Abstract
Background
Diabetic retinopathy (DR) may be asymptomatic in both mild and advanced stages. A patient’s accurate perception of their DR severity may therefore be critical for effective self-management behaviors and understanding the need for timely intervention and follow-up.
Purpose
To evaluate the relationship between self-reported and actual retinopathy severity in diabetic patients.
Methods
This study was a single-center cross-sectional survey. Diabetic patients identified by enterprise data warehouse were sent an online questionnaire where they were asked to self-assess for presence of DR and grade their severity. Actual DR grading was determined via chart review. The primary outcome measures were patient-assessed DR severity and agreement with actual DR severity.
Results
Of 3208 invitations sent, 324 (10%) patients responded and 319 responses were analyzed. The data showed that 39 of 253 (15%) with no DR, 26 of 40 (65%) with mild/moderate DR, and 24 of 26 (92%) with severe DR believed they had DR (p<0.001). Of those with no DR, 214 of 253 (85%) accurately assessed absence of DR. Of those with mild/moderate DR, 25 of 40 (63%) accurately assessed their severity, 14 of 40 (35%) believed they had no DR, and 1 of 40 (3%) believed they had severe DR. In patients with severe DR, 9 of 26 (35%) correctly assessed their severity, 15 of 26 (58%) believed they had mild/moderate DR, and 2 of 26 (8%) believed they had no DR.
Conclusion
Patients with severe DR were the most likely to report presence of DR, but often underestimated their disease severity. Many with mild/moderate DR did not realize they had DR. This consistent underestimation of severity across all a significant barrier to timely follow-up and treatment necessary to prevent future visual impairment.
Keywords: diabetic retinopathy, diabetes, survey, severity, perception, awareness, understanding, anti-VEGF, barriers, screening, accuracy, self-report
Background
Diabetes mellitus (DM) is the leading cause of blindness among working adults aged 20–64 in the United States.1 It is estimated that over 30 million people in the US suffer from diabetes, of which 28.5% have evidence of diabetic retinopathy (DR) and 4.4% have vision-threatening retinopathy.2,3
Overall compliance with DR screening is poor, with one study revealing only 34% of patients were screened in a given year.4 As many patients lack an accurate understanding of the severity of their diabetic disease in general,5 multiple studies have assessed awareness of the ocular complications amongst patients with diabetes.6–9 One such study identified minimal understanding of ocular complications as the primary reason for lack of screening.9 Indeed, patients’ own perceptions about diabetes and its consequences have a strong psychological link to self-management behaviors.10 Diabetic patients who demonstrated better compliance with weight loss measures and glycemic control perceived their diabetes to be of greater severity than less compliant patients.11 Several studies have further examined the accuracy of self-reporting of DR against the true presence or absence of retinopathy.12–14 However, there have been limited studies evaluating patients’ own self-assessment of the severity of their diabetic retinopathy.
Anti-vascular endothelial growth factor (anti-VEGF) therapy is the current gold standard for the management of exudative macular degeneration and venous occlusive disease.15–21 It has also revolutionized the management of diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR).22–26 Recently, there has been enthusiasm for earlier intervention designed to slow the progression of diabetic retinopathy and reduce the risk of vision-threatening complications.27 With these earlier interventions, it is imperative that patients have an accurate awareness of the severity of their condition, which can be asymptomatic even in advanced stages. The present study is designed to evaluate patients’ perceived severity of diabetic retinopathy compared to their actual disease severity. We hypothesized that patients with DR would be likely to underestimate the severity of their disease given its often asymptomatic nature, which could subsequently represent a major barrier to providing appropriate care to prevent future visual impairment in these patients.
Methods
This study was a single-center, cross-sectional electronic survey conducted between August and December 2019. The inclusion criteria were: (1) presence of type I or type II diabetes with or without retinopathy; (2) men and women 21 years of age or older; (3) eye exam performed by an ophthalmologist in the Northwestern University Department of Ophthalmology in the last 24 months; (4) English as primary language or no language specified; (5) email address on file.
