Abstract
Aims
The aim of this study is to determine factors associated with long‐term recovery of left ventricular ejection fraction (LVEF) in patients with heart failure with reduced EF (HFrEF) and if further recovery also occurs in this group.
Methods and results
Among 621 participants enrolled in the Alberta Heart Failure Etiology and Analysis Team (HEART) Study, 316 with Stage C HF underwent comprehensive imaging and biomarker testing at enrolment and at 1‐year follow up. Using pre‐enrolment data, HF with recovered EF (HFrecEF) was defined as an absolute improvement ≥5% in LVEF from the prior lowest LVEF value, with a final LVEF value > 35% at or prior to study baseline. Participants with all LVEF > 40% were included for comparison. Hospitalization‐free survival to 5 years was performed.
The median cohort age was 66 years, and time from diagnosis was 4 years; 82% were male patients. Of the 316 patients, 95 (30%) patients had HFrecEF and 56 (18%) patients pHFrEF. On multivariate analysis, only shorter duration of HF was predictive of HFrecEF status. Over 1 year, LVEF increased in the HFrecEF group 4.0% (0.15–7.90, P = 0.042) as compared with persistent HFrEF, who in turn demonstrated higher baseline serum high sensitivity Troponin‐T with further increase at follow up 0.55(0.33–0.86, P = 0.011). No change in any parameter in the HFpEF/HFmrEF group at follow up was observed.
Conclusions
Patients with HFrecEF demonstrate evidence of additional late improvement in LVEF and unchanged troponin levels, in contrast to those with persistent HFrEF, where LVEF does not improve and serum troponin rises over time. These data help to inform mechanisms relating to late LV remodelling.
Keywords: Heart failure, Left ventricular remodelling, Troponin, Biomarkers
Introduction
Treatment of patients with left ventricular (LV) systolic dysfunction using evidence‐based medical therapy is associated with improved symptoms and reduced morbidity and mortality. 1 , 2 , 3 , 4 , 5 Previous reports suggest that patients may demonstrate significant improvement in LV ejection fraction (EF) with evidence‐based medical therapy (i.e. reverse remodelling), also referred to as heart failure with recovered EF (HFrecEF). 2 , 6 , 7 This phenomenon occurs in 20–45% of patients with HF with reduced EF (HFrEF), is associated with improved prognosis, and is more commonly seen in patients of female gender, non‐ischaemic aetiology of HF, shorter duration of HF, and persistence of beta blocker use. 1 , 6 , 7 However, few reports detail serial clinical, biomarker, and imaging correlates of patients previously experiencing reverse remodelling. The Alberta Heart Failure and Etiology Analysis Team (HEART) study, patients with American Heart Association Stage B or C HF (irrespective of LVEF) were enrolled and followed with serial testing. 8 The aim of this analysis was to characterize and compare study participants with HFrecEF vs. those with persistent HFrEF regarding baseline characteristics, changes in imaging and laboratory markers, and quality of life and associate these with clinical outcomes.
Methods
Study cohort
The study was approved at both institutional Research Ethics Boards, and all study participants signed informed consent prior to enrolment. The investigation conforms with all principles outlined in the Declaration of Helsinki. Details of the study participants of the Alberta HEART study have been previously described. 8 In brief, participants were recruited in Alberta, Canada from 2010 to 2014 from a variety of outpatient clinics and the community at large. We prospectively enrolled 621 participants and classified each into one of five groups: (i) at risk of developing HFpEF/HFmrEF and no clinically overt HF or known cardiovascular disease; (ii) at risk of developing HFpEF/HFmrEF but no clinically overt HF and the presence of another symptomatic disease (e.g. chronic lung disease); (iii) clinical HFpEF/HFmrEF; (iv) clinical HFrEF; and (v) age‐matched and gender‐matched controls. For the purposes of this analysis, only groups 3 and 4 are included.
