ABSTRACT
Background
Microbial factors are likely to be involved in the recurrence of Crohn’s disease (CD) after bowel resection. We investigated the luminal microbiota before and longitudinally after surgery, in relation to disease recurrence, using 16S metagenomic techniques.
Methods
In the prospective Post-Operative Crohn’s Endoscopic Recurrence (POCER) study, fecal samples were obtained before surgery and 6, 12, and 18 months after surgery from 130 CD patients. Endoscopy was undertaken to detect disease recurrence, defined as Rutgeerts score ≥i2, at 6 months in two-thirds of patients and all patients at 18 months after surgery. The V2 region of the 16S rRNA gene was sequenced using Illumina MiSeq. Cluster analysis was performed at family level, assessing microbiome community differences between patients with and without recurrence.
Results
Six microbial cluster groups were identified. The cluster associated with maintenance of remission was enriched for the Lachnospiraceae family [adjusted OR 0.47 (0.27–0.82), P = .007]. The OTU diversity of Lachnospiraceae within this cluster was significantly greater than in all other clusters. The cluster enriched for Enterobacteriaceae was associated with an increased risk of disease recurrence [adjusted OR 6.35 (1.24–32.44), P = .026]. OTU diversity of Enterobacteriaceae within this cluster was significantly greater than in other clusters.
Conclusions
Luminal bacterial communities are associated with protection from, and the occurrence of, Crohn’s disease recurrence after surgery. Recurrence may relate to a higher abundance of facultatively anaerobic pathobionts from the Enterobacteriaceae family. The ecologic change of depleted Lachnospiraceae, a genus of butyrate-producing bacteria, may permit expansion of Enterobacteriaceae through luminal environmental perturbation.
KEYWORDS: Microbiota, microbiome, Crohn’s disease, surgery, enterobacteriaceae
Introduction
Crohn’s disease (CD) is believed to result from interactions between genetic, immune, microbial, and other environmental factors. Up to 70% of patients with CD require intestinal resection at some time in their life, of whom approximately two-thirds develop recurrence requiring further surgery.1–4 Just as the gut microbiota is the antigenic drive to inflammation in surgery-naïve disease, so it is likely to play a role in disease recurrence after surgery.5–7 While the contribution of the microbiome to CD has been investigated in a number of longitudinal disease cohorts (with and without surgical intervention),8–10 the post-operative setting offers the possibility of evaluating disease longitudinally from the time of active disease to macroscopic normality and then disease recurrence.
The major microbial communities that exist in the bowel are the mucosally associated microbiota and the fecal microbiota (the luminal stream). The abundance of bacterial families differs between these ecosystems, within healthy individuals11,12 and in disease.13 The overall diversity of species is greater in the fecal stream than at the mucosal surface, while the mucosally associated microbiota is relatively enriched for the Proteobacteria and Bacteroidetes phyla.14 Metagenomic studies of both the mucosal and luminal gut microbiome in Crohn’s disease have shown a decrease in the presence and diversity of Firmicutes (including the obligate anaerobes from the Clostridia class) and an increase in Gammaproteobacteria (particularly the facultatively anaerobic Enterobacteriaceae).8,15-17 In relation to surgery, Faecalibacterium prausnitzii is in low abundance in the ileal mucosa at the time of surgery,18 and luminal Streptococcus increases in its abundance from the time of surgery to 6 months after surgery, and is associated with disease recurrence.10
The process of luminal gut surgery is associated with an altered microbiome, not only in CD but also in relation to surgery for gastrointestinal cancer or small bowel transplant.5,6,19-21 These changes occur most likely as a result of structural alterations to gut continuity,22 preexisting and post-operative inflammation,23,24 and antibiotics22 administered in the immediate post-operative period.21 The local environment of the margins of resected bowel also changes, particularly in relation to oxygen exposure,25,26 and peritoneal pH related to CO2 insufflation during laparoscopic surgery.27,28 When differential bacterial abundance between post-operative and non-operative patients has been assessed in stool or tissue, significant relative reductions in the Faecalibacterium genus, the Lachnospiraceae family, taxa from the Ruminococcaceae family (including the Ruminococcus genus) and the Clostridiales order were observed.9,29 However, large-scale longitudinal surveys of the post-operative luminal microbiome have not been undertaken.
We have previously assessed the mucosa-associated microbiota in 34 CD patients from the Post-operative Crohn’s Endoscopic Recurrence (POCER) study.6,30 A low ileal abundance of Faecalibacterium prausnitzii (<0.1% 16S relative abundance; OR 14 [1.7–110], P = .013) and a detectable abundance of Proteus species (from the Enterobacteriaceae family; OR 13 [1.1 − 150], P = .039) were strongly associated with endoscopic disease recurrence.6
The luminal environment is likely to play a key role in the homeostatic balance between the luminal and mucosal compartments. Given the absence of data pertaining to the luminal environment after surgery, we have undertaken a large prospective study of the fecal microbiota of 130 CD patients following bowel resection and investigated its association with active disease.
Methods
Clinical characteristics
The POCER study was a prospective, multicenter, randomized controlled trial on post-operative strategies to reduce Crohn’s disease recurrence.30 The POCER study design is outlined in Figure 1, with patients stratified high or low risk on the basis of ≥1 known risk factors (smoking, previous surgery, penetrating disease). Patients’ disease phenotype was recorded at baseline using the Montreal Classification.31 Following surgical resection of macroscopic active Crohn’s disease, patients were randomly allocated (2:1) to active care or standard care. Patients in the active arm had a colonoscopy at 6 months; patients who had endoscopic recurrence (Rutgeerts score4 ≥i2) at 6 months stepped-up treatment according to their baseline therapy, to thiopurine, fortnightly adalimumab with thiopurine, or weekly adalimumab. Patients in the standard care arm did not have a colonoscopy at 6 months. Patients in both arms of the study had a colonoscopy at 18 months.
Figure 1.
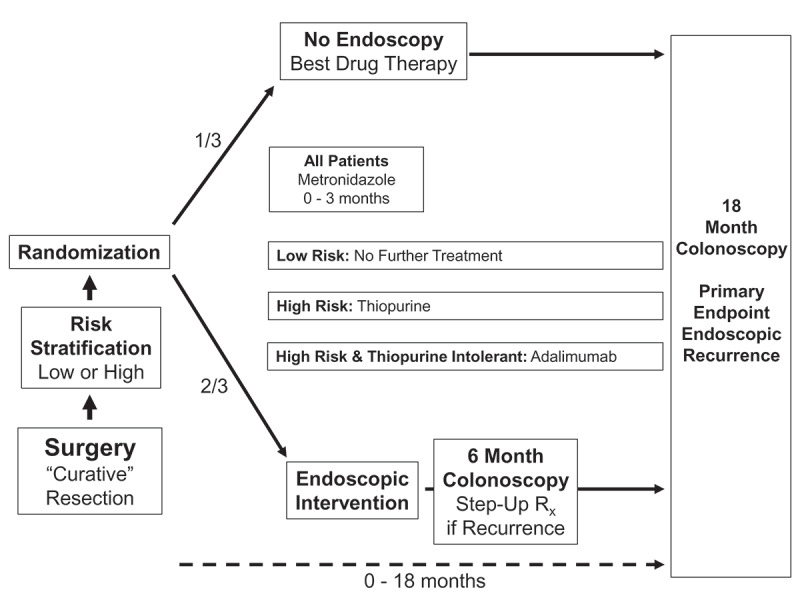
POCER study design.
Medication use, the Crohn’s Disease Activity Index32 and symptoms were recorded at each major visit. All patients were prescribed 3 months of metronidazole (400 mg twice per day) from the time of surgery; 77% (100/130 patients) of patients tolerated the dose (defined as ≥80% compliance); adherence to this dose was used as an adjusting factor in all analyses.
Study and ethical approval
The Post-Operative Crohn’s Endoscopic Recurrence (POCER) Study (Clinical Trial Registration: NCT00989560) was a multicenter, randomized controlled trial conducted in 17 clinical centers. It established the benefit of surveillance and treatment strategies for the prevention of post-operative Crohn’s disease recurrence.30,33
Ethical approval was obtained from the Human Research Ethics Committees of St Vincent’s Hospital, Melbourne, Australia, and all other participating hospitals.