With Institutional Review Board (IRB) approval, eligible patients meeting the inclusion criteria were identified using the Northwestern Medicine Enterprise Data Warehouse (EDW). The International Classification of Diseases, Tenth Revision (ICD-10) codes used for the inclusion criteria are provided in Appendix A.
Patients were invited to complete an online Research Electronic Data Capture (REDCap) survey via email. Informed consent was obtained via electronic signature, and participants were asked to provide identifying information that was used to link their survey response to study-specific data in the electronic medical record (EMR).
For the individuals who responded, presence or absence of DR along with stage of DR (mild NPDR, moderate NPDR, severe NPDR, PDR) were recorded by chart review of the most recent ophthalmology note and fundus exam in the EMR.
IRB
This study was approved by the Northwestern University Institutional Review Board Office (STU00209048). It adhered to the tenets of the Declaration of Helsinki.
Questionnaire
The questionnaire was 26 questions in length and took approximately 10 minutes to complete. It was divided into five sections: demographics, diabetes background, DR awareness, DR treatment, and compliance. While our questionnaire was not validated, several questions were based on a prior validated survey assessing general awareness of DR.8 Questions were multiple choice unless otherwise indicated. For a full version of the questionnaire, see Appendix B.
In the demographics section, participants were asked to provide information regarding ethnic origin, sex, and highest level of education.
In the diabetes background section, participants were asked about their known duration of diabetes and medication regimen for diabetes treatment.
In the DR awareness section, participants were asked a series of questions regarding their general understanding of DR. Specifically, they were asked to indicate “yes” or “no” regarding several questions, including whether or not they were aware diabetes could affect vision and whether they knew what “diabetic retinopathy” means. Next, participants were asked questions regarding their personal ocular complications of diabetes. Specifically, they were asked to indicate whether diabetes was affecting their eyes and, if so, to what extent (mild, moderate, or severe). Two additional “yes” or “no” questions asked whether or not they had been told that fluid was affecting their eyes or they had new or abnormal blood vessels.
In the DR treatment section, patients were asked to indicate whether or not they had received laser treatments or eye injections for diabetes and, if so, for what purpose.
Finally, in the compliance section, participants were asked to report control of blood sugar and approximate last hemoglobin A1c value. Respondents were asked to identify barriers to receiving routine eye exams (including a free response option).
Analysis
Descriptive statistics were conducted on the EDW data for responders and non-responders. The age of the two groups was compared using a t-test. All other p-values were either chi-square or Fisher’s exact test.
Self-reported “perceived” DR severity was categorized into three groups: none, mild/moderate, and severe. Chart-reviewed “actual” DR severity was categorized into three identical groups, with both PDR and severe NPDR being categorized as severe. This categorization scheme was adopted based on prior literature and for simplicity of reporting.28 Cohen’s weighted kappa was calculated to assess agreement between perceived and actual DR severities, and additional descriptive statistics were undertaken for this data.
Patients were stratified as “underestimators” (perceived severity less than actual severity), “accurate” (perceived severity equal to actual severity), and “overestimators” (perceived severity greater than actual severity). Again, descriptive statistics were conducted, the ages of the groups were compared using ANOVA, and remaining variables were compared using either chi-square or Fisher’s exact test.
All statistical analysis was performed with R (Version 3.6).
Results
Of the 3239 patients invited to participate in the study, 324 responded for a response rate of 10.0%. Table 1 shows the EDW-reported characteristics of patients who responded versus patients who did not respond. Responders differed from non-responders in terms of gender (p=0.015), race (p<0.001), and presence of type 1 diabetes (p<0.001). Compared to non-responders, a greater proportion of responders were male, white, and had type 1 diabetes.
Table 1.
