ABSTRACT
The incidence of gastrointestinal infections continues to increase, and infectious colitis contributes significantly to morbidity and mortality worldwide. Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) has been discovered to be strongly involved in the intestinal homeostasis. However, whether intestinal CEACAM1 expression has an impact on the control of infectious colitis remains elusive. Citrobacter rodentium (C. rodentium) is a gram-negative enteric pathogen that induces colonic inflammation in mice, with a critical role for CD4+ T cell but not CD8+ T cell immunity to primary infection. Here, we show that Ceacam1−/− mice are much more susceptible to C. rodentium infection than wildtype mice, which is mediated by a defect in the intestinal barrier and, surprisingly, by a dysregulated CD8+ T cell but not CD4+ T cell response in the colon. CEACAM1 expression is essential for the control of CD8+ T cell immunity, as CEACAM1 deficiency during C. rodentium infection inhibits CD8+ T cell exhaustion. We conclude that CEACAM1 is an important regulator of CD8+ T cell function in the colon, and blocking CEACAM1 signaling to activate CD8+ T cells may have unforeseen side effects.
KEYWORDS: CEACAM1, enteric infection, infectious colitis, CD8 T cells, inhibitory molecule
Introduction
Mucosal surfaces are important routes of entry for bacterial pathogens into the host. In the gastrointestinal tract, enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC) are major causes of infectious diarrheal diseases in humans, and continue to pose significant health burdens worldwide.1,2 Citrobacter (C.) rodentium is a mucosal pathogen of mice that shares several pathogenic mechanisms with EPEC and EHEC. Therefore, it has been widely used as a model organism to study the role of host-pathogen interaction in the colon.3 The mouse-restricted pathogen C. rodentium and the human enteric pathogens EPEC and EHEC infect the intestines of the hosts, where they intimately attach to the apical surface of intestinal epithelial cells in the lower gastrointestinal tract, induce the destruction of intestinal architecture and activate the mucosal immune system.4 Most importantly, infectious colitis still contributes significantly to morbidity and mortality worldwide.5
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), also known as CD66a or biliary glycoprotein-1, is a multifunctional transmembrane protein expressed in diverse cell types, including epithelial cells and certain cells of the immune system. CEACAM1 is a member of the CEA gene family and the Ig superfamily with a basic structure of sequentially ordered Ig-like domains followed by a transmembrane and a cytoplasmic domain.6,7 The cytoplasmic domain of CEACAM1 is differently spliced resulting in a cytoplasmic short (CEACAM1-S) and a cytoplasmic long (CEACAM1-L) isoform. CEACAM1-S was described to activate T cells and induce regulatory T cells (Tregs) while CEACAM1-L, containing two intracellular immune receptor tyrosine-based inhibitory motifs (ITIMs), can inhibit activated T-cell function. Both isoforms are usually co-expressed and the CEACAM1-L to S ratio alters depending on the cellular growth and activation stage.8,9 In general, CEACAM1 serves as an adhesion molecule via homophilic and heterophilic interactions and participates in multiple physiological and pathophysiological processes.7,10-13 CEACAM1 is typically involved in cell-cell attachment, epithelial differentiation, neo-vascularization and regulation of B- and T-cell proliferation.14-16 Moreover, direct immunomodulatory consequences have been suggested based on immune cell expression and the presence of ITIM motifs in the intracellular domain of the protein.17 For example, CEACAM1 has been considered to be an inhibitory receptor that suppresses the activation of CD4+ T cells.18,19 However, it has been shown that the CEACAM1-S expression in CD4+ T cells leads to enhanced Treg induction and subsequently to the protection from T-cell-mediated liver injury.20 Furthermore, in a mouse model of chronic viral infection, CEACAM1 activation strongly improved the antiviral CD8+ T cell response16 suggesting different functions of CEACAM1 depending on the expressing cell types and stimuli.
There is increasing evidence that CEACAM1 is strongly involved in the maintenance of intestinal homeostasis.18,19,21,22 CEACAM1 expression is increased on the cell surface of human T cells in Celiac disease and inflammatory bowel disease.23,24 Well in line, CEACAM1 was shown to promote the induction of Tregs and follicular helper T cells in the intestine.19 In vivo ligation of CEACAM1 with CEACAM1 homophilic ligands in T cells was able to prevent or block mucosal inflammation associated with either chemical-induced colitis or naïve T cell-transfer models of colitis.18,25
Beside the impact on multiple immunological processes in the intestine, CEACAM1 is also described as cellular receptor for a variety of Gram-negative bacterial pathogens associated with the human mucosa.26 Under homeostasis, CEACAM1 is expressed at low levels in intestinal epithelial cells, which prevents their use by opportunistic pathogenic bacteria for attachment.27 However, under inflammatory conditions, released pro-inflammatory molecules induce CEACAM1 expression, which promotes the adhesion of pathogenic bacteria.28,29 Together, the immunomodulatory function of CEACAM1 and the fact that CEACAM1 can function as a microbial receptor30 imply an important physiological role for CEACAM1 in mucosal tissues of the gastrointestinal tract.
In the present study, we determined the impact of CEACAM1 on the course of C. rodentium induced colitis. CEACAM1 deficiency strongly enhanced the susceptibility to enteric C. rodentium infection. Infected Ceacam1−/− mice developed a stronger pathology, were prone to bacterial dissemination to systemic organs, and showed a hyperactive CD8+ T cell response in the colon compared to infected wildtype mice. These findings identified CEACAM1 as a critical regulator of CD8+ T cell responses during infectious colitis.
Materials and methods
Mice
Wildtype (WT) and Ceacam1−/− mice were maintained on the C57BL/6 J genetic background and were bred as homozygous. WT (C57BL/6J) mice were obtained from Harlan Winkelmann GmbH (Borchen, Germany) or bred in-house. Ceacam1−/− (2D2) deficient mice were kindly provided by N. Beauchemin (Goodman Cancer Research Center, McGill University, Montreal, QC, Canada). Eight- to twelve-week-old age-matched animals were used for all experiments, and bred and co-housed under specific pathogen-free conditions in the Laboratory Animal Facility of the University Hospital Essen. All animal experiments were performed in strict accordance with the guidelines of the German Animal Protection Law and were approved by the state authorities for Ethics in Animal Experiments of North-Rhine Westphalia, Germany.
C. rodentium infection model
The study was performed with the WT strain ICC168 gifted by C. Riedel (University of Ulm).31 C. rodentium was cultured overnight in Luria-Bertani at 37°C, centrifuged at 3000 × g for 10 min, and washed with PBS. Mice were infected by oral gavage with 100 µl PBS containing approximately 2 × 109 CFUs of C. rodentium. After gavage, an aliquot of the remaining suspension was plated in serial dilutions on MacConkey agar to control the infective dose. Bacterial numbers in stool and liver were determined by collecting fecal pellets and livers at various time points after infection. Serial dilutions of the fecal und liver homogenates were plated on MacConkey agar to determine the CFUs after overnight incubation at 37°C. C. rodentium was identified by the color and shape of the colonies. In addition, colony PCR was performed to verify C. rodentium. Mice were analyzed at various time points post infection (p.i.), and the spleens and colon were removed and prepared for analysis as described below.