Fecal sample collection and DNA extraction
A total of 136 patients provided 314 fecal samples. Fecal samples were collected up to 2 weeks before surgery, and at 6, 12, and 18 months post-operatively. Samples were collected prior to the administration of bowel preparation, frozen initially at −20°C for transport, then stored at −80°C until analysis.
DNA was extracted from 100 mg of homogenized stool sample with mechanical disruption (bead beating) using the MoBio Powersoil DNA extraction Kit (Mo Bio Laboratories, Carlsbad, USA) as per manufacturer’s instructions.
PCR for 16 Sribosomal gene amplification
The bacterial 16S V2 region was PCR amplified with universal bacterial 16S primers F101, and 342R34,35 and barcoded with Illumina index/adaptors using the Expand High Fidelity PCR kit (Roche). PCR products were purified with the Wizard SV gel and PCR clean-up system (Promega, Madison, USA). The DNA was quantitated (NanoDrop, Thermo Scientific, Waltham, USA) and sequenced by the Australian Genome Research Facility (AGRF) using the Illumina Miseq platform producing 250 bp paired-end reads.
Bioinformatic and statistical analysis
All forward and reverse reads were trimmed to the first 200 bp using FASTX-Toolkit Version 0.0.14 (http://hannonlab.cshl.edu/fastx_toolkit/) and merged using FLASH Version 1.2.11.36 Merged reads were then quality filtered (allowed n’s = 1, ≤3 low-quality bp allowed before trimming) in Qiime version 1.91.37 Closed reference operational taxonomic unit (OTU) picking was performed for QC and sample selection. Technical issues affected the merge percentage of 23 samples from 1 of 4 sequencing plates; in this case, the R1 primer was clipped using FASTX-Toolkit Version 0.0.14 as above, and only the forward reads were used. A further quality threshold was set, including only samples with a merge percentage of >90% and at least 15,000 reads per sample prior to merging, leaving 288 of 314 samples from 130 patients for subsequent analysis. Sequencing plate was further included as a covariate in relevant statistical analyses. Chimeric reads were identified using the USEARCH Version 6.1,38 referenced against the rdp_Gold database and ~7% of reads were removed. The open reference method and the Greengenes 97% OTU reference set, version 13_839 was used to assign OTUs. Reads with less than 60% sequence identity to the Greengenes reference set were removed.
Taxonomy was assigned using the uclust consensus taxonomy assigner, and relative abundance calculated at family and genus level.38 An average of 78,095 reads per sample (range 10,701–665,671) were assigned to OTUs.
In total, 10,000 read subsamples were rarefied prior to diversity analysis. Alpha diversity was calculated using number of OTU’s, Shannon’s Diversity Index (SDI), and Chao1 Diversity Index. The Wilcoxon rank sum test or Kruskal–Wallis rank sum test (where there were more than two groups) was used to compare alpha diversity metrics.
Beta diversity was assessed by (i) presence or absence of the full range of OTUs – unweighted UniFrac, and (ii) composition and abundance – weighted UniFrac. Beta diversity comparisons were assessed using the adonis package in R, a non-parametric multivariate ANOVA on both the weighted and unweighted UniFrac distance matrices.40,41
Taxonomic analysis
Differential abundance of OTUs at family, genus, and species levels were assessed on cumulative sum scaled (CSS normalization) OTU tables by zero-inflated Gaussian (ZIG) modeling, using the MetagenomeSeq package in R.42 This two-step analysis method firstly uses raw counts to calculate a scaling factor (the sum of counts up to a quantile, optimal scaling factor for our data = 0.61), then analyzes the normalized count data using a zero-inflated Gaussian distribution mixture model, which accounts for under-sampling bias and allows inclusion of confounding factors.42 Taxa were excluded if not present in at least 20% of samples for each comparison. All taxonomic assessments were adjusted for age, gender, smoking status (except where smoking was the primary variable), metronidazole tolerance, body mass index (BMI), sequencing plate, and hospital of origin. The change of abundance is given as a positive (increase) or negative (decrease) log2 fold difference (LFD). To account for multiple comparison OTUs with an unadjusted P value of <0.05 were false-discovery rate adjusted (FDR, Benjamini & Hochberg method), and were then reported as significant for FDR P values <.05.
Cluster analysis
In addition to taxonomic individual family-level assessment, cluster analysis at family level was also performed to identify patterns of change associated with disease recurrence.
We performed hierarchical clustering (1-Pearson’s correlation, Ward’s minimum variance using function hclust in R) on the relative abundances of 23 common family level OTUs (comprising at least 10% relative abundance in one or more samples) across all samples. This accounted for 99.5% of all reads. We chose the number of clusters that maximized the average silhouette score. Each cluster was assessed for recurrence at 18 months using generalized estimating equations (GEE) adjusted for sample time point, smoking status, BMI, metronidazole use, age, gender, and sequencing plate. To account for multiple comparisons, P Values were further false-discovery rate adjusted (FDR, Benjamini & Hochberg method), and were then reported as significant for FDR P values <.05. Clustering summarizes the differences in the population structure of the total microbial community.
Baseline, 6, and 18 month samples were included in the comparisons for (i) predicting subsequent endoscopic recurrence at six and 18 months, and (ii) cross-sectional outcomes at each time-point.
Assessment of endoscopic disease recurrence
Anastomotic and ileal disease recurrence at colonoscopy was assessed using the Rutgeerts score,4,43 b4y the consensus of two investigators (PDC, MAK) blinded to patients’ treatment. Remission was defined by either mucosal normality (Rutgeerts i0) or ≤5 aphthous ulcers (i1), and recurrence defined as i2 (>5 aphthous ulcers or larger anastomotic lesions), i3 (diffuse ileitis) or i4 (large ulcers, narrowing or diffuse inflammation).4,43 Severe recurrence was defined as Rutgeerts i3 or i4.
Results
This studied cohort comprised 130 patients (44% male; median age 36) undergoing a bowel resection for Crohn’s Disease. Most patients (82%) were stratified as high risk for recurrence. The most common indication for surgery was a perforation (48%), with the majority of patients having a penetrating phenotype (53% B3 Montreal Phenotype). The most common surgery was an ileocecal resection (78%), followed by a small bowel resection (14%, with or without ileocecal resection). Further patient demographics and baseline cohort characteristics are shown in Table 1.
Table 1.
Demographics of the patient cohort – fecal microbiota studies.
| Demographics | ||
|---|---|---|
| n | % | |
| n [male] | 57 | 43.85 |
| Age, median | 36 | |
| Interquartile range [IQR] | (26–46.75) | |
| Active smoker | 38 | 29.23 |
| Body mass index, median | 23.58 | |
| Interquartile range [IQR] | (20.59–28.11) | |
| Age at diagnosis: | ||
| A1 ≤ 16 y | 16 | 12.31 |
| A2 17–40 | 94 | 72.31 |
| A3 > 40 | 20 | 15.38 |
| Duration of Crohn’s disease (years): | ||
| Median Duration | 10 | |
| Interquartile range [IQR] | (4–15) | |
| ≥10 y | 66 | 50.77 |
| Disease location at surgery: | ||
| L1 Ileum only | 69 | 53.08 |
| L2 Colon only | 9 | 6.92 |
| L3 Ileum and colon | 52 | 40.00 |
| L4 Upper GI | 7 | 5.38 |
| Disease phenotype at surgery: | ||
| B1 Inflammatory | 12 | 9.23 |
| B2 Stricturing | 49 | 37.69 |
| B3 Penetrating | 69 | 53.08 |
| P Perianal Disease | 16 | 12.31 |
| Indication for surgery: | ||
| Failure of drug therapy | 29 | 22.31 |
| Obstruction | 38 | 29.23 |
| Perforation | 63 | 48.46 |
| Type of surgical resection: | ||
| Ileocolic resection | 102 | 78.46 |
| Small bowel resection | 7 | 5.38 |
| Subtotal colectomy | 6 | 4.62 |
| Simultaneous ileocolic resection and colectomy | 4 | 3.08 |
| Simultaneous ileocolic and isolated small bowel resection | 11 | 8.46 |
| Number of previous surgical resection(s): | ||
| 0 | 91 | 70.00 |
| 1 | 28 | 21.54 |
| 2 | 6 | 4.62 |
| 3 or more | 5 | 3.85 |
| Immediate postoperative baseline drug therapy: | ||
| Metronidazole alone | 23 | 17.69 |
| Thiopurine | 72 | 55.38 |
| Adalimumab | 35 | 26.92 |
| 6-month endoscopic outcomes (n = 92): | ||
| Remission (Rutgeerts i0–i1) | 60 | 65.22 |
| Recurrence (Rutgeerts i2–i4) | 32 | 34.78 |
| 18-month endoscopic outcomes (n= 105): | ||
| Remission (Rutgeerts i0–i1) | 60 | 57.14 |
| Recurrence (Rutgeerts i2–i4) | 45 | 42.86 |
Ninety-two patients were randomized the active care arm and underwent a colonoscopy at 6 months; while all patients (n = 130) underwent a colonoscopy at 18 months post-operatively. At colonoscopy patients were assessed for endoscopic disease recurrence.
