Abstract
Purpose
Premenstrual symptoms comprise a wide range of mood, behavioral, and physical symptoms occurring during the luteal phase. Perceived injustice is a belief linked to unfairness (ie, unnecessary suffering caused by illness). This study aimed to assess the validity and reliability of the Premenstrual Symptoms Questionnaire (PSQ), a patient-reported outcome measurement tool, and to examine the association between perceived injustice/perception of menstruation and premenstrual symptoms, as measured by the PSQ.
Materials and Methods
Of 1388 female students, we analyzed 879 students with regular menstrual cycles who completed the PSQ, the premenstrual dysphoric disorder (PMDD) scale, the Somatic Symptom Scale-8 (SSS-8), and the Injustice Experience Questionnaire-chronic (IEQ-chr). First, the PSQ was examined for evidence of reliability and validity. Next, we used multiple regression and multivariate logistic regression to investigate the association between perceived injustice and premenstrual symptoms, using PSQ score as both a continuous variable and a dichotomous variable (premenstrual disorders or not). Moreover, the association between PSQ score and perceived menstruation was tested using student’s t-test and analysis of variance.
Results
In terms of reliability, Cronbach’s α for PSQ score was 0.93. To assess structural validity, we used confirmatory factor analysis, which showed that the one-factor model and the two-factor model were a good fit. The PSQ showed good agreement with the PMDD scale. In terms of concurrent validity, PSQ total score correlated strongly with PMDD scale score, SSS-8 score, and IEQ-chr score (r = 0.88, 0.69, 0.57, respectively). IEQ-chr score predicted PSQ score (standardized regression coefficient = 0.53; P < 0.0001) and higher prevalence of premenstrual disorders (odds ratio: 1.15; 95% confidence interval: 1.12–1.19). Negative perception of menstruation was associated with premenstrual symptoms.
Conclusion
The PSQ showed sound psychometric properties among the adolescents in our sample. Perceived injustice and negative perception of menstruation were associated with premenstrual symptoms.
Keywords: premenstrual syndrome, premenstrual disorders, injustice experience questionnaire-chronic, psychometric testing, validity
Introduction
Premenstrual symptoms comprise of a wide range of mood, behavioral, and physical symptoms that are limited to the luteal phase.1 Premenstrual disorders (PMDs) include premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD).2 Epidemiologic surveys have shown that the prevalence of premenstrual symptoms is high (8090%).3 PMDD is a severe form of PMS defined mainly by its psychiatric symptoms, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM).4 The burden of PMDs is high among women between menarche and menopause. We have previously reported that PMDs are common menstrual problems among adolescents and that one in nine Japanese female high school students had absences from school because of PMD symptoms.5
Although the precise pathophysiology of PMDs remains unknown, various causes have been suggested, including hormonal changes, serotonergic dysfunction, stress, and poor lifestyle habits.6 Previous studies have examined the relationships between PMDs and psychological factors.7–9 Perceived stress, neuroticism, and coping strategies have been reported to be strongly related to PMDs.10
Perceived injustice is a belief linked to unfair treatment and unnecessary suffering caused by illness.11 Perceived injustice has been extensively analyzed among people with chronic pain, including those with whiplash, fibromyalgia, and osteoarthritis.12 In a recent study, we explored the association between perceived injustice and severity of menstrual pain.13 Many women with premenstrual symptoms also suffer from related pain symptoms such as breast tenderness and abdominal pain.14 However, an association between perceived injustice and premenstrual symptoms has not yet been reported. A previous study on chronic pain treatment showed that cognitive behavioral therapy could be used to target perceived injustice.15 Cognitive behavioral therapy might be recommended as non-pharmacological medical approach to treat premenstrual symptoms without serious adverse effects.16 Accordingly, it may be fruitful to view perceived injustice as a new therapeutic target in the treatment of premenstrual symptoms.
The aims of this study were to assess the validity and reliability of a patient-reported outcome measurement (PROM) tool for premenstrual symptoms, the Premenstrual Symptoms Questionnaire (PSQ),17 and to examine the association between perceived injustice/perception of menstruation and premenstrual symptoms.