Macroscopic and histopathologic assessment of colitis
Macroscopic colonic damage was assessed on the day of euthanasia. Assessment was based on two main characteristics of the pathologic state: colon length shortening and colon weight gain. Colons were prepared as Swiss rolls, and stored in 4% paraformaldehyde until the tissue was embedded in paraffin for histologic scoring. Tissue sections (4 µm) were prepared from paraffin-embedded tissue blocks, H&E stained and evaluated histopathologically in a blinded manner. The rectal part of the colon was assessed for inflammatory cell infiltrates, epithelial damage, goblet cell depletion, neutrophil infiltration, crypt abscesses, and crypt hyperplasia, each in a 0 to 3 scoring system (0 = no change; 1 = mild change; 2 = moderate change; 3 = profound change). All evaluated parameters together gave rise to an overall inflammatory score (0–15) in the rectal colon region. Crypt heights were measured by micrometry; 30 measurements were taken in the distal colon for each mouse. Only well-oriented crypts were measured.
Immunohistochemical analysis of CEACAM1
The rectal area (~ 1 cm in length) of C. rodentium infected and naïve animals was embedded in Tissue-Tek and sections (5–7 μm) were made. The sections were fixed in acetone/ethanol and then blocked with 1% BSA/PBS. The antibodies dissolved in 0.5% BSA/PBS were incubated for 60 minutes in a moist chamber in the dark. After repeated washing with PBS, the sections were embedded in Fluoromount G. The immunohistochemical images were subsequently taken on a Zeiss laser scanning microscope. For the CEACAM1 staining, a fluorescein-labeled anti-CEACAM1 antibody (αmsCC1-FITC) was used in combination with DAPI. To visualize the infection with C. rodentium, a specific antibody against the strain Citrobacter (Abcam, Cambridge, UK) in combination with the secondary antibody mRuby was used for the immunohistochemical analysis.
C. rodentium pull down approach
C. rodentium were diluted in 600 µl 1% BSA/PBS containing 5 µg msCEACAM1-Fc, huCEACAM8-Fc, huCEACAM1-Fc and huCEACAM1dN-Fc, respectively. Control was left without recombinant protein. Samples were incubated rotating for 4 h at 4°C. Then 50 µl of each probe were collected and boiled for 5 min at 100°C together with 15 µl of 5x reducing Lämmli-buffer containing 5% β-mercaptoethanol to demonstrate applied CEACAM-Fc. Subsequently bacteria were washed 6 times by centrifugation and resuspension of the pellet with 600 µl PBS. After the last washing step, the dry pellets were boiled for 5 min at 100°C in 50 µl of 1x reducing Lämmli-buffer containing 5% β-mercaptoethanol. Samples were subjected to TRICIN-PAGE, blotted to nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany) and reacted with Ponceau Red staining to monitor total protein loading. Membranes were blocked in 5% milk/PBS solution and subsequently incubated with HRP-coupled goat anti-human Fc pAb (Jackson ImmunoResearch (West Grove, PA) for 1 h at RT. After 3 x washing with PBS protein detection on nitrocellulose filters were performed utilizing an ECL-substrate (Amersham Biosciences) and resulting chemiluminescence was detected by the Fujifilm LAS-3000 digital imaging system.
Intestinal permeability-assay
For in vivo analysis of the intestinal permeability fluorescein isothiocyanate-conjugated (FITC)-dextran beads have been used. Briefly, food and water were withdrawn for 4 h and mice were orally administrated with permeability tracer (60 mg/100 g body weight of FITC-labeled dextran, MW 4000; FD4, Sigma-Aldrich, St. Louis, USA). Serum was collected five hours later and fluorescence intensity was determined (excitation, 492 nm; emission, 525 nm; BioTek). FITC-dextran concentrations were determined using a standard curve generated by serial dilution of FITC-dextran.
Cytokine quantification
Cytokine levels in serum samples and cytokine secretion of in vitro cultured murine colonic explants were assessed using polystyrene bead-based Luminex technology (R&D Systems), according to the manufacturer’s instructions. The assay was carried out on a Luminex 200 system and cytokine concentrations were quantified using the Luminex IS software (Luminex Corporation).
Isolation of splenocytes
Spleens were rinsed with an erythrocyte lysis buffer (containing 0.15 M NH4Cl, 10 mM KHCO3, and 0.5 M EDTA), meshed through a 100-µm cell strainer, and washed with PBS containing 2 mM EDTA and 2% fetal calf serum.
Isolation of lamina propria lymphocytes from the colon
Lamina propria (LP) lymphocytes were isolated as described previously,32 with minor modifications. Colons were flushed with PBS to remove feces, opened longitudinally, and cut into 1-cm pieces. Tissue pieces were washed twice in PBS containing 3 mM EDTA for 10 min at 37°C with rotation. EDTA was removed by washing colon pieces twice in Roswell Park Memorial Institute (RPMI) medium containing 1% FCS, 1 mM EGTA, and 1.5 mM MgCl2 for 15 min at 37°C with rotation. Colon pieces were then subjected to intense vortexing, washed with PBS, and digested in RPMI containing 20% FCS and 100 U/mL collagenase (Clostridium histolyticum; Sigma-Aldrich, St. Louis, MO) for 90 min at 37°C. Remaining tissue was separated from cells by passing the cell suspension through a 40-µm cell strainer and washing it with culture medium.
Magnetic purification of splenic CD8+ T cells
For the specific enrichment of CD8+ T cells, the autoMACS® Separation System and the CD8+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) were used according to the manufacturer’s instructions.
In vitro CD8+ T cell proliferations assay
After magnetic separation, the cells were washed in IMDM medium, resuspended in 4 ml fresh IMDM medium and incubated with 2.5 μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE, CFSE purchased from Invitrogen, Karlsruhe) for 8 minutes at 37°C. After adding 4 ml of prewarmed FCS and re-incubating for 5 minutes at 37°C, the cells were washed with PBS. Afterward, 2.5 × 105 cells per well were plated into anti-CD3 antibody coated 96 well flat bottom plate. The proliferation capacity was measured daily by flow cytometry.
Antibodies and flow cytometry
LP lymphocytes were stained with fluorochrome-labeled anti-mouse CD4 (RM4-5), CD8 (53–6.7), CTLA-4 (UC10-4F10-11), CD62 L (MEL-14), CD3e (145–2 C11), PD-1 (RPM1-30), GzmB (GB11) and CD69 (H1.2F3) antibodies. All antibodies used in this study were obtained from either BD Biosciences (Heidelberg, Germany) or eBioscience (Frankfurt, Germany). For analysis of intracellular cytokines, lymphocytes (0.5 × 106 cells per well) isolated from LPLs were stimulated for 4 h with 10 ng/mL PMA and 1 µg/mL Ionomycin in the presence of 5 µg/mL Brefeldin A (all from Sigma-Aldrich). After the cell surface staining, cells were fixed with the Fixation/Permeabilization buffer set (eBioscience) according to the manufacturer’s instructions and stained for intracellular cytokines IL-17A (TC11-18H10.1) and IFN-γ (XMG1.2).