Baseline Characteristics of Survey Respondents versus Non-Respondents
| n | Level | Overall | No Response | Responded | p |
|---|---|---|---|---|---|
| 3239 | 2915 | 324 | |||
| Age (mean (SD)) | 63.03 (13.97) | 63.11 (14.04) | 62.26 (13.32) | 0.296 | |
| Gender (%) | Female | 1662 (51.3) | 1517 (52.0) | 145 (44.8) | 0.015 |
| Male | 1577 (48.7) | 1398 (48.0) | 179 (55.2) | ||
| Race (%) | No Data | 1 (0.0) | 1 (0.0) | 0 (0.0) | <0.001 |
| American Indian or Alaska Native | 17 (0.5) | 15 (0.5) | 2 (0.6) | ||
| Asian | 187 (5.8) | 183 (6.3) | 4 (1.2) | ||
| Black or African American | 953 (29.4) | 898 (30.8) | 55 (17.0) | ||
| Declined | 225 (6.9) | 205 (7.0) | 20 (6.2) | ||
| Native Hawaiian or Other Pacific Islander | 5 (0.2) | 5 (0.2) | 0 (0.0) | ||
| Other | 203 (6.3) | 187 (6.4) | 16 (4.9) | ||
| Unknown | 4 (0.1) | 4 (0.1) | 0 (0.0) | ||
| White | 1644 (50.8) | 1417 (48.6) | 227 (70.1) | ||
| Ethnicity (%) | Declined | 252 (7.8) | 226 (7.8) | 26 (8.0) | 0.262 |
| Hispanic or Latino | 312 (9.6) | 290 (9.9) | 22 (6.8) | ||
| Not Hispanic or Latino | 2674 (82.6) | 2398 (82.3) | 276 (85.2) | ||
| Unable to Answer | 1 (0.0) | 1 (0.0) | 0 (0.0) | ||
| Type 1 Diabetes (%) | 483 (14.9) | 413 (14.2) | 70 (21.6) | <0.001 | |
| Type 2 Diabetes (%) | 3150 (97.3) | 2837 (97.3) | 313 (96.6) | 0.471 | |
| Presence of diabetic retinopathy (%) | 510 (15.7) | 455 (15.6) | 55 (17.0) | 0.575 |
Notes: Presence of type 1 diabetes, type 2 diabetes, and diabetic retinopathy were determined by the International Classification of Diseases, Tenth Revision codes provided in Appendix A.
Three patients were excluded due to lack of a fundus exam in the past 24 months, and another 2 were excluded due to the presence of a background retinopathy ICD code in the chart with an absence of documented presence or absence of DR in clinic notes. Thus, 319 responses were analyzed. Two hundred and fifty-three patients (79%) had no DR, and 66 patients (21%) had DR of which 40 patients (13%) had mild/moderate DR and 26 patients (8%) had severe DR.
Perceived versus Actual Severity of DR
Table 2 shows the number of patients with each combination of perceived DR severity and actual DR severity. Cohen’s weighted kappa for agreement between these two variables was 0.51 (CI: 0.41–0.61). Figure 1 depicts the proportion of patients within each actual severity group that believed they had no DR, mild/moderate DR, and severe DR.
Table 2.
Perceived versus Actual Diabetic Retinopathy (DR) Severity Amongst Survey Respondents
| Perceived DR Severity | ||||
|---|---|---|---|---|
| None | Mild/Moderate | Severe | ||
| Actual DR Severity | None | 214 | 36 | 3 |
| Mild/moderate | 14 | 25 | 1 | |
| Severe | 2 | 15 | 9 | |
Notes: Each cell represents the number of patients with each combination of perceived versus actual DR severity. Underestimators are shaded in dark gray, accurate patients are shaded in light gray, and overestimators are shaded in white.
Abbreviation: DR, diabetic retinopathy.