Materials and Methods
Settings and Participants
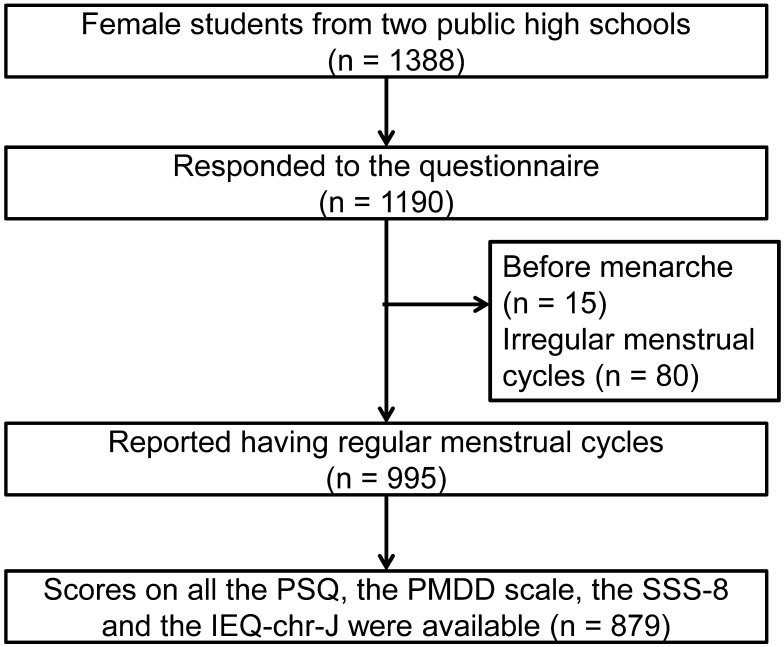
A school-based survey was conducted in December 2019 with a sample of 1388 Japanese female students from two public high schools in Sendai, the largest city in northeastern Japan. The study questionnaire was distributed to all female students at each school by their homeroom teachers. The questionnaires were completed, sealed in envelopes, and collected in the class. In total, 1190 students responded the questionnaire, including 995 who had regular menstrual cycles (25–38 days) (Figure 1). For this study, we selected the 879 students who completed all items on the PSQ, the PMDD scale, the Somatic Symptom Scale-8 (SSS-8), and the Japanese version of the Injustice Experience Questionnaire-chronic (IEQ-chr-J).
Figure 1.
Flow chart of the study.
Abbreviations: PSQ, Premenstrual Symptoms Questionnaire; PMDD, premenstrual dysphoric disorder; SSS-8, Somatic Symptom Scale-8; IEQ-chr-J, Japanese version of the Injustice Experience Questionnaire-chronic.
Questionnaire
The Premenstrual Symptoms Questionnaire and the Premenstrual Dysphoric Disorder Scale
In this study, we used the PSQ, which was developed in our previous study,17 and the PMDD scale, which was developed by Miyaoka et al to screen for premenstrual symptoms.18,19 In both PROM tools, the PMDD criteria from the DSM are translated into a rating scale with degrees of severity described in Japanese. The PSQ and the PMDD scale are therefore essentially identical to the Premenstrual Symptoms Screening Tool (PSST).20 The PSQ has been found to be useful in our previous studies,5,17 but its reliability and validity have not been systematically evaluated. The PMDD scale, in contrast, has been found to have high reliability and validity. Therefore, we selected the PMDD scale to study the concurrent validity of the PSQ.
The PSQ asks, Within the last 3 months, have you experienced the following premenstrual symptoms starting during the week before menses and stopping a few days after the onset of menses?
The premenstrual symptoms listed are (i) depressed mood, (ii) anxiety or tension, (iii) tearfulness, (iv) anger or irritability, (v) decreased interest in work, home, or social activities, (vi) difficulty concentrating, (vii) fatigue or lack of energy, (viii) overeating or food cravings, (ix) insomnia or hypersomnia, (x) feeling overwhelmed, and (xi) physical symptoms such as tender breasts, feeling of bloating, headache, joint or muscle pain, or weight gain.
These 11 symptoms are listed in the DSM criteria for PMDD. The PMDD scale consists of 12 symptoms, with “Insomnia or hypersomnia” divided into “Insomnia” and “Hypersomnia” as separate symptoms.
The PSQ also asks whether the premenstrual symptoms experienced interfere with (a) work efficiency or productivity, or home responsibilities; (b) social activities; or (c) relationships with coworkers or family. These three items measuring functional impairment of social and life activities were same as items in the Daily Record of Severity of Problems,21,22 the diary chart for PMDs with the strongest evidence of validity and reliability.23 The PMDD scale also included five items on functional impairment that were same as items in the PSST. In the present study, in both the PSQ and the PMDD scale, students were asked to rate the severity of premenstrual symptoms and these symptoms’ interference with activities as 1 – Not at all, 2 – Mild, 3 – Moderate, or 4 – Severe. The total scores on the PSQ and the PMDD scale were calculated as the sum of 14 items and 17 items, respectively. PSQ total score ranges from 14 to 56, and PMDD scale total score ranges from 17 to 68.
We divided the students into three groups on the basis of their premenstrual symptoms: PMDD, moderate-to-severe PMS, and no/mild PMS, according to the criteria reported by Steiner et al in 2003.20 We further divided the students into two groups: the PMDs group, who had moderate-to-severe PMS or PMDD, and the no PMDs group, who had no PMS or mild PMS, according to the PSQ criteria.