Of the 288 fecal samples from 130 patients, 61 were obtained immediately pre-operatively (baseline), 86 at 6 months, 80 at 12 months, and 61 at 18 months.
Of 130 patients with fecal microbiota results, 35 patients provided 1 stool sample at any time point, 46 patients samples at 2 time points, 35 patients at 3 time points, and 14 patients at all 4 time points (baseline, 6 months, 12 months, and 18 months). Only the 6 month and 18 month stool samples had time-matched endoscopic outcomes. Matched outcomes were available for 60 of 86 6-month samples, of which 21 (35%) had endoscopic recurrence, and for all of the 18 month samples, of which 20 (33%) had endoscopic recurrence. The definition of endoscopic recurrence at 6 or 18 months related to the endoscopic finding at each of those time points.
Taxonomic analysis at individual species and genus level showed heterogeneous and inconsistent log fold differences across all timepoints. Family level clustering on all samples revealed distinct clusters associated with high silhouette values, that had significant associations with recurrence at 18 months regardless of the timepoint at which samples were obtained. Hence, for consistency, analysis at family level only is reported.
Hierarchical clustering of the relative abundance of major bacterial families assigned each sample to one of six distinct groups. Samples within each cluster group were enriched for different bacterial families including Lachnospiraceae, Bacteroidaceae, Veillonellaceae, Enterobacteriaceae, Prevotellaceae and a mixed profile (Figure 4a).
Figure 4.
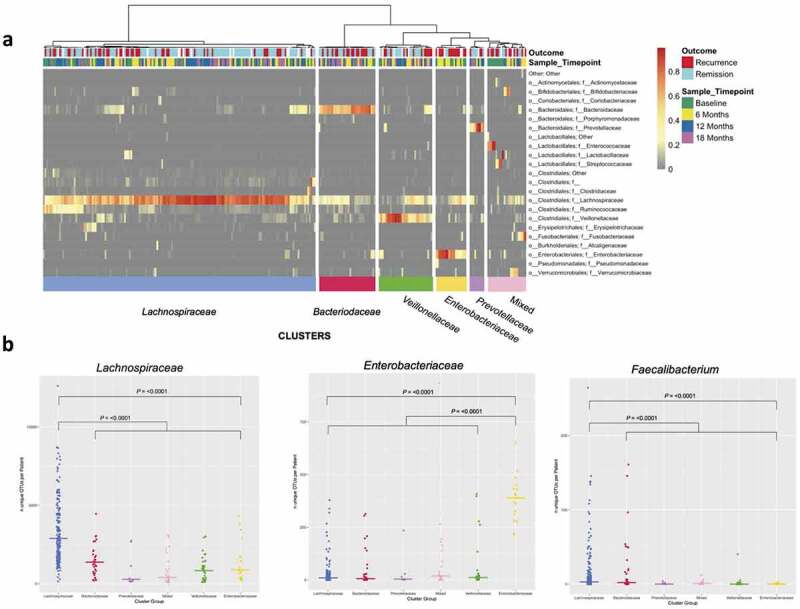
Panela: Hierarchical cluster analysis showing samples partitioning into six major groups based on relative abundance at family level. Annotations below the dendrogram are outcome at 18 months (Recurrence ≥ Rutgeerts i2, Remission ≤ Rutgeerts i1) and sample timepoint. Color bars below the panel denote the cluster group, named by the respective dominating bacterial family. Panel b: Number of unique OTUs within each cluster (x-axis) for Lachnospiraceae OTUs (left) and Enterobacteriaceae OTUs (middle) at family level, and Faecalibacterium OTUs at genus level (right). Bar represents median number of OTUs per cluster group.
The pre-operative microbiome and its association with patient demographics
Among the 61 baseline samples, we assessed for differences in alpha and beta diversity with patient smoking status and disease phenotype.
While no alpha diversity comparisons passed FDR-adjustment for multiple testing, nominally significant differences (P < .05) at baseline were observed for disease location, where ileal disease was associated with higher Shannon Diversity Index (SDI) than ileo-colonic or colonic disease, and for prior surgery, which was associated with a lower SDI. Alpha diversity results are shown in Table 2. Similar results were found for number of OTUs and Chao1 index (results not shown).
Table 2.
Alpha diversity comparisons between groups based on demographics and outcomes. SDI (Shannon Diversity Index), Wilcoxon rank sum test, or Kruskal–Wallis rank sum test. Top panel: Baseline demographic comparisons with three outcomes, Bottom Panel: Baseline demographic comparisons and metronidazole tolerance with two outcomes, and endoscopic outcomes at 6 and 18 months. Significant P values are shown in bold.
| Comparison | Timepoint | Group 1 | n | Median (IQR) | Group 2 | n | Median (IQR) | Group 3 | n | Median (IQR) | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoking | Baseline | Current | 13 | 4.75 (4.16–6.38) | Past | 18 | 5.54 (3.03–6.46) | Never | 30 | 5.20 (3.48–6.93) | 0.899 | ||||||||
| Disease Location | Baseline | Ileal | 30 | 6.10 (4.35–7.19) | Colonic | 4 | 4.13 (1.74–6.79) | Ileocolonic | 27 | 4.73 (2.36–5.99) | 0.032 | ||||||||
| Disease Behavior |
Baseline |
Inflammatory |
8 |
5.51 (5.04–6.52) |
Stricturing |
23 |
5.17 (3.56–6.42) |
Penetrating |
30 |
5.10 (3.13–6.62) |
0.693 |
||||||||
|
Comparison |
Timepoint |
Group 1 |
n |
Median (IQR) |
Group 2 |
n |
Median (IQR) |
P |
|||||||||||
| Prior Surgery | Baseline | No | 42 | 5.79 (3.80–6.88) | Yes | 19 | 4.73 (2.80–5.76) | 0.050 | |||||||||||
| Metronidazole Tolerance | 6 months | Tolerated | 66 | 5.24 | Not Tolerated | 20 | 5.14 | 0.679 | |||||||||||
| 6 Month Recurrence | 6 months | Remission | 40 | 5.27 (3.19–5.92) | Recurrence | 22 | 4.98 (3.74–6.66) | 0.256 | |||||||||||
| 18 Month Recurrence | 6 months | Remission | 42 | 5.69 (4.08–6.36) | Recurrence | 30 | 4.44 (3.14–5.67) | 0.041 | |||||||||||
| 18 Month Recurrence | 18 months | Remission | 41 | 6.10 (5.13–7.02) | Recurrence | 20 | 5.94 (3.81–6.63) | 0.186 | |||||||||||
Unweighted UniFrac, but not weighted UniFrac, showed FDR-significant differences according to smoking status (Table 3, all samples; current smoker vs past smoker vs never smoked, unweighted, Adonis FDR P = .009), and according to disease location (FDR P = .020).
