ABSTRACT
There is growing evidence of the interconnectivity between animals, humans, and the environment, which has manifested in the One Health perspective that takes all three into account for a more comprehensive vision of health. Over the past century, agriculture has become increasingly industrialized with a particular rise in the amount of livestock raised and meat produced. In order to fulfill such market demands, livestock farmers and agricultural corporations have artificially selected for and bred their cash animals to be more and more metabolically efficient via genetic and human-driven means. However, by selecting for more metabolically efficient animals, we may have inadvertently been selecting for obesogenic gut microbiota. This is further compounded by the potential obesogenic and microbiome-altering role antibiotics play in livestock. Evidence suggests that there is the potential for interspecies gut microbe transmissibility. It is notable that there has been a concurrent multispecies obesity epidemic across the same timeframe, which raises questions about potential connections between these epidemics. If it is the case that humans have inadvertently influenced their own obesity epidemic via the artificial selection of and antibiotic administration to livestock, then this holds significant ethical implications. This analysis considers current meat consumption trends, the impacts of livestock on climate change, and animal ethics. The paper concludes that due to the potential significant impact yet tenuous nature of the evidence on this subject stemming from research silos, there is a definitive ethical impetus for researchers to bridge these silos to better understand the true nature of the issue. This case is emblematic of an overarching ethics-driven need for deeper collaboration between isolated but related research disciplines to better characterize issues of public health relevance. It also raises concerns regarding inherent value-driven strife that may arise between competing One Health domains.
KEYWORDS: Microbiome, One Health, agriculture, livestock, ethics, obesity, antibiotics
Introduction
Humans have been omnivorous from the time we diverged from the genetic tree as our own species. Since that time, we have created increasingly more efficient ways of cultivating animals for consumption to satiate our carnivorous desires. However, we have found through empirical evidence that meat consumption incurs a multitude of negative effects for human health, animal treatment, and environmental well-being. Yet despite knowledge of these negative consequences, most of humanity continues to increase its meat consumption at an alarming rate. In order to fulfill market demands, livestock farmers and agricultural corporations over the past century have been artificially selecting for and breeding their cash animals to be more and more metabolically efficient through phenotypic selection, mass antibiotic administration, etc. Such metabolic efficiency allows livestock to achieve several times larger yields for the same amount of resource input. However, by artificially selecting for more metabolically efficient animals, we may inadvertently be pushing humanity toward greater metabolic efficiency. The present paper investigates the ethics of livestock artificial selection and antibiotic administration in the context of the obesity epidemic and global climate change from a One Health perspective. The paper also analyzes the role radical uncertainty plays in our appraisal of these hypothetical relationships and describes the ethical relevance research holds in our dealings with radically uncertain scenarios.
The hologenome, livestock, and obesity
Obesity is defined by the CDC as “weight that is higher than what is considered as a healthy weight for a given height,” and is multifactorial.1,2 It is a growing epidemic in the United States and across the world. Per the CDC’s National Center for Health Statistics 2015–2016 data, 71% of adults over 20 y old in the U.S. were living with overweight or obesity and 39.8% with obesity.3 These data represent a 50% increase in prevalences compared to just 20 y ago.4 Obesity has risen across all spectrums of age, gender, and race. Since the 1960s, not only has obesity tripled among adult populations, it has also nearly quadrupled in children and adolescents, as demonstrated by CDC data in Figures 1 and 2.5,6 Per most recent 2017–2018 data from the CDC, the crude prevalence of obesity in the U.S. has continued to increase to 42.5%.3 Further, projections by Ward, et al suggest with high predictive accuracy that by 2030, nearly 50% of adults in the U.S. will have obesity and that 25% will have severe obesity.7
Figure 1.
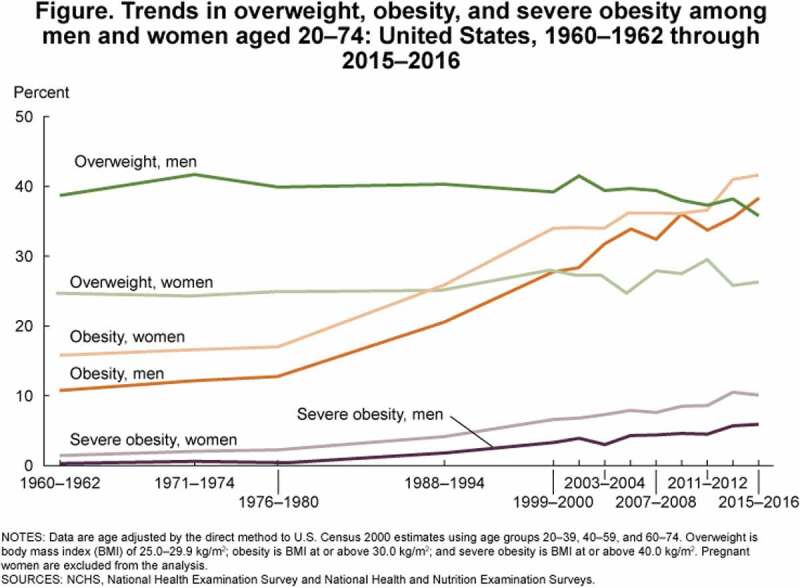
CDC data on overweight, obesity, and severe obesity in adults in the U.S.5
Figure 2.