Menstrual Pain Intensity
Menstrual pain intensity was evaluated using a numerical rating scale. On numerical rating scales, respondents rate their severity of menstrual pain from 0 (no pain) to 10 (the worst pain imaginable).
Injustice Experience Questionnaire-Chronic
To study perceived injustice, we used the IEQ-chr-J.13 The original version of the Injustice Experience Questionnaire was developed to assess perceived injustice among injured persons.11,24 This questionnaire was modified to measure chronic symptoms in the IEQ-chr.25 The IEQ-chr-J has been found to have high reliability and validity. The IEQ-chr-J comprises 12 items, with a five-point response format (0–4) for each item. Therefore, IEQ-chr-J score can range from 0 to 48.
Somatic Symptom Scale-8
We used the SSS-8 to further assess the concurrent validity of the PSQ.26 Somatic symptoms are common to many diseases and are present in psychiatric conditions such as depressive disorders and anxiety disorders.27,28 The Japanese version of the SSS-8 has been found to have high reliability and validity.29 The SSS-8 consists of eight items, each with a five-point response format (0–4). Therefore, SSS-8 score can range from 0 to 32.
Perception of Menstruation
We used two original questions to assess participants’ perceptions of menstruation. The first of these questions was “Do you agree with the statement that it is unjust that only women menstruate?” and the second question was “Do your male friends or family members feel empathy regarding the difficulty you experience related to menstruation?” The response options for the first question were “Yes” or “No,” and the response options for the second question were “Yes,” “No,” or “Other.” For each participant, we also collected information about age, school grade level, body weight, height, age at menarche, sleep latency time, and sleep duration. Body mass index (kg/m2) was calculated by dividing weight by height squared. We defined participants with sleep-onset insomnia as those who needed more than 15 minutes to fall asleep, in line with the criteria listed in the Japanese version of the Pittsburgh Sleep Quality Index.30
Statistical Analysis
Means and standard deviations were calculated for continuous variables, and proportions were calculated for categorical variables. To confirm the factorial validity, we performed a confirmatory factor analysis (CFA) with a one-factor structure and a two-factor structure. The second model was based on the PMDD criteria from the DSM, which consisted of premenstrual symptoms and impairment. In the CFA, model fit was evaluated using fit indices: the chi-square (χ2) statistic, the goodness-of-fit index (GFI), the adjusted GFI (AGFI), the Tucker–Lewis Index (TLI), the comparative fit index (CFI), the root mean square residual error of approximation (RMSEA), and the standardized root mean square residual (SRMR). GFI and AGFI values >0.90 indicate a good fit.31 TLI and CFI values close to 0.95 indicate a relatively good fit.32 RMSEA values <0.08 and SRMR values <0.09 suggest a good fit.31
Correlations were analyzed using Spearman’s rank correlation coefficient. The agreement between the PSQ and the PMDD scale was evaluated by the weighted kappa. Kappa values of 0.81–1.00 suggest almost perfect agreement, and kappa values of 0.61–0.80 suggest substantial agreement.33
Multiple regression analysis was used to explore the association between IEQ-chr-J total score and PSQ total score. The included covariates were school grade level, body mass index, age at menarche, menstrual pain intensity, and sleep-onset insomnia.
We examined the association between perceived injustice and PMDs. Odds ratios (ORs) of the prevalence of PMDs and covariates were calculated using multivariate logistic regression analysis. Here, the included covariates were school grade level, body mass index, age at menarche, menstrual pain, and sleep-onset insomnia.
Differences in PSQ total score were assessed using Student’s t-test or one-way analysis of variance followed by Dunnett’s test, as appropriate.
Statistical analyses except for the CFA and weighted kappa were performed using JMP Pro 15.1.0 (SAS, Cary, NC, USA). The CFA was performed using IBM SPSS Amos 26 (IBM Corp., New York, USA). Weighted kappa was calculated using the Excel add-in software BellCurve for Excel 3.2.0 (Social Survey Research Information Co., Ltd., Tokyo, Japan). Statistical significance was set at P < 0.05 (two-tailed tests).
Results
The characteristics of the study population are presented in Table 1. Cronbach’s α coefficient was calculated to assess reliability. The Cronbach’s α for PSQ was 0.93, indicating very good internal consistency.
Table 1.
Characteristics of the Study Participants (n = 879)
| Characteristic | |
|---|---|
| Age (years), mean (SD) School year, number (%) First year Second year Third year Missing |
16.7 (0.9) 290 (33.0) 283 (32.2) 303 (34.5) 3 (0.3) |
| Age at menarche (years), mean (SD) | 12.0 (1.4) |
| BMI (kg/m2), mean (SD) | 19.6 (5.0) |
| Menstrual pain intensity, mean (SD) | 4.6 (2.7) |
| Sleep-onset insomnia, n (%) | 291 (33.1) |
| Total sleep duration (minutes), mean (SD) | 368.7 (56.1) |
Abbreviations: BMI, body mass index; SD, standard deviation.
