated to be diagnosed with IBD,4 representing a 50% increase from 1999 (2 million).5 The rapid development supports that environmental factors, rather than genetic drift, are primarily responsible for the increased incidences of IBD.6-9 It is of practical importance to identify the environmental risk factors of IBD, which could lead to significant impact for public health and regulatory policy. However, the roles of environmental factors in IBD are under-studied, and represent a significant knowledge gap within the pathogenesis of IBD.
Triclocarban (3,4,4ʹ-trichlorocarbanilide, TCC) has been used as an antimicrobial ingredient for more than 60 years, and is incorporated into many consumer products such as bar soaps, deodorants, and detergents.10 Each year, U.S. consumers are exposed to approximate 500,000 pounds of TCC from personal care products.10 The 2013–2014 National Health and Nutrition Examination Survey showed that 36.9% of the urine samples in the U.S. contained TCC.11 The majority of used TCC is ultimately released into the environment leading to widespread pollution. As a result, TCC was listed as a top-10 contaminant in U.S. rivers.10 More alarmingly, recent studies showed that environmental TCC could be efficiently taken up by food crops, leading to the bioaccumulation of TCC and potential human exposure through food consumption. Notably, Mathews et al. showed that some common food crops, such as broccoli, potato, beat, cabbage, and pepper, can accumulate >100 ppm TCC in the root tissues, and onions can accumulate >800 ppm TCC in the bulbs.12 The results from this study are supported by many other investigations.13-20 Together, the ubiquitous presence of TCC has raised concern about its impact on the environment and human health.
The regulatory policy of TCC is an intensively debated topic now. In 2016, the FDA removed TCC from over-the-counter handwashing products.21 This decision was mainly based on recent studies which showed that compared with plain soaps, the antimicrobial soaps containing TCC did not provide additional health benefits;22 therefore, high-volume low-value use of TCC in handwashing products was not further allowed by the FDA.21 This ruling only affects over-the-counter handwashing products, but TCC remains approved by the FDA and the EPA for use in many other consumer products. A better understanding of the impact of TCC on human health could be important to prepare possible further regulatory policies of this compound.
Previous studies for TCC toxicology have focused on endocrine function,23-29 however the effects of TCC on other human disorders are largely unknown. Our recent studies showed that exposure to other consumer antimicrobials, such as triclosan (TCS), benzalkonium chloride (BAC), and benzethonium chloride (BET), exaggerates the severity of colitis and exacerbates the development of colitis-associated colon tumorigenesis in mouse models, through gut microbiota-dependent mechanisms.30,31 To date, the effects of TCC on colitis are unknown. Here we studied the actions and mechanisms of TCC on colitis and colitis-associated colon tumorigenesis in animal models.
Methods and materials
Animal experiments
All animal experiments were conducted in accordance with the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Massachusetts Amherst. Six-week-old male C57BL/6 mice were housed in a 24°C standard specific-pathogen-free (SPF) animal room with 12-h light/dark cycle and given food and water ad libitum. To facilitate the microbiota study, after mouse arrival, the mice were rotated between different cages during the adaptation period, then the mice were randomized to different treatment groups (2–3 mice per cage). These procedures are expected to mitigate potential cage effects on gut microbiota.32,33
Dextran sodium sulfate (DSS)-induced colitis
C57BL/6 male mice (six-week-old, Charles River, Wilmington, MA) were randomly divided into control and treatment groups (n = 8 for each group). The mice in the control group were treated with a modified AIN-93G diet (Table S1) containing 0.5% v/w polyethylene glycol 400 (PEG 400, EMD Millipore, Billerica, MA), and the mice in the treatment group were treated with the diet containing TCC (99%, Sigma-Aldrich, St. Louis, MO) dissolved in PEG 400 during the whole experiment. After 3 weeks, the mice were treated with 2% DSS (36–50 kDa, MP Biomedicals, Solon, OH) in drinking water to induce acute colitis. After 9 days, the mice were sacrificed, and the blood and colon tissues were collected for analysis.
Interleukin-10 (Il-10)−/− colitis model
Il-10−/− male mice (five-week-old, stock No. 002251, JAX, Bar Harbor, ME) were randomly divided into control and treatment groups (n = 8 for each group). The mice were fed with the modified AIN-93G diet containing PEG 400 (0.5% v/w) or 80 ppm TCC dissolved in PEG 400 during the whole experiment. Standard-sterilized water was supplied in bottles ad libitum. After 12 weeks, the mice were treated with 200 ppm piroxicam (Sigma-Aldrich, St. Louis, MO) via diet to accelerate development of colitis.34 After 1 week, the mice were sacrificed, and the blood and colon tissues were collected for analysis.
