ABSTRACT
The significance of maternal appropriate calcium intakes for energy metabolism in the offspring has been recognized. Nonalcoholic fatty liver disease (NAFLD) is considered as the hepatic manifestation of metabolic syndrome. So in this study, we proposed that there were long-term effects of maternal calcium status on the progress of NAFLD by altering the intestinal microbiota and lipid metabolism with attention to potential sex differences among the mouse offspring. Thirty-four-week female C57BL/6 J mice were subjected to obtain low, normal and high calcium reproductive diets throughout the gestation and lactation. After weaning, both the male and female mouse offspring were fed with the high-fat diet for 16 weeks, with the normal diet as control. Biochemical indicators in the plasma and hepatic tissue were measured using ELISA or enzymatic methods. The expression of lipid metabolism, inflammatory and fibrosis related genes was determined by RT-PCR. The intestinal microbiota was analyzed by 16S rRNA high-throughput sequencing. Maternal normal and low calcium intake could, respectively, inhibit the progress of high-fat diet induced NAFLD in the male and female mouse offspring, which was characterized by the least lipid droplets, inflammatory infiltration and fibrosis, the lowest concentrations of free fatty acids and triglyceridethe lowest expression of genes involving in de novo lipogenesis and the highest expression of genes related to lipid oxidation and hydrolysis, inflammatory, and fibrosis. Pyrosequencing of 16S rRNA genes revealed that the male mouse offspring with maternal normal calcium intake and the female mouse offspring with maternal low calcium intake, after the high-fat diet feeding, had distinct intestinal microbiota, which was closer to thosein mice with the normal diet feeding. Analysis of the functional features for the different microbiota was compatible with the expression of genes associated with lipogenesis, lipid oxidation and hydrolysis. Thus, there is a sex-specific manner for maternal calcium requirement to inhibit the progress of offspring NAFLD, that might be less for the female offspring and more for the male offspring.
KEYWORDS: Calcium, maternal, sex-specific, nonalcoholic fatty liver disease, intestinal, microbiota; lipid metabolism
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a growing cause of chronic liver injury by excessive fat deposition that has become a worldwide health concern with one billion individuals globally.1 The clinical spectrum of NAFLD is comprised by a varity of diseases ranging from steatohepatitis, fibrosis, and even cirrhosis and hepatocellular carcinoma.2 Despite the clinical and public health is important, there are few effective therapeutic prevention and clearly pathogenic mechanisms. Notably, many experimental evidence has suggested a complex interplay of multiple biological processes involving in the occurrence and progress of NAFLD such as intestinal microbiota dysbiosis, heightened intestinal barrier permeability, abnormal lipid metabolism, metabolic endotoxemia, and various inflammatory processes.3–6 During the past decade, the disruptions of intestinal microbiota as the indicator of gut-liver-axis have received more attention on the progression of NAFLD owing to its roles on modulating the energy homeostasis, increasing the contents of short-chain fatty acids, endotoxemia, endogenous ethanol and other microbe products and affecting the choline and bile acids metabolism in the hepatic tissue.7–10 Moreover, the results from the epidemiological and clinical studies have proved that the dysbiotic gut ecosystem might further disrupt the integrity of the intestinal barrier to exacerbate the lipid accumulation in the hepatic tissue to increase the risk of NAFLD.11
The colonization and development of a stable intestinal flora in the infants are regulated by many elements, including maternal nutritionand diets, use of antibiotics, probiotics and other drugs, host’s genetics, and etc. Recently, a growing body of evidence have demonstrated that not only over-nutrition or under-nutrition but also deficiency of micronutrients are critical in the colonization of intestinal microbiota, which is associated with the alterations of intestinal barrier function and elevated levels of bacterial endotoxin. Calcium, an essential mineral nutrient, plays an important role in many physiological processes including bone construction, weight reduction, energy metabolism, and hormone secretion as a second messenger and/or signal transduction.12 Epidemiological and animal studies have demonstrated that the physiological effects of calcium are mediated primarily by the changes of intracellular Ca2+ ([Ca2+]i) under the condition of unsuitable calcium intake, which could induce the abnormal colonization of intestinal flora by modulating the intestinal pH, promoting the excretion of bile and fatty acidsand affecting the intestinal stability.13 On the other hand, it can also exert coordinated regulatory effects on the adipocyte lipid metabolism and triacylglycerol storage by increasing the gene expressions on the lipogenic and de novo lipogenesis and inhibiting the lipolysis and thermogenesis to result in excessive lipid accumulation in the hepatic tissueand associated NAFLD.14,15 Recently, emerging evidence has shown that the first 1,000 d in life is a critical period for the colonization of intestinal flora and prevention of many chronic non-communicable diseases (NCD) such as NAFLD.16 Thus, keeping adequate calcium intake in this period of the life is of a great importance for a positive calcium balance, good bone density necessary for the skeletal consolidation, and reducing the occurrence of NCD in the later.17 However, the daily calcium intake is inadequacy among the most Chinese people including the pregnant woman based on comparison between the actual intake and the Chinese Dietary Reference Intakes (DRIs).18 So one purpose of this study was to discuss whether this unsuitable intake of calcium in early life could influence the development of NAFLD in later life stage by altering the intestinal microbiota and lipid metabolism.
Up to now, there have been some researches that try to discuss the effects of maternal calcium supplementation on the healthy outcomes in the offspring. However, they mainly focused on offspring bone and body composition, and produced mixed results for different population and intervention. Ward KA et al. indicated that calcium supplementation of pregnant women with low calcium intakes could only accelerate the childhood trajectories of growth, bone, and body composition development among males aged 8–12 y, but result in slower growth among females.19 The above evidence implies that maternal calcium demand during the pregnancy and lactation is different depending on sex of the offspring, but the underlying mechanisms are not clear. This discovery provides new challenges for the existing maternal and child health care, and also gives powerful evidence for individualized nutrition, which is of great significance to improve the children’s health. Therefore, the other important hypothesis of this study is that whether this unsuitable intake of calcium in early life can influence the development of NAFLD in later life stage in a sex-specific manner. In this present work, we investigated the effects of maternal insufficient and excessive calcium intake during the pregnancy and lactation on the development of NAFLD in response to a high-fat diet by examining the intestinal microbiota and lipid metabolism with attention to potential sex differences among the mouse offspring.
2. Materials and methods
2.1. Diets
Three types of reproductive diets with low (LC, 0.25%), normal (NC, 0.70%), and high calcium (HC, 1.20%) concentrations were designed according to the gestating and growing formula (D10012G) from the Research Diets, Inc (New Brunswick, NJ, USA). Meanwhile, a high-fat diet (HFD) (34.9% fat by weight, 60% kcal, No. H10060) and a normal-fat diet (4.3% fat by weight, 10% kcal, No. H10010) with normal calcium content were designed based on the formula for mature rodents (D10012M) from Research Diets, Inc. All these diets were prepared by Beijing HFK Bioscience Co., Ltd. (http://www.hfkbio.com/) and stored at −20°C until use.
2.2. Animal experiments
Four-week-old C57BL/6J female mice were purchased fromBeijing HFK Bioscience Co., Ltd. After 3 d of recovery, these mice were randomly divided into three groups (n = 10/group, n = 5/cage) and fed with the LC, NC, and HC reproductive diets, respectively, for 8 weeks. Then, the female mice were mated with the 12-week-old C57BL/6J male mice following two females to one male per cage and continued on their own diets throughout the whole gestation and lactation. The male and female mice offspring from maternal different calcium intervention groups (LC, NC, and HC) (n = 8/group, n = 4/cage) were both fed with the HFD diet for 16 weeks after weaning at 21 d old and named LC-HFD, NC-HFD, and HC-HFD groups, respectively. Meanwhile, the male and female pups from the NC group were still fed with the normal-fat diet after weaning as the NC-C group. The specific diagram for this study design was shown in Supplemental Figure 1.
In this feeding process, the body weight was recorded weekly, food intake was measured monthly, then the energy intake was calculated according to the food intake. All mice were fed at the Animal Facility in the Laboratory Animal Center of Fourth Medical Center of the PLA General Hospital of China according to the standard animal feeding methods with the 12 hour (h) light and 12 h dark cycle at 22–25°C and humidity of 40–60%.
2.3. Sample collection
The feces samples from the male and female mice offspring were collected at the end of feeding procedure in the day before they were weaned and executed, and then stored at −80°C. The mouse offspring were anesthetized by intraperitoneal injection of Avertin (2,2,2-tribromoethanol) (T-4840-2; Aldrich Chemical, Munich, Bavaria, Germany) (125 mg/kg) after 8-h fasting. The blood samples were obtained by heart puncture and then sacrificed by injection of an overdose of Avertin (500 mg/kg) to minimize the suffering. Immediately, the epididymal white adipose tissue (eWAT) and hepatic tissue were dissected free of the surrounding tissues, which were partly fixed by immersion in 10% buffered formalin, the remaining tissues were frozen in liquid N2 and stored at −80°C cryogenic refrigerator until use.