To analyze the factorial validity of the PSQ, first, we performed a CFA with a one-factor structure including the six error covariance terms (Supplemental Material 1). Second, we performed a CFA with a two-factor structure including the five error covariance terms (Supplemental Material 2). These two models of the PSQ showed similarly good fit (Table 2).
Table 2.
Goodness-of-Fit Summary of the PSQ (One-Factor Model and Two-Factor Model)
| Model | χ2(df) | GFI | AGFI | TLI | CFI | RMSEA (90% CI) | SRMR |
|---|---|---|---|---|---|---|---|
| One-factor (14 items + six error covariance) | 425.1 (71) | 0.93 | 0.90 | 0.94 | 0.95 | 0.08 (0.07–0.08) | 0.04 |
| Two-factor (11 items + 3 items + five error covariance) | 403.7 (71) | 0.94 | 0.91 | 0.94 | 0.96 | 0.07 (0.07–0.08) | 0.04 |
Abbreviations: PSQ, Premenstrual Symptoms Questionnaire; χ2, chi-square; df, degrees of freedom; GFI, goodness-of-fit index; AGFI, adjusted GFI; TLI, Tucker–Lewis Index; CFI, comparative fit index; RMSEA, root mean square residual error of approximation; CI, confidence interval; SRMR, standardized root mean square residual.
Next, we analyzed the consistency of the evaluation of premenstrual symptoms between the PSQ and the PMDD scale. PSQ total score was found to be very highly correlated with PMDD scale total score (Spearman’s r = 0.88, P < 0.0001). Severity of PMDs as evaluated by the PSQ and the PMDD scale is shown in Table 3. In terms of the measure of agreement between the two scales, weighted kappa was 0.65 (95% confidence interval: 0.58–0.72). This means that the strength of agreement between the PSQ and the PMDD scale was substantial. We further analyzed the correlation coefficients and the agreement for individual premenstrual symptoms (Table 4). The strongest correlation was for “anger or irritability” and “overeating or food cravings” (r = 0.80), and the weakest correlation was for “Insomnia” (r = 0.44). In terms of agreement, the highest value was for “overeating or food cravings” (kappa = 0.81), and the lowest value was for “Insomnia” (kappa = 0.36).
Table 3.
Cross-Tabulation of Severity of Premenstrual Disorders Assessed by the PSQ and the PMDD Scale (n = 879 Students)
| PMDD Scale | |||||
|---|---|---|---|---|---|
| No/Mild PMS | Moderate to Severe PMS | PMDD | Total | ||
| PSQ | No/mild PMS | 694 | 64 | 5 | 763 |
| Moderate-to-severe PMS | 19 | 58 | 8 | 85 | |
| PMDD | 3 | 13 | 15 | 31 | |
| Total | 716 | 135 | 28 | 879 | |
Note: The relevant number of students in each table cell are presented here.
Weighted kappa (95% confidence interval): 0.65 (0.58–0.72), P < 0.001
Abbreviations: PSQ, Premenstrual Symptoms Questionnaire; PMDD, premenstrual dysphoric disorder; PMS, premenstrual syndrome.
Table 4.
Correlation Coefficients and Agreement Between the PSQ and the PMDD Scale
| Depressed Mood | Anxiety or Tension | Tearful | Anger or Irritability | Decreased Interest | Difficulty Concentrating | Fatigue or Lack of Energy | Overeating or Food Cravings | Insomnia or Hypersomnia (PSQ) |
Feeling Overwhelmed | Physical Symptoms | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypersomnia Insomnia (PMDD scale) | ||||||||||||
| Spearman’s r | 0.72* | 0.63* | 0.77* | 0.80* | 0.71* | 0.70* | 0.71* | 0.80* | 0.53* | 0.44* | 0.67* | 0.70* |
| Weighted kappa | 0.72* | 0.57* | 0.80* | 0.79* | 0.70* | 0.70* | 0.71* | 0.81* | 0.51* | 0.36* | 0.72* | 0.68* |
Note: *P < 0.0001
Abbreviations: PSQ, Premenstrual Symptoms Questionnaire; PMDD, premenstrual dysphoric disorder.
Table 5 shows the correlation coefficients for the PSQ, the SSS-8, and the IEQ-chr-J. PSQ total score was correlated with SSS-8 total score (r = 0.69) and with IEQ-chr-J score (r = 0.57). Severity of PMDs evaluated by the PSQ was modestly correlated with SSS-8 total score (r = 0.46) and with IEQ-chr-J score (r = 0.46). In terms of the individual items in the PSQ, the correlation coefficients ranged from 0.38 to 0.60 for SSS-8 total score and from 0.28 to 0.54 for the IEQ-chr-J score.