Azoxymethane (AOM)/DSS-induced colorectal tumorigenesis in mice
C57BL/6 male mice (six-week-old) were acclimated for 1 week and randomized into control and treatment groups (n = 16 for each group). The mice were fed with the modified AIN-93G diet containing PEG 400 (0.5% v/w) or 80 ppm TCC dissolved in PEG 400 during the whole experiment. After 3 weeks, the mice were treated with 10 mg/kg AOM (Sigma-Aldrich, St. Louis, MO) via intraperitoneal injection. After 1 week, they were given 2% DSS in drinking water for 1 week. At day 50 post the AOM injection, the mice were sacrificed, and the blood and colon tissues of the mice were collected for analysis. The colon tissues were cut open longitudinally, the tumor numbers were counted. The diameter of each tumor was measured and the tumor size was calculated using the formula tumor size = π/4 x diameter,2 as we described.30
Antibiotic cocktail-mediated suppression of gut microbiota
C57BL/6 male mice (five-week-old) were treated with drinking water with or without a broad-spectrum antibiotic cocktail (1.0 g/L ampicillin and 0.5 g/L neomycin) during the whole experiment.35,36 After 4 days, the mice were treated with the modified AIN-93G diet containing 80 ppm TCC or vehicle (PEG 400) until the end of the experiment. After 3 weeks of diet treatment, the mice were stimulated with 2% DSS for 8 days in drinking water to induce colitis. At the end of the experiment, the mice were sacrificed, and the blood and colon tissues were collected for analysis.
Flow cytometry analysis
The distal colon tissues from the mice were dissected, washed with cold PBS, and digested with Hank’s-balanced salt solution (HBSS, Lonza, Basel, Switzerland) supplemented with 1 mM dithiothreitol (DTT) and 5 mM ethylenediaminetetraacetic acid (EDTA) for 2 h at 4°C. The single-cell suspensions were filtered through 70 μm cell filters (BD Biosciences, San Jose, CA). The cells were stained with FITC-conjugated anti-mouse CD45 antibody, PerCP/Cy5.5-conjugated anti-mouse F4/80 antibody, and isotype control antibody according to the manufacturer’s instructions (BioLegend, San Diego, CA). The stained cells were analyzed using BD LSRFortessa™ cell analyzer (BD Biosciences, San Jose, CA) and data were analyzed using FlowJo software (FlowJo LLC, Ashland, OR). In our analysis, leukocytes were identified as CD45+ cells and macrophages were identified as CD45+ F4/80+ cells.
ELISA analysis of cytokines in plasma
The blood samples were harvested via cardiac puncture, and the plasma fractions were prepared by centrifugation of the blood at 1,500 g for 10 min at 4°C. The concentration of IL-6 in plasma was determined using a CBA Mouse Inflammation Kit (BD Biosciences) according to the manufacturer’s instruction.
Quantitative reverse transcription PCR (RT-qPCR) analysis
The colon tissues were frozen by liquid nitrogen and ground. Total RNA was isolated from the colon tissues using TRIzol reagent (Ambion, Austin, TX) according to the manufacturer’s instructions. The RNA was reverse transcribed into cDNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instruction. RT-qPCR was carried out with a DNA Engine Opticon system (Bio-Rad Laboratories, Hercules, CA) with Maxima SYBR-green Master Mix (Thermo Fisher Scientific). The sequences of mouse-specific primers (Thermo Fisher Scientific) were listed in Table S2. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an internal control.
Hematoxylin and eosin (H&E) staining
The dissected colon tissues were fixed in 4% formalin (Thermo Fisher Scientific) for 24 h, embedded into paraffin (Thermo Fisher Scientific), and sliced into 5-μm sections. The slides were stained with hematoxylin and eosin (Sigma-Aldrich), and examined with a light microscope. The histological scores were evaluated by observers who were blinded as to which treatment group the mice belonged to. The histological damage score is the sum of evaluation based on crypt architecture, degree of inflammatory cell infiltration, muscle thickening, and goblet cell depletion of the tissue.37
Immunohistochemistry (IHC)
The sections were prepared and heated in 0.01 M citrate buffer (pH 6.0) for 20 min in a PT Module antigen retrieval device (Thermo Fisher Scientific). The antibodies against mouse PCNA and β-catenin (Cell Signaling Technology) were incubated overnight at 4°C. Horseradish peroxidase (HRP)-conjugated secondary antibodies were then applied to the sections, followed by chromogen 4-diaminobenzidine (Dako, Carpinteria, CA) staining. Sections were then counterstained with hematoxylin for 2 min. Positive expression was observed under light microscope.