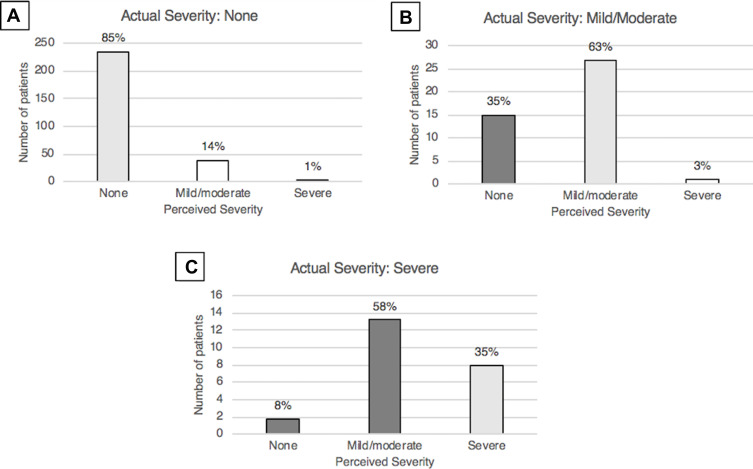
Figure 1.
Bar graphs representing the perceived severity of diabetic retinopathy (DR) in patients with no DR (A), mild/moderate DR (B), and severe DR (C).
Thirty-nine of 253 (15%) with no DR, 26 of 40 (65%) with mild/moderate DR, and 24 of 26 (92%) with severe DR believed they had DR (p<0.001). Of those with no DR, 214 of 253 (85%) accurately assessed absence of DR. Of those with mild/moderate DR, 25 of 40 (63%) accurately assessed their severity, 14 of 40 (35%) believed they had no DR, and 1 of 40 (3%) believed they had severe DR. In patients with severe DR, 9 of 26 (35%) correctly assessed their severity, 15 of 26 (58%) believed they had mild/moderate DR, and 2 of 26 (8%) believed they had no DR. In total, 31 of 66 (47%) of all patients with DR underestimated the stage of their disease. Patients with severe DR were the least accurate in self-assessing DR severity (p<0.001).
Characteristics of Underestimators, Overestimators, and Accurate Patients
Table 3 shows the characteristics of “underestimators” who underestimated their DR severity, “overestimators” who overestimated their DR severity, and “accurate” patients who correctly assessed their DR severity. Two hundred and forty-eight patients were accurate, 40 were overestimators, and 31 were underestimators.
Table 3.
Self-Reported Characteristics of Overestimators, Underestimators, and Accurate Patients
| n | Overall | Accurate | Overestimator | Underestimator | p | |
|---|---|---|---|---|---|---|
| 319 | 248 | 40 | 31 | |||
| Age (mean (SD)) | 62.26 (13.32) | 62.28 (13.05) | 66.30 (14.43) | 56.84 (12.46) | 0.012 | |
| Ethnic origin (%) | Asian or Pacific Islander | 10 (3.1) | 5 (2.0) | 4 (10.0) | 1 (3.2) | 0.099 |
| Black not Hispanic | 59 (18.5) | 49 (19.8) | 5 (12.5) | 5 (16.1) | ||
| Hispanic | 24 (7.5) | 16 (6.5) | 5 (12.5) | 3 (9.7) | ||
| Other | 3 (0.9) | 2 (0.8) | 0 (0.0) | 1 (3.2) | ||
| Prefer not to answer | 2 (0.6) | 1 (0.4) | 1 (2.5) | 0 (0.0) | ||
| White not Hispanic | 221 (69.3) | 175 (70.6) | 25 (62.5) | 21 (67.7) | ||
| Sex (%) | Female | 144 (45.1) | 115 (46.4) | 14 (35.0) | 15 (48.4) | 0.389 |
| Male | 175 (54.9) | 133 (53.6) | 26 (65.0) | 16 (51.6) | ||
| Highest level of education completed (%) | Elementary/Middle School | 2 (0.6) | 2 (0.8) | 0 (0.0) | 0 (0.0) | 0.717 |
| High School or GED equivalent | 49 (15.4) | 41 (16.5) | 3 (7.5) | 5 (16.1) | ||
| Bachelor’s degree | 119 (37.3) | 91 (36.7) | 19 (47.5) | 9 (29.0) | ||
| Master’s degree or above | 145 (45.5) | 110 (44.4) | 18 (45.0) | 17 (54.8) | ||
| Prefer not to answer | 4 (1.3) | 4 (1.6) | 0 (0.0) | 0 (0.0) | ||
| Duration of diabetes (%) | Less than 5 years | 53 (16.6) | 48 (19.4) | 5 (12.5) | 0 (0.0) | <0.001 |
| 5–10 years | 78 (24.5) | 64 (25.8) | 13 (32.5) | 1 (3.2) | ||