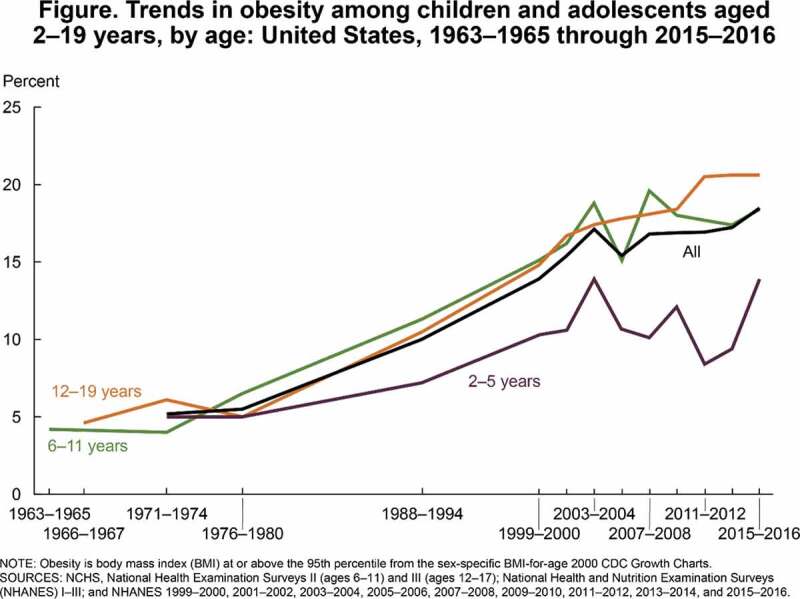
CDC data on overweight, obesity, and severe obesity in children and adolescents in the U.S.
Obesity’s harmful health consequences are striking. It affects the body negatively in a wide variety of ways by increasing the risk of mortality, hypertension, hyperlipidemia, type 2 diabetes, coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, some cancers, poorer quality of life, depression, anxiety, and difficulty with physical functioning.1 As is likely evident, this incurs a tremendous cost in terms of economic impacts from direct and indirect costs. In 2008, the medical care costs of obesity in the United States were 147 USD billion, and the annual nationwide productivity costs related to absenteeism secondary to obesity ranged from 3.38 USD billion to 6.38 USD billion.1 Obesity is, therefore, one of the most critically important health afflictions affecting the U.S. and global populations today.
Obesity is not just limited to humans. There appears to be a concurrent plurality of obesity epidemics across species and across the same time frame, which has raised questions for some researchers about potential connections between these epidemics. One study analyzing historic weight records for 24 animal populations that interact with humans (including domesticated animals, rodents, and primates) showed the same pattern of weight gain as humans over the past century, which cannot be explained by statistical chance alone.8 The symmetry of selection phenomena is important when considering a common thread between convergent phenotypes, as they can emerge independently in different species. One critical selection modality is the human element, as anthropogenic influence is now a salient component of many selection phenomena.9,10 As such, there are a variety of anthropogenic mechanisms through which animal obesity may be influencing human obesity. What accounts for this phenotype diffusion across species? One explanation is that this plurality of obesity across species may be reflective of a shared vulnerability, such as constitutional or genetic changes within gut microbiomes.
The human gut microbiota consists of the trillions of symbiotic microorganisms harbored by an individual, including bacteria, viruses, eukaryotic microbes, and archaea.11 The gut microbiome refers to the collective genomes within these microorganisms.12 They contribute metabolic functions, inform our immune systems, and protect against other pathogens.11 The hologenome is a conceptual construct that considers a host organism and all of its associated symbiotic microbiota (e.g. flora of the gut, skin, mouth, pulmonary tree, genitourinary tracts, etc.) as a single unit. The human genome is comprised of around 23,000 genes, and a human microbiome encodes about 3,000,000 genes, which produce thousands of metabolites that replace important host functions.13 A complex symbiosis between a human body and its microbiome exists, and if this interaction becomes disrupted, it can have detrimental, pathologic effects on both.14 Studies have demonstrated that dysbiosis (i.e. microbial imbalance) between humans and their gut microbiome can hold significant pathologic health impacts in terms of risk of obesity, respiratory diseases, irritable bowel disease, cardiovascular disease, and even mental health and brain disorders.14
In general, microbiota diversity is an approximate surrogate for the health of a microbiome, and lower diversity is considered a marker for dysbiosis.15 However, researchers are finding that the presence or absence of certain key microbes may influence human metabolism and health in specific ways. Further, the bacteria that comprise the gut microbiome have been shown to be transferrable not only from person to person but also zoonotically, lending credence to a One Health perspective regarding gut microbiota.16,17 Per the CDC, One Health is defined as “a collaborative, multisectoral, and transdisciplinary approach with the goal of achieving optimal health outcomes recognizing the interconnection between people, animals, plants, and their shared environment”.18 Taking a hologenome perspective of the human body allows us to conceptually account for both our own genomes and these additional >3,000,000 “foreign” genes whose products influence each other, and which, in tandem, directly or indirectly impact health and well-being.