16S rRNA sequencing of fecal microbiota
C57BL/6 male mice (six-week-old) were maintained on a modified AIN-93G diet containing PEG 400 (0.5% v/w) or 80 ppm TCC dissolved in PEG 400 for 3 weeks. The feces were collected for microbiota analysis. The total fecal DNA was extracted using QIAamp DNA Stool Mini Kit (Qiagen) following the manufacturer’s instruction with the addition of the bead-beating step. The quality of the extracted DNA was measured using a NanoDrop Spectrophotometer (Thermo Scientific) and verified using gel electrophoresis. PCRs were performed in a 96-well format on a Veriti thermal cycler (Life Technology) with 2 × KAPA HiFi Hotstart ReadyMix (KAPA Biosystem) using primers specific for the V3-V4 region of the 16s rRNA gene (see Table S2). After purification with AMPure XP beads (Beckman Coulter), a limited cycle PCR was performed using the Nextera XT Index Kit (Illumina) to attach dual indices and Illumina sequencing adapters, followed by an additional purification with AMPure XP bead. The quantity of the purified PCR products was measured using a Qubit dsDNA BR Assay kit (Life technology) and the amplicon quality was estimated by ScreenTape Assay on Tape Station 2200 (Agilent). After quantification and qualification, samples were pooled in equimolar amount and pair-end 2*300 bp sequencing was performed on an Illumina MiSeq platform using a Miseq reagent kit V3 (8% PhiX) (Illumina). The sequencing data were processed by QIIME software pipeline v1.9.1. In general, the high-quality sequence data (quality value ≥ 30) was demultiplexed. Sequences were then clustered into operational taxonomic units (OTUs) using Open reference OTU picking against Greengenes bacterial 16S rRNA database (13_8 release) with a 97% similarity threshold. The α-diversity (the diversity within sample community species richness) was determined with 10 iterations at a maximal sequence depth where all samples could be included. The β-diversity (dissimilarity among different treatment groups) was calculated using weighted and unweighted UniFrac distances.
Culture of bifidobacterium infantis 272
B. infantis 272 (ATCC, Manassas, VA) was subcultured at 37°C in MRS broth (Thermo Fisher Scientific) containing 0.5 g/L L-cysteine in an anaerobic cabinet (Whitley A35 anaerobic workstation, Don Whitley Scientific, Shipley, England) under an atmosphere of 85% N2, 10% CO2, and 5% H2. The B. infantis 272 were inoculated 1:100 into MRS broth containing TCC or DMSO vehicle and then incubated at 37°C in anaerobic conditions for 48 h. Bacterial growth was monitored by measuring the turbidity at 600 nm.
Data analysis
Data are expressed as the mean ± standard error of the mean (SEM). Statistical comparison of two groups was performed using Student’s t-test or Wilcoxon-Mann-Whitney test, and comparison of three or more groups was analyzed by one-way ANOVA followed by Tukey’s post hoc test. Analysis of inflammation in antibiotics-treated mouse experiments was performed by two-way ANOVA, followed by Tukey–Kramer’s method, and H&E histology data were analyzed by two-way ANOVA Poisson Generalized Linear Model, followed by the Tukey–Kramer’s multiple comparison method. P < .05 were considered statistically significant.
Results
TCC increased DSS-induced colitis in mice
To determine the effect of TCC on colonic inflammation, we studied its effect on colitis using a well-established DSS-induced colitis model in C57BL/6 mice.38 Treatment with TCC via diet (80 ppm in diet, administering TCC at a dose of ~8 mg/kg/day, based on a diet of 3 g daily chow) exaggerated DSS-induced colitis in mice (Figure 1). Compared with vehicle control, treatment with TCC exacerbated body weight loss (Figure 1a, P< .05), exacerbated colon length reduction (Figure 1b, P < .01), increased plasma concentrations of the pro-inflammatory cytokine IL-6 (Figure 1c, P < .01), up-regulated the gene expression of Il-6 in the colon (Figure 1d, P < .05), increased infiltration of leukocytes (CD45+) into the colon (Figure 1e), and exaggerated crypt damage in the colon (Figure 1f, P < .01). We also tested the effect of a low-dose TCC (10 ppm in diet) and found that TCC also exaggerated DSS-induced colitis (Figure S1). Compared with treatment with 10 ppm TCC via diet, treatment with 80 ppm TCC did not further increase DSS-induced colitis in a statistically significant manner. Together, these results demonstrate the pro-colitis effect of TCC in vivo.
Figure 1.
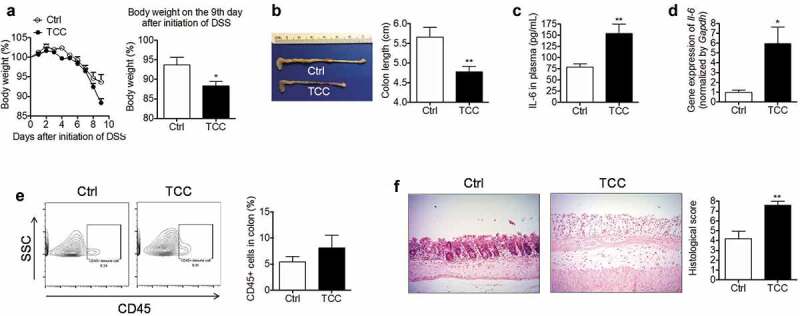
TCC increased DSS-induced colonic inflammation in C57BL/6 mice. (a) Bodyweight. Left: time-course of body weight; Right: quantification of mouse body weight on the final day. (b) Colon length. (c) Concentration of IL-6 in plasma. (d) Gene expression of Il-6 in colon. (e) Quantification of immune cell infiltration into the colon by flow cytometry analysis. (f) H&E staining of the colon. The data are mean ± SEM, * P < .05, ** P < .01, n = 8 mice per group.