Results
Ceacam1−/− mice are strongly susceptible to C. rodentium infection
To obtain insights into the function of CEACAM1 during infectious colitis, we infected wildtype (WT) and Ceacam1−/− mice via oral gavage with ~ 2 × 109 CFU C. rodentium per mouse and characterized the inflammatory response in the colon. First, we determined the bacterial burden in the fecal pellets of infected mice. C. rodentium infected Ceacam1−/− mice exhibited significantly higher bacterial numbers at day 3 and 5 post infection than did infected WT mice Figure 1a, suggesting a faster colonization of the pathogen. Of note, the reduced bacterial eradication in Ceacam1−/− mice at day 10 was associated with exaggerated inflammation, which was characterized histologically by a higher inflammation score and more severe crypt elongation compared to infected WT mice (Figure 1b). To determine the specific localization of C. rodentium in the colonic tissue, we infected WT and Ceacam1−/− mice with C. rodentium and stained colonic biopsies for CEACAM1 and C. rodentium at day 10 post infection. In line with the CFU in feces, we detected a higher bacterial burden in Ceacam1−/− mice compared to WT mice with a strong accumulation at the epithelial cells Figure 1c. Interestingly, the immunofluorescence analysis revealed a co-localization of epithelial CEACAM1 and C. rodentium in WT mice Figure 1c. It is well established that human CEACAM1 serves as cellular receptor for a variety of Gram-negative bacterial pathogens.26 To rule out that a potential binding of C. rodentium to CEACAM1 could be responsible for the severe pathological phenotype seen in Ceacam1−/−- mice, we performed a bacterial pull-down approach. As shown in Figure 1d neither mouse CEACAM1-Fc nor human CEACAM1-Fc was pulled down by C. rodentium. Thus, the phenotypical differences in mice with and without CEACAM1 expression were not caused by direct interaction of C. rodentium with CEACAM1 expressed on epithelia, endothelia and leukocytes. In summary, these findings indicate that CEACAM1 expression is involved in the eradication of C. rodentium and important for the control of infection-associated inflammation.
Figure 1.
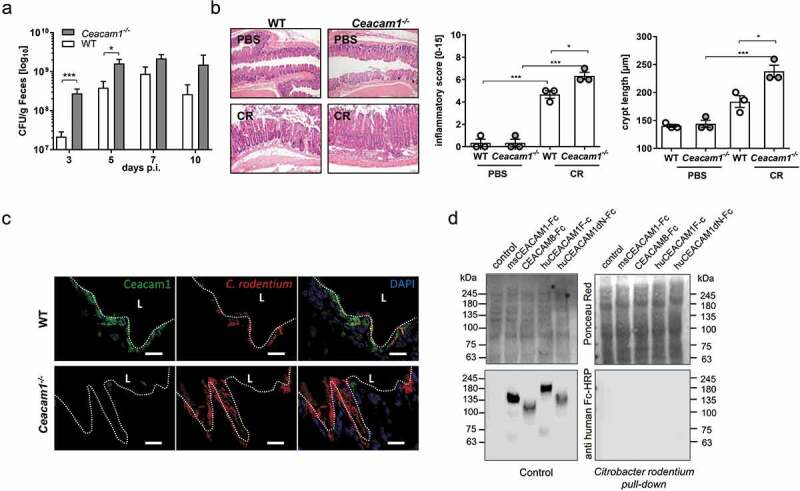
CEACAM1 deficiency leads to enhanced susceptibility to C. rodentium induced colitis. Ceacam1−/− and C57Bl/6 mice were orally infected with ~ 2 × 109 colony forming units (CFU) of C. rodentium. (a) At indicated days post infection (days p.i.), fecal pellets were collected to determine the colony forming units (CFU) per gram feces. (n = 31 each group). (b) Representative H&E staining of colon sections from PBS or C. rodentium infected WT or Ceacam1−/− mice 10 days post infection. Inflammatory score and crypt length of uninfected WT or Ceacam1−/− mice, and infected WT and Ceacam1−/− mice 10 days post C. rodentium infection. (c) Frozen section (5 µm) of the rectal colon tissue harvested from mice 10 days post infection. C. rodentium (red), CEACAM1 (green) and DAPI (blue). (One representative of n = 4 is shown, scale bar = 20 µm.) (d) The potential interaction of C. rodentium to human and mouse CEACAMs were analyzed by pull down experiments followed by western blot analysis. CEACAM8-Fc and the CEACAM1dN-Fc lacking the N-domain served as negative controls for this experiment. Ponceau Red staining was used to demonstrate loading of the bacterial extracts. One representative of three independent experiments is shown. All data are presented as mean ± SEM. Statistics were performed using the one-way ANOVA test followed by Bonferroni’s multiple comparison test (*, p < .05; **, p < .01; ***, p < .001).
CEACAM1 provides host resistance to bacterial dissemination
CEACAM1 overexpression was described to enhance the intestinal barrier function.33 Thus, we tested whether CEACAM1 deficiency affects the intestinal permeability under homeostasis or whether a stress signal such as a pathogen is involved. WT and Ceacam1−/− mice were gavaged with FITC-labeled dextran beads and intestinal permeability was assessed as concentration of serum FITC-dextran. Detection of FITC concentrations in the serum of Ceacam1−/− mice was significantly increased when compared to evaluated WT controls Figure 2a. Moreover, the difference between WT and Ceacam1−/− mice was even stronger when mice were infected for 10 days with C. rodentium before oral gavage of FITC-labeled dextran beads Figure 2b.
Figure 2.
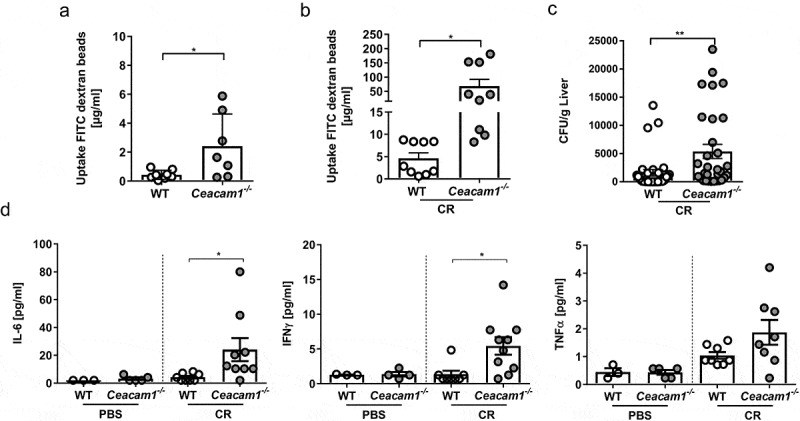
CEACAM1 deficiency impairs the intestinal permeability. (a) Naïve Ceacam1−/− and WT mice were orally administrated with FITC-labeled dextran beads. Serum was collected five hours later, and fluorescence intensity was determined (n = 7). (b) Ceacam1−/− and WT mice were orally gavaged with ~ 2 × 109 CFU of C. rodentium. At day 10 post infection FITC-labeled dextran beads were orally administrated, and the fluorescence intensity was determined 5 h later. (c) Mice were orally gavaged with PBS or ~ 2 × 109 CFU of C. rodentium. Bacterial load (CFU/g) in the liver 10 days post infection. (d) Concentration of the cytokines IL-6, IFNγ and TNFα were measured in the sera of Ceacam1−/− and WT mice 10 dpi using Luminex technologies. All data are presented as mean ± SEM. Statistics were performed using the Student’s t-test (*, p < .05; **, p < .01).
As a consequence, we hypothesized that CEACAM1 deficiency in intestinal epithelial cells may lead to bacterial dissemination from the gut to the systemic organs of a host. Thus, we infected WT and Ceacam1−/− mice with C. rodentium, harvested livers 10 days post infection and analyzed the presence of viable bacteria. Interestingly, a higher bacterial burden was observed in the liver of infected Ceacam1−/− mice compared to infected WT mice Figure 2c The production of cytokines is a hallmark of immune responses being mounted toward the infection. To assess whether the systemic distribution of C. rodentium in Ceacam1−/− mice altered the cytokine profile, we infected WT and Ceacam1−/− mice and measured cytokine levels in the sera. Of note, we found significantly elevated levels of IL-6 and IFNγ, and a trend of more TNFα in the serum of infected Ceacam1−/− mice 10 days post infection compared to infected WT mice (Figure 2(d)), demonstrating a systemic immune response in infected Ceacam1−/− mice.