Over the past 150 y, humans have artificially selected many livestock for their metabolic phenotype, such as cattle and chickens.19,20 For example, a chicken breed that was commercialized in 1957 and another breed from 2005 were raised under the same conditions with the same food. At the conclusion of this experiment, the 2005 chicken breed weighed four times more than the 1957 breed.20 By selecting for obesity in livestock animals, we may have also been inadvertently selecting for gut microbiota that are obesogenic. Similar to the relationship between plants and soil, artificial selection of the gut microbiome can act on animals on short timescales (e.g. lifetimes) and is supported by experimental evidence in pigs, rats, and voles.21 We also have human twin and metagenomic data indicating that there are microbial genes associated with obesity and network-level differences in microbial metabolic genes between lean and obese characteristics.22-25 We have even discovered specific gut microbes that are associated with more obese or leaner individuals, e.g. Christensenella and Akkermansia.26-28 In fact, there are animal experiments demonstrating that a fecal microbiota transplant from an obese animal to a lean animal will trigger the lean animal to become obese.29 This phenomenon has inspired researcher clinicians to begin trials on treating obesity with fecal microbiota transplants from lean individuals.30
Much research has also been conducted looking into the relationship between antibiotics, microbiome dysbiosis, and obesity as well. Approximately 80% of the antibiotics sold in the U.S. are for use in animal agriculture.31 Antibiotics are used to such a wide extent in animal agriculture because farmers have discovered that subtherapeutic continuous dosing of various antibiotics allows their livestock to gain more weight with less food.32 The antibiotics are not administered to animals that are sick; they are provided in the animals’ feed generally in a prophylactic manner without regard to animal health status. It is possible that by administering antibiotics so broadly to livestock animals in the name of metabolic efficiency that we have not only altered their microbiomes but done so in a way that artificially selects for obesogenic gut microbes. There is evidence in murine models that low-dose antibiotic regimens mimicking those in farm use both alter the gut microbiome and increase adiposity and body fat composition changes.33 It has further been posited that antibiotic use in humans may be associated with the development of obesity.34-36 There are several intriguing pathways by which antibiotic administration may lead to gut microbe dysbiosis and ultimately impact the development of obesity.
Around 60% of human pathogens have zoonotic (primarily livestock) origins.37 It is not a far reach to think that gut bacteria are similarly transferrable between animals and humans. In fact, there are studies that support this idea and demonstrate that domesticated animals and humans share gut microbes with one another.16,17 We also have several mechanisms through which livestock gut microbiota can be transferred to humans. First, U.S. livestock animals alone produce 2 billion tons of manure per year, management of which is variably regulated state by state.38 Raw cattle manure is frequently spread over fields harvested for human food, and many farms reuse animal feces as components of feed for their livestock.
Second, cattle gut microbes are also found in food consumed by humans (e.g. as seen in outbreaks of Escherichia coli). The classic One Health example offered by the CDC follows: Cows may graze in a pasture next to a lettuce farm. Cattle may contain guts populated by E. coli but remain asymptomatic. E. coli may be found present in their feces, and their manure may contaminate the nearby lettuce field. Humans may then eat the contaminated lettuce and become infected with E. coli, resulting in morbidity. A similar mechanism may also be at play for obesogenic gut microbes.
Third, it is possible that certain microbes are transmitted through milk as well.39 Further, veterinary antibiotic metabolites associated with adiposity have been found in the urine of Chinese school children.40 County-level usage of veterinary antibiotics has also been demonstrated to overlay with county-level obesity in the U.S.41 Both of these examples from Wang, et al. and Riley, et al. further demonstrate potential transferrable pathways from livestock to humans in addition to other potential obesogens (i.e. substances that cause obesity). Finally, it is quite possible that horizontal gene transfer may be occurring between related animal and human gut bacteria, allowing for increased propensities for and susceptibilities to obesity.
Therefore, there may be hidden harms when a commercially favorable trait such as metabolic efficiency is selected for in livestock. An individual’s genome resides within a hologenome, which in turn resides within the environmental metagenome.42 Even without selecting for an individual’s genome, the genetic milieu a person lives within (i.e. genetic and epigenetic effects) could be augmented by a selection of the microbiome or environmental metagenome, and this selection could impact the individual’s phenotype via transferable mechanisms. If a microbial extended phenotype (e.g. obesity) was selected for artificially in an agricultural species, and that phenotype was then transferred to humans, then the extended phenotype in the human could be obscured partially by epistasis and pleiotropy.43 A conceptual diagram representing the hypothesis of obesogenic gut microbiome transfer between livestock and humans is represented in Figure 3.
Figure 3.
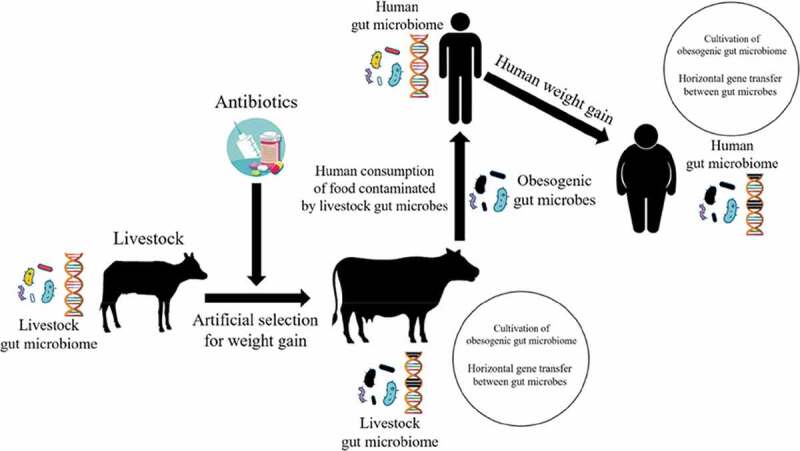
Illustration of the potential genesis of obesogenic gut microbiota in livestock via artificial selection and widespread antibiotic administration along with their likely transmissible pathways to humans, possibly contributing to weight gain.
In effect, humans may have inadvertently contributed to their own obesity epidemic via the artificial selection of metabolically efficient, obese livestock animals and through the transfer of their obesogenic gut microbiota.
Ethical analysis
There have been several articles investigating potential ethical concerns related to the microbiome in terms of research considerations.44,45 However, this ethical analysis will focus on the potential public health implications and extrapolate to wider ethical consequences for research under speculative circumstances.