Table 5.
Correlation Coefficients for the PSQ, the SSS-8, and the IEQ-Chr-J
| SSS-8 Spearman’s r |
IEQ-chr-J Spearman’s r |
|
|---|---|---|
| Total score | 0.69* | 0.57* |
| Severity of PMDs | 0.46* | 0.46* |
| Depressed mood | 0.52* | 0.54* |
| Anxiety or tension | 0.57* | 0.49* |
| Tearful | 0.52* | 0.46* |
| Anger or irritability | 0.50* | 0.43* |
| Decreased interest | 0.45* | 0.46* |
| Difficulty concentrating | 0.56* | 0.43* |
| Fatigue or lack of energy | 0.60* | 0.47* |
| Overeating or food cravings | 0.40* | 0.28* |
| Insomnia or hypersomnia | 0.48* | 0.34* |
| Feeling overwhelmed | 0.47* | 0.49* |
| Physical symptoms | 0.52* | 0.31* |
| Work efficiency or productivity | 0.55* | 0.47* |
| Social activities | 0.43* | 0.37* |
| Relationships with coworkers or family | 0.38* | 0.40* |
Note: *P < 0.0001
Abbreviations: PSQ, Premenstrual Symptoms Questionnaire; SSS-8, Somatic Symptom Scale-8; IEQ-chr-J, Japanese version of the Injustice Experience Questionnaire-chronic; PMDs, premenstrual disorders.
To analyze the association between premenstrual symptom severity and perceived injustice in more detail, multiple regression analysis was performed (Table 6). Menstrual pain and IEQ-chr-J score were associated with PSQ total score. The results of the variance inflation factor showed that there was no multicollinearity problem in this analysis (1.01 to 1.13). The multivariate logistic analysis revealed that the risk factors for PMDs were menstrual pain (OR = 1.16; 95% confidence interval [CI]: 1.06–1.27) and IEQ-chr-J score (OR = 1.15; 95% CI: 1.12–1.19) (Table 7). Late age at menarche was associated with lower risk of PMDs (OR = 0.81; 95% CI: 0.69–0.94).
Table 6.
Multiple Regression Analysis Calculating the Associations Between Sample Characteristics and Severity of Premenstrual Symptoms
| β | 95% CI | p | Standardized β | VIF | |
|---|---|---|---|---|---|
| School grade level | 1.10 | −0.14 to 2.34 | 0.08 | 0.05 | 1.01 |
| BMI (kg/m2) | 0.03 | −0.17 to 0.24 | 0.74 | 0.01 | 1.03 |
| Age at menarche | −0.71 | −1.45 to 0.03 | 0.06 | −0. 05 | 1.05 |
| Menstrual pain | 1.71 | 1.32 to 2.10 | < 0.0001 | 0.24 | 1.13 |
| Sleep-onset insomnia | 0.90 | −0.21 to 2.01 | 0.11 | 0.04 | 1.07 |
| IEQ-chr-J | 1.29 | 1.15 to 1.42 | < 0.0001 | 0.53 | 1.12 |
Abbreviations: β, regression coefficient; CI, confidence interval; VIF, variance inflation factor; BMI, body mass index; IEQ-chr-J, Japanese version of the Injustice Experience Questionnaire-chronic.
Table 7.
Multivariate Analyses of Risk Factors for Premenstrual Disorders
| Multivariate | |||
|---|---|---|---|
| OR | 95% CI | P | |
| School grade level | 1.16 | 0.89–1.51 | 0.274 |
| BMI (kg/m2) | 1.00 | 0.96–1.04 | 0.834 |
| Age at menarche | 0.81 | 0.69–0.94 | 0.008 |
| Menstrual pain | 1.16 | 1.06–1.27 | 0.001 |
| Sleep-onset insomnia | 1.10 | 0.70–1.72 | 0.677 |
| IEQ-chr-J | 1.15 | 1.12–1.19 | < 0.0001 |
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; IEQ-chr-J, Japanese version of the Injustice Experience Questionnaire-chronic.
We then analyzed how many students felt a sense of injustice regarding their menstruation (Table 8). A total of 40.4% students answered “Yes” to the question of whether it is unjust that only women menstruate. PSQ total score was significantly higher among students who perceived injustice than among those who did not. When asked whether they felt that their male friends or family members felt empathy regarding the difficulty they experienced related to menstruation, 17.7% of the students responded “No” (Table 8). PSQ total score was significantly higher among the students who did not think that their male friends or family members felt empathy about this than among those who felt that their male friends or family did feel this kind of empathy.
Table 8.