The data clearly indicate that CEACAM1 expression is essential for an intact intestinal barrier, and CEACAM1 deficiency results in an enhanced systemic bacterial distribution.
CEACAM1 deficiency does not alter CD4+ T cell immunity but boosts CD8+ T cell activity
Infection with C. rodentium and clearance of the pathogen are associated with the induction of Th1 and Th17 adaptive immune responses.34 To determine the CD4+ T cell response during infection of WT and Ceacam1−/− mice, colonic lamina propria (LP) cells were isolated and characterized by flow cytometry. As reported before, slightly enhanced frequencies of CD4+ T cells were detected in Ceacam1−/− mice compared to WT mice Figure 3a. However, neither differences in the frequencies of IFNγ and IL-17 producing CD4+ T cells nor in the production level of IFNγ and IL-17 were observed between infected WT and Ceacam1−/− mice Figure 3b,c. By analyzing the frequencies and the phenotype of CD8+ T cells in the LP of non-infected and infected WT and Ceacam1−/− mice we detected a significant alteration in the T cell activity. Even though, no differences in the colonic CD8+ T cell frequencies were detected Figure 4a, CD8+ T cells isolated from the LP of non-infected Ceacam1−/− mice expressed significantly more CD69 and less CD62L compared to CD8+ T cells isolated from the LP of WT mice Figure 4b,c. In addition, CD8+ T cells from non-infected Ceacam1−/− mice seem to produce more GzmB than WT mice under homeostasis. Importantly, these differences in the phenotype were even stronger during infection, suggesting that CD8+ T cells in the colonic LP of Ceacam1−/− mice exhibit a more hyperactive phenotype than the CD8+ T cells in the WT mice.
Figure 3.
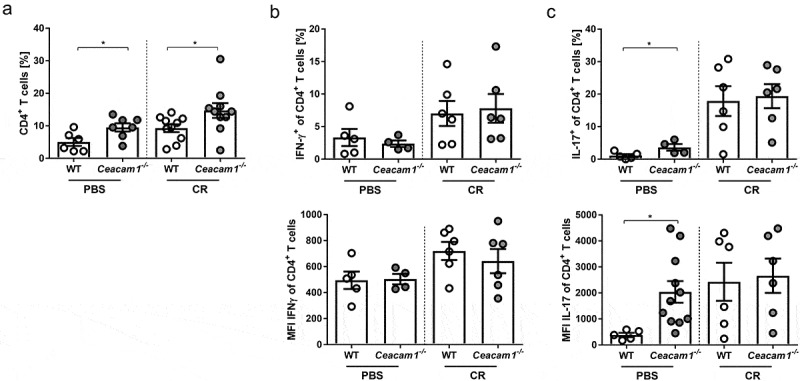
CD4+ T cell activation in the lamina propria of Ceacam1−/− mice is not altered. Ceacam1−/− and WT mice were orally infected with ~ 2 × 109 CFU of C. rodentium or gavaged with PBS. At day 10 post infection, lamina propria cells of the colon were isolated and characterized by flow cytometry. (a) Percentage and MFI of living CD4+ T cells, (b) Th1 (IFN-γ) and (c) Th17 (IL-17) cells. All data are presented as mean ± SEM. Statistics were performed using the Student’s t-test (*, p < .05; **, p < .01; ***, p < .001).
Figure 4.
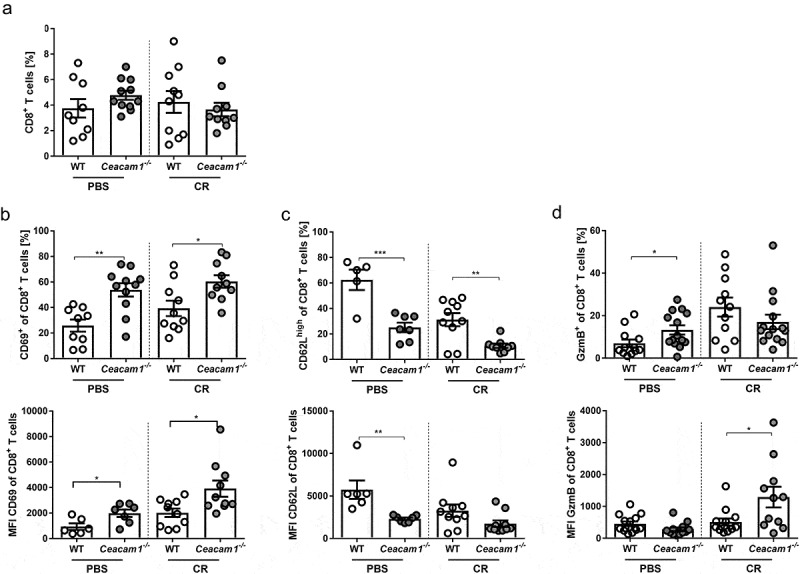
Altered CD8+ T cell activation in the lamina propria of Ceacam1−/− mice. Ceacam1−/− and WT mice were orally infected with ~ 2 × 109 CFU of C. rodentium or gavaged with PBS. At day 10 post infection, lamina propria cells of the colon were isolated and characterized by flow cytometry. (a) Percentage of living CD8+ T cells and MFI of (b) activation marker CD69, (c) L-Selectin (CD62L) and (d) GzmB. All data are presented as mean ± SEM. Statistics were performed using the Student’s t-test (*, p < .05; **, p < .01; ***, p < .001).
CEACAM1 deficiency inhibits the expression of inhibitory receptors
CEACAM1 was described previously as an activation-induced inhibitory molecule on T cells similar to PD-1 and CTLA-4.35,36 Thus, we analyzed whether the absence of CEACAM1 interferes with the expression of PD-1 or CTLA-4. We infected WT and Ceacam1−/− mice with C. rodentium and determined the frequencies and the expression levels of PD-1 and CLTA-4 on CD8+ T cells isolated from the LP 10 days post infection. As expected, PD-1 and CTLA-4 expression were strongly upregulated on CD8+ T cells, isolated from infected WT mice Figure 5a,b. In contrast, no upregulation of these molecules was observed on CD8+ T cells of infected Ceacam1−/− mice, neither in the frequency nor in the expression level per cell. These results suggest that either CD8+ T cells in the LP of infected Ceacam1−/− mice are not activated, or the cells are hyperactive due to a defect in T cell exhaustion.
Figure 5.
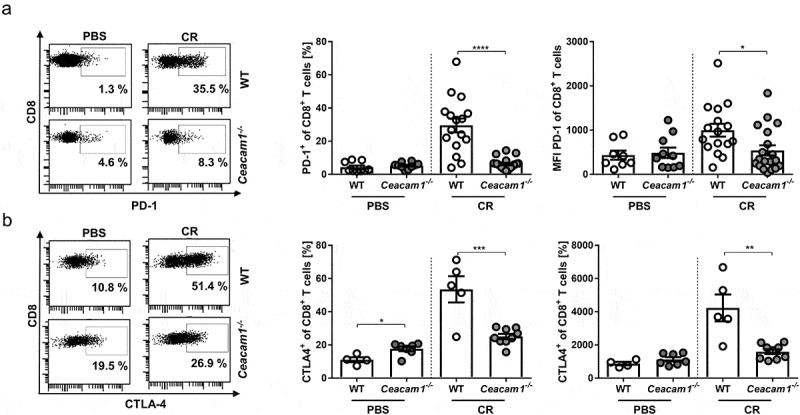
Dysregulated expression of co-inhibitory molecules in Ceacam1−/− mice. Ceacam1−/− and WT mice were orally infected with ~ 2 × 109 CFU of C. rodentium or gavaged with PBS. At day 10 post infection, lamina propria cells of the colon were isolated and characterized by flow cytometry for the frequency and MFI of (a) of PD1+ CD8+ T cells and (b) CTLA-4+ CD8+ T cells. All data are presented as mean ± SEM. Statistics were performed using the Student’s t-test (*, p < .05; **, p < .01; *** p < .001, ****, p < .0001).