All these sample collections were performed from 08:00 to 12:00 in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of National Administration Regulations on Laboratory Animals of China. The animal protocol used in this study was approved by the Ethics of Animal Experiments of Fourth Medical Center of PLA General Hospital in China.
2.4. Measurements of biochemical parameters in the plasma and hepatic tissue
The concentrations of calcium (Cat. no. C004-2-1, Nanjing Jiancheng Bioengineering Institute, China), parathyroid hormone (PTH) (Cat. no. H207-96t, Nanjing Jiancheng Bioengineering Institute, China), triglyceride (TG) (Cat. no. CH0101151, Sichuan Maker Science Tech. Co., LTD, China), total cholesterol (TC) (Cat. no. CH0101160, Sichuan Maker Science Tech. Co. LTD, China) in the plasma and/or hepatic tissue were, respectively, assayed by colorimetric or enzymatic methods. The levels of inflammatory cytokines TNF-ɑ, IL-1β, IL-4, and IL-10 in the plasma were also determined using ELISA kits, according to the manufacturer’s guidelines (Invitrogen, ThermoFisher Scientific, CN).
2.5. Hepatic tissue histology
The hepatic tissues of the mouse offspring, fixed in 10% buffered formalin by immersion, were chosen to make the histology analysis. The hepatic histopathologies were evaluated by hematoxylin and eosin (H&E) staining, the accumulation of lipid drops in the hepatic tissue was stained with Oil red O for histopathological assessment. The degree of fibrosis in the hepatic tissue was measured by Masson staining. Then all samples above were examined under light microscope at magnification of 400×. Then the Image-pro Plus 6 (IPP 6) was used to quantitatively analyze the amount of lipid droplets in each slice.
2.6. Gene expression analysis by RT-PCR in the hepatic tissue
Total RNA of the hepatic tissue was extracted using Trizol Reagents (Cat. No. 15596-206, Invitrogen, Carlsbad, CA, USA), and the cDNA was reversely transcribed by SuperScriptTM III First-Strand Synthesis System (Cat. No. 18080-051, Invitrogen, Carlsbad, CA, USA). Expressions of the target genes involved in lipid oxidation (PPAR-α, CPT-1a, CPT-1c, CPT2, PGC-1α, UCP1, and PRDM16), hydrolysis (ATGL and HSL), lipogenesis (DGAT2, Plin2, Fasn, Acc1, LPL, and SREBP-1c), synthesis (PPAR-γ, C/EBP-ɑ, and Fabp4), hepatic inflammation (TGFɑ, IL-1β, ccl2, ccl4, IL4, and IL10), and fibrosis (TGF-β and Collagen) were measured using the quantitative RT-PCR with GAPDH as the invariant internal control. The oligonucleotide primers for all these targeted genes were found in the Primer-Bank (https://pga.mgh.harvard.edu/primerbank/) following the NCBI gene ID, checked by the Primer-Blast (http://www.ncbi.nlm.nih.gov/tools/primer blast/) and composed by the Sangon Biotech (Shanghai) Co., Ltd, which were shown in Supplemental Table 1.
Table 1.
Body weight and metabolic indicators in the plasma of male and female mice offspring under maternal different calcium intake.
| NC-C (n = 8) | NC-HFD (n = 8) | LC-HFD (n = 8) | HC-HFD (n = 8) | P | |
|---|---|---|---|---|---|
| Male Initial body weight (g) |
9.68 ± 1.09 | 10.00 ± 1.62 | 10.10 ± 1.95 | 9.28 ± 1.93 | .373 |
| Final body weight (g) | 27.45 ± 1.21 | 38.34 ± 2.10* | 43.77 ± 2.90*# | 44.63 ± 3.42*# | <.001 |
| Food intake (g/d) | 2.33 ± 0.11 | 2.21 ± 0.19 | 2.07 ± 0.24 | 2.25 ± 0.13 | .172 |
| Energy intake (kcal/d) | 8.96 ± 0.43 | 11.52 ± 0.98* | 10.78 ± 1.21* | 11.73 ± 0.68* | .021 |
| Calcium (mmol/L) | 2.06 ± 0.66 | 2.19 ± 0.65 | 2.42 ± 0.62 | 2.22 ± 0.53 | .629 |
| PTH (μg/L) | 1.25 ± 0.079 | 1.19 ± 0.12 | 1.45 ± 0.076*# | 1.28 ± 0.094 | .041 |
| FFA (Emq/L) | 6.09 ± 1.84 | 13.74 ± 2.10* | 28.70 ± 3.48*# | 16.83 ± 7.05* | <.001 |
| TG (mmol/L) | 0.17 ± 0.069 | 0.27 ± 0.098* | 0.31 ± 0.092* | 0.37 ± 0.13*# | .003 |
| TC (mmol/L) | 2.21 ± 0.78 | 2.84 ± 0.56* | 3.69 ± 0.91*# | 4.05 ± 0.79*# | <.001 |
| IL6 (pg/mL) | 10.42 ± 1.01 | 11.28 ± 1.17 | 11.12 ± 1.36 | 11.64 ± 0.37 | .762 |
| TNF-α (pg/mL) | 15.88 ± 0.95 | 16.32 ± 0.26 | 16.67 ± 0.40* | 16.29 ± 0.24 | .045 |
| IL-4 (pg/mL) | 25.25 ± 4.49 | 13.04 ± 2.32* | 10.85 ± 1.54*# | 9.11 ± 0.82*# | <.001 |
| IL-10 (pg/mL) | 197.17 ± 20.78 | 142.33 ± 17.29* | 102.58 ± 14.17*# | 108.65 ± 14.57*# | .029 |
| Female Initial body weight (g) |
8.89 ± 0.90 | 8.77 ± 0.78 | 9.18 ± 1.03 | 8.74 ± 0.82 | .325 |
| Final body weight (g) | 21.86 ± 1.20 | 35.21 ± 2.10* | 29.38 ± 1.90*# | 34.87 ± 3.40* | <.001 |
| Food intake (g/d) | 2.15 ± 0.12 | 2.09 ± 0.32 | 2.23 ± 0.21 | 2.07 ± 0.16 | .802 |
| Energy intake (kcal) | 8.28 ± 0.46 | 10.96 ± 1.67* | 11.69 ± 1.10* | 10.85 ± 0.84* | .033 |
| Calcium(mmol/L) | 2.17 ± 0.44 | 2.53 ± 0.49 | 2.68 ± 0.41 | 2.55 ± 0.58 | .133 |
| PTH (μg/L) | 1.35 ± 0.30 | 1.22 ± 0.43 | 1.33 ± 0.14 | 1.34 ± 0.23 | .214 |
| FFA(Emq/L) | 4.28 ± 1.04 | 14.23 ± 2.53* | 9.17 ± 1.91*# | 13.82 ± 2.45* | <.001 |
| TG (mmol/L) | 0.15 ± 0.091 | 0.36 ± 0.13* | 0.24 ± 0.054*# | 0.33 ± 0.11* | .003 |
| TC (mmol/L) | 1.74 ± 0.56 | 3.35 ± 0.54* | 2.96 ± 0.61*# | 3.06 ± 0.38* | <.001 |
| IL-6 (pg/mL) | 16.53 ± 0.38 | 17.26 ± 0.73* | 17.33 ± 0.28* | 17.30 ± 0.32* | .013 |
| TNF-α (pg/mL) | 20.32 ± 0.23 | 21.50 ± 0.19* | 21.43 ± 0.21* | 21.30 ± 0.14* | .034 |
| IL-4 (pg/mL) | 17.16 ± 0.19 | 17.01 ± 0.41 | 16.91 ± 0.25 | 17.30 ± 0.25 | .051 |
| IL-10 (pg/mL) | 61.57 ± 24.66 | 38.05 ± 18.86* | 42.79 ± 11.18* | 39.08 ± 12.13* | .041 |
Four-week-old C57BL/6 J female mice were fed with low (LC, 0.25%), normal (NC, 0.70%), and high (HC, 1.20%) calcium reproductive diets, respectively, starting 2 months before the conception, which were continued throughout the whole pregnancy and lactation. After weaning, the pups were fed with a normal (NC-C) or high-fed diets (NC-HFD, LC-HFD and HC-HFD) for 16 weeks. All values are shown as means ± SD, n = 8 in each group for both the male and female mouse offspring. *Compared with the NC-C group, P < .05. #Compared with the NC-HFD group, P < .05.