We are ethically obligated to investigate and mitigate harms to human health, and the argument supporting this follows. We create metabolically efficient livestock animals in order to satiate our meat consumption desires. This artificial selection for metabolically efficient livestock animals may be indirectly linked to obesity in humans. Obesity is an epidemic in the U.S. and globally and is associated with an extensive list of serious and debilitating disease conditions. These diseases, in turn, would be of our own making, and disease is a harm. We have an ethical responsibility to appropriately address and curtail diseases that cause harm and suffering not only on an individual patient basis, but on public health and societal levels as well. Thus, we are ethically obligated to investigate the appropriateness of selecting livestock animals for obesity as it relates to human health.
The newfound information previously outlined concerning the human hologenome and how it interacts with livestock animals and the environmental metagenome add another piece of ethical complexity to the context. A prima facie ethical argument in environmental and food ethics states that we should all decrease our meat consumption because it benefits human health, the environment, and animal welfare.46 It helps humans (bioethics) by improving human health through decreasing cardiovascular disease, colorectal cancer, obesity, and other health maladies associated with meat consumption.40,47,48,49 It helps the environment (environmental ethics) by reducing harmful climate change through decreasing deforestation, reducing methane production, freeing land for conservation, decreasing resources required for livestock, etc. It helps animals (animal ethics) by reducing cruel living conditions, decreasing slaughtering, freeing space for conservation of wild animals, etc.
The benefits of decreased meat consumption at a societal level are ethically obvious and inarguable. However, suppose humans were more inclined to continue their current meat consumption habits rather than curtail it. It is not a difficult leap to assume that those who advocate for significantly decreasing our meat consumption face an incredible amount of inertia. The evidence affirms this, as livestock animals and global meat production have continued to increase rapidly over the past 50 y, with total production having grown four- to five-fold since 1961, as demonstrated in Figures 4 and 5.50
Figure 4.
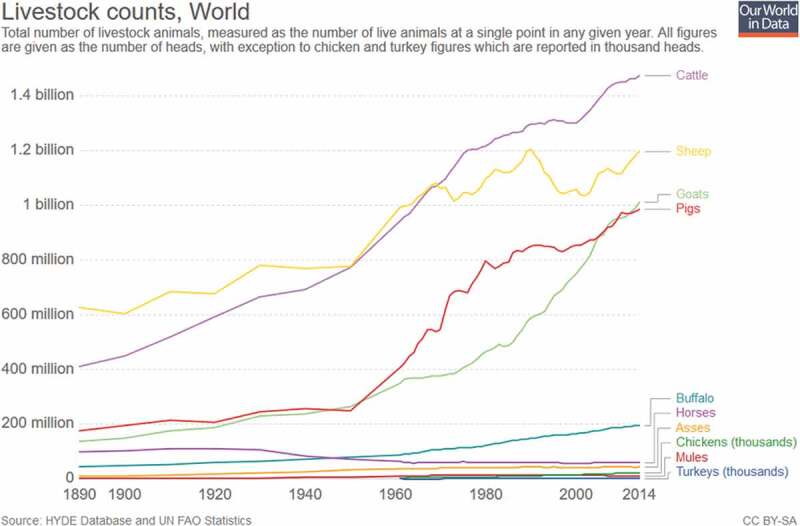
Global livestock counts.50
Figure 5.
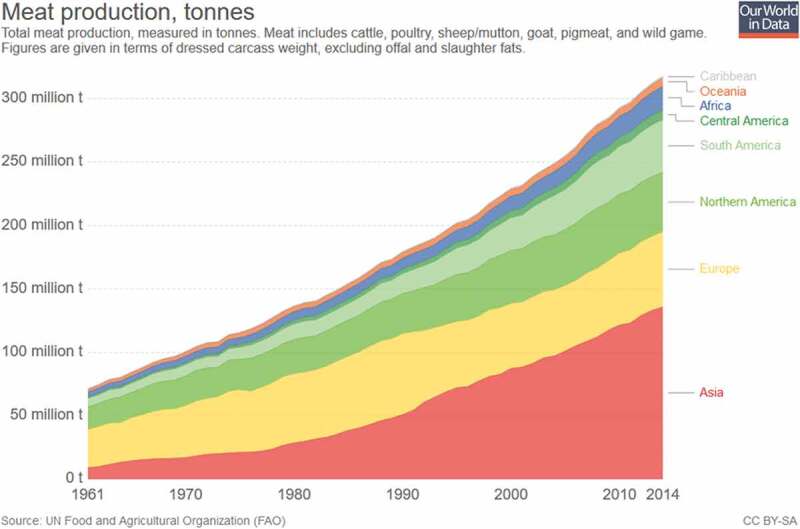
Global meat production.50
This analysis will, therefore, examine the ethics of meat production and consumption in the context of the hologenome interaction based on the assumption that human behavior regarding meat consumption will not change in a meaningful way. Another critical consideration in ethically analyzing this situation is that the world currently faces a looming global catastrophe manifested by climate change, to which livestock animals significantly contribute in a detrimental fashion. The meat produced for consumption by livestock animals leaves a significantly higher greenhouse gas footprint when compared to vegetable-based food options, as shown in Figure 6.50
Figure 6.