Data sources
Standard baseline demographics, medical history, physical examination, quality of life (using the Kansas City Cardiomyopathy Clinical Summary Score), medication use, and laboratory and imaging data were collected at study enrolment and 1‐year follow up.
Additional data on clinical outcomes were extracted from administrative databases by the regional health authority, Alberta Health Services. These include (i) the Discharge Abstract Database, which contains diagnostic and treatment information and discharge status for patients admitted to any acute care hospital in Alberta; (ii) the National Ambulatory Care Reporting System database, which records all outpatient clinic visits (including emergency department visits) in Alberta; and (iii) the Alberta Health Care Insurance Registry database, which tracks the vital status of all residents of Alberta. These databases are linkable to our study cohort using a unique and anonymous patient identifier. The primary study data were collected and managed using RedCap electronic data management tools hosted at the University of Alberta. All patients were followed for clinical outcomes for a period of 5 years.
Ejection fraction data and classification of responder status
Historic echocardiographic LVEF measurements clinically performed prior to study enrolment were collected from the medical record. Participants for this analysis were included if they were determined at study baseline to have (i) Stage C HF, (ii) at least two prior adequate studies with a recorded LVEF, and (ii) at least 1 year between first and most recent measurements (including baseline). Historical LVEF measurements were not adjudicated. Reported values for LVEF were recorded; if a range was reported in any report, we recorded the mean value and rounded and decimal > 0.5 to the next highest (i.e. 32.5% was recorded as 33%) and 0.4 or less to the lower number (i.e. 32.4% to 32%). Duration of HF was determined by using the earliest date of HF diagnosis from any available clinic note or by direct patient history.
At study baseline and 1‐year follow up, all participants underwent 2D echocardiography. All measurements were obtained using a commercially available ultrasound system equipped with a 1–5 MHz transducer (iE33, Phillips Medical Systems, Andover, Massachusetts) and archived for offline assessment. All images were acquired according to American Society of Echocardiography recommendations. LVEF was quantified using the 2D biplane Simpson's rule.
All prior historical LVEF values, together with the study baseline LVEF, were used for participant classification. Study subjects with recovered LVEF (HFrecEF) if at least one historical LVEF measured <35% and a subsequent or study baseline LVEF measured >35% and an absolute improvement ≥5% in LVEF from the prior lowest LVEF value. Participants were classified as persistent HFrEF if any prior LVEF measured < 35% and they did not experience improvement as previously mentioned and as HF with preserved ejection (HFpEF/HFmrEF) fraction if no prior or baseline LVEF measured < 40%.
Outcomes
The outcomes of interest included change from study enrolment to 1‐year follow‐up for (i) echocardiographic LVEF; and (ii) serum biomarkers, inclusive of B‐type natriuretic peptide (BNP), N‐terminal pro‐BNP (NT‐proBNP), and high sensitivity Troponin‐T (hs‐TnT). Clinical outcomes included hospitalization‐free survival at 1 year and change from baseline Kansas City Cardiomyopathy Questionnaire Clinical Summary Score.
Statistical analysis
Patient characteristics at enrolment were compared for each of the HFrecEF, persistent HFrEF, and HFpEF/HFmrEF groups. Median and interquartile interval (IQR) for continuous variables and frequency (%) for categorical variables were estimated and compared between the groups using the Kruskal‑Wallis one‐way analysis of variance and the Pearson χ 2 test, respectively. For comparisons of change from baseline, HFrecEF and HFrEF groups were compared with each other. For participants in the HFpEF/HFmrEF group, only in‐group baseline to 1‐year follow up changes were evaluated. We used logistic regression models to examine the unadjusted and independent factors associated with the likelihood to respond (HFrecEF vs. persistent HFrEF). Odds ratios were calculated with 95% confidence intervals (CIs). Modelling assumption of linearity and normality of continuous risk factors were assessed. Flexible modelling that relaxes the linearity and/or appropriate transformation for skewed variables has been applied when the assumptions failed.