2.7. DNA extraction and 16S rRNA high-throughput sequencing
About 80-mg fecal samples of male and female mice offspring were were used to extract the bacterial DNA using the QIAamp Fast DNA Stool Mini Kit (Cat. No. 51504, Qiagen, Germany) according to the manufacture’s protocol. The concentrations of bacterial DNA were measured using Nanodrop 2000 (Thermo Scientific, USA).
The final DNA purification and microbiota determination were measured by Shanghai Majorbio Bio-pharm Technology Co., Ltd. In this process, the V3-V4 region of the bacteria’s 16S ribosomal RNA (rRNA) gene was amplified by PCR with bar-code-indexed primers (338 F and 806 R) using Fastpfu Polymerase. Amplicons were purified by gel extraction (AxyPrep DNA Gel Extraction Kit, Axygen Bio-sciences, Union City, California, USA) and quantified using QuantiFluor-ST (Promega, USA). The purified amplification was then pooled in equimolar concentrations, and paired-end sequencing was performed using an Illumina MiSeq instrument (Illumina, San Diego, California, USA).
2.8. Microbial analysis
All the microbial data were analyzed on the free online platform of Majorbio I-Sanger Cloud Platform (www.i-sanger.com).20 To clarify the similarities of intestinal flora composition among all the experimental groups (NC-C, NC-HFD, LC-HFD, and HC-HFD), the estimators of α-diversity (Shannon, Simpson, Ace and Chao indexes) were calculated based on rarefied OTU tables. Principal Coordinates Analyses (PCA) of unweighted UniFrac distance matrix is displayed as the β-diversity. Weighted UniFrac distance matrices were computed to detect global variations in the composition of microbial communities at the phylum and genus levels by one-way analysis of variance (ANOVA). PICRUST (phylogenetic investigation of communities by reconstruction of unobserved states, V1.0) was used to predict the 16S rRNA based high-throughput sequencing data for functional features from the phylogenetic information with an estimated accuracy of 0.8. The Cluster of Orthologous Groups (COG) database was obtained through the Green-gene ID corresponding to each OTU in the EGGNOG database (Evolutionary Genealogy of Genes: Non-supervised Orthologous Groups). Then the predicted functional composition profiles were collapsed into level 2 of KEGG (Kyoto Encyclopedia of Genes and Genomes) database pathways. Pathways that were presented in <10% of the samples were not included in the comparison analysis. The correlations between the microbiota composition of the top 10 richness genus and the significantly different expression of lipid related genes among the mouse offspring with different maternal calcium intake during the pregnancy and lactation were determined by RDA/CCA analysis and the related heatmap figures.
2.9. Statistical analysis
All statistical analyses were conducted by SPSS 21.0. ANOVA was performed to compare the means of indexes among different groups with normally distributed data. The differences between these groups in data with the non-normal distribution were assessed using Mann–Whitney U-test or Wilcoxon Signed-Rank test. Then the Student–Newman–Keuls (SNK) test was used to determine where the differences existed between each two groups. Linear relationships between these variables were tested by Spearman’s correlations. P < .05 was considered to be statistically significant.
3. Results
3.1. Body weight and energy intake in the male and female mice offspring
As shown in Table 1, the body weight and energy intake in the HFD groups (NC-HFD, LC-HFD, and HC-HFD) were significantly higher as compared to the NC-C group in both male and female mice offspring (P < .05). Further comparisons among these three HFD groups showed that the body weight in the LC-HFD and HC-HFD groups was heavier than those in the NC-HFD group (P < .05) with no changes of the energy intake in the male mouse offspring. While among the female mouse offspring, the body weight from the NC-HFD and HC-HFD groups was heavier than those in the LC-HFD group (P < .05) with no changes in energy intake.
3.2. The changes of biochemical parameters and inflammation-related cytokines in the plasma among the male and female mice offspring
As shown in Table 1, in the male mouse offspring, the contents of FFA, TC and TG in the HFD groups were higher as compared to the NC-C group, while the concentrations of IL-4 and IL10 were lower (P < .05). Further comparisons among these three HFD groups showed that FFA, TC, and TG in the LC-HFD or/and HC-HFD groups were higher, while the IL4 and IL10 were lower than those in the NC-HFD group (P < .05). Meanwhile, the concentrations of PTH and TNFɑ were higher in the LC-HFD group than those in the NC-C and NC-HFD groups. In the female mouse offspring, the contents of FFA, TC, TG, TNFɑ, and IL-6 in the HFD groups were higher, while the concentrations of IL10 were lower as compared to the NC-C group (P < .05). Further comparison showed that FFA, TG, and TC in the LC-HFD group were lower than those in the NC-HFD group (P < .05).
3.3. Effects of maternal different calcium intake on the progress of HFD-induced hepatic steatosis in the male/female mouse offspring
In the male mouse offspring, the contents of hepatic TG (Figure 1(e)) among the HFD groups were higher as compared to the NC-C group. Further comparisons among the three HFD groups showed that hepatic TG in the LC-HFD or/and HC-HFD groups were higher than that in the NC-HFD group (P < .05). The histological analysis also showed the accumulation of lipid droplets, in the hepatic tissue was more heavier in the LC-HFD and HC-HFD groups than that in the NC-HFD group (P < .05).20 The inflammation and fibrosis has been clearly shown in Figures 2 and 3. In the mice fed with the HFD, local inflammation and fibrosis were more serious than those in the NC-C group (Figures 2(a) and 3(a)), with higher expressions of TNF-α (Figure 2(c)), IL-1β (Figure 2(d)), ccl2 (Figure 2(e)), ccl4 (Figure 2(i)), TGFβ (Figure 3(b)) and Collagen (Figure 3(c)), and lower expressions of IL-4 (Figure 2(j)), and IL-10 (Figure 2(k)). Further comparisons among these three HFD groups proved that local inflammation and fibrosis were more serious in the LC-HFD and HC-HFD groups than those in the NC-HFD group, with the higher expressions of TNF-α, IL-1β, ccl2, ccl4 and Collagen, and lower expressions of IL-4.
Figure 1.
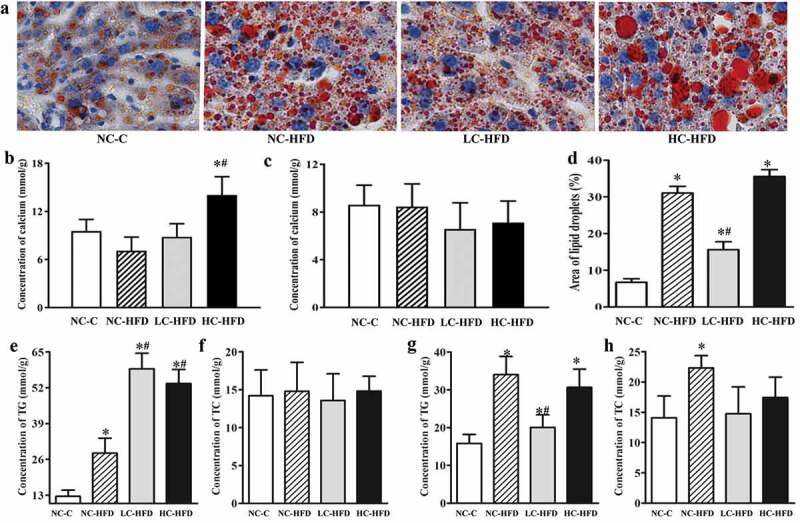
Maternal calcium intake during the pregnancy and lactation affected the lipid deposition and accumulation in the hepatic tissue for the male and female mouse offspring. Lipid deposition and accumulation in the hepatic tissue among the female mouse offspring (a and d) were shown by Oil red O staining and quantitatively analyzed using the Image-pro Plus. The concentrations of calcium, TG and TC in the hepatic tissue from the male (b, e, and f) and female mouse offspring (c, g, and h) were measured by colorimetric or enzymatic methods. Data are presented as means with standard deviation (SD), n = 8 mice/group. One-way analysis of variance (ANOVA) was performed to compare the indexes among different groups. Then, the Student–Newman–Keuls (SNK) test was used to determine where the differences exist between each two groups. *Compared to the NC-C group, P < .05; #compared to the NC-HFD group, P < .05.
Figure 2.