Table 3.
Beta diversity outcomes – weighted and unweighted UniFrac Index, with significance using the adonis function; a permutational multivariate analysis of variance (MANOVA) performed on distance matrices. FDR significant P values are shown in bold.
| Weighted UniFrac |
Unweighted UniFrac |
|||||
|---|---|---|---|---|---|---|
| Beta Diversity | R2 | P Value | FDR P Value | R2 | P Value | FDR P Value |
| Baseline Characteristics | ||||||
| By Smoking Status (Current vs. Past vs. Never Smokers) | ||||||
| All Samples | 0.003 | 0.513 | 0.763 | 0.006 | 0.001 | 0.009 |
| Baseline | 0.012 | 0.644 | 0.763 | 0.017 | 0.395 | 0.594 |
| 6 Months | 0.015 | 0.27 | 0.598 | 0.017 | 0.009 | 0.033 |
| 18 Months | 0.008 | 0.811 | 0.843 | 0.017 | 0.398 | 0.594 |
| By Disease Location (Ileal, Ileocolonic and Colonic) | ||||||
| All Samples | 0.010 | 0.13 | 0.483 | 0.010 | 0.003 | 0.020 |
| Baseline | 0.050 | 0.084 | 0.437 | 0.041 | 0.021 | 0.068 |
| 6 Months | 0.017 | 0.749 | 0.843 | 0.023 | 0.445 | 0.609 |
| 18 Months | 0.027 | 0.557 | 0.763 | 0.036 | 0.097 | 0.229 |
| Remission vs. Recurrence | ||||||
| Baseline samples for 6 month outcomes | 0.013 | 0.792 | 0.843 | 0.019 | 0.943 | 0.957 |
| 6 Month samples for 6 month outcomes | 0.020 | 0.264 | 0.598 | 0.015 | 0.684 | 0.756 |
| Baseline samples for 18 month outcomes | 0.016 | 0.595 | 0.763 | 0.019 | 0.698 | 0.756 |
| 6 Month samples for 18 month outcomes | 0.014 | 0.372 | 0.722 | 0.016 | 0.091 | 0.229 |
| 18 Month samples for 18 month outcomes | 0.074 | 0.008 | 0.069 | 0.019 | 0.111 | 0.241 |
| Mucosal Normality (i0) vs. Severe Recurrence (i3/4) at 6 months | ||||||
| All Samples | 0.013 | 0.389 | 0.722 | 0.014 | 0.474 | 0.616 |
| Baseline | 0.085 | 0.212 | 0.598 | 0.060 | 0.553 | 0.685 |
| 6 Months | 0.041 | 0.446 | 0.763 | 0.046 | 0.208 | 0.386 |
| Mucosal Normality (i0) vs. Severe Recurrence (i3/4) at 18 months | ||||||
| All Samples | 0.013 | 0.228 | 0.598 | 0.015 | 0.006 | 0.026 |
| Baseline | 0.037 | 0.646 | 0.763 | 0.063 | 0.25 | 0.433 |
| 6 Months | 0.031 | 0.561 | 0.763 | 0.034 | 0.632 | 0.747 |
| 18 Months | 0.060 | 0.276 | 0.598 | 0.053 | 0.204 | 0.386 |
The cluster groups (Figure 4a) were assessed for association with baseline patient phenotype according to Montreal Classification,31 as well as other baseline characteristics. The mixed cluster group was associated with the penetrating disease phenotype (Montreal B3) at baseline (OR 15; 95%CI 1.81–671.98, P = .002). Baseline samples assigned to the mixed cluster group were enriched for facultative anaerobic and oxygen-tolerant bacterial families (Enterococcaceae, Lactobacillaceae, and Streptococcaceae), likely reflecting active inflammation (Figure 4a). The Lachnospiraceae cluster was associated with lower rates of previous surgery (baseline; no prior surgery vs. one or more previous operations; OR 0.23, 95%CI 0.06–0.84, P = .022). No cluster group was associated with smoking status.
The fecal microbiota at the time of surgery was not predictive of endoscopic disease recurrence at 6 or 18 months.
The early post-operative period
At 6 months post-operatively there was no significant difference in alpha diversity seen between patients who did or did not take the prescribed dose of post-operative metronidazole.
There were no differences in baseline alpha diversity between patients in remission vs recurrence, or between mucosal normality (i0) vs severe recurrence (i3/4), at the 6 month colonoscopy.
When the 6 month fecal samples were assessed based on the 6 month endoscopic outcome there were also no differences in alpha diversity (remission vs recurrence, mucosal normality vs severe recurrence). However, alpha diversity at 6 months in patients who subsequently developed endoscopic recurrence at 18 months was lower than in those with subsequent remission (median SDI 4.44 vs 5.69; P = .041).
There were also no significant differences at any subsequent time-point in beta diversity (composition) between patients based on metronidazole treatment.
The differential abundance of taxa at family level for patients with endoscopic recurrence compared to remission are shown in Figure 3. At 6 months, these bacteria were more abundant in patients with disease recurrence: Odoribacteraceae (FDR P = .0001), Barnesiellaceae (FDR P = .001), and Mogibacteriaceae (FDR P = .001); whereas Peptostreptococcaceae (FDR P = .036) was reduced relative to those in remission.
Figure 3.
Cumulative Sum Scaled (CSS) normalized relative count distributions for endoscopic remission and recurrence (left of each figure), and Log2 Fold Difference (right of each figure) of statistically significant taxa at family level for patients with disease recurrence at each endoscopically assessed time point (6 and 18 months). Zero-inflated Gaussian modeling on cumulative sum scaled and normalized data at OTU level, further aggregated at family level, adjusted for age, gender, smoking status, metronidazole tolerance, body mass index, sequencing plate, hospital of origin, and sample timepoint where appropriate. Families that met FDR P Value threshold are shown in purple. Only taxa that met P ≤ 0.05 are reported, FDR Adjusted P Values in are adjusted for number of comparisons.
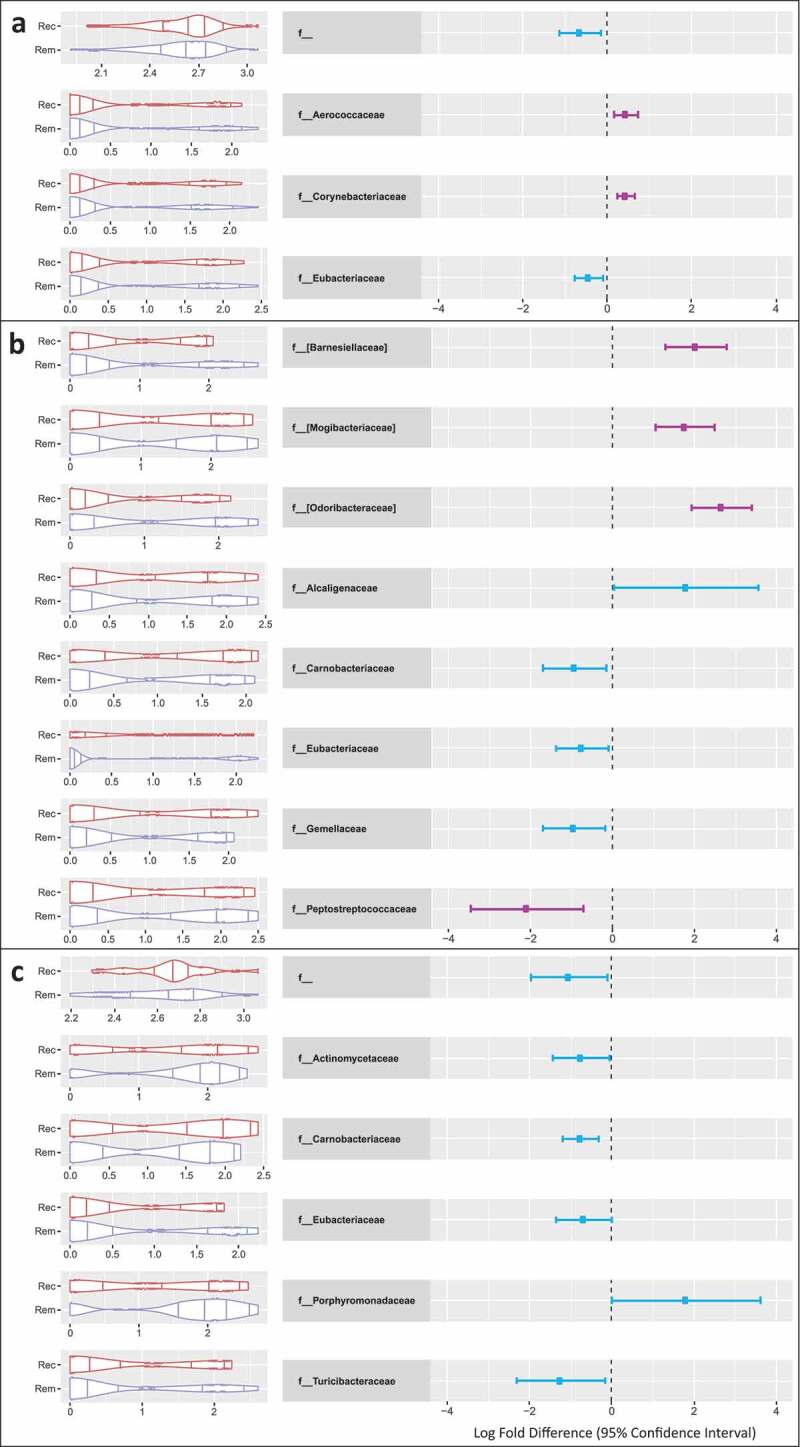
Panel A: Significant taxonomic findings at family level at all time points in relation to 18 month endoscopic outcome. Panel B: Significant taxonomic findings at family level at 6 months in relation to endoscopic findings at 6 months. Panel C: Significant taxonomic findings at family level at 18 months in relation to endoscopic findings at 18 months.