| More than 10 years | 182 (57.1) | 132 (53.2) | 20 (50.0) | 30 (96.8) | ||
| Unsure | 6 (1.9) | 4 (1.6) | 2 (5.0) | 0 (0.0) | ||
| Insulin use (%) | 75 (23.5) | 46 (18.5) | 8 (20.0) | 21 (67.7) | <0.001 | |
| Are you aware that diabetes can affect your vision? (%) | Yes | 315 (98.7) | 244 (98.4) | 40 (100.0) | 31 (100.0) | 1.000 |
| Do you know what diabetic retinopathy means? (%) | Yes | 235 (73.7) | 177 (71.4) | 31 (77.5) | 27 (87.1) | 0.157 |
| Have you ever been told that fluid is affecting your vision? (%) | Yes | 39 (12.2) | 19 (7.7) | 13 (32.5) | 7 (22.6) | <0.001 |
| Have you ever been told that you have new or abnormal blood vessels? (%) | Yes | 52 (16.3) | 31 (12.5) | 7 (17.5) | 14 (45.2) | <0.001 |
| Have you ever received laser treatment for diabetes? (%) | Yes | 40 (12.5) | 16 (6.5) | 7 (17.5) | 17 (54.8) | <0.001 |
| Have you ever received eye injections for diabetes? (%) | Yes | 22 (6.9) | 11 (4.4) | 2 (5.0) | 9 (29.0) | <0.001 |
| Is your blood sugar glucose well controlled? (%) | Fluctuates | 135 (42.3) | 104 (41.9) | 15 (37.5) | 16 (51.6) | 0.161 |
| No | 15 (4.7) | 12 (4.8) | 0 (0.0) | 3 (9.7) | ||
| Yes | 169 (53.0) | 132 (53.2) | 25 (62.5) | 12 (38.7) | ||
| What was your last A1c measurement? (%) | Do not know | 21 (6.6) | 17 (6.9) | 4 (10.0) | 0 (0.0) | 0.226 |
| Greater than 7 | 124 (38.9) | 93 (37.5) | 14 (35.0) | 17 (54.8) | ||
| Less than 7 | 174 (54.5) | 138 (55.6) | 22 (55.0) | 14 (45.2) |
Abbreviation: SD, standard deviation.
The three groups differed with regards to age (p=0.012), with underestimators younger on average with a mean age of 56.84 years and overestimators older on average with a mean age of 66.30 years. Ethnic origin (p=0.099), sex (p=0.389), and education level (p=0.717) were not significantly different between the groups. Duration of diabetes (p<0.001) and use of insulin (p<0.001) varied between the groups. The majority of underestimators (96.8%) had been diagnosed with diabetes for greater than 10 years compared to approximately half (53.2%) of patients that responded accurately. Additionally, 67.7% of underestimators used insulin, compared to 18.5% of accurate patients.
Self-reported awareness that diabetes can affect vision (p=0.560) and self-reported knowledge of the term “diabetic retinopathy” (p=0.145) were not significantly different between groups. 7.7% of accurate patients, 32.5% of overestimators, and 22.6% of underestimators reported they had been told that fluid was affecting their vision (p<0.001). Underestimators were more likely to report being told they have new or abnormal blood vessels, or to have received laser treatment or eye injections (p<0.001). Self-reported blood sugar control (p=0.180) and A1c (p=0.227) were not significantly different between the groups.