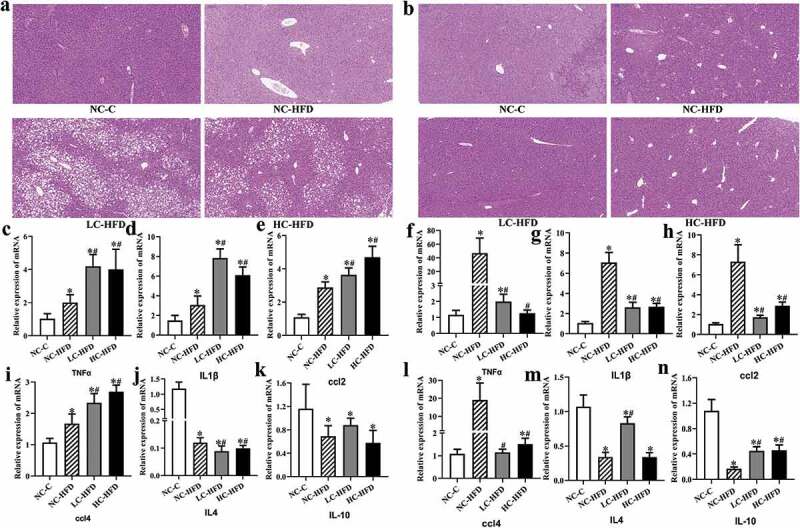
Effects of maternal different dietary calcium intake on the HFD-induced inflammatory infiltration and expression of related genes in the hepatic tissue of the male and female mouse offspring. The inflammatory infiltration in the hepatic tissue was shown by the H&E staining in the male (a) and female mouse offspring (b). The expression of proinflammatory genes (TNF-α, IL-1β, ccl2, and ccl4) and anti- inflammatory genes (IL-4 and IL-10) in the hepatic tissue among the male (c, d, e, i, j, and k) and female mouse offspring (f, g, h, l, m, and n) was measured by quantitative RT-PCR. Data are presented as means with standard deviation (SD), n = 8 mice/group. One-way analysis of variance (ANOVA) was performed to compare the indexes among different groups. Then the Student–Newman–Keuls (SNK) test was used to determine where the differences exist between each two groups. *Compared to the NC-C group, P < .05; #compared to the NC-HFD group, P < .05.
Figure 3.
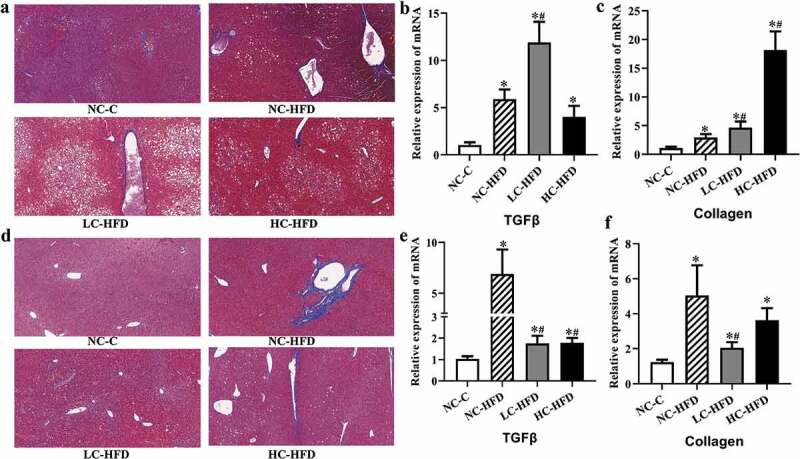
Effects of maternal different dietary calcium intake on the HFD-induced hepatic fibrosis and expression of related genes in the hepatic tissue of the male and female mouse offspring. The hepatic fibrosis in the hepatic tissue was shown by the Masson staining in the male (a) and female mouse offspring (d). The expression of genes associated with hepatic fibrosis (TGFβ and Collagen) in the male (b and c) and female mouse offspring (e and f) was measured by the quantitative RT-PCR. Data are presented as means with standard deviation (SD), n = 8 mice/group. One-way analysis of variance (ANOVA) was performed to compare the indexes among different groups. Then the Student–Newman–Keuls (SNK) test was used to determine where the differences exist between each two groups. *Compared to the NC-C group, P < .05; #compared to the NC-HFD group, P < .05.
In the female mouse offspring, the concentration of hepatic TG (Figure 1(g)) among the HFD groups was higher as compared to the NC-C group (P < .05). Further comparisons showed that the hepatic TG in the LC-HFD group was lower than those in the NC-HFD group (P < .05). The histological analysis also proved the accumulation of lipid droplets in the hepatic tissue was more serious in the NC-HFD and HC-HFD groups than that in the LC-HFD group (Figure 1(a,d)) (P < .05). The mice fed with HFD had more serious local inflammation and fibrosis compared to the NC-C group (Figures 2(b) and 3(d)), with the higher expressions of TNF-α (Figure 2(f)), IL-1β (Figure 2(g)), ccl2 (Figure 2(h)), ccl4 (Figure 2(l)), TGFβ (Figure 3(e)) and Collagen (Figure 3(f)) and lower expressions of IL-4 (Figure 2(m)), and IL-10 (Figure 2(n)). Further comparisons among the three HFD groups proved that local inflammation and fibrosis had been improved to a greater extent in the LC-HFD group than those in the NC-HFD group, with lower expressions of TNF-α, IL-1β, ccl2, ccl4, TGFβ and Collagen, and lower expressions of IL-4, and IL-10.
3.4. Effects of maternal different calcium intake on the expressions of genes related with the lipid metabolism in the male/female mouse offspring
In the male mouse offspring, RT-PCR analysis confirmed that the expressions of genes related to lipid oxidation and hydrolysis such as PPAR-α, CPT-1α, CPT-1c, PGC-1α, UCP1, PRDM16, ATGL, and HSL were downregulated (Figure 4(a,b)); whereas the expression of genes involving in de novo lipogenesis including DGAT2, Plin2, Fasn, Acc1, LPL, SREBP-1c, PPAR-γ, and FABP4 were upregulated in the HFD groups as compared to the NC-C group (P < .05) (Figure 5(a,b)). Further analysis of these genes' expression in three HFD groups indicated that there were lower expressions of PPAR-α, CPT-1c, PGC-1α, UCP1, ATGL, and HSL, higher expressions of DGAT2, Fasn, Acc1, LPL, SREBP-1c, PPAR-γ, and FABP4 in the LC-HFD or/and HC-HFD groups than those in the NC-HFD group (P < .05).
Figure 4.
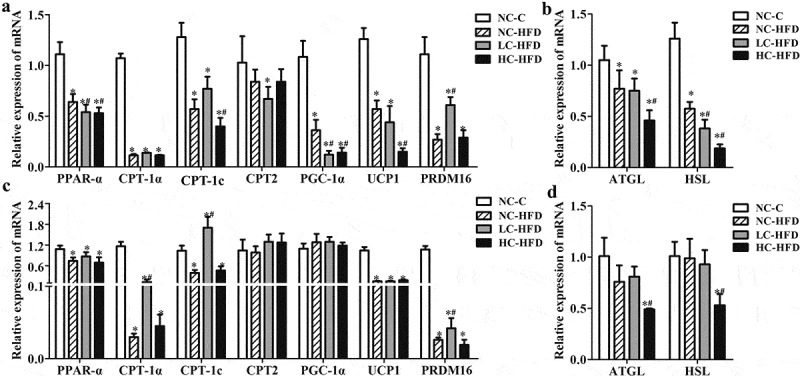
Effects of maternal different dietary calcium intake on the expression of genes involving in the lipid oxidation and hydrolysis in the hepatic tissue of the male and female mouse offspring. The expression of genes associated with lipid oxidation, lipolysis (PPAR-α, CPT-1a, CPT-1 c, CPT2, PGC-1α, UCP1, and PRDM16) and hydrolysis (ATGL and HSL) in the hepatic tissue among the male (a, b) and female mouse offspring(c, d) was measured by the quantitative RT-PCR. Data are presented as means with standard deviation (SD), n = 8 mice/group. One-way analysis of variance (ANOVA) was performed to compare the indexes among different groups. Then the Student–Newman–Keuls (SNK) test was used to determine where the differences exist between each two groups. *Compared to the NC-C group, P < .05; #compared to the NC-HFD group, P < .05.
Figure 5.