PSQ Total Score by Perception of Menstruation
| Mean PSQ Total Score (95% CI) | P | |
|---|---|---|
| “Do you agree with the statement that it is unjust that only women menstruate?” | (Student’s t-test) | |
| Yes (n = 355, 40.4%) | 39.7 (37.6–41.7) | 0.030 |
| No (n = 498, 56.7%) | 36.7 (35.0–38.4) | |
| Missing (n = 26, 2.9%) | ||
| “Do your male friends or family members feel empathy regarding the difficulty you experience related to menstruation?” | (one-way ANOVA followed by Dunnett’s test) | |
| Yes (n = 538, 61.2%) | 35.8 (34.2–37.5) | 1 |
| No (n = 156, 17.7%) | 45.6 (42.5–48.5) | < 0.0001 |
| Other (n = 157, 17.9%) | 36.8 (33.8–39.9) | 0.802 |
| Missing (n = 28, 3.2%) |
Abbreviations: PSQ, Premenstrual Symptoms Questionnaire; CI, confidence interval; ANOVA, analysis of variance.
Discussion
The present study evaluated the PSQ, a PROM tool for premenstrual symptoms. We found strong evidence of validity and reliability for the questionnaire. Additionally, our findings show a correlation between premenstrual symptoms and perceived injustice.
CFA demonstrated that the PSQ fit a one-factor model and two-factor model well. The PMDD scale has been reported to fit a three-factor model identified by CFA.18 One previous report on the results of a CFA of another PMDD questionnaire based on the DSM showed that both a one-factor model and a two-factor model produced a good fit to the data for several indicators.34 This discrepancy with our findings may be because of differences in the questionnaires used to record premenstrual symptoms and in the studies’ recruitment methods.
The Cronbach’s α for PSQ (0.93) showed a high degree of internal consistency. This value was as high as that reported previously for the PMDD scale (0.91).18
The PMDD scale and the PSST have the same structure, with 12 items on premenstrual symptoms and five items on functional impairment. The PSQ, which comprises 11 items on premenstrual symptoms and three items on functional impairment, could be considered the short forms of the other two scales. Although the PMDD scale has already been used as a PROM tool for premenstrual symptoms in Japan with strong evidence of reliability and validity, the PSQ would be more convenient because of its briefness.
We also showed the association of premenstrual symptoms and somatic symptoms for the first time. As mentioned above, somatic symptoms are present in psychiatric conditions such as depressive disorders and anxiety disorders27,28 and are considered to be manifestations of underlying psychiatric disorders.35 Because depressive mood and anxiety are also premenstrual symptoms, this association is reasonable.
Our data showed that both the severity of premenstrual symptoms as assessed by the PSQ total score and the risk of PMDs were positively associated with perceived injustice. A previous study in a chronic pain treatment setting found that cognitive behavioral therapy could be used to target perceived injustice.15 Accordingly, perceived injustice may also be a therapeutic target among individuals experiencing premenstrual symptoms.
Women’s perceptions regarding menstruation have been shown to vary by age, with younger adolescents tending to show more negative attitudes.36,37 Adolescence is a unique time in human development, both psychologically and physiologically. It is a vulnerable period between childhood and adulthood. Adolescents tend to have more negative attitudes toward menstruation. A previous study reported that more than half of female adolescents had a negative attitude toward menstruation.36 Our data showed that approximately 40% of the students perceived injustice regarding their menstruation and that about 18% felt that their male friends or family members lacked understanding of their menstruation difficulties. Those who had such opinions also had a higher severity of premenstrual symptoms. These students might feel injustice that only women menstruate and, consequently, have more premenstrual symptoms. However, the questions used to measure perception of menstruation in this study were potentially leading. Among adolescent girls, having a strongly negative perception of menstruation is generally associated with having insufficient information.38 Health education programs for adolescent girls including psychological stress management during menstruation might be helpful to alleviate premenstrual symptoms in this population.
Our study had several limitations. The main limitation was that the study was cross-sectional in design. It was therefore impossible to determine causality between premenstrual symptoms and perceived injustice. Second, we screened for PMDs by retrospective self-report, making the study susceptible to recall bias. The diagnosis of PMDs requires the prospective daily charting over a period of two consecutive symptomatic cycles.23 However, prospective daily charting is difficult in large samples. Third, the study was conducted only in Sendai, the largest city in northeastern Japan, which might limit the generalization of the findings to the total population of adolescent girls in Japan. A related limitation was that the study participants were all high school students. Our results can thus be generalized only to adolescent girls with PMDs. Further research is necessary to determine whether similar results would be found among adult women.
Conclusions
The present study presented evidence of the validity and acceptability of the PSQ as a measure of premenstrual symptoms in our study sample. We further found that premenstrual symptoms are associated with perceived injustice.
Acknowledgment
We thank Jennifer Barrett, PhD, from Edanz Group for editing a draft of this manuscript.