Rem: Endoscopic Remission (Rutgeerts score i0-1), Rec: Endoscopic Recurrence (Rutgeerts score ≥i2).
To address the changing bacterial population over time, we addressed whether certain clusters were associated with particular time points (Figure 4a). The Enterobacteriaceae cluster was enriched for samples in the early post-operative period (baseline and 6-month vs. 12 and 18 month samples; Fisher’s exact P = .0005).
The late post-operative period
Alpha diversity increased from the time of surgery to 18 months post-operatively (Figure 2; median SDI 5.23 vs 6.10; paired Wilcoxon test, P = .048); with the greatest increase in diversity occurring between 12 and 18 months post-operatively (median SDI 5.26 vs 6.10; P = .009), but no significant difference between baseline and 12 months.
Figure 2.
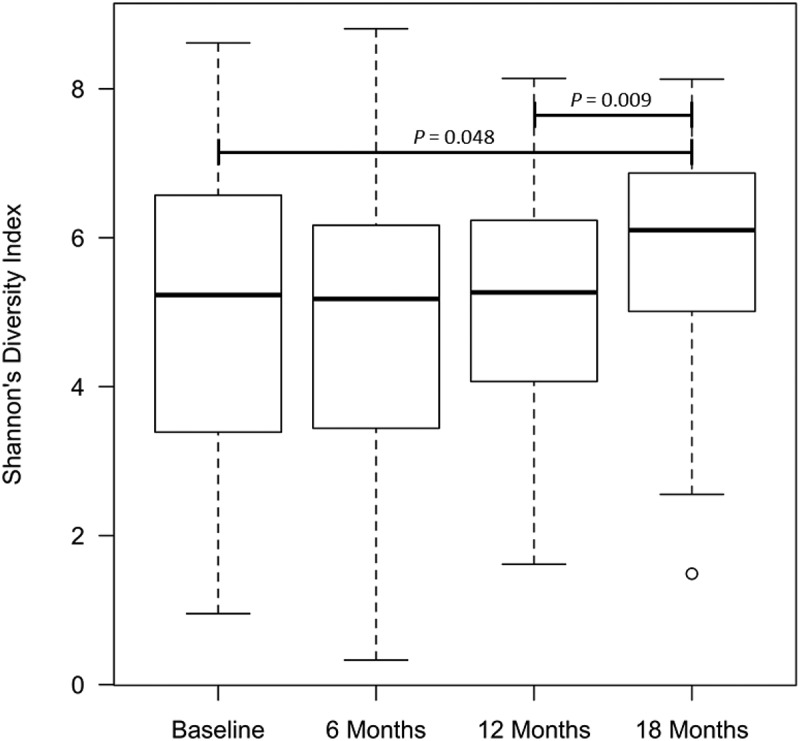
Alpha diversity for the whole patient cohort over time as measured by the Shannon’s Diversity index, using Kruskal–Wallis test for overall comparison of time points, and Wilcoxon Rank sum test between individual time points.
At 18 months alpha diversity did not differ between recurrence and remission (median SDI 5.94 vs 6.10; P = .186; Table 2). Further, there were no differences in alpha diversity when comparing extremes in outcome (mucosal normality – i0 vs. severe recurrence – i3/4) among baseline samples for 18 month endoscopic outcome.
Beta diversity differed significantly between baseline and 18 months, but the magnitude of the difference was small and accounted for only a small amount of overall variation (unweighted UniFrac R2 0.021, FDR P = .009 and weighted UniFrac R2 0.069, FDR P = .013). Weighted UniFrac analysis was not different at FDR significance between patients in endoscopic remission and those with recurrence at any time-point (Table 3). Samples within patients were significantly more similar than between patients (weighted UniFrac P < .001; unweighted UniFrac P < .001).
When combing baseline, 6 and 18 month time point samples, Corynebacteriaceae (FDR P = .001), and Aerococcaceae (FDR P = .034) were significantly increased in patients with disease recurrence at 18 months (Figure 3).
The Lachnospiraceae cluster was associated with a reduced risk of recurrence at 18 months (Table 4; adjusted odds ratio 0.47, 95%CI 0.27–0.82; P = .007, FDR P = .042). Conversely, the Enterobacteriaceae cluster was associated with an increased risk of recurrence at 18 months (adjusted odds ratio 6.35, 95%CI 1.24–32.44; P = .026, FDR P = .078). There was no significant association of any cluster with outcome (recurrence or remission) at 6 months.
Table 4.
Odds ratios based on hierarchical clustering groups at family level using generalized estimating equations. Clusters are colored by cluster assignment (color bars; Figure 4a).
 |
The predominant families identified in the cluster analysis did not show a significant log fold difference in the taxonomic analysis (all time point samples for 18 month outcomes: Lachnospiraceae LFD 0.07; FDR P = .825, Enterobacteriaceae LFD 0.34; FDR P = .685) suggesting that the average abundance of these taxa was not different between outcome but the number of samples at the extreme end of the distribution of these taxa (i.e. dominated by these taxa) was significantly different between patients in remission versus those with endoscopic recurrence (Figure 5).
Figure 5.
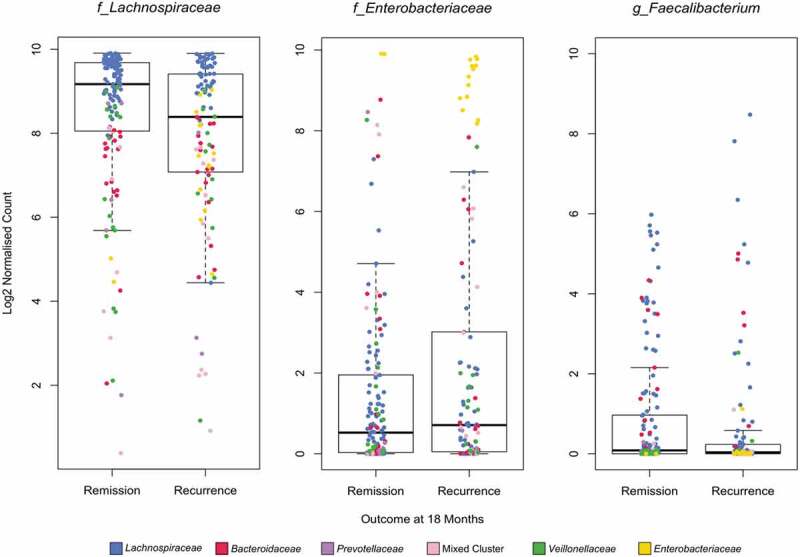
Cumulative Sum Scaled (CSS) normalized Log2 relative count derived from all samples (0,6,12 and 18 months) for Lachnospiraceae, Enterobacteriaceae (family level), and Faecalibacterium (genus level) by outcome at 18 months. Individual samples are colored by cluster assignment as per legend and figure 4A.