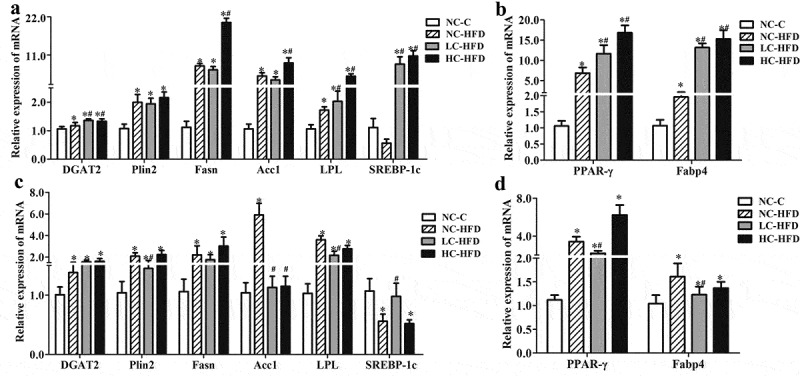
Effects of maternal different dietary calcium intake on the expression of genes involving in the lipogenesis and lipid synthesis in the hepatic tissue of the male and female mouse offspring. The expression of genes associated withlipid synthesis and lipogenesis (DGAT2, Plin2, Fasn, Acc1, LPL and SREBP-1c, PPAR-γ, C/EBP-ɑ and Fabp4) in the hepatic tissue among the male (a, b) and female mouse offspring (c, d) was measured by the quantitative RT-PCR. Data are presented as mean with standard deviation (SD), n = 8 mice/group. One-way analysis of variance (ANOVA) was performed to compare the indexes among different groups. Then the Student–Newman–Keuls (SNK) test was used to determine where the differences exist between each two groups. *Compared to the NC-C group, P < .05; #compared to the NC-HFD group, P < .05.
In the female mouse offspring, the expression of PPAR-α, CPT-1α, CPT-1c, UCP1, PRDM16, ATGL, and HSL was downregulated (Figure 4(c,d)); whereas the expression of DGAT2, Plin2, Fasn, Acc1, LPL, PPAR-γ, and FABP4 was upregulated in the HFD groups as compared to the NC-C group (P < .05) (Figure 5(c,d)). Further analysis of these genes' expression in three HFD groups indicated that, compared to the NC-HFD group, there was an increased expression of CPT-1α, CPT-1c and PRDM16, and decreased expression of Plin2, Acc1, LPL, PPAR-γ, and FABP4 in the LC-HFD group (P < .05).
3.5. Gut microbiota dysbiosis among the male and female mice offspring affected by maternal different calcium intake
The bacterial community relative abundance (Ace and Chao indexes) and diversity (Shannon and Simpson indexes), measured by numbers of observed OTUs, were compared among the mouse offspring in maternal different calcium intake groups. We confirmed Shannon index was increased and Simpson was decreased in three HFD groups as compared with the NC-C group in the male mouse offspring, which were also affected by maternal insufficient calcium intake (LC-HFD) as compared to the NC-HFD group (P < .05) (Figure 6(e,f)). However, Chao index among the male mouse offspring and all α-diversity indexes(ACE, Shannon and Simpson) in the female mouse offspring were not significantly different among the four groups (Figures 6(d) and 7(d–f)). The beta diversity index as an unweighted UniFrac-based principal analysis (PCA) revealed that the overall microbial composition of HFD groups could be deviated from the NC-C group at the phylum and genus levels, either in the male or female mouse offspring (P < .05). Further analysis found that the clusters in the male mouse offspring from the LC-HFD and HC-HFD groups were significantly different from those in the NC-HFD fed mice at the genus level (P < .05; Figure 6(a,b)). While in the female mouse offspring, the clusters in samples from the LC-HFD group was significantly different from those in the NC-HFD and HC-HFD groups at the genus level (P < .05; Figure 7(a,b)).
Figure 6.
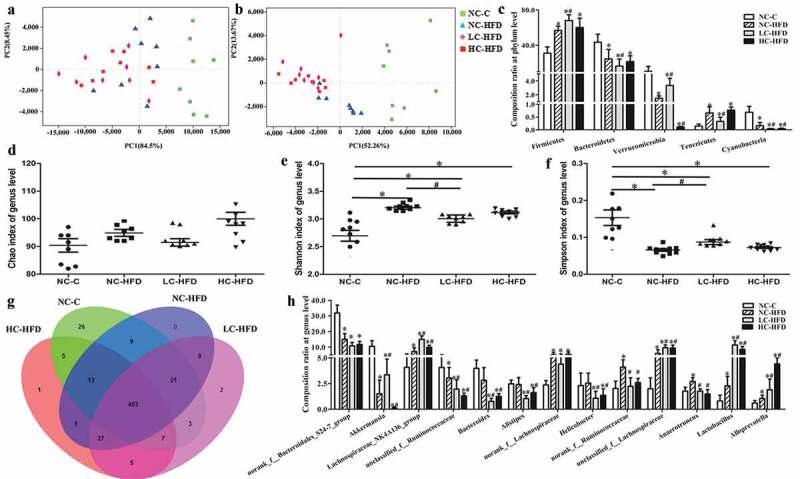
Microbiota composition and diversity of the male mouse offspring affected by maternal different calcium intake during pregnancy and lactation. Bacterial communities at the phylum and genus levels based on weighted UniFrac distance were shown using the PCA analysis (a and b). Estimate of bacterial richness was assessed by Chao index at the genus level (d). Estimates of bacterial diversity were assessed by Shannon and Simpson indexes of genus level (e and f). Different fecal microbiota compositions were, respectively, shown at the levels of the phylum (c) and genus (g, h). Data are presented as median and quartile, n = 8 mice/group. The differences of the data among the different calcium intake groups during the pregnancy and lactation were assessed using Mann–Whitney U-test and Wilcoxon signed-rank test. *Compared to the NC-C group, P < .05; #compared to the NC-HFD group, P < .05.
Figure 7.
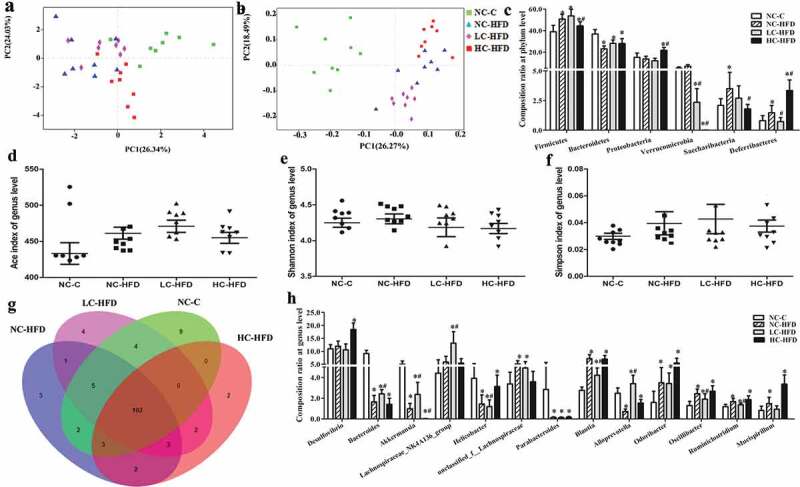
Microbiota composition and diversity of the female mouse offspring affected by maternal different calcium intake during the pregnancy and lactation. Bacterial communities at the phylum and genus levels based on weighted UniFrac distance were shown using the PCA analysis (a and b). Estimate of bacterial richness was assessed by Ace index at the genus level (d). Estimates of bacterial diversity were assessed by Shannon and Simpson indexes of genus level (e and f). Different fecal microbiota compositions were, respectively, shown at the levels of the phylum (c) and genus (g, h). Data are presented as median and quartile, n = 8 mice/group. The differences of the data among the different calcium intake groups during the pregnancy and lactation were assessed using Mann–Whitney U-test and Wilcoxon signed-rank test. *Compared to the NC-C group, P < .05; #compared to the NC-HFD group, P < .05.
To investigate the specific changes of microbiota and differences between the male and female mouse offspring affected by maternal different dietary calcium intake, we assessed the gut microbiota compositions at the phylum and genus levels. In the male mouse offspring, at the phylum level, the proportions of Firmicutes, and Tenericutes were increased, and those of Bacteroidetes, Verrucomicrobia, and Cyanobacteria were decreased in the HFD groups as compared to the NC-C group (P < .05). Meanwhile, there were much higher contents of Firmicutes and lower percents of Bacteroidetes, Verrucomicrobia and Cyanobacteria in the LC-HFD or/and HC-HFD groups than those in the NC-HFD group (Figure 6(c)). Different bacterial groups at the genus level showed the effects of maternal insufficient (LC-HFD group) or/and excessive calcium intake (HC-HFD group) on the offspring, characterizedby the reductions in proportions of unclassified_f_Ruminococcaceae, Bacteroides, Alistipes, Helicobacter, norank_f_Ruminococcaceae, and Anaerotruncus, and increases in proportions of Akkermansia, Lachnospiraceae_NK4A136_group, unclassified_f_ Lachnospiraceae, Lactobacillus and Alloprevotella as compared with the NC-HFD group (P < .05) (Figure 6(g,h)).