CEACAM1 controls CD8+ T cell activity
To get more insight into the function of CEACAM1 on CD8+ T cells, we performed in vitro stimulation studies. CD8+ T cells from the spleen of WT and Ceacam1−/− mice were purified and stimulated with plate-bound anti-CD3 antibody for at least 3 days. Interestingly, we observed no differences in the proliferation capacity of CD8+ T cells between WT and Ceacam1−/− mice Figure 6a. However, CD8+ T cells from Ceacam1−/− mice showed a significant delay in the upregulation of PD-1 expression Figure 6b but an enhanced secretion of proinflammatory cytokines such as IL-6, IL-17 and TNFα Figure 6c. These data suggest that CEACAM1 deficiency on CD8+ T cells might lead to intestinal inflammation as observed with other checkpoint inhibitors.37,38 To determine the impact of CD8+ Ceacam1−/− T cells on infectious colitis, we infected WT and Ceacam1−/− mice with C. rodentium and depleted the CD8+ T cells during the course of infection Figure 7a. Interestingly, depletion of CD8+ T cells neither reduced the bacterial burden in the feces of infected WT mice nor in the feces of infected Ceacam1−/− mice Figure 7b. As seen before, we observed an enhanced spleen weight, higher colon weight to length ratio and stronger crypt hyperplasia in Ceacam1−/− mice compared to infected WT mice Figure 7c–e. Of note, depletion of CD8+ T cells in WT mice during infection did not alter the inflammatory response. In contrast, depletion of CD8+ T cells from Ceacam1−/− mice normalized the disease activity to the level seen in infected WT mice Figure 7c–e. These data clearly show that CEACAM1 expression is essential to control CD8+ T cells immunity during infectious colitis.
Figure 6.
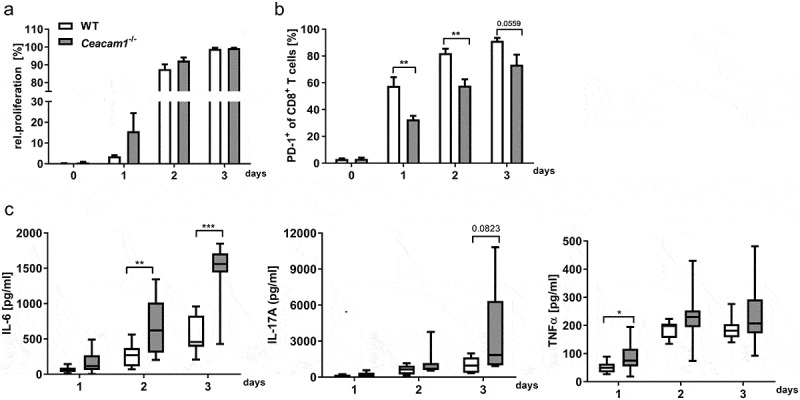
CD8+ T cells from Ceacam1−/− mice show an enhanced activity in vitro. CD8+ T cells were isolated from the spleen of naïve Ceacam1−/− or WT mice, CFSE-labeled and stimulated with anti-CD3 antibody. (a) Proliferation of CD8+ T cells was determined at indicated time points by flow cytometry and the loss of CFSE dye (n = 4). (b) Percentages of PD1+ CD8+ T cells were measured at indicated time points. (c) Concentration of IL-6, IL-17A and TNFα in the cell culture supernatants was analyzed at indicated time points using Luminex technology (n = 9–10). All data are presented as mean ± SEM. Statistics were performed using the Student’s t-test for individual time points (*,p< .05; **,p< .01; ***,p< .001).
Figure 7.
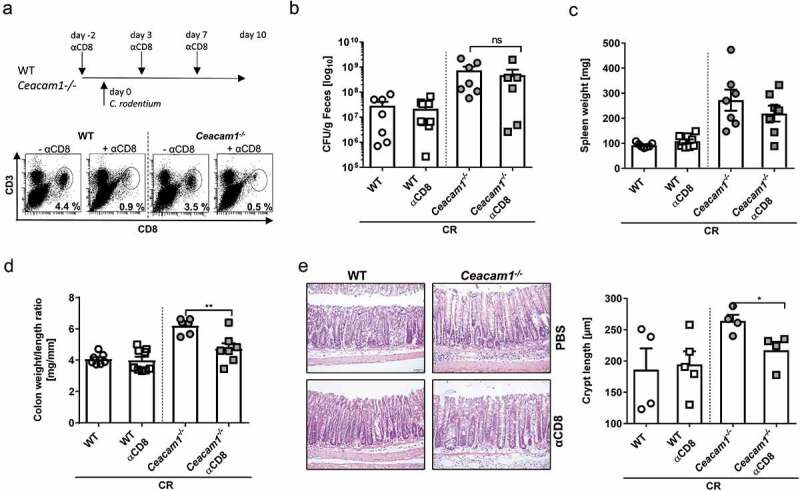
CEACAM1 controls the CD8+ T cells activity during bacterial induced colitis. Ceacam1−/− and WT mice were orally infected with ~ 2 × 109 CFU of C. rodentium and either injected with PBS or depleting anti-CD8 antibodies. (a) Experimental setup and efficacy of CD8+ T cell depletion in the colonic lamina propria at day 10 post infection was measured. (b) CFU/g feces at day 10 of infection. (c) Spleen weight and (d) colon weight/length ratio at 10 days post infection is indicated. (e) Representative H&E staining of colon sections (left graph) and crypt length (right graph) from infected Ceacam1−/− or WT mice either with or without CD8+ T cell depletion. All data are presented as mean ± SEM. Statistics were performed using the Student’s t-test (*, p < .05; **, p < .01; ***, p < .001).
To check whether we can transfer the phenotype of infected Ceacam1−/− mice to WT mice or if a smaller population of CD8+ Ceacam1−/− T cells in WT mice may enhance the defense against C. rodentium infection and protect from severe infectious colitis we isolated CD8+ T cells from the spleen of either naïve WT or Ceacam1−/− mice. 5 × 106 purified CD8+ T cells were adoptively transferred into WT mice (WT CD8+, Ceacam1−/− CD8+) prior to infection with ~ 2 × 109 CFU of C. rodentium Figure 8a. First, the expression of PD-1 and CEACAM1 was determined in the colon of infected mice. Interestingly, the expression of PD-1 in the colonic tissue was strongly upregulated when infected recipient mice were adoptively transferred with CD8+ T cells from WT mice compared to non-infected WT mice. In contrast, in infected mice that received Ceacam1−/− CD8+ T cells the upregulation of PD-1 expression was less strong compared to the infected controls which is well in line with the phenotype seen in infected Ceacam1−/− mice Figure 8b. In addition, the expression of CEACAM1 was reduced in infected mice, which received CD8+ T cells from Ceacam1−/− mice compared to the control mice. To assess whether the CD8+ T cell transfer also altered the colonic cytokine secretion, we measured cytokine levels in the supernatant of colonic explant culture. Interestingly, we found significantly reduced levels of IFNγ and TNFα in the colon of mice, which were adoptively transferred with CD8+ T cells from Ceacam1−/− mice compared to mice transferred with CD8+ T cells from WT mice Figure 8c. Moreover, the crypt hyperplasia was higher in the mice with higher cytokine secretion. Thus, the adoptive transfer of CEACAM1 deficient CD8+ T cells into WT mice with endogenous CEACAM1 sufficient CD8+ T cells mice did not accelerate C.rodentium colitis but support the fight against the pathogen.