For the comparison of the change in the diagnostic imaging parameters, biomarker values, and the quality of life measures, the linear mixed effects model for repeated measure outcomes was applied. The model comprises the responses of the three groups and its interaction with time (baseline, 12 months) as fixed‐effects component and patient‐specific random intercept to account for the correlation of repeated measurements within a subject. The distribution of the measurements was assessed for violation of the normality assumption and was log transformed if necessary.
Proportional hazards Cox regression was used to evaluate whether the HFrecEF patients differ from persistent HFpEF/HFmrEF regarding hospitalization and the composite of hospitalization and emergency department visit at 1 year. Both univariable and multivariable regression models were used to determine the unadjusted and adjusted effect estimates of hazard ratios (HRs) and 95% CI. Baseline risk factors that were different expressed between the groups in a univariable analysis were considered in the adjusted model. The factors include age (per 10 years and split at 65 years), gender, aetiology of HF (ischaemic vs. non‐ischaemic), duration of HF, history of coronary artery disease, and previous coronary revascularization (coronary artery bypass grafting or percutaneous coronary intervention). Kaplan–Meier method was used to estimate the event‐free survival rates, and the log‐rank test was applied to evaluate significance of the difference between the three groups. No imputation for missing data was performed for any variable. A two‐sided test with P value < 0.05 was regarded as significant. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Study cohort
The cohort distribution is shown in Figure 1 . Among the 621 patients, 261 without Stage C HF were excluded and an additional 44 were subsequently excluded for either insufficient echocardiographic data (29 patients) or a bidirectional LVEF pattern (15 patients, including 4 with initial EF > 40% falling to below 40% and 11 with initial improvement in LVEF and subsequent fall to below 40%), leaving 316 for this analysis. The median age (IQR) was 69 (60.78) years. Most of our cohort were Caucasian (91%), male patients (63%), suffered from non‐ischaemic HF aetiology, (64%) and reported HF as a diagnosis for median 4 (IQR: 1.5–8) years duration at the time of enrolment. We identified 95 participants with HFrecEF, 56 who did not improve LVEF (persistent HFrEF) and 165 with persistent HFpEF/HFmrEF.
FIGURE 1.
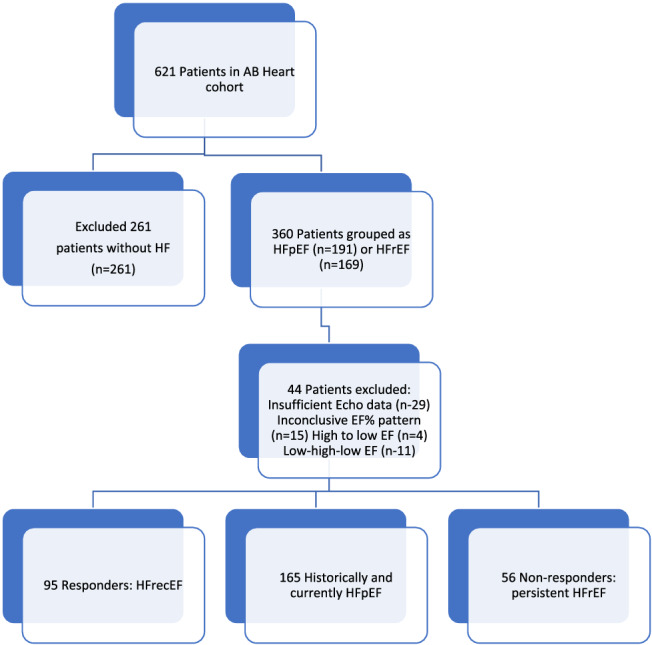
CONSORT diagram of patient disposition. Measurements of left ventricular ejection fraction (LVEF) included all obtained historical data (not evaluated in the core lab) and the study baseline echo (evaluated in the core lab). Participants were included if there were at least two LVEF measurements of adequate quality, measured at least 1 year apart. They were classified as heart failure with recovered ejection fraction (HFrecEF) if at least one historical LVEF measured <35% and a subsequent or study baseline LVEF measured >35% and was at least 5% higher than the lowest previous LVEF. Participants were classified as persistent heart failure with reduced ejection fraction if any prior LVEF measured <35% and they did not experience improvement as previously mentioned and as heart failure with preserved ejection (heart failure with mid‐range ejection fraction) fraction if no prior or baseline LVEF measured <40%.