Funding Statement
This work was supported in part by grants from JSPS KAKENHI (19K09792), Tokyo, Japan and Cyclic Innovation for Clinical Empowerment, AMED (19pc0101040h0001), Tokyo, Japan.
Ethics Approval and Informed Consent
The study was carried out in accordance with the principles outlined in the Declaration of Helsinki. The trial protocol was approved by the Ethics Committee of Kindai University (approval number: 31-149). Participating students provided informed consent before completing the survey. The ethics committee approved a waiver of parental informed consent because the students’ intention to participate could be confirmed, and the data were anonymized and contained no identifiable personal information about the respondents. The decision not to obtain parental informed consent was in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by Japan’s Ministry of Education, Culture, Sports, Science and Technology and Japan’s Ministry of Health, Labour and Welfare.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of the data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Yonkers KA, O’Brien PM, Eriksson E. Premenstrual syndrome. Lancet. 2008;371(9619):1200–1210. doi: 10.1016/S0140-6736(08)60527-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien PM, Backstrom T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Women’s Mental Health. 2011;14(1):13–21. doi: 10.1007/s00737-010-0201-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angst J, Sellaro R, Merikangas KR, Endicott J. The epidemiology of perimenstrual psychological symptoms. Acta Psychiatr Scand. 2001;104(2):110–116. doi: 10.1034/j.1600-0447.2001.00412.x [DOI] [PubMed] [Google Scholar]
- 4.Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 5.Takeda T, Koga S, Yaegashi N. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in Japanese high school students. Arch Women’s Mental Health. 2010;13(6):535–537. doi: 10.1007/s00737-010-0181-3 [DOI] [PubMed] [Google Scholar]
- 6.Grady-Weliky TA. Clinical practice. Premenstrual dysphoric disorder. N Engl J Med. 2003;348(5):433–438. doi: 10.1056/NEJMcp012067 [DOI] [PubMed] [Google Scholar]
- 7.Ramcharan S, Love EF, Fick GH. The epidemiology of premenstrual symptoms in a population-based sample of 2650 urban women: attributable risk and risk factors. J Clin Epidemiol 1992;45(4):377–92. doi: 10.1016/0895-4356(92)90039-p [DOI] [PubMed] [Google Scholar]
- 8.Gannon L, Luchetta TF, Pardie L. Perimenstrual symptoms: relationships with chronic stress and selected lifestyle variables.Behav Med. 1989;15(4):149–59. doi: 10.1080/08964289.1989.9934578 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto K, Okazaki A, Sakamoto Y, Funatsu M. The relationship between premenstrual symptoms, menstrual pain, irregular menstrual cycles, and psychosocial stress among Japanese college students. J Physiol Anthropol. 2009;28(3):129–136. doi: 10.2114/jpa2.28.129 [DOI] [PubMed] [Google Scholar]
- 10.Del Mar Fernandez M, Regueira-Mendez C, Takkouche B. Psychological factors and premenstrual syndrome: A Spanish case-control study. PLoS One. 2019;14(3):e0212557. doi: 10.1371/journal.pone.0212557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan MJ, Adams H, Horan S, Maher D, Boland D, Gross R. The role of perceived injustice in the experience of chronic pain and disability: scale development and validation. J Occup Rehabil. 2008;18(3):249–261. doi: 10.1007/s10926-008-9140-5 [DOI] [PubMed] [Google Scholar]
- 12.Carriere JS, Donayre Pimentel S, Yakobov E, Edwards RR, Systematic A. Review of the association between perceived injustice and pain-related outcomes in individuals with musculoskeletal pain. Pain Med. 2020;21:1449–1463. doi: 10.1093/pm/pnaa088 [DOI] [PubMed] [Google Scholar]
- 13.Yamada K, Adachi T, Kubota Y, Takeda T, Iseki M. Developing a Japanese version of the injustice experience questionnaire-chronic and the contribution of perceived injustice to severity of menstrual pain: a web-based cross-sectional study. Biopsychosoc Med. 2019;13:17. doi: 10.1186/s13030-019-0158-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser G, Janda C, Kleinstauber M, Weise C. Clusters of premenstrual symptoms in women with PMDD: appearance, stability and association with impairment. J Psychosom Res. 2018;115:38–43. doi: 10.1016/j.jpsychores.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Raftery MN, Murphy AF, O’Shea E. Effectiveness of a cognitive behavioural therapy-based rehabilitation programme (Progressive Goal Attainment Program) for patients who are work-disabled due to back pain: study protocol for a multicentre randomised controlled trial. Trials. 2013;14:290. doi: 10.1186/1745-215-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busse JW, Montori VF, Krasnik C. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6–15. doi: 10.1159/00062296 [DOI] [PubMed] [Google Scholar]