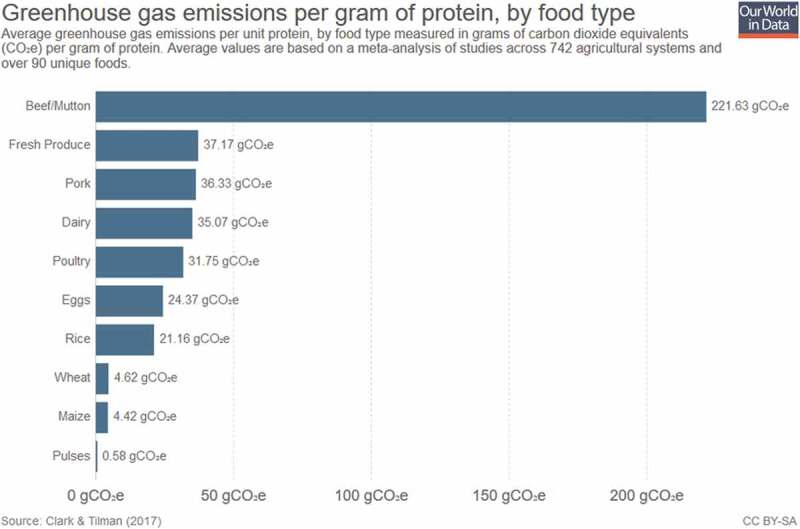
Greenhouse gas emissions by food type.50
By creating more metabolically efficient livestock animals, we create animals that are less harmful to the environment and climate change. We can decrease livestock impacts on climate change by selecting for animals that grow faster and grow larger. The less time to large size, the less time the animals produce a strain on the environment. The greater the efficiency ratio of input to output, the fewer resources and animals required for output. Rather than needing to feed and care for four chickens, a person can raise one chicken with the same meat output. This obesity selection approach requires less land and resource input as well as limiting greenhouse gas emissions compared to a lean animal approach, assuming meat consumption rates remain the same or will continue to increase globally (which data suggests will be the case, as shown in the previous figures). Therefore, the ethical dilemma is that obese animals appear to be a partial solution to feeding the meat demands of the world with climate change-limiting effects, yet the process itself may be playing a role in human obesity, leaving us vulnerable to serious diseases. We are trading one public health disaster for another.
This scenario also reveals an inherent tension within the One Health concept. The ideal for One Health emphasizes that pursuing actions that benefit humans, animals, and the environment is preferable. Though this ideal is strong and ought to be pursued, this scenario reveals that there will inevitably arise situations in which we must make value-based decisions that act more so in the health and well-being interests of one domain more so than another. While the ideal is that we optimize the health and well-being of all three domains, it might not always be possible to do so, and in such cases, competing ethical values will come into conflict with each other. It is therefore evident that the hologenome-livestock-obesity problem is one that holds potentially significant ethical implications.
Implications for research and ethics
This problem also vividly illustrates a case of radical uncertainty. Radical uncertainty is a phenomenon in which there is a considerable amount of ambiguity or a significant lack of clarity regarding the evidence for a situation or relationship. Yet, practically speaking, we must nevertheless decide how we ought to respond when presented with such evidence. Radically uncertain scenarios such as the one illustrated previously can drive our planning and focus for research endeavors. However, it is essential to appropriately weigh the level of threat that a scenario as radically uncertain as this presents. As such, there are two primary considerations in assessing the ethical threat level in cases of radical uncertainty: the strength of the evidence at hand and the potential magnitude of the issue.
First, in assessing the strength of evidence of the problem, there are several key knowledge gaps that would require investigation as to whether the proposed association is in fact robust. One gap is the demonstration of the transmission of livestock gut microbiota to human populations. Though we have tangential evidence of such, as discussed through various metabolite surveys and ecologic-level data, there must be more vigorous data to confirm this transmission pathway. Another key area to define is the role of animal metabolism and whether obesity is truly at play with respect to metabolic efficiency. It could be the case that the two are minimally, if at all, related to each other. Another gap is identifying a more robust catalog of obesogenic microbes in livestock as well as in humans, and then confirming if these same microbes are capable of zoonotic and/or reverse-zoonotic transmission. It would also be worthwhile to demonstrate if horizontal gene transfer between zoonotic gut microbiota would be possible and to document whether obesogenic genes were subjected to this transfer. One must also consider information regarding the epigenetic interactions between a host’s genome and their microbiome to determine if obesogenic microbes selectively impact some individuals or populations more than others. Much more substantial research must be conducted to confirm the hypothetical biologic and transmission mechanisms offered in this proposed scenario, as much of them are based upon related or tangential mechanisms but little direct evidence. Thus, the strength of evidence is weak.
The second step is to determine or estimate the magnitude of impact of the hologenome-livestock-obesity interaction. Given the previous discussion, the potential impacts in responding to this are substantial. We also have extensive data regarding human obesity and its detrimental health effects. However, we are still lacking in knowing just how extensively the gut microbiome impacts human obesity. It would be necessary to conduct further studies regarding specific gut microbiota species and their obesogenic potential. It would also require an estimation of obesogenic gut microbiota on human obesity itself in terms of risk. In addition, one would need to know the number of people such gut microbiota would impact, which would necessitate host gene and gut microbe gene interactions prevalence and details regarding exposure (e.g. geography, intensity, dose-response, population sizes). Without more precise estimates regarding the individual-level impact of gut microbiota on obesity and the populations affected, it is difficult to accurately assess how impactful it is. However, given that obesity is so widespread and causes significant morbidity and mortality, even a small decrease would yield very meaningful results. Therefore, the potential magnitude of impact at this stage is substantial.