Baseline characteristics by heart failure group
Overall, participants with persistent HFpEF/HFmrEF were older, more likely to be female participants, to have a non‐ischaemic aetiology of HF, a history of hypertension, atrial fibrillation, and less likely to be treated with beta blockers when compared with pooled participants in both HFrecEF and persistent HFrEF groups (Table 1 ). There was no difference in baseline medical therapy, quality of life scores, biomarker values, or left atrial volume index between groups (Table 2 ).
TABLE 1.
Patient characteristics by the response group (n = 316)
| Baseline characteristics | Response status | |||
|---|---|---|---|---|
|
HFrecEF (n = 95) |
Persistent HFrEF (n = 56) |
HFpEF/HFmrEF (n = 165) |
Overall P value |
|
| Age, median (IQR) | 66 (55, 74) | 65 (58, 74) | 73(64,81) *** | <.0001 |
| Gender (female), % | 28 (29.5) | 9 (16.1) | 80(48.5) *** | <.0001 |
| Ethnicity | 0.66 | |||
| Caucasian | 82 (86.3) | 53 (94.6) | 151(91.5) | |
| Aboriginal | 3 (3.2) | 1 (1.8) | 3(1.8) | |
| South Asian | 7 (7.4) | 1 (1.8) | 6(3.6) | |
| Other | 3 (3.2) | 1 (1.8) | 5(3.0) | |
| CCS angina classification | 0.43 | |||
| 0 | 82(86.3) | 43(76.8) | 126(76.4) | |
| >1 | 11(11.6) | 10(17.9) | 26(15.8) | |
| Not available | 2(2.1) | 3(5.3) | 13(7.9) | |
| NYHA functional classification | 0.49 | |||
| Class I | 26 (27.4) | 11 (19.6) | 38(23.0) | |
| Class II‐IV | 67 (70.5) | 45 (80.4) | 125(75.8) | |
| Not available | 2 (2.1) | 0 (0.0) | 2(1.2) | |
| Quality of life | ||||
| KCCQ‐CSS | 77.0 ± 2.2 | 75.8 ± 2.8 | 69.1 ± 1.67 | |
| Primary aetiology of heart failure | 0.0003 | |||
| Ischaemic | 32 (33.7) ** | 32 (57.1) | 45(27.3) *** | |
| Non‐ischaemic | 62 (65.3) | 23 (41.1) | 116(70.3) | |
| Not available | 1(1.1) | 1(1.8) | 4(2.4) | |
| Duration of HF diagnosis, (median IQR, months) | 24 (12, 72)** | 78 (24, 120) | 48(18,96) * | 0.0015 |
| Medical comorbidity | ||||
| Hypertension | 50 (52.6) | 28 (50.0) | 128 (77.6) *** | <.0001 |
| Diabetes | 33 (34.7) | 20 (35.7) | 65(39.4) | 0.73 |
| Coronary artery disease | 37 (38.9) * | 33 (58.9) | 65(39.4) * | 0.026 |
| Atrial fibrillation | 34 (35.8) | 26 (46.4) | 85(51.5) | 0.049 |
| Chronic obstructive pulmonary disease | 19 (20.0) | 10 (17.9) | 36(21.8) | 0.81 |
| Previous CABG | 10 (10.5) * | 14 (25.0) | 24(14.5) | 0.054 |
| Previous PCI | 19 (20.0) * | 22 (39.3) | 30(18.2) ** | 0.0038 |
| Medication use, % | ||||
| Beta blocker | 93 (97.9) | 55 (98.2) | 136(82.4) ** | <.0001 |