TCC increased colitis in Il-10−/− mice
To further validate the pro-colitis effect of TCC, we tested its action on spontaneous colitis using a genetically engineered Il-10−/− mouse model.34 Treatment with TCC via diet (80 ppm in diet) exaggerated colitis in Il-10−/− mice (Figure 2). Compared with the vehicle control, treatment with TCC reduced the colon length (Figure 2a, P < .05), increased the gene expression of Il-6 in the colon (Figure 2b, P < .05), enhanced infiltration of leukocytes (CD45+) into the colon (Figure 2c, P < .01), and exaggerated crypt damage in the colon (Figure 2d, P < .05). These results further validate that exposure to TCC exaggerated colitis in vivo.
Figure 2.
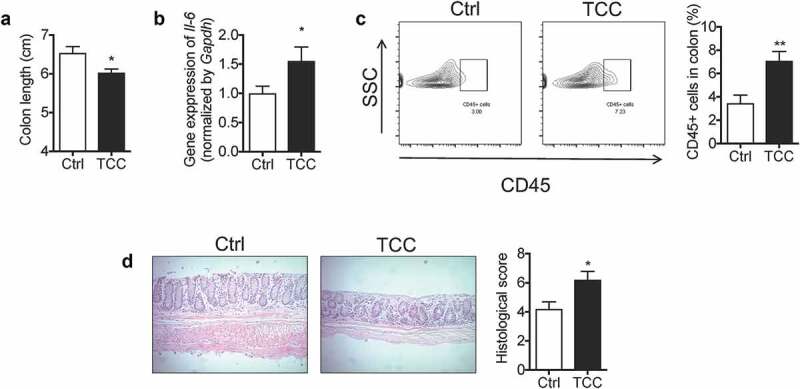
TCC increased the colonic inflammation in Il-10−/- mice. (a) Colon length. (b) Gene expression of Il-6 in colon. (c) Quantification of immune cell infiltration into the colon by flow cytometry analysis. (d) H&E staining of the colon. The data are mean ± SEM, * P < .05, ** P < .01, n = 8 mice per group.
TCC increased AOM/DSS-induced colon tumorigenesis in mice
We tested the effect of TCC on colitis-associated colon tumorigenesis using a well-established AOM/DSS-induced colon cancer model in C57BL/6 mice.39 Treatment with TCC via diet (80 ppm in diet) increased AOM/DSS-induced colon tumorigenesis in mice (Figure 3). Compared with the vehicle control, treatment with TCC reduced overall survival of the mice (Figure 3a). Regarding colon tumorigenesis, TCC increased the tumor number, tumor size, and total tumor burden in mice (Figure 3b, P < .01), illustrating its pro-tumorigenic effect. Consistent with enhanced colon tumorigenesis, immunohistochemical staining showed that TCC increased protein levels of proliferating cell nuclear antigen (PCNA, a marker of tumor proliferation, P < .01) and β-catenin (a marker of the pro-tumorigenic Wnt pathway, P < .001) in colon tumors (Figure 3c). In addition, RT-qPCR showed that TCC treatment increased expressions of c-Myc and Axin2 (markers of Wnt pathway) in colon tumors (Figure 3d, P < .05), further supporting that TCC enhanced activation of the pro-tumorigenic Wnt pathway in vivo. Inflammation plays a central role in colon tumorigenesis.1 Compared with the vehicle control, TCC increased gene expressions of Il-6 and Tnf-α in colon tumors (Figure 3d, P < .05), and enhanced infiltration of CD45+ and CD45+ F4/80+ immune cells into colon tumors (Figure 3e, P < .01), illustrating its enhancing effect on tumor inflammation. Together, these results demonstrate that exposure to TCC exaggerated colitis-associated colon tumorigenesis in vivo.
Figure 3.