In the female mouse offspring, at the phylum level, the proportions of Firmicutes, Saccharibacteria, and Deferribacteres were over-represented, and those of Bacteroidetes and Verrucomicrobia were decreased in the HFD groups relative to the NC-C group (P < .05). Further analysis showed there were much higher contents of Proteobacteria and Deferribacteres and lower proportions of Firmicutes, Verrucomicrobia and Saccharibacteria in the HC-HFD than those in the NC-HFD group, while in the LC-HFD group, the proportions of Verrucomicrobia and Deferribacteres were lower (Figure 7(c)). At the genus level, we observed 13 bacterial taxa that displayed different abundance among the four groups. Compared to the NC-HFD and/or HC-HFD groups, there were significant reductions in the proportions of Helicobacter, Blautia, Oscillibacter, Ruminiclost-ridium and Mucispirillum, and increases in the proportions of Bacteroides, Akkermansia, Lachnospiraceae_NK4A136 _ group and Alloprevotella in the LC-HFD group (Figure 7(g,h)).
3.6. Functional features of the gut microbiota isolated from the male and female mouse offspring affected by maternal different calcium intake
To better understand the important role of the gut microbiota in the male and female mouse offspring affected by maternal different calcium intake, PICRUST1.0 program was used to predict our 16S rRNA based high-throughput sequencing data and further analyze the data in the context of COG database and KEGG (level 2) categories. Using the above methods, we obtained a microbial COG profile, KEGG and correlated the microbial functional features with the important genes found in the mouse offspring (Figure 4), which may predict the metabolic functions enriched in our samples.
In the male mouse offspring, the top 10 vigorous functional features of COG profile included carbohydrate transport and metabolism, general function prediction only, amino acid transport and metabolism, Transcription; cell wall/membrane/envelope biogenesis, replication, recombination and repair, translation, ribosomal structure and biogenesis, signal transduction mechanisms, energy production and conversion, and inorganic ion transport and metabolism (Figure 8(a,c)). While, in the female mouse offspring, the major function features were carbohydrate transport and metabolism, general function prediction only, amino acid transport and metabolism, transcription, cell wall/membrane/envelope biogenesis, replication, recombination and repair, signal transduction mechanisms, translation, ribosomal structure and biogenesis, energy production and conversion and inorganic ion transport and metabolism (Figure 8(b,d)).
Figure 8.
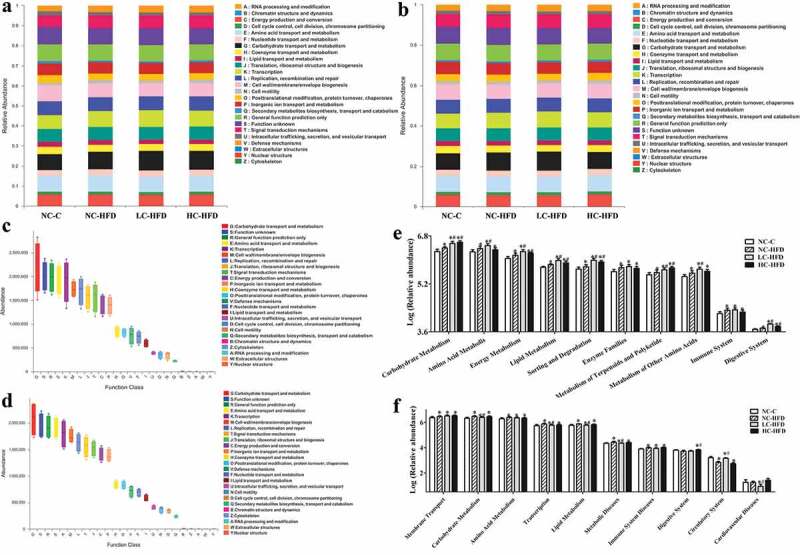
The microbial functional features among the male and female mouse offspring with maternal different calcium intake during the pregnancy and lactation The Cluster of Orthologous Groups (COG) and KEGG (Kyoto Encyclopedia of Genes and Genomes) database pathways were, respectively, used to predict the microbial functional features among the male (a, c, and e) and female mouse offspring (b, d, and f) with maternal different calcium intake during the pregnancy and lactation. One-way analysis of variance (ANOVA) was performed to compare the KEGG pathways among the different groups. Then, the Student–Newman–Keuls (SNK) test was used to determine where the differences existed between each two groups. *Compared to the NC-C group, P < .05; #Compared to the NC-HFD group, P < .05.
Multiple KEGG (level 2) categories were disturbed in the mouse offspring affected by maternal different dietary calcium. In the male mouse offspring, the pathways enriched in HFD groups (LC-HFD, NC-HFD, and HC-HFD) highlighted carbohydrate metabolism, amino acid metabolism, energy metabolism, lipid metabolism, sorting and degradation, enzyme families, metabolism of terpenoids and polyketide, metabolism of other amino acids, immune system and digestive system relative to the NC-C group. Further comparison showed that comparing with those in the NC-HFD group, the pathways involving in carbohydrate metabolism, energy metabolism, lipid metabolism, sorting and degradation, metabolism of terpenoids and polyketide, and digestive system were higher in the LC-HFD and HC-HFD groups (Figure 8(e)). The gut microbiome of the HFD groups among the female mouse offspring was characterized by over-representation of pathways involved in membrane transport, carbohydrate metabolism, amino acid metabolism, transcription, lipid metabolism, metabolic diseases, immune system diseases, and low-representation of pathways in the circulatory system than those in the NC-C group. Strikingly, bacterial invasion in the LC-HFD group was more significantly lower in the predicted pathways for carbohydrate metabolism, transcription, lipid metabolism, metabolic diseases, cardiovascular diseases, and higher level of pathways in the circulatory system than those in the NC-HFD group, in which the pathways in the LC-HFD were similar to those in the NC-C group.
3.7. Relationships between the microbiota community structure and the characteristics of lipid metabolism
Spearman correlation test was performed to evaluate the relationships between the different maternal dietary calcium status-associated genera and the characteristics of lipid metabolism. In the male mouse offspring, RDA/CCA analysis and related heat-map figures indicated that the differentially expressed genes for lipid oxidation and hydrolysis (PGC-1ɑ, HSL, and UCP1), being positively correlated with the lower body weight gain in the NC-HFD group, were in a better fitting degree, andthese genes were also positively related with norank_f_Ruminococcaceae and norank_f_Bacteroidales_S24_7_group, and negatively related with Alloprevotella, Lactobacillus, Desulfovibrio, and Lachnospiraceae_NK4A136_group. The fat synthesis related genes including Fasn, Acc1, PPAR-γ, LPL, SREBP-1c, and FABP4 were positively correlated with the higher body weight gain in the LC-HFD and HC-HFD groups, and these genes were positively correlated with Alloprevotella, Lactobacillus, Lachnospiraceae_NK4A136_group, and Candidatus_Saccharimonas, and negatively correlated with norank_f_Ruminococcaceae, norank_f_Bacteroidales_S24_7_group and Ruminiclostridium_9 (Figure 9(a,c)).
Figure 9.
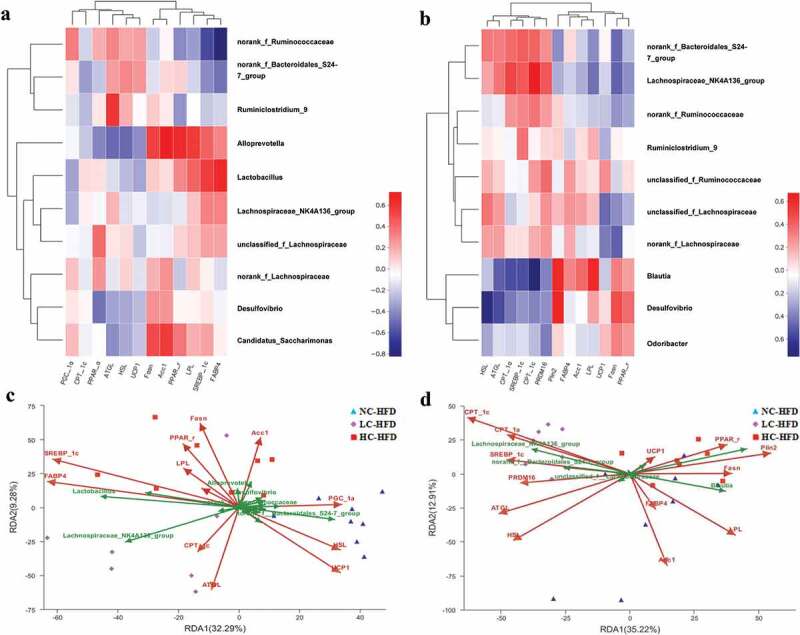
Correlations between the different microbiota composition of the top 10 richness genus and the expressions of lipid related genes among the male and female mouse offspring with maternal different calcium intake during the pregnancy and lactation. RDA/CCA analysis and related heatmaps were used to, respectively, discuss the correlations between the different microbiota composition of the top 10 richness genus and the expressions of lipid-related genes among the male (a and c) and female (b and d) mouse offspring at the genus level.