Samples within the Lachnospiraceae and Enterobacteriaceae clusters had significantly higher abundance and species diversity (as measured by the number of unique OTUs) (Figure 4b). We then tested if the diversity of Lachnospiraceae and Enterobacteriaceae families was associated with recurrence in all patients regardless of cluster assignment. Patients with greater OTU diversity in the Lachnospiraceae family were less likely to recur regardless of cluster group (median number of OTUs within the family at 18 months: Remission 2197 vs Recurrence 1657, P = .005).
As a lower abundance and diversity of Faecalibacterium prausnitzii has been associated with the presence of active Crohn’s disease and post-operative recurrence,6,9,18,20,33,44 we investigated diversity of OTUs within this genus in all clusters (Figure 4b, right panel). There was a significant increase in the number of unique OTUs mapped to the Faecalibacterium genus (from the family Ruminococcaceae) in the Lachnospiraceae cluster compared to other clusters (P = <0.001); however, there were no significant log fold differences in Faecalibacterium at genus level for any comparison (all samples for 18 month outcomes; LFD 0.28, FDR P = .722). The diversity of OTUs within the Faecalibacterium genus was associated with recurrence at 18 months (median number of OTUs at 18 months; Remission 2 vs Recurrence 1, P = .04).
Discussion
We have studied a large prospective, well-characterized cohort of 130 Crohn’s disease patients longitudinally from the time of surgery to address differences between patients who remained well after surgery and those who experienced recurrent macroscopic disease. The known predictability of post-operative recurrence occurring at the anastomosis and neo-terminal ileum allowed for reliable assessment of disease recurrence so that the relationship between luminal microbial changes and recurrence could be established.
We identified microbial profiles that are associated with disease recurrence (dominance of Enterobacteriaceae) and remission (dominance and increased diversity of Lachnospiraceae) after surgery. Enterobacteriaceae are associated with active intestinal inflammation,45–48 while Lachnospiraceae (in particular Anaerostipes, Roseburia, Coprococcus, Eubacterium, and other butyrate-producing genera within this family) are associated with diminished inflammation via anti-inflammatory short-chain fatty acid production.49,50
Dominance of Enterobacteriaceae at baseline and 6 months post-operatively was associated with an increased risk of disease recurrence at 18 months, even when adjusted for patient factors. While the overall sample size within the Enterobacteriaceae cluster was modest, the adjusted odds ratio was high (6.35), the level of significance before and after false discovery correction was high, the finding has biological plausibility, and these findings concur with previously reported results from our group on the analysis of the mucosal microbiome from a subset of these patients.6
Enrichment of this family has been associated previously with active Crohn’s disease,29,51 and genera within this family (Proteus and Escherichia species) have been linked with post-operative disease recurrence in the mucosal microbiome.6 There was also a decrease in the OTU diversity of the Faecalibacterium genus in the Enterobacteriaceae cluster associated with disease recurrence, consistent with observations that diversity within the Faecalibacterium genus is reduced in active Crohn’s disease.44 A recent study of mucosal biopsies has demonstrated that patients who recurred at 6-months post-operatively had a lower proportion of the spore-forming Clostridiales at the time of surgery than those without recurrence (8.8% recurrence vs 19.5% remission; P = .02), which was postulated to relate to these spore-forming species being resistant to the insult of surgery.52 Furthermore, those with subsequent recurrence showed expansion of the Enterobacteriaceae family at the time of surgery (44.3% recurrence vs 16.9% remission; P = .03).52
Our results are consistent with those of Dey et al.20 and others,52–54 that reported reduced abundance of genera within the Lachnospiraceae family at the time of surgery and the association with subsequent disease recurrence. Similar work using fecal samples by Halfvarson et al. has also demonstrated that the abundance of Lachnospiraceae is reduced in CD patients who have undergone a resection compared to those that have not had surgery and to healthy controls.9 Abundance of the Lachnospiraceae has been associated with response to anti-TNF-α therapy and an increased abundance of Enterobacteriaceae with the need for surgery.29 However, within-family diversity of unique OTU’s was predictive of response in our cohort, implying that while relative abundance of these families is important, the species diversity within the family also contributes to the outcome.
The ecology of butyrate-producing genera is altered by surgery; obligate anaerobes are depleted in the immediate post-operative period by local environmental changes (exposure to oxygen, altered pH, and mucosal changes in active inflammation) combined with the effect of antibiotics.15 The Lachnospiraceae family reside within the Clostridium cluster XIVa, and contain many key butyrate-producing genera (such as Blautia, Butyrivibrio, Anaerostipes, Dorea, Roseburia, Coprococcus, and Eubacterium).50 Depletion of these obligate anaerobes alters the availability and downstream metabolism of microbiota-derived butyrate by colonocytes. The switch by colonocytes from butyrate oxidation (when butyrate is depleted) to fermentation of glucose results in increased luminal oxygen availability, which in turn allows the facultatively anaerobic Enterobacteriaceae to expand.15,29,48 There is evidence for post-surgical “blooms” of the Enterobacteriaceae family, even in patients without inflammatory bowel disease. A study addressing microbial alterations in patients with an ileostomy (following small bowel transplant) demonstrated a significant expansion in Enterobacteriaceae and Lactobacillaceae, as a result of increased oxygen favoring the facultative anaerobes, and reducing populations of obligate anaerobes.21 Antibiotics used in these patients also influence microbial ecology via respiratory pathways and depletion of key bacterial families; some antibiotics have been linked with enterobacterial expansion.55,56
In addition to alterations in oxygen availability, there are also perioperative increases in the availability of terminal electron acceptors and donors for microbial facultative anaerobic metabolism by the Enterobacteriaceae, such as Nitrate, Nitrite, S-oxides (tetrathionate), N-oxides, and fumarate.46,57 This occurs as a result of host inflammatory responses and is postulated to be a consequence of inflammation rather than a cause.23,58 Recruitment of neutrophils and subsequent respiratory burst in the inflamed bowel releases reactive oxygen species (ROS) that, with endogenous sulfur-containing compounds, react to produce tetrathionate, an electron acceptor for Salmonella and Proteus spp.59,60 Breakdown of colonocytes provides further alternative respiratory electron acceptors via ethanolamine obtained from phospholipid/cell-wall breakdown,61 as well as trimethylamine derived from phosphatidylcholine.62
We undertook two different analytic approaches with regards to association with recurrence, namely differential abundance of normalized counts of individual taxa, and association of cluster groups based on hierarchical clustering on relative abundance. These approaches are complementary. The former has highlighted rare taxa that were not identified in the cluster analysis due to abundance cutoffs (at least 10% relative abundance in one or more samples), whereas the clustering allows comparisons of community structure, particularly where a certain bacterial family dominates samples.
The fecal and mucosal-associated microbiota constitute different ecological environments. We have previously identified Proteus spp. in the mucosa-associated with recurrent disease.6 The current findings of altered community structure, and differences between patients with and without disease recurrence, are likely to be complementary and related to our previous mucosal microbial findings. The exact relationship between these compartments remains to be determined.
Analysis of the post-operative microbiome in Crohn’s disease is complicated by necessary variability in the administration of antibiotics and other post-operative therapies. However, in this cohort the use of antibiotics post-operatively was protocolized and consistent across the majority of patients. The use of metronidazole in 80% of patients post-operatively was a correcting factor but may confound some comparisons.63–65 Nonetheless, disease recurrence in the presence of antibiotics may still reflect important microbial differences related to recurrent disease.