| ACEi/ARB | 85 (89.5) | 52 (92.9) | 136(82.4) | 0.083 |
| Diuretic | 75 (78.9) | 44 (78.6) | 136(82.4) | 0.72 |
| Statins | 59 (62.1) | 37 (66.1) | 111(67.3) | 0.70 |
| ASA | 60 (63.2) | 41 (73.2) | 93(56.4) * | 0.075 |
| Digoxin | 17 (17.9) | 9 (16.1) | 16(9.7) | 0.14 |
| Antiplatelet | 11 (11.6) | 9 (16.1) | 11(6.7) * | 0.097 |
| Anticoagulant | 41 (43.2) | 27 (48.2) | 79(47.9) | 0.73 |
ACEi/ARB, angiotensin II converting enzyme inhibitor/angiotensin II receptor blocker; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; HFrecEF, heart failure with recovered ejection fraction (responder); HFrEF, persistent heart failure with reduced ejection fraction; HFpEF/HFmrEF, heart failure with preserved ejection fraction; IQR, interquartile interval; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
P < 0.05 compared with HFrEF.
P < 0.01 compared with HFrEF.
P < 0.001 compared with non‐responders.
TABLE 2.
Heart failure with recovered ejection fraction and heart failure with preserved ejection fraction vs. persistent heart failure with reduced ejection fraction: change from baseline to 1‐year follow up according to heart failure with recovered ejection fraction, persistent heart failure with reduced ejection fraction, and heart failure with preserved ejection fraction; echocardiographic, biomarker, and quality of life parameters
| Parameter (mean ± SE) | Difference/ratio in rate of change (Δβ1 a ) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparators | HFrecEF (n = 95) | Persistent HFrEF (n = 56) | HFpEF/HFmrEF (n = 165) | HFrecEF vs. HFrEF | HFpEF/HFmrEF vs HFrEF | |||||
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Δβ1(95%CI) | P value | Δβ1 (95% CI) | P value | |
| Echocardiographic measurements | ||||||||||
| LV ejection fraction (%) | 44.8 ± 1.0 | 49.9 ± 1.0 | 24.7 ± 1.2 | 25.8 ± 1.6 | 58.0 ± 0.7 | 59.6 ± 0.8 | 4.0(0.15–7.90) | 0.042 | 0.51(−3.11–4.13) | 0.78 |
| RVSP+ | 31.9 ± 2.0 b | 32.8 ± 2.1 | 40.2 ± 2.8 | 39.5 ± 3.3 | 34.4 ± 1.6NS | 33.7 ± 1.6 | 1.04(0.82–1.33) | 0.72 | 1.0(0.80–1.24) | 0.96 |
| LVIDd (cm) | 5.37 ± 0.080 b | 5.32 ± 0.082 | 6.39 ± 0.10 | 6.33 ± 0.12 | 4.77 ± 0.06 b | 4.76 ± 0.062 | 0.013(−0.20–0.23) | 0.90 | 0.05(−0.15–0.25) | 0.62 |
| LVIDs (cm) | 4.09 ± 0.090 b | 3.94 ± 0.088 | 5.57 ± 0.11 | 5.50 ± 0.13 | 3.17 ± 0.064 b | 3.15 ± 0.067 | −0.088(−0.36–0.18) | 0.52 | 0.054(−0.19–0.30) | 0.67 |