Since at this point, the strength of evidence is weak and the potential magnitude of impact is substantial, there is a strong impetus for conducting more research in this area, though it must be done so judiciously. It is still far too early with too great of uncertainty to be seriously considered in discussions about obesity prevention and control in humans. By investing more research into bridging existing research silos that limit our knowledge of this issue, we may not just reveal more about the potential associations between artificial selection and antibiotic use in livestock and obesity in humans, we may reveal other potentially important One Health insights with important public health implications. Further research could also lead to new and innovative research ideas by focusing on bridging research silos. The mere process of developing the bridges between research silos could create new avenues and models for longitudinal research collaboration.
This scenario highlights an issue inherent to emerging technologies and newly developing scientific understandings. It is emblematic of a radically uncertain condition. Our decisions to act are dependent upon the information and data we have available to us to aid us in our decision-making process. By increasing the amount and accuracy of information we have about a radically uncertain issue, (1) we can be more assured that an issue either does or does not warrant further investigation and/or an actionable response, and (2) we will have a greater probability that our decisions and actions will have the desired result (e.g. curtailing the public health problem). Both are important points in terms of distributive justice, as we live in a world of limited resources, whether they be in research (e.g. opportunity costs taking away from more deserving research endeavors, funds, researcher manpower) or in actions (e.g. distractions from more impactful or important topics, resources).
When we discuss the results of our decisions in public health or One Health contexts, this necessarily involves human morbidity, mortality, and quality of life. This inherently denotes ethical implications stemming from our decisions regarding these radically uncertain issues of potential public health importance. For example, if we choose to respond to the hologenome-livestock-obesity problem, then our decisions will potentially impact the lives and well-being of millions of individuals. It is a set of decisions and actions that holds significant ethical weight. Yet this assumes that the problem is true and is a non-insignificant factor in obesity along the causal chain or associative milieu. Should it be the case that we decide to act hastily with the little information before us, we may respond improperly (and thereby waste resources by acting inefficiently and/or ineffectively) or respond when we ought not have (e.g. if it is the case that there is minimal impact from this hologenome-livestock-obesity relationship). Whenever we make a decision, we are making a probability-based calculation based upon available information. Epistemological responsibility in this context, therefore, is an ethical responsibility.
If we can be more certain that our decisions and actions will yield worthwhile and desired results (i.e. improving morbidity, mortality, and quality of life), then we have an ethical obligation to improve the probability (i.e. certainty) that our decisions will yield the desired result. By deciding or acting too hastily with too little information, we are taking unnecessary risks that may manifest unfortunate consequences for those in whom it holds an impact, which would be an unethical decision or action. It is an issue of ethical risk mitigation. By decreasing our uncertainties, we mitigate our risk with respect to the decisions and actions we take, which have real consequences with ethical implications for the well-being of others. We improve the probability of decision-result congruity and appraisal of radically uncertain context gravity by conducting research to better understand the problem at hand. Therefore, we have an ethical obligation to conduct research to better inform our decisions.
One of the primary weaknesses in this hologenome-livestock-obesity concept is the issue of research isolation in silos, which is reflective of an overarching weakness within One Health’s ecosystem of ideas.51 This scenario is emblematic of the necessity of connecting research silos across all domains as an ethical issue in need of correction. We must forge bridges between isolated research silos; by neglecting to draw linkages between different research domains, we retain blind spots in our knowledge base of various topics that may hold tremendous consequences for human health and in the ethical implications for how we address such potential issues.
Another important ethical consideration that arises from radically uncertain problems is that with uncertainty comes fear and conjecture. Some of these fears may be dangerous, unfounded, or even outrageous. As we have seen with the advent and proliferation of social media, it is increasingly facile for erroneous claims to be distributed widely and rapidly. Without appropriately researching concepts that truly warrant further investigation, this leaves the door open for potentially dangerous conjecture without readily available evidence to solidly refute such claims. We must beware of such dubious claims based upon epistemologically shaky information. For example, in the case of the hologenome-livestock-obesity scenario, some individuals might conclude that they ought not to interact with people with obesity or those who consume meat because they “might catch fat” from their gut microbes as one catches a cold. In better understanding worthwhile radically uncertain problems, we may be better equipped to put such troubled ideas to bed.
Conclusions
It should be emphasized that the hologenome-livestock-obesity hypothesis is a radically uncertain proposition. We should approach it with a significant amount of scrutiny. It could be the case that the causal mechanism between livestock animal metabolic efficiency, obesity, and their gut microbes is not accurate or consistent. For example, it may be that there is an obesogenic gut microbe association between animals and humans, but the primary culprit is not actually through the artificial selection of livestock animals. Rather, it could instead be due to the widespread use of antibiotics in livestock which has significantly altered animal gut microbiome compositions and has since transferred to humans with potential obesogenic properties. This thought exercise of the hologenome-livestock-obesity connection is currently fraught and filled with unanswered questions, potential conceptual pitfalls, and holes in evidence at this point in time. However, by performing more research into the area, we could discover interesting and important information regarding the gut microbiome in addition to testing the veracity and impact of the hologenome-livestock-obesity claim.
The overarching takeaways from this paper remain consistent regardless of the veracity of the hypothesis. First, the hologenome-livestock-obesity scenario illustrates potential internal value-based strife that may arise in the One Health concept. Second, the fact that there still remain a considerable number of unknowns regarding the veracity of this concept illustrates several issues related to newly emerging or potential problems of unknown significance. It demonstrates the necessity of performing research to better characterize these radically uncertain concerns based upon an ethical impetus emerging from epistemological responsibility of decision-making. Finally, there is an ethical justification for why we must perform research to substantiate our decision-making processes and why we must be judicious in how we decide which concepts warrant further investigation in considering research as a limited resource. We must continue to pursue research endeavors with outside the box thinking and increase research silo bridging in order to broaden our thought horizons and fill our gaps in knowledge. Ultimately, we must remain vigilant as to how we appraise evidence and how data and information inform our decision-making, particularly as it pertains to public health. When considering the health and well-being of humans and the environment, epistemological responsibility is an ethical responsibility.