- 17.Takeda T, Tasaka K, Sakata M, Murata Y. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in Japanese women. Arch Women’s Mental Health. 2006;9(4):209–212. doi: 10.1007/s00737-006-0137-9 [DOI] [PubMed] [Google Scholar]
- 18.Miyaoka YAY, Kamo T, Ueda K. The reliability and validity of the newly developed PMDD scale. J Jp Soc Psychosom Obstet Gynecol. 2009;14:194–201. [Google Scholar]
- 19.Miyaoka Y, Akimoto YF, Ueda K, et al. Fulfillment of the premenstrual dysphoric disorder criteria confirmed using a self-rating questionnaire among Japanese women with depressive disorders. Biopsychosoc Med. 2011;5:5. doi: 10.116/1751-075-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Women’s Mental Health. 2003;6(3):203–209. doi: 10.1007/s00737-003-0018-4 [DOI] [PubMed] [Google Scholar]
- 21.Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Women’s Mental Health. 2006;9(1):41–49. doi: 10.1007/s00737-005-0103-y [DOI] [PubMed] [Google Scholar]
- 22.Takeda T, Shiina M, Yamada K. Development of the Japanese version of the Daily Record of Severity of Problems (DRSP): translation and linguistic validation. Clinical Gynecol Obstet. 2019;73(8):807–811. [Google Scholar]
- 23.Management of premenstrual syndrome: green-top guideline no. 48. BJOG. 2017;124(3):e73–e105. doi: 10.1111/1471-0528.14260 [DOI] [PubMed] [Google Scholar]
- 24.Yamada KA-O, Adachi T, Mibu A, et al. Injustice experience questionnaire, japanese version: cross-cultural factor-structure comparison and demographics associated with perceived injustice. PLoS One. 2016;11(8):e0160567. doi: 10.1371/journal.pone.0160567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakobov E. Validation of the injustice experiences questionnaire adapted for use with patients with severe osteoarthritis of the knee. J Arthritis. 2014;03:02. doi: 10.4172/2167-7921.1000130 [DOI] [Google Scholar]
- 26.Gierk B, Kohlmann S, Kroenke K, et al. The somatic symptom scale-8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med. 2014;174(3):399–407. doi: 10.1001/jamainternmed.2013.12179 [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Jackson JF, Chamberlin J. Depressive and anxiety disorders in patients presenting with physical complaints: clinical predictors and outcome. [DOI] [PubMed] [Google Scholar]
- 28.Simon GE, VonKorff MF, Piccinelli M. An international study of the relation between somatic symptoms and depression. N Engl J Med. 1999;341(18):1329–35. [DOI] [PubMed] [Google Scholar]
- 29.Matsudaira K, Oka H, Kawaguchi M, et al. Development of a Japanese version of the somatic symptom scale-8: psychometric validity and internal consistency. Gen Hosp Psychiatry 2017;45:7–11. doi: 10.1016/j.genhosppsych.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Doi Y, Minowa MF, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res 2000;97(2-3):165–72. doi: 10.101/s0165-1781(00)00232-8 [DOI] [PubMed] [Google Scholar]
- 31.Hooper DCJ, Mullen MR. Structural equation modelling: guidelines for determining model fit. Electron J Bus Res Methods. 2008;6(1):53–60. [Google Scholar]
- 32.Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- 33.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 34.Wang YP, Teng CF, Vieira Filho AHG. Dimensionality of the premenstrual syndrome: confirmatory factor analysis of premenstrual dysphoric symptoms among college students). Brazilian J Med Biol Res. 2007;40:639–647 doi: 10.1590/S0100-879X2007000500006 [DOI] [PubMed] [Google Scholar]
- 35.Tomenson B, Essau CF, Jacobi F, et al. Total somatic symptom score as a predictor of health outcome in somatic symptom disorders. British J Psychiatry. 2013;203:373–380. doi: 10.1192/bjp.bp.112.114405. [DOI] [PubMed] [Google Scholar]
- 36.Serret-Montoya J, Villasis-Keever MA, Mendoza-Rojas MO, Granados-Canseco F, Zuniga-Partida EA, Zurita-Cruz JN. Factors that impact on the perception of menstruation among female adolescents. Arch Argent Pediatr. 2020;118(2):e126–e134. doi: 10.5546/aap.2020.eng.e126 [DOI] [PubMed] [Google Scholar]
- 37.Marván ML, Molina-Abolnik M. Mexican adolescents’ experience of menarche and attitudes toward menstruation: role of communication between mothers and daughters. J Pediatric Adolescent Gynecol.2012;25:358–363. doi: 10.1016/j.jpag.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 38.Chandra-Mouli V, Patel SV. Mapping the knowledge and understanding of menarche, menstrual hygiene and menstrual health among adolescent girls in low- and middle-income countries. Reprod Health. 2017;14(1):30. doi: 10.1186/s12978-017-0293-6 [DOI] [PMC free article] [PubMed] [Google Scholar]