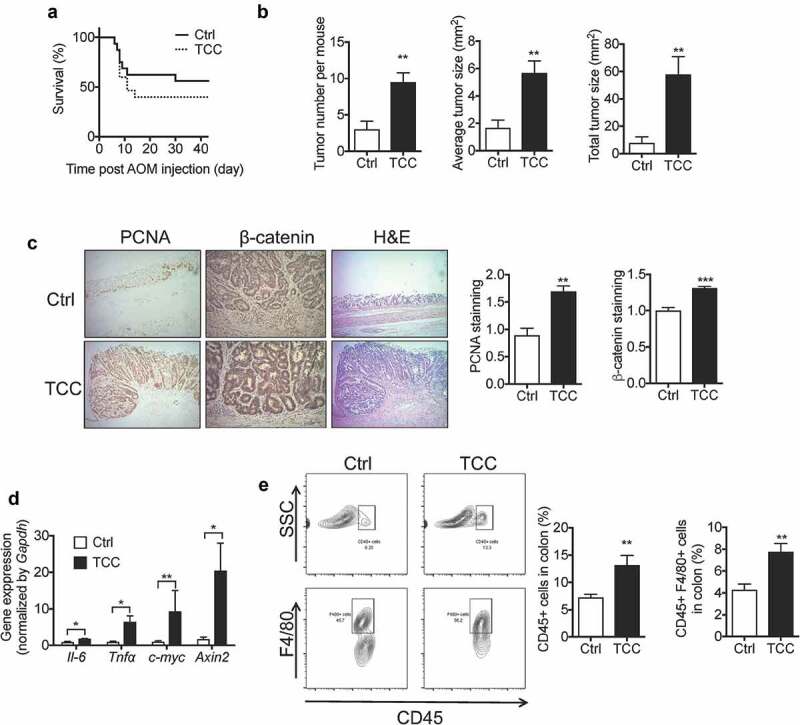
TCC increased AOM/DSS-induced colon cancer in C57BL/6 mice. (a) Survival curve. (b) Quantification of colon tumors in mice. (c) IHC staining of PCNA and β-catenin in colon tumors from the mice treated with vehicle or TCC. (d) Gene expressions in colon tumors. (e) Quantification of immune cell infiltration into colon tumors by flow cytometry analysis. The data are mean ± SEM, * P < .05, ** P < .01, *** P < .001, n = 16 mice per group.
TCC reduced the diversity and changed the composition of gut microbiota in mice
Gut microbiota plays a central role in regulating colonic inflammation and colon tumorigenesis.40 We studied the effect of TCC on gut microbiota in C57BL/6 mice. Treatment with TCC via diet (80 ppm in diet) for 3 weeks decreased the α-diversity of the gut microbiota, as assessed by PD-whole tree analysis (Figure 4a, P < .001), and modulated the β-diversity of the microbiota, as assessed by principal coordinate analysis (Figure 4b, P < .01). Regarding the composition of the gut microbiota, exposure to TCC altered the relative bacterial abundance at both phylum and genus levels (Figure 4c–d, Table S3–4). Notably, TCC increased the abundance of Proteobacteria (Figure 4c, Table S3, P < .001), which has been shown to be increased in IBD patients and is associated with the pathogenesis of IBD.41 TCC also reduced the abundance of Bifidobacterium (Figure 4d, Table S4, P < .05), which has been shown to have anti-inflammatory effects.42 Together, these results showed that exposure to TCC could cause adverse effects on gut microbiota.
Figure 4.

TCC reduced the diversity and altered the composition of gut microbiota in C57BL/6 mice. (a) α-diversity of the gut microbiota. (b) β-diversity of the gut microbiota, calculated by Principle Coordinate Analysis (PCoA) based on weighted UniFrac distance. (c) Relative abundance of gut bacteria at phylum levels. (d) Relative abundance of gut bacteria at genus levels. The data are mean ± SEM, *** P < .01, n = 16 mice per group.
TCC inhibited growth of bifidobacterium infantis in vitro
Given our findings that TCC reduced the relative abundance of Bifidobacterium in gut microbiota in vivo, we studied whether it could directly inhibit the growth of Bifidobacterium in vitro. Compared with the vehicle control (DMSO), TCC at a concentration of 100 nM inhibited ~30% of the growth of B. infantis 272 (Figure S2, P < .05). This result supports that TCC could have direct effects on gut bacteria.
TCC increased DSS-induced colitis via gut microbiota-dependent mechanisms
To validate the roles of gut microbiota in the biological actions of TCC, we tested whether antibiotic cocktail-mediated suppression of gut microbiota modulates the pro-colitis effect of TCC (see scheme of animal experiment in Figure 5a). We used an antibiotic cocktail from previous studies.35,36 We found that treatment with this cocktail caused a > 99% reduction of fecal bacteria, as assessed by RT-qPCR analysis of the 16S rRNA gene (Figure S3), validating that this antibiotic cocktail suppressed gut microbiota. Before the DSS stimulation, treatment with TCC and/or the antibiotic cocktail had little impact on mouse body weight (Figure S4).
Figure 5.