Acknowledgements
The author would like to express his sincere gratitude to Dr. Angie Boyce, Dr. Gail Geller, and Dr. Travis Rieder from the Johns Hopkins Berman Institute of Bioethics for their thoughtful guidance in the development of this manuscript. He would also like to thank Dr. Rachel Wright for her invaluable review and edits. Finally, he would like to thank Dr. Jameson Voss for opening the wide world of the gut microbiome to him and inspiring the genesis of this project.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Government disclaimer statement
The contents, views, or opinions expressed in this publication or presentation are those of the author(s) and do not necessarily reflect official policy or position of Uniformed Services University of the Health Sciences, the Department of Defense (DoD), or Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
References
- 1.CDC . Overweight and obesity. Centers for disease control and prevention. 2020. https://www.cdc.gov/obesity/adult/defining.html.
- 2.Dhurandhar EJ, Keith SW.. The aetiology of obesity beyond eating more and exercising less. Best Pract Res Clin Gastroenterol. 2014;28(4):533–13. doi: 10.1016/j.bpg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. Hyattsville (MD): National Center for Health Statistics; 2020. NCHS Data Brief, no. 360. [Google Scholar]
- 4.CDC . Obesity and overweight. National center for health statistics. Centers for Disease Control and Prevention. 2017. https://www.cdc.gov/nchs/fastats/obesity-overweight.htm. [Google Scholar]
- 5.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960-1962 through 2015-2016. National Center for Health Statistics. Centers for Disease Control and Prevention. 2018a. https://www.cdc.gov/nchs/data/hestat/obesity_adult_15_16/obesity_adult_15_16.htm. [Google Scholar]
- 6.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2015-2016. National center for health statistics. Centers for Disease Control and Prevention. 2018b. https://www.cdc.gov/nchs/data/hestat/obesity_child_15_16/obesity_child_15_16.htm [Google Scholar]
- 7.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. state-level prevalence of adult obesity and severe obesity. NEJM. 2019;381(25):2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 8.Klimentidis YC, Beasley TM, Lin HY, Murati G, Glass GE, Guyton M, Allison DB, Jorgensen M, Heymsfield SB, Kemnitz J. Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proc R Soci London B: Biol Sci. 2011;278(1712):1626–1632. doi: 10.1098/rspb.2010.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson JN. Relentless evolution. Chicago (IL): University of Chicago Press; 2013. [Google Scholar]
- 10.Whitham TG, Bailey JK, Schweitzer JA, Shuster SM, Bangert RK, LeRoy CJ, Wooley SC, Allan GJ, DiFazio SP, Potts BM. A framework for community and ecosystem genetics: from genes to ecosystems. Nat Rev Genet. 2006;7(7):510–523. doi: 10.1038/nrg1877. [DOI] [PubMed] [Google Scholar]
- 11.Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2016;31(1):69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faloney G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 14.Scotti E, Boué S, Sasso GL, Zanetti F, Belcastro V, Poussin C, … Hoeng J. Exploring the microbiome in health and disease: implications for toxicology. Toxicol Res Appl. 2017; doi:. [DOI] [Google Scholar]
- 15.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Knight R, Knights D, Clemente JC, Nakielny S. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao L, Estelle J, Kiilerich P, Ramayo-Caldas Y, Xia Z, Feng Q, Wang J, Pedersen AØ, Kjeldsen NJ, Liu C. A reference gene catalogue of the pig gut microbiome. Nat Microbiol. 2016;1(12):16161. doi: 10.1038/nmicrobiol.2016.161. [DOI] [PubMed] [Google Scholar]
- 18.CDC . One health. Centers for disease control and prevention. 2020. Accessed April 4, 2020. https://www.cdc.gov/onehealth/index.html.