One limitation of this study was the heterogeneity of anti-inflammatory drug treatment provided in the post-operative period, limiting our ability to correct for therapy. Pre-operative therapy is likely to have a lesser confounding effect on the post-operative microbiome than the act of surgery itself, which induces changes in the microbiome even in healthy people.6,19 While there are few data reported on the effect of thiopurines on the microbiome, there is evidence that the anti-TNF drugs are associated with an increase in short-chain fatty acid-producing genera.66 This includes members of the Lachnospiraceae family such as Anaerostipes, Roseburia, Coprococcus, and Lachnospira, as well as the Ruminococcus and Faecalibacerium genera.66 There is also a decrease over time in Proteobacteria (including Enterobacteriaceae species)66–68 and a shift toward the microbiota of healthy control subjects.69 Given that these anti-TNF drugs are effective for post-operative endoscopic recurrence in our30 and other cohorts,70,71 this microbial pattern is consistent with post-operative remission.
In this multi-center study, most patients provided one or more stool samples. A stool sample was not obtained for every time for every patient – clustering methodology took this into account. Colonoscopic outcome measurements at two timepoints were available for the majority of patients. We would like to have also adjusted microbiota findings according to drug regime. However, the number of patients on each drug regimen, and the changes in drug therapy within patients according to the study protocol for disease recurrence, provided numbers that were too small to make these analyses meaningful. This study has not addressed the functional correlates of the identified microbial changes. However, the functional capacity of some of these bacteria whose abundance is changed is known and discussed above.
In conclusion, this study highlights structural community changes in the luminal microbiota after surgery for Crohn’s disease and its association with disease recurrence. This information now needs to be considered in relation to the mucosal changes after surgery, to provide an integrated overview about the microbiota in relation to disease.
Acknowledgments
AbbVie, Gutsy Group, Gandel Philanthropy, Angior Foundation, and Crohn’s Colitis Australia provided funding for the main clinical study. The National Health and Medical Research Council (NHMRC) supported A.L.H., S-M.T., P.D.C., M.A.K., M.I., and C.D.K. MI was supported by Fellowship #1061435, co-funded between the NHMRC and Australian Heart Foundation.
Funding Statement
This work was supported by Crohn’s and Colitis Australia; The National Health and Medical Research Council of Australia l; National Heart Foundation of Australia [1061435]; AbbVie (US); The Angior Family Foundation; Gandel Philanthropy; Gutsy Group.
Author contributions
A.L.H., S-M.T., M.I., E.K.W., P.D.C., M.A.K. – study concept and design; acquisition of data; data interpretation; drafting of the manuscript; critical revision of the manuscript; obtained funding. H.F., M.I., J.W., and C.D.K. – acquisition of data; critical revision of the manuscript. J.J.Y.S - critical revision of the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Bernell O, Lapidus A, Hellers G.. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231(1):38–45. doi: 10.1097/00000658-200001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV Jr.. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol. 2012;107(11):1693–1701. doi: 10.1038/ajg.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33(3):331–335. doi: 10.1136/gut.33.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99(4):956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 5.Mondot S, Lepage P, Seksik P, Allez M, Treton X, Bouhnik Y, Colombel JF, Leclerc M, Pochart P, Dore J, et al. Structural robustness of the gut mucosal microbiota is associated with Crohn’s disease remission after surgery. Gut. 2016;65(6):954–962. doi: 10.1136/gutjnl-2015-309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright EK, Kamm MA, Wagner J, Teo SM, Cruz P, Hamilton AL, Ritchie KJ, Inouye M, Kirkwood CD. Microbial factors associated with postoperative Crohn’s disease recurrence. J Crohns Colitis. 2017;11(2):191–203. doi: 10.1093/ecco-jcc/jjw136. [DOI] [PubMed] [Google Scholar]
- 7.Wlodarska M, Kostic Aleksandar D, Xavier Ramnik J. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe. 2015;17(5):577–591. doi: 10.1016/j.chom.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gevers D, Kugathasan S, Denson Lee A, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song Se J, Yassour M, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2(17004). doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pascal V, Pozuelo M, Borruel N, Casellas F, Campos D, Santiago A, Martinez X, Varela E, Sarrabayrouse G, Machiels K, et al. A microbial signature for Crohn’s disease. Gut. 2017;66(5):813–822. doi: 10.1136/gutjnl-2016-313235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araújo-Pérez F, McCoy AN, Okechukwu C, Carroll IM, Smith KM, Jeremiah K, Sandler RS, Asher GN, Keku TO. Differences in microbial signatures between rectal mucosal biopsies and rectal swabs. Gut Microbes. 2012;3(6):530–535. doi: 10.4161/gmic.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haberman Y, Tickle TL, Dexheimer PJ, Kim M-O, Tang D, Karns R, Baldassano RN, Noe JD, Rosh J, Markowitz J, et al. Pediatric Crohn’s disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest. 2014;124(8):3617–3633. doi: 10.1172/JCI75436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringel Y, Maharshak N, Ringel-Kulka T, Wolber EA, Sartor RB, Carroll IM. High throughput sequencing reveals distinct microbial populations within the mucosal and luminal niches in healthy individuals. Gut Microbes. 2015;6(3):173–181. doi: 10.1080/19490976.2015.1044711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux -J-J, Blugeon S, Bridonneau C, Furet J-P, Corthier G, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn’s disease patients. Proc Natl Acad Sci. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neut C, Bulois P, Desreumaux P, Membree J-M, Lederman E, Gambiez L, Cortot A, Quandalle P, Kruiningen H, Colombel J-F. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am J Gastroenterol. 2002;97(4):939–946. doi: 10.1111/j.1572-0241.2002.05613.x. [DOI] [PubMed] [Google Scholar]
- 20.Dey N, Soergel DAW, Repo S, Brenner SE. Association of gut microbiota with post-operative clinical course in Crohn’s disease. BMC Gastroenterol. 2013;13(1):1–11. doi: 10.1186/1471-230X-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman AL, Lough DM, Barupal DK, Fiehn O, Fishbein T, Zasloff M, Eisen JA. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc Natl Acad Sci U S A. 2009;106(40):17187–17192. doi: 10.1073/pnas.0904847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devine AA, Gonzalez A, Speck KE, Knight R, Helmrath M, Lund PK, Azcarate-Peril MA. Impact of ileocecal resection and concomitant antibiotics on the microbiome of the murine jejunum and colon. PLoS One. 2013;8(8):e73140. doi: 10.1371/journal.pone.0073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2(2):119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15(8):1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 25.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147(5):1055–1063.e1058. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera Chávez F, Lopez C, Bäumler A. Oxygen as a driver of gut dysbiosis. Free Radic Biol Med. 2017;105(93):93–101. doi: 10.1016/j.freeradbiomed.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Wong YT, Shah PC, Birkett DH, Brams DM. Peritoneal pH during laparoscopy is dependent on ambient gas environment: helium and nitrous oxide do not cause peritoneal acidosis. Surg Endosc. 2004;19(1):60–64. doi: 10.1007/s00464-003-9291-6. [DOI] [PubMed] [Google Scholar]
- 28.O’May GA, Reynolds N, Smith AR, Kennedy A, Macfarlane GT. Effect of pH and antibiotics on microbial overgrowth in the stomachs and duodena of patients undergoing percutaneous endoscopic gastrostomy feeding. J Clin Microbiol. 2005;43(7):3059–3065. doi: 10.1128/JCM.43.7.3059-3065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz B, Juillerat P, Øyås O, Ramon C, Bravo FD, Franc Y, Fournier N, Michetti P, Mueller C, Geuking M, et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med. 2019;25(2):323–336. doi: 10.1038/s41591-018-0308-z. [DOI] [PubMed] [Google Scholar]