TCC increased DSS-induced colitis via gut microbiota-dependent mechanisms. (a) Scheme of animal experiment. (b) Colon length. (c) FACS quantification of immune cell infiltration into colon. (d) H&E staining of colon. The data are mean ± SEM. The statistical significance (P-value) of the interaction effect between TCC treatment (TCC versus vehicle control in the diet) and antibiotic treatment (antibiotic cocktail versus no antibiotic cocktail in the drinking water) on colitis was determined by two-way ANOVA analysis. * P < .05, ** P < .01, *** P < .001, n = 8–10 mice per group.
Regarding DSS-induced colitis, two-way ANOVA analysis showed that there was a significant interaction (P < .05) between TCC treatment (TCC versus vehicle) and antibiotic treatment (antibiotic cocktail versus no antibiotic cocktail) on colonic inflammation (Figure 5b–d). Notably, without antibiotic treatment, TCC exposure enhanced crypt damage in the colon while, with antibiotic treatment, the pro-colitis effect of TCC was abolished (Figure 5d). These results support that gut microbiota play a critical role in the pro-colitis effect of TCC in vivo.
Discussion
To date, the effects of TCC on human health are not well understood. Previous studies showed that TCC could be a potential endocrine-disrupting compound;23-29 besides endocrine function, the effects of TCC on other human disorders are largely unknown. Here our central finding is that exposure to TCC exaggerated colonic inflammation and colitis-associated colon tumorigenesis in mice. We found that exposure to relatively low-dose TCC via diet (10–80 ppm in diet, administering TCC at a dose of ~1–8 mg/kg/day, based on a diet of 3 g daily chow) increased disease developments in multiple animal models, including DSS-induced acute colitis in C57BL/6 WT mice, chronic colitis in genetically engineered Il-10−/− mice, and AOM/DSS-induced colon tumorigenesis in C57BL/6 WT mice, illustrating its pro-colitis and pro-neoplastic actions. This finding is largely in agreement with our previous studies which showed that other commonly used antimicrobials, such as TCS, BAC, and BET, also exaggerated colitis and colon tumorigenesis in mouse models.30,31 At a level of 80 ppm in diet, treatment with TCC, TCS, BAC, and BET increased the severity of DSS-induced colitis,30,31 suggesting the potent pro-colitis effects of these compounds, though detailed dose–response studies are needed to better characterize their potencies.
A previous study showed that the no-observed-adverse-effect-level (NOAEL) of TCC was 75 mg/kg/day,43 which leads to a calculated acceptable daily intake (ADI) of TCC to be 0.75 mg/kg/day. This ADI value is comparable to the dose used in our study, as we showed that TCC at a dose of ~1 mg/kg/day exacerbated DSS-induced colitis in mice, supporting the notion that the observed adverse effects of TCC in animal experiments could mimic responses in human exposure to TCC. In addition, previous studies showed that many common food crops could accumulate 100–800 ppm TCC,12 therefore, the administration method (oral administration) and dose regime (10–80 ppm in diet) used in our studies could reflect potential human exposure to TCC. We have to point out that there are many challenges to using animal models to study human exposure to TCC: there could be significant differences when exposed to TCC via oral intake (e.g. consumption of TCC-contaminated water or food) or dermal application (e.g. usage of TCC-containing washing products), and there could be significant inter-individual variations in exposure level, absorption, and metabolism of TCC. In addition, we only used male mice in the animal experiments. Previous studies have shown that male and female mice have different responses in experimental colitis models;44 furthermore, TCC has potent effects on endocrine function.23-29 It is feasible that TCC exposure could cause different effects on colitis in male versus female mice. Together, our results suggest that TCC could be a novel environmental risk factor for colitis and colitis-associated colon cancer. Due to the ubiquitous presence of TCC in our environment and possibly in our food system, it is of critical importance to better understand the actions of TCC on colonic inflammation and colitis-associated colon cancer, in order to prepare for further regulation policies of this compound.
Our studies support that gut microbiota contributes to the pro-colitis effects of TCC. First, we found that exposure to TCC reduced the diversity of the gut microbiota in mice. This finding is in agreement with a previous study which showed that exposure to TCC caused dysbiosis in rats.45 Previous studies have constantly shown that compared with healthy individuals, IBD patients have reduced diversity of gut microbiota, suggesting that a reduction of microbial diversity could be correlated with adverse outcomes of gut health.46 Second, we found that exposure to TCC increased abundance of potentially harmful bacteria, and reduced abundance of beneficial bacteria in mouse gut microbiota. Notably, TCC increased the abundance of Proteobacteria phylum, which has been shown to be expanded in the gut microbiota of IBD patients, and associated with the pathogenesis of IBD.41 In addition, TCC treatment caused a ~ 75% reduction of the abundance of Bifidobacterium, which has been shown to have anti-inflammatory effects.42 We further found that treatment with TCC at a concentration of 100 nM inhibited the growth of B. infantis in vitro, suggesting that TCC could have a direct effect on Bifidobacterium. Though there is no study of colonic concentrations of TCC in humans, previous studies showed that after a routine usage of TCC-containing personal care products, the blood concentrations of TCC in humans can reach up to ~500 nM,47 supporting that the dose used in our in vitro experiment (100 nM) is biologically relevant. The TCC-induced changes of Bifidobacterium and Proteobacteria are consistent with the pro-colitis effect of TCC, but more studies are needed to validate the contributions of these gut bacteria in the biological actions of TCC. Finally, we showed that TCC failed to promote DSS-induced colitis in antibiotic cocktail-treated mice, supporting that gut microbiota is required for the pro-colitis effect of TCC. We have to mention that there are limitations to using the antibiotic cocktail strategy to study the roles of gut microbiota involved.48 More studies, notably fecal transplant in germ-free mice, are needed to determine whether TCC exposure-induced alterations in the composition of the microbiota contributes to the colitis- and colon tumorigenesis-enhancing effects of TCC.