Figure 8.
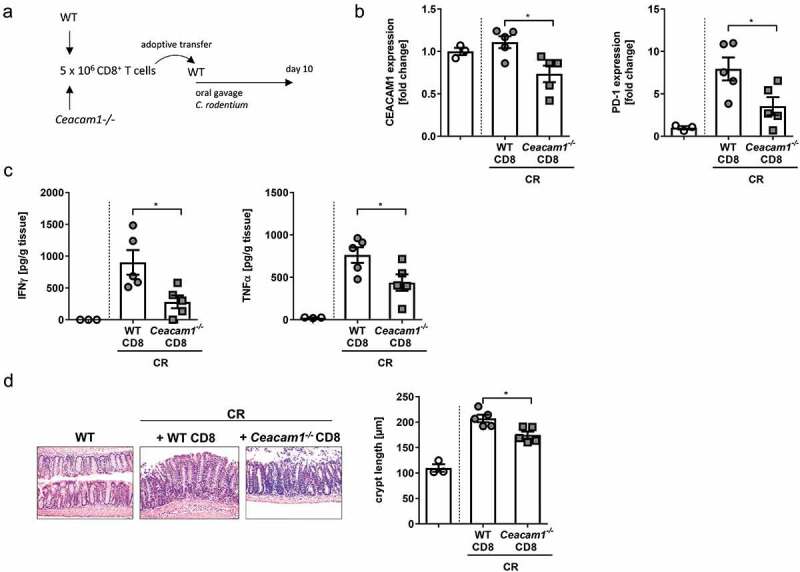
Adoptive transfer of CEACAM1 deficient CD8+ T cells modulates C.rodentium induced colitis. CD8+ T cells were isolated from the spleen of either Ceacam1−/− or WT mice and 5 × 106 purified CD8+ T cells were adoptively transferred into WT mice prior to infection with ~ 2 × 109 CFU of C.rodentium. (a) Experimental setup. (b) PD-1 and CEACAM1 expression was analyzed in the colon of infected mice 10 days post infection. (c) Colonic cytokine secretion was determined 10 days post infection. (d) Representative H&E staining of colon sections and crypt length from infected mice either after transfer of WT CD8+ T cells or transfer of CD8+ T cells from Ceacam1−/− mice. All data are presented as mean ± SEM. Statistics were performed using the Student’s t-test (*,p<.05;).
Discussion
The gastrointestinal tract is the largest mucosal surface in the body and thus constitute major sites of contact with bacteria. Therefore, the gastrointestinal tract is one route used by pathogens to enter the body. In this study, we describe the involvement of CEACAM1 in the control of infectious colitis. Interestingly, we found that Ceacam1−/− mice are highly susceptible to C. rodentium induced colitis, suggesting a protective function of CEACAM1 in the colon.
In humans, CEACAM1 is the target of several Gram-negative commensal and pathogenic bacteria that inhabit the mucosal surfaces. In particular, pathogenic Neisseria, Haemophilus influenzae, Moraxella catarrhalis, Helicobacter pylori, Escherichia coli and Fusobacterium spp. strains have been found to associate via a highly specific protein-protein interaction with CEACAM1.2,39-42 Although the CEACAM-binding bacteria differ in their pathogenic potential, they share the same ecological niche. Thus, it is likely that CEACAM-binding promotes colonization of the mucosa. Interestingly, colons of Ceacam1−/− mice are much stronger colonized with C. rodentium compared to CEACAM1 sufficient WT mice, especially in the early phase of infection. Our fluorescence analysis revealed an enhanced accumulation of C. rodentium at the epithelial cells. However, we observed that, in contrast to other Gram-negative bacteria, C. rodentium is not binding to CEACAM1 and therefore CEACAM1 is not involved in the attachment of C. rodentium to intestinal epithelial cells.
Besides a higher CFU in the feces, infected Ceacam1−/− mice are prone to bacterial dissemination to systemic organs, as we found a higher bacterial burden in the liver of infected Ceacam1−/− mice compared to infected WT mice. The intestinal barrier function plays a critical role in the defense against invading pathogens.43,44 Intestinal tight junction (TJ) proteins are a key component of the intestinal barrier.45-47 TJ damages lead to an increase of the intestinal permeability and result in the access of intraluminal antigens or bacteria into the mucosa.48 CEACAM1 overexpression in the colon was shown to modulate TJ protein expression and strengthen the intestinal barrier.33 Consistently, we detected a defect in the intestinal integrity in Ceacam1−/− mice under homeostasis and even stronger during infectious colitis. The oral gavage of non-infected and infected Ceacam1−/− mice with FITC-labeled dextran beads revealed a massive translocation of the beads to the serum, significantly stronger as seen in WT mice. Thus, our data clearly indicate that CEACAM1 expression is essential for an intact intestinal barrier and deficiency of epithelial CEACAM1 pave the way for bacterial translocation and systemic dissemination.
Interestingly, Ceacam1−/− mice do not develop spontaneous intestinal inflammation but are predisposed to excessive intestinal inflammation upon initiation of a gut immune response. Several studies have indicated that the activation of CD4+ and CD8+ T cells and the differentiation into effector T cells initiate intestinal diseases and promote intestinal inflammation.49-51 Furthermore, the intestinal induction or expansion of regulatory T cells counterbalance the inflammatory response.52 Interestingly, CEACAM1 was identified to be involved in regulating T cell-dependent immunopathology in murine colitis models.21,35 CEACAM1 expression is mostly excluded from naïve T cells but is expressed on T cells activated by stimulation with IL-2 or antiCD3 antibodies.53,54 CEACAM1-S expression in CD4+ T cells was shown to enhance Treg induction and stability, which confers protection from T-cell-mediated injury.20 Furthermore, deficiency in CEACAM1 results in the lack ofactivation of CD8+ regulatory T cells in the human mucosa.55,56 However, in our experimental setup we could not detect any difference in the frequency or phenotype of regulatory T cells between C. rodentium infected WT and Ceacam1−/− mice (data not shown). CEACAM1-L, the dominant isoform expressed in most T cells, was shown to act as an inhibitory receptor downregulating T cell activation and suppressing T cell functions,24 similar to the function of the inhibitory receptors CTLA-4 or PD-1.57,58 Blocking CTLA-4 and PD-1 signaling by immune checkpoint inhibitors has been successfully introduced for the reactivation of exhausted T cells and established as anticancer treatment, which significantly improved the survival of patients with advanced cancer.59 Although efficacy and durability of responses with checkpoint inhibitors has been well established, one of the major concerns is the high rate of adverse events that are predominantly immune related. One of the most common and severe immune-related adverse events is immune checkpoint inhibition-related colitis.60,61 Thus, CEACAM1 inhibition or ablation might lead to the same excessive intestinal inflammation as observed with CTLA-4 or PD-1 inhibitors.37,38
In this study, we show that CD8+ T cells in the lamina propria of Ceacam1−/− do not upregulate PD-1 or CTLA-4 expression during infection with C. rodentium, although CD8+ T cells from Ceacam1−/− express higher levels of CD69 and GzmB compared to WT mice. In general, PD-1 expression is induced after CD8+ T cell activation to avoid uncontrolled cytotoxicity.62,63 Our in vitro studies reveal a delay in the upregulation of PD-1 expression after anti-CD3 stimulation in Ceacam1−/− mice but an enhanced cytokine production. Hence, our data indicate a hyperactive phenotype of CD8+ T cells in Ceacam1−/− mice compared to WT mice. This is well in line with a study from Zhang et al., which demonstrates that co-expression and ligation of CEACAM1 and TIM-3, another inhibitory receptor expressed on activated T cells, promotes T cell exhaustion in colorectal cancer patients.36 Vice versa, in patients suffering from multiple sclerosis the percentage and expression of CEACAM1 and TIM-3 on CD8+ and CD4+ T cells was decreased suggesting uncontrolled T cells activation.64
In immunocompetent mice, CD8+ T cells are dispensable for the eradication of C. rodentium and the control of immune pathology. Mice depleted of CD8+ T cells were as competent as control mice in their ability to eradicate C. rodentium from mucosal and systemic tissue. In contrast, mice depleted of CD4+ T cells were shown to be highly susceptible to infection and developed severe colitis.65 We found, that the depletion of CD8+ T cells from infected Ceacam1−/− mice normalized the disease activity to WT level but the transfer of low numbers of CEACAM1 deficient CD8+ T cells to WT mice with CEACAM1 sufficient cells reduced intestinal inflammation. Thus, in our experimental setting, CEACAM1 expression seems to be essential to control CD8+ T cell immunity during infectious colitis. However, a tightly controlled CD8+ T cell response could be beneficial during bacteria driven colitis.