| LAVi (mL/m2)+ | 31.8 ± 1.5 b | 33.1 ± 1.6 | 42.7 ± 2.6 | 45.0 ± 3.1 | 36.1 ± 1.3 b | 36.4 ± 1.3 | 0.99(0.86–1.13) | 0.86 | 0.95(0.84–1.08) | 0.46 |
| Biomarkers | ||||||||||
| BNP+ (pmol/mL) | 96.7 ± 11.84 b | 87.0 ± 11.0 | 282.7 ± 41.8 | 269.9 ± 43.6 | 121.4 ± 10.7 b | 117.6 ± 10.6 | 0.94(0.72–1.24) | 0.67 | 1.01(0.79–1.30) | 0.89 |
|
NT‐proBNP+ (pmol/mL) |
61.2 ± 9.1 b | 50.8 ± 7.8 | 174.0 ± 31.3 | 170.3 ± 33.2 | 64.6 ± 7.0 b | 67.3 ± 7.4 | 0.85(0.62–1.16) | 0.30 | 1.07(0.80–1.42) | 0.66 |
|
Troponin+ (ng/L) |
0.012 ± 0.0017 b | 0.0096 ± 0.0014 | 0.017 ± 0.0028 | 0.025 ± 0.0048 | 0.01 ± 0.001 b | 0.011 ± 0.0011 | 0.55(0.33–0.86) | 0.010 | 0.76(0.48–1.14) | 0.17 |
| Haemoglobin (mg/dL) | 140.4 ± 1.9NS | 139.9 ± 2.0 | 137.2 ± 2.6 | 137.1 ± 2.9 | 133.1 ± 1.5NS | 132.0 ± 1.5 | −0.37(−7.06–6.32) | 0.91 | −0.93(−3.25–9.56) | 0.33 |
| ALT+ (IU/mL) | 23.3 ± 1.5NS | 26.9 ± 6.3 | 27.5 ± 2.4 | 23.2 ± 5.5 | 22.1 ± 1.1NS | 25.8 ± 3.7 | 1.36(0.56–3.31) | 0.41 | 1.41(0.68–2.93) | 0.28 |
|
Creatinine+ (umol/L) |
92.7 ± 3.5 b | 96.5 ± 3.6 | 107.3 ± 5.3 | 105.5 ± 5.6 | 104.3 ± 2.9NS | 106.4 ± 3.1 | 1.06(0.96–1.17) | 0.26 | 1.04(0.95–1.14) | 0.43 |
|
Potassium (mmol/L) |
4.45 ± 0.05NS | 4.55 ± 0.05 | 4.56 ± 0.07 | 4.47 ± 0.08 | 4.37 ± 0.038 b | 4.33 ± 0.04 | 0.19(−0.03–0.41) | 0.09 | 0.06(−0.14–0.27) | 0.55 |
|
Sodium+ (pmol/mL) |
139.8 ± 0.4 | 139.4 ± 0.4 | 139.4 ± 0.5 | 139.2 ± 0.6 | 140.0 ± 0.3 | 139.4 ± 0.3 | 1.00(0.99–1.01) | 0.82 | 1.00(0.99–1.01) | 0.63 |
| Quality of life | ||||||||||
| KCCQ Clinical Score | 77.0 ± 2.2 | 77.0 ± 2.2 | 75.8 ± 2.8 | 77.7 ± 3.2 | 69.1 ± 1.67 | 68.3 ± 1.73 | −1.83(−7.6–3.92) | 0.53 | −2.69(−8.09–2.72) | 0.33 |
| KCCQ Overall Score | 73.6 ± 2.3 | 74.5 ± 2.4 | 72.5 ± 3.0 | 74.0 ± 3.3 | 67.9 ± 1.74 | 67.4 ± 1.8 | −0.68(−6.66–5.30) | 0.82 | −2.08(−7.7–3.54) | 0.47 |
ALT, alanine aminotransferase; BNP, brain natriuretic peptide; HFpEF/HFmrEF, heart failure with preserved ejection fraction; HFrecEF, heart failure with recovered left ventricular ejection fraction; HFrEF, heart failure with reduced left ventricular ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LAVi, left atrial volume index; LVESVi, left ventricular end systolic volume index; LVIDd, left ventricular end diastolic dimension; LVIDs, end systolic left ventricular dimension; NS, not significant baseline difference compared with persistent HFrEF group; NT‐proBNP, N‐Terminal brain natriuretic peptide; RVESVi, right ventricular end systolic volume index; RVSP, estimated right ventricular systolic pressure.