As shown in Figure 9(b,d), in the female mouse offspring, there were better fitting degrees at the differentially expressed genes related to lipid oxidation and hydrolysis (CPT-1ɑ, CPT-1c, PRDM16, ATGL, and HSL) contributing to the lower body weight gain in the LC-HFD group. There were positive correlations between these genes with the microbiota compositions at the genus level including norank_f_Bacteroidales_S24_7_group, Lachnospiraceae_NK4A136_ group and norank_f_Ruminococcaceae, and negative correlations between gene expressions with Blautia, Desulfovibrio and Odoribacter. The fat synthesis related genes including Fasn, Acc1, PPAR-γ, LPL, and Plin2 were positively correlated with the higher body weight gain in the NC-HFD and HC-HFD groups, and further these genes were positively correlated with Blautia, Desulfovibrio and Odoribacter, and negatively correlated with norank_f_Bacteroidales_S24_7_group, Lachnospiraceae_NK4A136_ group and norank_f_Ruminococcaceae.
4. Discussion
NAFLD, which is defined as the associations of hepatic steatosis, hepatocellular injury, inflammation and fibrosis, is associated with many metabolic syndromes such as obesity, insulin resistance, type 2 diabetes, and other chronic non-communicable diseases.21,22 So understanding the prevention and mechanisms of NAFLD is of the utmost importance. Recently, many evidences have shown that the disorder of the gut microbiota, and resulted disturbance in intestinal barrier function, could lead to the increment of hepatic fat accumulation.23 Interestingly, Le Roy T. and et al. also proved that the gut microbiota dysbiosis contributed to the development of NAFLD through affecting the fatty acid oxidation and lipogenesis by comparing the germ-free (GF) and gut microbiota transplant mice.24 Thus, these results demonstrated that the “gut-liver axis” is exerted to maintain the homeostasis of the gut microbiota and hepatic lipid metabolism.25,26 In our study, compared with the normal-fat diet feeding, high-fat diet feeding could result in the NAFLD by altering the gut bacterial communities and lipid metabolism both in the male and female mice, which is completely consistent with the previous researches.23–26
The onset and progression of NAFLD are closely associated with the insufficient (or excessive) intake of specific nutrients, such as marine n-3 fatty acids, soybean oil-derived n-6 fatty acids, sugars, folate and vitamin B12, and certain dietary patterns that composed of highly processed foods, sweets, and sugar-sweetened beverages as well as high-fat diets.27-30 However, there were few researches to discuss the associations between NAFLD and the dietary supplementation of mineral substance. Calcium, as the fifth most abundant element in human body with more than 99% residing in the skeleton, plays a central role in a wide range of essential functions including bone formation, muscle contraction, neurotransmitter release and lipid metabolism.31 it has been demonstrated that many functions of the liver could be regulated by the homeostasis between the intracellular and extracellular Ca2+, which can be affected by inappropriate calcium intake through the modulation of regulatory enzyme activity.32,33 During the pregnancy and lactation, maternal calcium metabolism should adapt to the demand created by the fetus and placenta, which could be drawn together with other minerals to mineralize the development of fetal skeleton.34 So it is a priority for mothers-to-be to obtain a balanced profile of calcium to ensure optimal fetal growth and development and productivity later in the life.35 Our previous epidemiological and animal experiments have confirmed that maternal calcium intake could promote the growth and development of the infants, while imbalance in maternal calcium intake would aggravate the high-fat induced obesity of the adult male mouse offspring through modulating on the gut microbiota and lipid metabolism.20 In addition, our animal study found that maternal insufficient or excessive calcium status during pregnancy and lactation programmed an abnormal expression of hepatic and adipose genes in the offspring, leading to dyslipidemia and hepatic lipid accumulation with the normal diets36 In the current study, maternal inappropriate calcium supplementation has long-term effects on the progress of high-fat induced NAFLD among the mouse offspring by altering the intestinal microbiota and lipid metabolism. Specifically, maternal normal and low calcium intake could respectively have the greatest inhibition on the progress of NAFLD in the male and female mouse offspring, which was characterized by the least lipid droplets, inflammatory infiltration and fibrosis, the lowest concentrations of free fatty acids and triglycerides, the lowest expression of genes involving in de novo lipogenesis and the highest expression of genes related to lipolysis. Pyrosequencing of 16S rRNA genes also revealed that the male mouse offspring in the NC-HFD group and the female mouse offspring in the LC-HFD group had distinct intestinal microbiota, which were closer to those in the NC-C group. Analysis of the functional features for the different microbiota was compatible with the gene expressions in de novo lipogenesis, lipid oxidation and hydrolysis.
To be noteworthy, our study indicated a significant sex-specific differences for the maternal calcium demand to inhibit the progress of NAFLD between the male and female mice offspring, showing that less calcium might be needed during maternal pregnancy and lactation for beneficial effects in the female than male offspring. Our findings of sex differences in response to maternal calcium supplementation on the progress of NAFLD by HFD induction have similarities to those seen in the previous clinical investigations, which all demonstrate different maternal calcium needs to promote health between the male and female offspring. Kate AW et al. found that maternal calcium supplementation affected childhood growth and bone development in a sex-specific manner, resulting in slower growth among females compared to placebo and accelerated growth among males at age 8–12 y in the rural African with daily extremely low intake.19 In contrast, the results from longitudinal follow-up trial were shown that pre-pubertal males aged 8–12 y who received calcium supplements for a year and who were followed to the end of growth have gone into their pubertal growth spurt earlier and so reached peak velocities for height and bone development at an earlier age than those who were in the placebo group.37,38 However, there is no clear and definite evidence that pregnant women with different genders of babies should be given different amount of calcium supplementation during the pregnancy and lactation. In this study, male and female offspring mice were conducted to discuss the effects of maternal calcium supplementation during pregnancy and lactation on offspring adult health, compensating for the short-term and uncontrollable for epidemiological study.
All these results proved the hypothesis that long-term effects of maternal calcium status on the progress of NAFLD could be regulated by altering the intestinal microbiota and lipid metabolism with attention to potential sex differences among the mouse offspring. As we all know, sex, an important biological variable, can markedly impact diverse physiological and pathological processes, as well as response to treatment in various diseases including heart disease, hypertension, obesity, and etc 39. Recently, more attentions have been paid to discuss the sex differences in many situations which demonstrate male and female infants respond differentially to same environmental stimuli, with different growth and neurodevelopmental trajectories.40–43 Male infants are also more likely to be disadvantaged when subjected to adversity. It has been demonstrated that animal milk composition differs depending on the offspring sex, suggesting that the tailoring of early life nutrition may be one mechanism to maximize health protection and development to infants of both sexes. Thus, the early life nutritional care may be different and optimism to minimize the “male disadvantage”.43 It is active calcium transport from the mother placenta to fetus during pregnancy that meets fetal more requirements for skeletal formation and mineralization through epithelial calcium channels by regulating many placental calcium binding proteins and plasma membrane calcium ATPase.44,45 Both “male disadvantage” and higher bone mineral density imply that male mice need more calcium during the early life and can not adapt to low-calcium environment for their adult health. Our findings are modest, yet significant, and need to be proved by the birth cohort studyin the future.
5. Conclusions
Insufficient or excessive intake of calcium during maternal pregnancy and lactation adversely affected the expression of genes associated with lipid hydrolysis, oxidation, synthesis and lipogenesis, and gut microbiota, resulting in more severity of NAFLD, and the effects of maternal calcium were sex specific for the offspring mice. Less calcium might be needed during maternal pregnancy and lactation for the beneficial effects on high-fat diet associated NAFLD in the female than the male offspring.
Supplementary Material
Acknowledgments
The authors would like to thank KY and DZ for animal feeding and Shanghai Majorbio Bio-pharm Technology Co., Ltd for microbial community analysis service.