Taken together, our results show that CEACAM1 can determine the host susceptibility to C. rodentium infection. As blocking inhibitory receptors can render the host more susceptible to acute infection with enteric pathogens, in some situations, this could represent an unanticipated consequence of immune checkpoint inhibitor targeting therapies.
Acknowledgments
We kindly thank Mechthild Hemmler-Roloff and Patrick Juszczak for excellent technical assistance. We are thankful to Daniela Catrini for the critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (DFG –RTG 1949 and RTG 2098).
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft [RTG2098, RTG1949].
Disclosure of Potential Conflicts of Interest
The authors report no conflict of interest.
References
- 1.Pearson JS, Giogha C, Ong SY, Kennedy CL, Kelly M, Robinson KS, Lung TWF, Mansell A, Riedmaier P, Oates CVL, et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature. 2013;501:247–251. doi: 10.1038/nature12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virji M, Makepeace K, Ferguson DJ, Watt SM.. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 3.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 4.Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039. doi: 10.1128/IAI.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navaneethan U, Giannella RA. Infectious colitis. Curr Opin Gastroenterol. 2011;27:66–71. doi: 10.1097/MOG.0b013e3283400755. [DOI] [PubMed] [Google Scholar]
- 6.Zebhauser R, Kammerer R, Eisenried A, McLellan A, Moore T, Zimmermann W. Identification of a novel group of evolutionarily conserved members within the rapidly diverging murine Cea family. Genomics. 2005;86:566–580. doi: 10.1016/j.ygeno.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Helfrich I, Singer BB. Size matters: the functional role of the CEACAM1 isoform signature and its impact for NK Cell-mediated killing in melanoma. Cancers (Basel). 2019;11:356. doi: 10.3390/cancers11030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, Obrink B. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol. 2002;168:5139–5146. doi: 10.4049/jimmunol.168.10.5139. [DOI] [PubMed] [Google Scholar]
- 9.Brewer ML, Dymock D, Brady RL, Singer BB, Virji M, Hill DJ. Fusobacterium spp. target human CEACAM1 via the trimeric autotransporter adhesin CbpF. J Oral Microbiol. 2019;11:1565043. doi: 10.1080/20002297.2018.1565043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watt SM, Teixeira AM, Zhou GQ, Doyonnas R, Zhang Y, Grunert F, Blumberg RS, Kuroki M, Skubitz KM et al. Homophilic adhesion of human CEACAM1 involves N-terminal domain interactions: structural analysis of the binding site. Blood. 2001;98:1469–1479. doi: 10.1182/blood.V98.5.1469. [DOI] [PubMed] [Google Scholar]
- 11.Tan K, Zelus BD, Meijers R, Liu JH, Bergelson JM, Duke N, Zhang R, Joachimiaka, Holmes KV, Wang JH. Crystal structure of murine sCEACAM1a[1,4]: a coronavirus receptor in the CEA family. Embo J. 2002;21:2076–2086. doi: 10.1093/emboj/21.9.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapoznik S, Ortenberg R, Schachter J, Markel G. CEACAM1 in malignant melanoma: a diagnostic and therapeutic target. Curr Top Med Chem. 2012;12:3–10. doi: 10.2174/156802612798919259. [DOI] [PubMed] [Google Scholar]
- 13.Farre D, Martinez-Vicente P, Engel P, Angulo A. Immunoglobulin superfamily members encoded by viruses and their multiple roles in immune evasion. Eur J Immunol. 2017;47:780–796. doi: 10.1002/eji.201746984. [DOI] [PubMed] [Google Scholar]
- 14.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. [DOI] [PubMed] [Google Scholar]
- 15.Khairnar V, Duhan V, Maney SK, Honke N, Shaabani N, Pandyra AA, Seifert M, Pozdeev V, Xu HC, Sharma P, et al. CEACAM1 induces B-cell survival and is essential for protective antiviral antibody production. Nat Commun. 2015;6:6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khairnar V, Duhan V, Patil AM, Zhou F, Bhat H, Thoens C, Sharma P, Adomati T, Friendrich SK, Bezgovsek J, et al. CEACAM1 promotes CD8(+) T cell responses and improves control of a chronic viral infection. Nat Commun. 2018;9:2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Chen L, Qiao SW, Nagaishi T, Blumberg RS. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol. 2008;180:6085–6093. doi: 10.4049/jimmunol.180.9.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, Qiao SW, Chen Z, Glickman J, Najjar SM, Nakajima A, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25:769–781. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Chen Z, Baker K, Halvorsen EM, da Cunha AP, Flak MB, Gerber G, Huang Y-H, Hosomi S, Arthur J, et al. The short isoform of the CEACAM1 receptor in intestinal T cells regulates mucosal immunity and homeostasis via Tfh cell induction. Immunity. 2012;37:930–946. doi: 10.1016/j.immuni.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horst AK, Wegscheid C, Schaefers C, Schiller B, Neumann K, Lunemann S, Langeneckert AE, Oldhafer KJ, Weiler-Normann C, Lang KS, et al. Carcinoembryonic antigen-related cell adhesion molecule 1 controls IL-2-dependent regulatory T-cell induction in immune-mediated hepatitis in mice. Hepatology. 2018;68:200–214. doi: 10.1002/hep.29812. [DOI] [PubMed] [Google Scholar]
- 21.Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN, et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471–482. doi: 10.1084/jem.20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, Rudd CE, Utku N, Blumberg RS, et al. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol. 2004;172:3535–3543. doi: 10.4049/jimmunol.172.6.3535. [DOI] [PubMed] [Google Scholar]
- 23.Donda A, Mori L, Shamshiev A, Carena I, Mottet C, Heim MH, Beglinger C, Grunert F, Rochlitz C, Terracciano L, et al. Locally inducible CD66a (CEACAM1) as an amplifier of the human intestinal T cell response. Eur J Immunol. 2000;30:2593–2603. doi:. [DOI] [PubMed] [Google Scholar]
- 24.Morales VM, Christ A, Watt SM, Kim HS, Johnson KW, Utku N, Texieira AM, Mizoguchi A, Mizoguchi E, Russell GJ, et al. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a). J Immunol. 1999;163:1363–1370. [PubMed] [Google Scholar]
- 25.Nakajima A, Iijima H, Neurath MF, Nagaishi T, Nieuwenhuis EE, Raychowdhury R, Glickman J, Blau DM, Russell S, Holmes KV, et al. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J Immunol. 2002;168:1028–1035. doi: 10.4049/jimmunol.168.3.1028. [DOI] [PubMed] [Google Scholar]