- 19.Bovine HapMap C, Gibbs RA, Taylor JF, Van Tassell CP, Barendse W, Eversole KA, Dodds KG, Hamernik DL, Kappes SM, Lien S. Genome-wide survey of SNP variation uncovers the genetic structure of cattle breeds. Science. 2009;324(5926):528–532. doi: 10.1126/science.1167936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuidhof MJ, Schneider BL, Carney VL, Korver DR, Robinson FE. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult Sci. 2014;93(12):2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyte M, Fodor AA, Chapman CD, Martin GG, Perez-Chanona E, Jobin C, Dess NK. Gut microbiota and a selectively bred taste phenotype: a novel model of microbiome-behavior relationships. Psychosom Med. 2016;78(5):610–619. doi: 10.1097/PSY.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 22.Greenblum S, Turnbaugh PJ, Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. PNAS. 2012;109(2):594–599. doi: 10.1073/pnas.1116053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute of Medicine (US) Food Forum . The human microbiome, diet, and health: workshop summary. Washington (DC): National Academies Press (US). 4, Influence of the Microbiome on the Metabolism of Diet and Dietary Components; 2013. https://www.ncbi.nlm.nih.gov/books/NBK154098/. [PubMed] [Google Scholar]
- 24.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Quince C, Faith JJ, McHardy AC, Yatsunenko T, Niazi F, Affourtit J, Egholm M, Henrissat B, Knight R, et al. Organismal, genetic, and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. PNAS. 2010;107(16):7503–7508. doi: 10.1073/pnas.1002355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaumont M, Goodrich JK, Jackson MA, Yet I, Davenport ER, Vieira-Silvas S, Debelius J, Pallister T, Mangino M, Raes J, et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 2016;17(1):189. doi: 10.1186/s13059-016-1052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 29.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marotz CA, Zarrinpar A. Treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J Biol Med. 2016;89:383–388. [PMC free article] [PubMed] [Google Scholar]
- 31.Martin MJ, Thottathil SE, Newman TB. Antibiotics overuse in animal agriculture: A call to action for health care providers. Am J Public Health. 2015;105(12):2409–2410. doi: 10.2105/AJPH.2015.302870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blaser MJ. Stop the killing of beneficial bacteria. Nature. 2011;476(7361):393–394. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 33.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11(3):182–190. doi: 10.1038/nrendo.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furlong M, Deming-Halverson S, Sandler DP, Meyre D. Chronic antibiotic use during adulthood and weight change in the sister study. PLoS One. 2019;14(5):e0216959. doi: 10.1371/journal.pone.0216959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turta O, Rautava S. Antibiotics, obesity and the link to microbes - what are we doing to our children? BMC Med. 2016;14(1):57. doi: 10.1186/s12916-016-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardeh M, Risley C, McIntyre MK, Setzkorn C, Baylis M. Database of host-pathogen and related species interactions, and their global distribution. Sci Data. 2015;2(1):150049. doi: 10.1038/sdata.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Main D (2015). Two numbers: animal manure a growing headache in America. Newsweek Magazine. Accessed April 25, 2019. https://www.newsweek.com/2015/12/18/two-numbers-animal-manure-growing-headache-america-402205.html.
- 39.Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11(8):e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Wang N, Wang B, Fang H, Fu C, Tang C, Jiang Q, Zhou Y, He G, Zhao Q. Antibiotics detected in urines and adipogenesis in school children. Environ Int. 2016;89:204–211. doi: 10.1016/j.envint.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Riley LW, Raphael E, Faerstein E. Obesity in the United States – dysbiosis from exposure to low-dose antibiotics? Frontiers in Public Health. 2013;1:69. doi: 10.3389/fpubh.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theis KR, Dheilly NM, Klassen JL, Brucker RM, Baines JF, Bosch TC, Bordenstein SR, Gilbert SF, Goodnight CJ, Lloyd EA. Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes. mSystems. 2016;1(2):e00028–16. doi: 10.1128/mSystems.00028-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voss JD, Goodson MS, Leon JC. Phenotype diffusion and one health: a proposed framework for investigating the plurality of obesity epidemics across many species. Zoonoses Public Health. 2017;65(3):279–290. doi: 10.1111/zph.12445. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y, Chen H, Lan C, Ren J. Help, hope and hype: ethical considerations of human microbiome research and applications. Protein Cell. 2018;9(5):404–415. doi: 10.1007/s13238-018-0537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhodes R. Ethical issues in microbiome research and medicine. BMC Med. 2016;14(156). doi: 10.1186/s12916-016-0702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickles M. The ethical arguments against eating meat. University of Oxford. 2017. Accessed April 17, 2019. http://www.ox.ac.uk/news/arts-blog/ethical-arguments-against-eating-meat.
- 47.Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016;19(5):893–905. doi: 10.1017/S1368980015002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richi EB, Baumer B, Conrad B, Darioli R, Schmid A, Keller U. Health risks associated with meat consumption: A review of epidemiological studies. Int J Vitam Nutr Res. 2015;85(1–2):70–78. doi: 10.1024/0300-9831/a000224. [DOI] [PubMed] [Google Scholar]
- 49.Rouhani MH, Salehi-Abargouei A, Surkan PJ, Azadbakht L. Is there a relationship between red or processed meat intake and obesity? A systematic review and meta-analysis of observational studies. Obes Rev. 2014;15(9):740–748. doi: 10.1111/obr.12172. [DOI] [PubMed] [Google Scholar]
- 50.Ritchie H, Roser M. (2017). Meat and seafood production and consumption. Our World in Data. Accessed April 21, 2019. https://ourworldindata.org/meat-and-seafood-production-consumption#how-are-land-use-requirements-and-greenhouse-gas-emissions-calculated-for-food-products.
- 51.Manlove KR, Walker JG, Craft ME, Huyvaert KP, Joseph MB, Miller RS, Nol P, Patyk KA, O’Brien D, Walsh DP, et al. ‘One health’ or three? Publication silos among the one health disciplines. PLoS Biol. 2016;14(4):e1002448. doi: 10.1371/journal.pbio.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- CDC . Overweight and obesity. Centers for disease control and prevention. 2020. https://www.cdc.gov/obesity/adult/defining.html.
- CDC . One health. Centers for disease control and prevention. 2020. Accessed April 4, 2020. https://www.cdc.gov/onehealth/index.html.
- Main D (2015). Two numbers: animal manure a growing headache in America. Newsweek Magazine. Accessed April 25, 2019. https://www.newsweek.com/2015/12/18/two-numbers-animal-manure-growing-headache-america-402205.html.
- Pickles M. The ethical arguments against eating meat. University of Oxford. 2017. Accessed April 17, 2019. http://www.ox.ac.uk/news/arts-blog/ethical-arguments-against-eating-meat.
- Ritchie H, Roser M. (2017). Meat and seafood production and consumption. Our World in Data. Accessed April 21, 2019. https://ourworldindata.org/meat-and-seafood-production-consumption#how-are-land-use-requirements-and-greenhouse-gas-emissions-calculated-for-food-products.