Funding Statement
This work was supported by National Natural Science Foundation of China (#81602859 to PL) and Research Funds of Profession Quota Budget from Beijing Municipal Science and Technology Commission (#2019 and 2020-bjsekyjs to KQ).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author contributors
PL and KQ participated in the study design, acquisition, analysis and interpretation of the data. XLC and XYC performed the mRNA extraction from the hepatic tissue. RW and TT did the whole RT-PCR experiments. KY and DZ carried out the animal feeding,, XF performed the bioinformatics of the microbial community analysis. PL and KQ drafted the manuscript, which all authors had commented on.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Rinella ME. Will the increased prevalence of nonalcoholic steatohepatitis (NASH) in the age of better hepatitis C virus therapy make NASH the deadlier disease? Hepatology. 2011;54:1118–1120. [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, et al. Loss of junctional adhesion molecule A promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology. 2016;151:733–746. doi: 10.1053/j.gastro.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 5.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 6.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, Chan CC, Lawson MJ, Klarquist J, et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med. 2017;23:829–838. doi: 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: a review of the literature. Int J Mol Sci. 2019;20:395. doi: 10.3390/ijms20020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 9.Su D, Nie Y, Zhu A, Chen Z, Wu P, Zhang L, Luo M, Sun Q, Cai L, Lai Y, et al. Vitamin D signaling through induction of paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models. Front Physiol. 2016;7:498. doi: 10.3389/fphys.2016.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sajjad A, Mottershead M, Syn WK, Jones R, Smith S, Nwokolo CU. Ciprofloxacin suppresses bacterialovergrowth, increases fasting insulin but does not correctlow acylated ghrelin concentration in non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;22:291–299. doi: 10.1111/j.1365-2036.2005.02562.x. [DOI] [PubMed] [Google Scholar]
- 11.Machado MV, Cortez-Pinto H. Diet, microbiota, obesity, and NAFLD: a dangerous quartet. Int J Mol Sci. 2016;17:481. doi: 10.3390/ijms17040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Pourmozaffar S, Hajimoradloo A, Paknejad H, Rameshi H. Effect of dietary supplementation with apple cider vinegar and propionic acid on hemolymph chemistry, intestinal microbiota and histological structure of hepatopancreas in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019;86:900–905. doi: 10.1016/j.fsi.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Alomaim H, Griffin P, Swist E, Plouffe LJ, Vandeloo M, Demonty I, Kumar A, Bertinato J. Dietary calcium affects body composition and lipid metabolism in rats. PLoS One. 2019;14:e0210760. doi: 10.1371/journal.pone.0210760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones KW, Eller LK, Parnell JA, Doyle-Baker PK, Edwards AL, Reimer RA. Effect of a dairy- and calcium-rich diet on weight loss and appetite during energy restriction in overweight and obese adults: a randomized trial. Eur J Clin Nutr. 2013;67:371–376. doi: 10.1038/ejcn.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziauddeen N, Roderick PJ, Macklon NS, Alwan NA. Predicting childhood overweight and obesity using maternal and early life risk factors: a systematic review. Obes Rev. 2018;19:302–312. doi: 10.1111/obr.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matkovic V, Heaney RP. Calcium balance during human growth: evidence for threshold behavior. Am J Clin Nutr. 1992;55:992–996. doi: 10.1093/ajcn/55.5.992. [DOI] [PubMed] [Google Scholar]
- 18.Cormick G, Betrán AP, Romero IB, Lombardo CF, Gülmezoglu AM, Ciapponi A, Belizán JM. Global inequities in dietary calcium intake during pregnancy: a systematic review and meta-analysis. BJOG. 2019;126:444–456. doi: 10.1111/1471-0528.15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward KA, Jarjou L, Prentice A. Long-term effects of maternal calcium supplementation on childhood growth differ between males and females in a population accustomed to a low calcium intake. Bone. 2017;103:31–38. doi: 10.1016/j.bone.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Tang T, Chang X, Fan X, Chen X, Wang R, Fan C, Qi K. Abnormality in maternal dietary calcium intake during pregnancy and lactation promotes body weight gain by affecting the gut microbiota in mouse offspring. Mol Nutr Food Res. 2019;63:e1800399. doi: 10.1002/mnfr.201800399. [DOI] [PubMed] [Google Scholar]
- 21.Younossi ZM. Non-alcoholic fatty liver disease-A global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359–370. doi: 10.1136/gutjnl-2018-316307. [DOI] [PubMed] [Google Scholar]
- 24.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 25.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajaj JS, Hylemon PB. Gut-liver axis alterations in alcoholic liver disease: are bile acids the answer? Hepatology. 2018;67:2074–2075. doi: 10.1002/hep.29760. [DOI] [PubMed] [Google Scholar]
- 27.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, et al., VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahamid M, Mahroum N, Bragazzi NL, Shalaata K, Yavne Y, Adawi M, Amital H, Watad A. Folate and b12 levels correlate with histological severity in nash patients. Nutrients. 2018;10:440. doi: 10.3390/nu10040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henkel J, Alfine E, Saín J, Jöhrens K, Weber D, Castro JP, König J, Stuhlmann C, Vahrenbrink M, Jonas W, et al. Soybean oil-derived poly-unsaturated fatty acids enhance liver damage in NAFLD induced by dietary cholesterol. Nutrients. 2018;10:1326. doi: 10.3390/nu10091326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss IN, Webb M, Orenstein D, Shibolet O, Kariv R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68:1239–1246. doi: 10.1016/j.jhep.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461–468. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grancara S, Battaglia V, Martinis P, Viceconte N, Agostinelli E, Toninello A, Deana R. Mitochondrial oxidative stress induced by Ca2+ and monoamines: different behaviour of liver and brain mitochondria in undergoing permeability transition. Amino Acids. 2012;42:751–759. doi: 10.1007/s00726-011-0991-2. [DOI] [PubMed] [Google Scholar]
- 33.Antonenko YN, Rokitskaya TI, Cooper AJ, Krasnikov BF. Minocycline chelates Ca2+, binds to membranes, and depolarizes mitochondria by formation of Ca2+-dependent ion channels. J Bioenerg Biomembr. 2010;42:151–163. doi: 10.1007/s10863-010-9271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang XL, Shang Y, Liu YJ, Li P, Wang YY, Liang AM, Qi KM. Effects of calcium supplementation during the pregnancy and early infancy stage on the body mass index and gut microbiota in the infants. Chin J Prevent Med. 2018;52:642–646. doi: 10.3760/cma.j..0253-9624.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Chang X, Fan X, Fan C, Tang T, Wang R, Qi K. Dietary calcium status during maternal pregnancy and lactation affects lipid metabolism in mouse offspring. Sci Rep. 2018;8:16542. doi: 10.1038/s41598-018-34520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prentice A, Dibba B, Sawo Y, Cole TJ. The effect of prepubertal calcium carbonate supplementation on the age of peak height velocity in Gambian adolescents. Am J Clin Nutr. 2012;96:1042–1050. doi: 10.3945/ajcn.112.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward KA, Cole TJ, Laskey MA, Ceesay M, Mendy MB, Sawo Y, Prentice A. The effect of prepubertal calcium carbonate supplementation on skeletal development in Gambian boys-a 12-year follow-up study. J Clin Endocrinol Metab. 2014;99:3169–3176. doi: 10.1210/jc.2014-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol. 2017;37:746–756. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geary MP, Pringle PJ, Rodeck CH, Kingdom JC, Hindmarsh PC. Sexual dimorphism in the growth hormone and insulin-like growth factor axis at birth. J Clin Endocrinol Metab. 2003;88:3708–3714. doi: 10.1210/jc.2002-022006. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, Donovan EF, Wright LL, Lemons JA, Tyson JE, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83:F182–5. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng TS, Loy SL, Cheung YB, Chan JK, Pang WW, Godfrey KM, Gluckman PD, Kwek K, Saw SM, Chong YS, et al. Sexually dimorphic response to feeding mode in the growth of infants. Am J Clin Nutr. 2016;103:398–405. doi: 10.3945/ajcn.115.115493. [DOI] [PubMed] [Google Scholar]
- 43.Galante L, Milan AM, Reynolds CM, Cameron-Smith D, Vickers MH, Pundir S. Sex-specific human milk composition: the role of infant sex in determining early life nutrition. Nutrients. 2018;10:1194. doi: 10.3390/nu10091194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayward CE, Renshall LJ, Sibley CP, Greenwood SL, Dilworth MR. Adaptations in maternofetal calcium transport in relation to placental size and fetal sex in mice. Front Physiol. 2017;8:1050. doi: 10.3389/fphys.2017.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burren CP, Caswell R, Castle B, Welch CR, Hilliard TN, Smithson SF, Ellard S. TRPV6 compound heterozygous variants result in impaired placental calcium transport and severe undermineralization and dysplasia of the fetal skeleton. Am J Med Genet A. 2018;176:1950–1955. doi: 10.1002/ajmg.a.40484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