Barriers to DR Screening
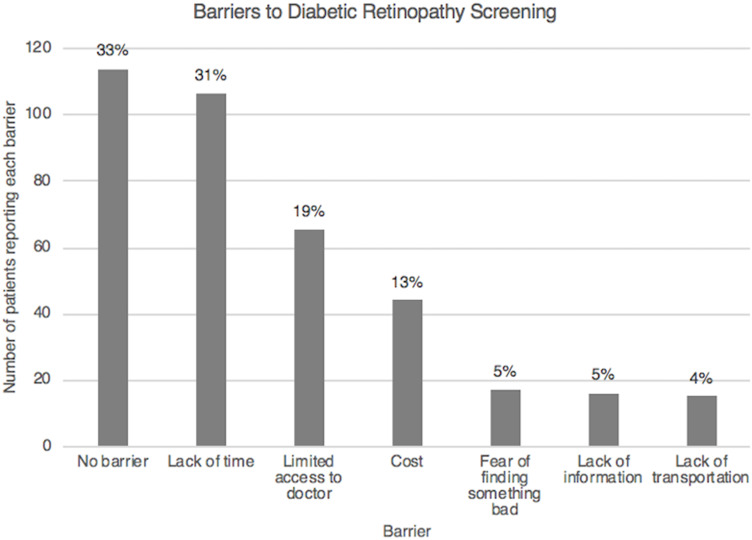
Figure 2 shows the percentage of patients reporting several different barriers to DR screening. One hundred and six patients (33%) reported they did not have difficulty receiving routine eye exams. Ninety-nine patients (31%) reported lack of time, 61 patients (19%) reported limited access to a doctor, and 41 patients (13%) reported cost as a barrier. Less reported barriers were fear of finding an abnormality (5%), lack of information (5%), and lack of transportation (4%).
Figure 2.
Bar graph illustrating the number and percentage of patients reporting each barrier to diabetic retinopathy screening.
Discussion
DR may be asymptomatic in both mild and advanced stages. There has been much interest in shifting treatment paradigms to earlier intervention with anti-VEGF therapy in order to prevent vision-threatening complications of proliferative disease and DME.27 However, it is crucial for clinicians to ensure their patients have an accurate understanding of their DR severity in order to reinforce effective management behaviors and the need for timely intervention and follow-up.
A study by Willis et al revealed that patients with more severe NPDR/PDR were more likely to be aware that diabetes had impacted their eyes compared to those with mild/moderate NPDR (81.5% versus 21.7%, respectively).28 Other studies examining the accuracy of self-reporting for the true presence or absence of DR have found generally poor sensitivity (9–42%) but high specificity (94–98%) for the accuracy of self-reporting.12–14
Beyond the diabetes literature, an analogous study in patients with chronic kidney disease (CKD) found that individuals with advancing stage of disease were more likely to self-report the presence of CKD.29
In our current study, patients with advanced stage of retinopathy were more likely to self-report the presence of DR, with 92% of the severe group compared to 65% of the mild/moderate group reporting DR. In contrast, when examining the accuracy of self-reported disease severity, 85% of those with no retinopathy, 63% with mild/moderate retinopathy, and 35% with severe retinopathy correctly identified their actual stage of retinopathy. Those with severe DR were the least accurate in self-assessing their severity, with 65% underestimating disease stage compared to 35% of those with mild/moderate disease. Compared to patients who accurately self-reported, underestimators were more likely to be younger individuals with longer-standing diabetes requiring insulin. They were also more likely to report being told they have “fluid or abnormal blood vessels” and to have received laser treatment or eye injections. This implies that despite escalation of care to such interventions, patients may not appreciate the severity of their disease. Interestingly, our study did not detect any differences between the underestimators and those who were accurate in terms of education level or control of diabetes.
While underestimators have a clear clinical relevance, overestimators also represent an important subgroup. The belief that one has more severe disease than actually exists may lead to an unfortunate burden of unwarranted anxiety. Forty-one of 319 patients (13%) were overestimators. Overestimators were older and more likely to report being told that “fluid” is affecting their vision than compared to those who reported accurately. As almost all of these patients had no actual DR, this may suggest a breakdown of communication between the ophthalmologist and this subset of patients.