- 26.Voges M, Bachmann V, Kammerer R, Gophna U, Hauck CR. CEACAM1 recognition by bacterial pathogens is species-specific. BMC Microbiol. 2010;10:117. doi: 10.1186/1471-2180-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ou G, Baranov V, Lundmark E, Hammarstrom S, Hammarstrom ML. Contribution of intestinal epithelial cells to innate immunity of the human gut–studies on polarized monolayers of colon carcinoma cells. Scand J Immunol. 2009;69:150–161. doi: 10.1111/j.1365-3083.2008.02208.x. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura Y, Murata Y, Park JH, Kotani T, Imada S, Saito Y, Okazawa H, Azuma T, Matozaki T. Regulation by gut commensal bacteria of carcinoembryonic antigen-related cell adhesion molecule expression in the intestinal epithelium. Genes Cells. 2015;20:578–589. doi: 10.1111/gtc.12247. [DOI] [PubMed] [Google Scholar]
- 29.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel J-F, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–571. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riedel CU, Casey PG, Mulcahy H, O’Gara F, Gahan CG, Hill C. Construction of p16Slux, a novel vector for improved bioluminescent labeling of gram-negative bacteria. Appl Environ Microbiol. 2007;73:7092–7095. doi: 10.1128/AEM.01394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 33.Jin Y, Lin Y, Lin L, Sun Y, Zheng C. Carcinoembryonic antigen related cellular adhesion molecule 1 alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Life Sci. 2016;149:120–128. doi: 10.1016/j.lfs.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 34.Seiffart V, Zoeller J, Klopfleisch R, Wadwa M, Hansen W, Buer J, Riedel C, Westendorf AM. IL10-deficiency in CD4+ T cells exacerbates the IFNγ and IL17 response during bacteria induced colitis. Cell Physiol Biochem. 2015;36:1259–1273. doi: 10.1159/000430295. [DOI] [PubMed] [Google Scholar]
- 35.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen B-S, Melum E, Pertel T, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Cai P, Li L, Shi L, Chang P, Liang T, Yang Q, Liu Y, Wang L, Hu L, et al. Co-expression of TIM-3 and CEACAM1 promotes T cell exhaustion in colorectal cancer patients. Int Immunopharmacol. 2017;43:210–218. doi: 10.1016/j.intimp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe N, Kaminuma O, Kitamura N, Hiroi T, Shiku H. Induced treg cells augment the Th17-mediated intestinal inflammatory response in a CTLA4-dependent manner. PLoS One. 2016;11:e0150244. doi: 10.1371/journal.pone.0150244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song MY, Hong CP, Park SJ, Kim JH, Yang BG, Park Y, Kim SW, Kim KS, Lee JY, Lee S-W, et al. Protective effects of Fc-fused PD-L1 on two different animal models of colitis. Gut. 2015;64:260–271. doi: 10.1136/gutjnl-2014-307311. [DOI] [PubMed] [Google Scholar]
- 39.Javaheri A, Kruse T, Moonens K, Mejias-Luque R, Debraekeleer A, Asche CI, Tegtmeyer N, Kalali B, Bach NC, Sieber SA, et al. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat Microbiol. 2016;2:16189. doi: 10.1038/nmicrobiol.2016.189. [DOI] [PubMed] [Google Scholar]
- 40.Tchoupa AK, Schuhmacher T, Hauck CR. Signaling by epithelial members of the CEACAM family - mucosal docking sites for pathogenic bacteria. Cell Commun Signal. 2014;12:27. doi: 10.1186/1478-811X-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill DJ, Virji M. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol Microbiol. 2003;48:117–129. doi: 10.1046/j.1365-2958.2003.03433.x. [DOI] [PubMed] [Google Scholar]
- 42.Virji M, Evans D, Griffith J, Hill D, Serino L, Hadfield A, Watt SM. Carcinoembryonic antigens are targeted by diverse strains of typable and non-typable Haemophilus influenzae. Mol Microbiol. 2000;36:784–795. doi: 10.1046/j.1365-2958.2000.01885.x. [DOI] [PubMed] [Google Scholar]
- 43.Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- 44.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 45.Kupfer K. Palmar dislocation of scaphoid and lunate as a unit: case report with special reference to carpal instability and treatment. J Hand Surg Am. 1986;11:130–134. doi: 10.1016/S0363-5023(86)80120-4. [DOI] [PubMed] [Google Scholar]
- 46.Weber CR. Dynamic properties of the tight junction barrier. Ann N Y Acad Sci. 2012;1257:77–84. doi: 10.1111/j.1749-6632.2012.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Q, Cao XF, Xu KZ. One stage resection of esophageal cancer and pulmonary bulla–a report of three patients. Zhonghua Zhong Liu Za Zhi. 1986;8:231–232. [PubMed] [Google Scholar]
- 48.Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10:a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tindemans I, Joosse ME, Samsom JN. Dissecting the heterogeneity in T-cell mediated inflammation in IBD. Cells. 2020;9:110. doi: 10.3390/cells9010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wadwa M, Klopfleisch R, Adamczyk A, Frede A, Pastille E, Mahnke K, Hansen W, Geffers R, Lang KS, Buer J, et al. IL-10 downregulates CXCR3 expression on Th1 cells and interferes with their migration to intestinal inflammatory sites. Mucosal Immunol. 2016;9:1263–1277. doi: 10.1038/mi.2015.132. [DOI] [PubMed] [Google Scholar]
- 51.Westendorf AM, Fleer D, Deppenmeier S, Gruber AD, Bruder D, Hansen W, Liblau R, Buer J. Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific CD8+ T cells. Gastroenterology. 2006;131:510–524. doi: 10.1053/j.gastro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 53.Moller MJ, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer. 1996;65:740–745. doi:. [DOI] [PubMed] [Google Scholar]
- 54.Kammerer R, Hahn S, Singer BB, Luo JS, von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol. 1998;28:3664–3674. doi:. [DOI] [PubMed] [Google Scholar]
- 55.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 56.Roda G, Dahan S, Mezzanotte L, Caponi A, Roth-Walter F, Pinn D, Mayer L. Defect in CEACAM family member expression in Crohn’s disease IECs is regulated by the transcription factor SOX9. Inflamm Bowel Dis. 2009;15:1775–1783. doi: 10.1002/ibd.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Schwartz JC, Guo X, Bhatia S, Cao E, Lorenz M, Zhang Z-Y, Edidin MA, Nathenson SG, Almo SC, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/S1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 58.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. Pillars article: CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. J Immunol 2011; 187:3466-74. doi: 10.1016/1074-7613(94)90071-X. [DOI] [PubMed] [Google Scholar]
- 59.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, van Leerdam ME, van den Heuvel M, Blank CU, van Dieren J, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3:e000278. doi: 10.1136/esmoopen-2017-000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 63.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piancone F, Saresella M, Marventano I, La Rosa F, Caputo D, Mendozzi L, Rovaris M, Clerici M. A deficit of CEACAM-1-expressing T lymphocytes supports inflammation in primary progressive multiple sclerosis. J Immunol. 2019;203:76–83. doi: 10.4049/jimmunol.1801625. [DOI] [PubMed] [Google Scholar]
- 65.Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003;71:5077–5086. doi: 10.1128/IAI.71.9.5077-5086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