In summary, here our studies showed that exposure to TCC, a widely used antimicrobial ingredient and a ubiquitous contaminant in the environment, exaggerated colonic inflammation and colitis-associated colon tumorigenesis in mice, through the modulation of gut microbiota. These results showed that TCC could be a novel risk factor for IBD and colon cancer. Further studies are needed to better characterize the impact of TCC exposure on gastrointestinal diseases in humans in order to prepare for possible further regulation of this compound.
Supplementary Material
Funding Statement
This research is supported by a new faculty start-up from the University of Massachusetts Amherst.
Competing financial interest declaration
The authors declare no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Terzic J, Grivennikov S, Karin E, Karin M.. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114 e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Van Der Kraak L, Gros P, Beauchemin N. Colitis-associated colon cancer: is it in your genes? World J Gastroenterol. 2015;21:11688–12. doi: 10.3748/wjg.v21.i41.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged > 18 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166–1169. doi: 10.15585/mmwr.mm6542a3. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen GC, Chong CA, Chong RY. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J Crohns Colitis. 2014;8:288–295. doi: 10.1016/j.crohns.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:367–374. [PMC free article] [PubMed] [Google Scholar]
- 7.Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halden RU. On the need and speed of regulating triclosan and triclocarban in the United States. Environ Sci Technol. 2014;48:3603–3611. doi: 10.1021/es500495p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye X, Wong L-Y, Dwivedi P, Zhou X, Jia T, Calafat AM. Urinary concentrations of the antibacterial agent triclocarban in United States residents: 2013–2014 national health and nutrition examination survey. Environ Sci Technol. 2016;50:13548–13554. doi: 10.1021/acs.est.6b04668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews S, Henderson S, Reinhold D. Uptake and accumulation of antimicrobials, triclocarban and triclosan, by food crops in a hydroponic system. Environ Sci Pollut Res Int. 2014;21:6025–6033. doi: 10.1007/s11356-013-2474-3. [DOI] [PubMed] [Google Scholar]
- 13.Higgins CP, Paesani ZJ, Chalew TE, Halden RU. Bioaccumulation of triclocarban in Lumbriculus variegatus. Environ Toxicol Chem. 2009;28:2580–2586. doi: 10.1897/09-013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiaia-Hernandez AC, Ashauer R, Moest M, Hollingshaus T, Jeon J, Spaak P, Hollender J. Bioconcentration of organic contaminants in Daphnia resting eggs. Environ Sci Technol. 2013;47:10667–10675. doi: 10.1021/es401763d. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Ernst F, Conkle JL, Gan J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ Int. 2013;60:15–22. doi: 10.1016/j.envint.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Aryal N, Reinhold DM. Phytoaccumulation of antimicrobials from biosolids: impacts on environmental fate and relevance to human exposure. Water Res. 2011;45:5545–5552. doi: 10.1016/j.watres.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Snyder EH, O’Connor GA, McAvoy DC. Toxicity and bioaccumulation of biosolids-borne triclocarban (TCC) in terrestrial organisms. Chemosphere. 2011;82:460–467. doi: 10.1016/j.chemosphere.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Wu C, Spongberg AL, Witter JD, Sridhar BB. Transfer of wastewater associated pharmaceuticals and personal care products to crop plants from biosolids treated soil. Ecotoxicol Environ Saf. 2012;85:104–109. doi: 10.1016/j.ecoenv.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Ismail NS, Muller CE, Morgan RR, Luthy RG. Uptake of contaminants of emerging concern by the bivalves Anodonta californiensis and Corbicula fluminea. Environ Sci Technol. 2014;48:9211–9219. doi: 10.1021/es5011576. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Spongberg AL, Witter JD, Fang M, Czajkowski KP. Uptake of pharmaceutical and personal care products by soybean plants from soils applied with biosolids and irrigated with contaminated water. Environ Sci Technol. 2010;44:6157–6161. doi: 10.1021/es1011115. [DOI] [PubMed] [Google Scholar]
- 21.Food & Drug Adminnistration, H.H.S. Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use. Final rule. Fed Regist. 2016;81:61106–61130. [PubMed] [Google Scholar]