- 30.De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM, et al. Crohn’s disease management after intestinal resection: a randomised trial. The Lancet. 2015;385(9976):1406–1417. doi: 10.1016/S0140-6736(14)61908-5. [DOI] [PubMed] [Google Scholar]
- 31.Silverberg MS. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal world congress of gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- 32.Best WR, Becktel JM, Singleton JW, Kern F, Jr. Development of a Crohn’s disease activity index: National cooperative Crohn’s disease study. Gastroenterology. 1976;70(3):439–444. [PubMed] [Google Scholar]
- 33.De Cruz P, Kang S, Wagner J, Buckley M, Sim WH, Prideaux L, Lockett T, McSweeney C, Morrison M, Kirkwood CD, et al. Association between specific mucosa-associated microbiota in Crohn’s disease at the time of resection and subsequent disease recurrence: a pilot study. J Gastroenterol Hepatol. 2015;30(2):268–278. doi: 10.1111/jgh.12694. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Heazlewood SP, Krause DO, Florin THJ. Molecular characterization of the microbial species that colonize human ileal and colonic mucosa by using 16S rDNA sequence analysis. J Appl Microbiol. 2003;95(3):508–520. doi: 10.1046/j.1365-2672.2003.02005.x. [DOI] [PubMed] [Google Scholar]
- 35.Sundquist A, Bigdeli S, Jalili R, Druzin ML, Waller S, Pullen KM, El-Sayed YY, Taslimi MM, Batzoglou S, Ronaghi M. Bacterial flora-typing with targeted, chip-based pyrosequencing. BMC Microbiol. 2007;7(1):108. doi: 10.1186/1471-2180-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Meth. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 39.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. Isme J. 2012;6(3):610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. Isme J. 2011;5(2):169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- 42.Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Meth. 2013;10(12):1200–1202. doi: 10.1038/nmeth.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25(6):665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez-Siles M, Martinez-Medina M, AbellC C, Busquets D, Sabat-Mir M, Duncan SH, Aldeguer X, Flint HJ, Garcia-Gil L. Mucosa-associated Faecalibacterium prausnitzii phylotype richness is reduced in patients with inflammatory bowel disease. Appl Environ Microbiol. 2015;81(21):7582–7592. doi: 10.1128/AEM.02006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8(3):292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, Gillis CC, Büttner L, Smoot MP, Behrendt CL, et al. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe. 2017;21(2):208–219. doi: 10.1016/j.chom.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Litvak Y, Byndloss MX, Tsolis RM, Baumler AJ. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr Opin Microbiol. 2017;39(1):1–6. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, Flint HJ, Louis P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8(1323):1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajilic-Stojanovic M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38(5):996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papa E, Docktor M, Smillie C, Weber S, Preheim SP, Gevers D, Giannoukos G, Ciulla D, Tabbaa D, Ingram J, et al. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS One. 2012;7(6):e39242. doi: 10.1371/journal.pone.0039242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laffin MR, Perry T, Park H, Gillevet P, Sikaroodi M, Kaplan GG, Fedorak RN, Kroeker K, Dieleman LA, Dicken B, et al. Endospore forming bacteria may be associated with maintenance of surgically-induced remission in crohn’s disease. Sci Rep. 2018;8(1):9734. doi: 10.1038/s41598-018-28071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machiels K, Pascal V, Sabino J, Santiago A, Campos D, Wolthuis A, De Buck van Overstraeten A, D’Hoore A, Van Assche G, Ferrante M, et al. DOP044 relationship between microbiota and development of early postoperative Crohn’s disease recurrence. J Crohn’s Colitis. 2017;11(suppl_1):S53–S53. doi: 10.1093/ecco-jcc/jjx002.081. [DOI] [Google Scholar]
- 54.Sokol H, Brot L, Stefanescu C, Auzolle C, Barnich N, Buisson A, Fumery M, Pariente B, Le Bourhis L, Treton X, et al. Prominence of ileal mucosa-associated microbiota to predict postoperative endoscopic recurrence in Crohn’s disease. Gut. 2019;69(3):462–472. [DOI] [PubMed] [Google Scholar]
- 55.Raymond F, Ouameur AA, Deraspe M, Iqbal N, Gingras H, Dridi B, Leprohon P, Plante PL, Giroux R, Berube E, et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. Isme J. 2016;10(3):707–720. doi: 10.1038/ismej.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faber F, Tran L, Byndloss MX, Lopez CA, Velazquez EM, Kerrinnes T, Nuccio S-P, Wangdi T, Fiehn O, Tsolis RM, et al. Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature. 2016;534(7609):697–699. doi: 10.1038/nature18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winter SE, Lopez CA, Bäumler AJ. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013;14(4):319–327. doi: 10.1038/embor.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. Host-derived nitrate boosts growth of E. Coli in the inflamed gut. Science. 2013;339(6120):708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenner DJ, Farmer JJ. Family i. Enterobacteriaceae. In: Brenner DJ, Krieg NR, Staley JT, editors. Volume two, part B - The Gammaproteobacteria. Bergey’s manual of systematic bacteriology. New York: Springer; 2005. p. 605. [Google Scholar]
- 61.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A. 2011;108(42):17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jameson E, Fu T, Brown IR, Paszkiewicz K, Purdy KJ, Frank S, Chen Y. Anaerobic choline metabolism in microcompartments promotes growth and swarming of Proteus mirabilis. Environ Microbiol. 2016;18(9):2886–2898. doi: 10.1111/1462-2920.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glick LR, Sossenheimer PH, Ollech JE, Cohen RD, Hyman NH, Hurst RD, Rubin DT. Low-dose metronidazole is associated with a decreased rate of endoscopic recurrence of Crohn’s disease after ileal resection: a retrospective cohort study. J Crohns Colitis. 2019;13(9):1158–1162. doi: 10.1093/ecco-jcc/jjz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108(6):1617–1621. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 65.D’Haens GR, Vermeire S, Van Assche G, Noman M, Aerden I, Van Olmen G, Rutgeerts P. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: a controlled randomized trial. Gastroenterology. 2008;135(4):1123–1129. doi: 10.1053/j.gastro.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Gao X, Ghozlane A, Hu H, Li X, Xiao Y, Li D, Yu G, Zhang T. Characteristics of faecal microbiota in paediatric Crohn’s disease and their dynamic changes during infliximab therapy. J Crohn’s Colitis. 2017;12(3):337–346. doi: 10.1093/ecco-jcc/jjx153. [DOI] [PubMed] [Google Scholar]
- 67.Ribaldone DG, Caviglia GP, Abdulle A, Pellicano R, Ditto MC, Morino M, Fusaro E, Saracco GM, Bugianesi E, Astegiano M. Adalimumab therapy improves intestinal dysbiosis in Crohn’s disease. J Clin Med. 2019;8(10):1646–undefined. doi: 10.3390/jcm8101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busquets D, Mas-de-Xaxars T, Lopez-Siles M, Martinez-Medina M, Bahi A, Sabat M, Louvriex R, Miquel-Cusachs JO, Garcia-Gil JL, Aldeguer X. Anti-tumour necrosis factor treatment with adalimumab induces changes in the microbiota of Crohn’s disease. J Crohns Colitis. 2015;9(10):899–906. doi: 10.1093/ecco-jcc/jjv119. [DOI] [PubMed] [Google Scholar]
- 69.Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, Bittinger K, Bailey A, Friedman ES, Hoffmann C, et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2015;18(4):489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Regueiro M, Feagan BG, Zou B, Johanns J, Blank MA, Chevrier M, Plevy S, Popp J, Cornillie FJ, Lukas M, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology. 2016;150(7):1568–1578. doi: 10.1053/j.gastro.2016.02.072. [DOI] [PubMed] [Google Scholar]
- 71.Aguas M, Bastida G, Cerrillo E, Beltran B, Iborra M, Sanchez-Montes C, Munoz F, Barrio J, Riestra S, Nos P. Adalimumab in prevention of postoperative recurrence of Crohn’s disease in high-risk patients. World J Gastroenterol. 2012;18(32):4391–4398. doi: 10.3748/wjg.v18.i32.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]