Δ β1 is the derived from fixed effect slope in the random effects model and it measures the difference in the rate of change or the ratio of the rates of changes (for variables indicated ‘+’ for which the model was fitted in a log‐scale).
Significant difference (P < 0.05) at baseline compared with persistent HFrEF.
As shown in Tables 1 and 2 , participants with HFrecEF were more likely than those with persistent HFrEF to be female participants (28% vs. 16%; P = 0.064), present with non‐ischaemic HF (65% vs. 41%; P = 0.0041), reported shorter duration of HF [median (IQR): 24 (12.72) months vs. 78 (24.120); P = 0.0001], and a lower prevalence of prior revascularization (coronary artery bypass grafting: 10.5% vs. 25% and percutaneous coronary intervention: 20% vs. 39.3%). At study baseline, LVEF was higher in the HFrecEF group while RVSP, LV diameter, and end‐systolic and end‐diastolic volume index were lower. In addition, baseline serum BNP, NT‐proBNP, and hs‐TnT were all lower (Table 3 ) in the HFrecEF group. There were no differences in medical therapy or quality of life scores observed between the two groups.
TABLE 3.
Baseline characteristics associated with responder (heart failure with recovered ejection fraction) status (n = 95)
| Factors | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, per 10 years | ||||
| ≤65 | 0.72(0.43–1.26) | 0.26 | 1.17(0.58–2.37) | 0.66 |
| >65 | 1.42(0.74–2.75) | 0.29 | 1.37(0.61–3.10) | 0.45 |
| Gender: female vs. male | 2.18(0.94–5.05) | 0.068 | 2.37(0.75–7.53) | 0.14 |
| CCS angina classification | 0.25 | |||
| 0 | ref | ‐ | ||
| ≥1 | 0.58(0.23–1.47) | ‐ | ||
| NYHA functional class | 0.19 | |||
| Class I | ref | ‐ | ||
| Class II‐IV | 0.59(0.27–1.29) | ‐ | ||
| Primary aetiology of HF | 0.004 | 0.82 | ||
| Ischaemic vs. non‐ischaemic | 0.37(0.19–0.74) | 0.80(0.12–5.42) | ||
|
Duration of HF (months log scale) |
0.48(0.32–0.73) | 0.0001 | 0.48(0.31–0.76) | 0.0016 |
| Medical comorbidity | ||||
| Hypertension | 1.11(0.57–2.15) | 0.75 | ‐ | |
| Diabetes | 0.96(0.48–1.91) | 0.90 | ‐ | |
| Coronary artery disease | 0.45(0.23–0.87) | 0.018 | 0.64(0.12–3.35) | 0.60 |
| Atrial fibrillation | 0.64(0.33–1.26) | 0.20 | ‐ | |
| COPD | 1.15(0.49–2.67) | 0.75 | ‐ | |
| Previous CABG or PCI | 0.36(0.18–0.72) | 0.0034 | 0.68(0.17–2.72) | 0.58 |
| Medication use | ||||
| Beta blocker | 0.85(0.08–9.54) | 0.89 | ‐ | |
| ACEi/ARB | 0.65(0.20–2.19) | 0.48 | ‐ | |
| Diuretic | 1.02(0.46–2.29) | 0.96 | ‐ | |
| Statins | 0.84(0.42–1.68) | 0.62 | ‐ | |
| ASA | 0.63(0.30–1.29) | 0.20 | ||
| Digoxin | 1.14(0.47–2.76) | 0.77 | ‐ | |
| Antiplatelet | 0.68(0.26–1.77) | 0.44 | ‐ | |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; COPD, chronic obstructive pulmonary disease; HF, heart failure; HFrecEF, heart failure with recovered ejection fraction (responder); NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Table 3 summarizes the association of baseline characteristics to response status. In the adjusted analysis, only the duration of HF was found to be independently associated with recovery of LVEF [adjusted odds ratios (95% CI): 0.48(0.31–0.76)].