With regards to barriers to DR screening, the most prevalent in descending order were lack of time, limited access to a doctor, and cost. Approximately one-third of patients reported no barrier. This is consistent with what has been previously identified in the literature. A systematic review of 69 primary studies reported the most common category of barriers was “environmental context and resources,” which encompassed time, accessibility to screening services, scheduling issues, and financial concerns.30 Another large study undertaken at a predominantly indigent clinic found cost to be the principal barrier, followed by access and “personal reasons.”31
Our study had several inherent limitations. It was conducted via email invitation with a response rate of 10% resulting in a small overall sample of 324 patients. This may have further led to associated non-response bias. Table 1 shows that men, white individuals, and those with type 1 diabetes were more likely to respond. The prevalence of DR as assessed by ICD codes was similar between responders and non-responders; the chart-reviewed prevalence of DR in the responders (21%) was also similar to the US population prevalence of 28.5%. The accuracy of the diabetes and DR diagnoses in Table 1 was limited by the reliance on ICD codes identified by EDW report. Severity of DR amongst non-responders was unknown, and the accuracy of severity assessment amongst responders was limited by the use of retrospective chart review. Additionally, the majority of patients who responded to the study had no DR and accurately self-reported their absence of DR. Thus, the group of accurate patients was dominated by this subgroup of patients. The sample size of patients with other stages of DR was particularly limited. Lastly, the frequency of underestimation of disease severity may vary with the specific population, with our results reflective of a tertiary care center in an urban setting.
This study is the first to examine the accuracy of patients’ self-reporting of diabetic retinopathy severity. We found that a large proportion of individuals with DR underestimate the severity of their disease via self-reporting. Although patients with severe DR were most likely to report presence of retinopathy as compared to their milder counterparts, they often underestimated disease severity. Similarly, many patients with mild/moderate disease were unaware that they were affected by retinopathy. Improved patient education and physician-to-patient communication is crucial to ensuring that patients have an accurate understanding of their disease severity, thus facilitating early intervention and consistent follow-up to prevent vision loss particularly as retinopathy advances. Future studies should focus on further defining the population at risk and barriers that exist to accurate understanding of their disease to allow for effective educational practices to bridge this gap in diabetic care.
Funding Statement
Northwestern Medicine Enterprise Data Warehouse Pilot Data Program Chicago, IL. Illinois Society for the Prevention of Blindness Chicago, IL. The sponsor or funding organization had no role in the design or conduct of this research.
Abbreviations
DM, diabetes mellitus; DR, diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; DME, diabetic macular edema; EDW, enterprise data warehouse; EMR, electronic medical record; ICD-10, International Classification of Diseases, Tenth Revision; IRB, Institutional Review Board.
Ethics Approval and Informed Consent
This study was prospectively approved by the Northwestern University Institutional Review Board Office (STU00209048). Informed consent was obtained from all participants via electronic signature.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
No conflicting relationship exists for any author and the authors report no conflicts of interest in this work.
References
- 1.Klein BEK. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720 [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA; 2020. [Google Scholar]
- 4.Mukamel DB, Bresnick GH, Wang Q, Dickey CF. Barriers to compliance with screening guidelines for diabetic retinopathy. Ophthalmic Epidemiol. 1999;6(1):61–72. doi: 10.1076/opep.6.1.61.1563 [DOI] [PubMed] [Google Scholar]
- 5.Harwell TS, Dettori N, McDowall JM, et al. Do persons with diabetes know their (A1C) number? Diabetes Educ. 2002;28(1):99–105. doi: 10.1177/014572170202800111 [DOI] [PubMed] [Google Scholar]