- 22.Aiello AE, Larson EL, Levy SB. Consumer antibacterial soaps: effective or just risky? Clin Infect Dis. 2007;45(Suppl 2):S137–147. doi: 10.1086/519255. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL. Triclocarban enhances testosterone action: a new type of endocrine disruptor?. Endocrinology. 2008;149:1173–1179. doi: 10.1210/en.2007-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giudice BD, Young TM. The antimicrobial triclocarban stimulates embryo production in the freshwater mudsnail Potamopyrgus antipodarum. Environ Toxicol Chem. 2010;29:966–970. doi: 10.1002/etc.v29:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinther A, Bromba CM, Wulff JE, Helbing CC. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol. 2011;45:5395–5402. doi: 10.1021/es1041942. [DOI] [PubMed] [Google Scholar]
- 26.Duleba AJ, Ahmed MI, Sun M, Gao AC, Villanueva J, Conley AJ, Turgeon JL, Benirschke K, Gee NA, Chen J, et al. Effects of triclocarban on intact immature male rat: augmentation of androgen action. Reprod Sci. 2011;18:119–127. doi: 10.1177/1933719110382581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yueh MF, Li T, Evans RM, Hammock B, Tukey RH. Triclocarban mediates induction of xenobiotic metabolism through activation of the constitutive androstane receptor and the estrogen receptor alpha. PLoS One. 2012;7:e37705. doi: 10.1371/journal.pone.0037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christen V, Crettaz P, Oberli-Schrammli A, Fent K. Some flame retardants and the antimicrobials triclosan and triclocarban enhance the androgenic activity in vitro. Chemosphere. 2010;81:1245–1252. doi: 10.1016/j.chemosphere.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Du G, Zhang W, Hu J, Wu D, Song L, Xia Y, Wang X. The in vitro estrogenic activities of triclosan and triclocarban. J Appl Toxicol. 2014;34:1060–1067. doi: 10.1002/jat.v34.9. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Wang W, Romano KA, Gu M, Sanidad KZ, Kim D, Yang J, Schmidt B, Panigrahy D, Pei R, et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci Transl Med. 2018;10. doi: 10.1126/scitranslmed.aan4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanidad KZ, Yang H, Wang W, Ozay EI, Yang J, Gu M, Karner E, Zhang J, Kim D, Minter LM, et al. Effects of consumer antimicrobials benzalkonium chloride, benzethonium chloride, and chloroxylenol on colonic inflammation and colitis-associated colon tumorigenesis in mice. Toxicol Sci. 2018;163:490–499. doi: 10.1093/toxsci/kfy045. [DOI] [PubMed] [Google Scholar]
- 32.Goodrich JK, Di Rienzi S, Poole A, Koren O, Walters W, Caporaso J, Knight R, Ley R. Conducting a microbiome study. Cell. 2014;158:250–262. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore RJ, Stanley D. Experimental design considerations in microbiota/inflammation studies. Clin Transl Immunol. 2016;5:e92. doi: 10.1038/cti.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 35.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 36.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozlowski C, Jeet S, Beyer J, Guerrero S, Lesch J, Wang X, DeVoss J, Diehl L. An entirely automated method to score DSS-induced colitis in mice by digital image analysis of pathology slides. Dis Model Mech. 2013;6:855–865. doi: 10.1242/dmm.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 39.Johnson RL, Fleet JC. Animal models of colorectal cancer. Cancer Metastasis Rev. 2013;32:39–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- 42.Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol. 2006;12:5941–5950. doi: 10.3748/wjg.v12.i37.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monsanto . Subchronic toxicity study in rats TCC (triclocarban). Report no. HL-84-208; (1985).
- 44.Babickova J, Tóthová Ľ, Lengyelová E, Bartoňová A, Hodosy J, Gardlík R, Celec P. Sex differences in experimentally induced colitis in mice: a role for estrogens. Inflammation. 2015;38:1996–2006. doi: 10.1007/s10753-015-0180-7. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy RC, Fling RR, Robeson MS, Saxton AM, Donnell RL, Darcy JL, Bemis DA, Liu J, Zhao L, Chen J, et al. Temporal development of gut microbiota in triclocarban exposed pregnant and neonatal rats. Sci Rep. 2016;6:33430. doi: 10.1038/srep33430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schebb NH, Ahn KC, Dong H, Gee SJ, Hammock BD. Whole blood is the sample matrix of choice for monitoring systemic triclocarban levels. Chemosphere. 2012;87:825–827. doi: 10.1016/j.chemosphere.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundberg R, Toft MF, August B, Hansen AK, Hansen CHF. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes. 2016;7:68–74. doi: 10.1080/19490976.2015.1127463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