- 6.Hussain R, Rajesh B, Giridhar A, et al. Knowledge and awareness about diabetes mellitus and diabetic retinopathy in suburban population of a South Indian state and its practice among the patients with diabetes mellitus: a population-based study. Indian J Ophthalmol. 2016;64(4):272–276. doi: 10.4103/0301-4738.182937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston PM, Wood CA, McCarty CA, Harper CA, Keeffe JE, Taylor HR. Awareness of diabetic retinopathy among people who attended a diabetic retinopathy screening program. Med J Aust. 1998;169(2):117. doi: 10.5694/j.1326-5377.1998.tb140205.x [DOI] [PubMed] [Google Scholar]
- 8.Bakkar MM, Haddad MF, Gammoh YS. Awareness of diabetic retinopathy among patients with type 2 diabetes mellitus in Jordan. Diabetes Metab Syndr Obes. 2017;10:435–441. doi: 10.2147/DMSO.S140841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajunisah I, Wong P, Tan L, Rokiah P, Reddy S. Awareness of eye complications and prevalence of retinopathy in the first visit to eye clinic among type 2 diabetic patients. Int J Ophthalmol. 2011;4(5):519–524. doi: 10.3980/j.issn.2222-3959.2011.05.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter CM. Understanding diabetes and the role of psychology in its prevention and treatment. Am Psychol. 2016;71(7):515–525. doi: 10.1037/a0040344 [DOI] [PubMed] [Google Scholar]
- 11.Alogna M. Perception of severity of disease and health locus of control in compliant and noncompliant diabetic patients. Diabetes Care. 1980;3(4):533–534. doi: 10.2337/diacare.3.4.533 [DOI] [PubMed] [Google Scholar]
- 12.Foreman J, Xie J, Keel S, van Wijngaarden P, Taylor HR, Dirani M. The validity of self-report of eye diseases in participants with vision loss in the National Eye Health Survey. Sci Rep. 2017;7(1):8757. doi: 10.1038/s41598-017-09421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacLennan PA, McGwin GJ, Searcey K, Owsley C. Medical record validation of self-reported eye diseases and eye care utilization among older adults. Curr Eye Res. 2013;38(1):1–8. doi: 10.3109/02713683.2012.733054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patty L, Wu C, Torres M, Azen S, Varma R. Validity of self-reported eye disease and treatment in a population-based study: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119(9):1725–1730. doi: 10.1016/j.ophtha.2012.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korobelnik J-F, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the Phase 3 GALILEO Study. Ophthalmology. 2014;121(1):202–208. doi: 10.1016/j.ophtha.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 16.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS Study. Ophthalmology. 2014;121(7):1414–1420.e1. doi: 10.1016/j.ophtha.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 18.Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III Study. Ophthalmology. 2010;117(6):1124–1133.e1. doi: 10.1016/j.ophtha.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 19.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a Phase III Study. Ophthalmology. 2010;117(6):1102–1112.e1. doi: 10.1016/j.ophtha.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 21.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR Study. Ophthalmology. 2009;116(1):57–65.e5. doi: 10.1016/j.ophtha.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 23.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375–381. doi: 10.1016/j.ophtha.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross JG, Glassman AR, Jampol LM, Writing Committee for the Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137–2146. doi: 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heier JS, Korobelnik J-F, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123(11):2376–2385. doi: 10.1016/j.ophtha.2016.07.032 [DOI] [PubMed] [Google Scholar]
- 27.Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina. 2018;2(10):997–1009. doi: 10.1016/j.oret.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 28.Willis JR, Doan QV, Gleeson M, et al. Self-reported healthcare utilization by adults with diabetic retinopathy in the United States. Ophthalmic Epidemiol. 2018;25(5–6):365–372. doi: 10.1080/09286586.2018.1489970 [DOI] [PubMed] [Google Scholar]
- 29.Saunders MR, Kim SD, Patel N, Meltzer DO, Chin MH. Hospitalized patients frequently unaware of their chronic kidney disease. J Hosp Med. 2015;10(9):619–622. doi: 10.1002/jhm.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham-Rowe E, Lorencatto F, Lawrenson JG, et al. Barriers to and enablers of diabetic retinopathy screening attendance: a systematic review of published and grey literature. Diabet Med. 2018;35(10):1308–1319. doi: 10.1111/dme.13686 [DOI] [PubMed] [Google Scholar]
- 31.Hartnett ME, Key IJ, Loyacano NM, Horswell RL, DeSalvo KB. Perceived barriers to diabetic eye care: qualitative study of patients and physicians. Arch Ophthalmol. 2005;123(3):387–391. doi: 10.1001/archopht.123.3.387 [DOI] [PubMed] [Google Scholar]