Cardiac imaging and biomarkers: change from baseline to 1 year
Participants with HFrecEF demonstrated an increase in LVEF at 1 year (44.9 ± 1.0 to 49.9 ± 1.0, P < 0.001) and a decrease in LV end systolic diameter (4.09 ± 0.09 to 3.94 ± 0.088 P = 0.043. Compared with that of the persistent HFrEF, the mean change in LVEF was significantly higher [Δβ1(95% CI): 4.0% (0.15–7.90), P = 0.042] while there were not significant changes nor difference of changes in right ventricular systolic pressure or left atrial volume index (Table 2 ). Participants with persistent HFrEF did not demonstrate change from baseline in any of these parameters. There was no difference in change from baseline to 1 year in BNP or NT‐proBNP in either group. However, baseline hs‐TnT did not change from baseline in the HFrecEF group (12 ± 17 to 9 ± 14) while it increased at 1‐year follow up in the persistent HFrEF group (17 ± 3 to 25 ± 5), a difference of 6 (3–9, P = 0.01). Participants with persistent HFpEF/HFmrEF did not demonstrate significant change from baseline in any echocardiographic, or biomarker parameter.
Clinical outcomes at 1 year
Figure 2 shows higher hospitalization‐free survival in the HFrecEF group with similar event rates compared with the persistent HFrEF and HFpEF/HFmrEF groups [adjusted HR: 0.40 (0.18–0.90)], while event rates between persistent HFrEF and HFpEF/HFmrEF groups were similar [HR, 1.16 (0.59–2.29)]. No differences in the change in quality of life over time were observed in any of the three groups.
FIGURE 2.
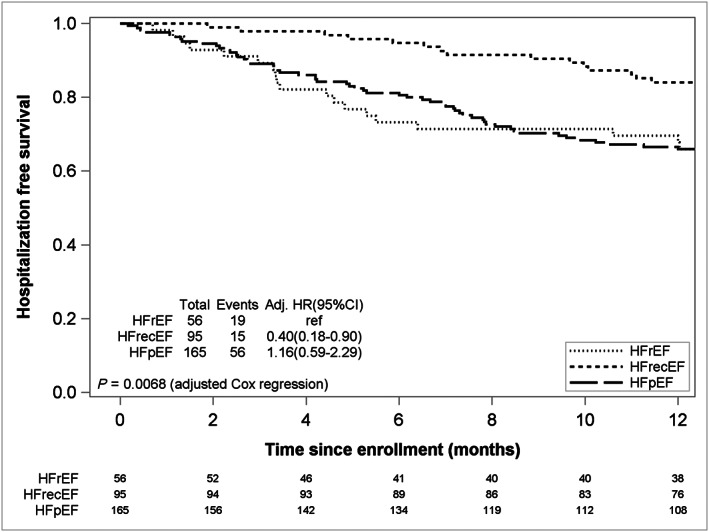
Hospitalization free survival at 1 year by response. HFrecEF, heart failure with recovered ejection fraction (responder); HFrEF, persistent heart failure with reduced ejection fraction; HFpEF/HFmrEF, heart failure with preserved ejection fraction. Ref: Reference group,(HFrEF); *adjusted for age per 10 years and split at 65, gender, type of HF (ischaemic vs. non‐ischaemic), duration of heart failure, coronary artery disease, previous coronary artery bypass grafting, or percutaneous coronary intervention.
Discussion
Our data confirm the association of baseline patient characteristics related to LVEF recovery 1 , 5 , 6 , 9 , 10 , 11 , and demonstrate two novel findings in patients with pre‐existing HFrecEF: (i) evidence of additional LVEF improvement at a median 2 years following initial diagnosis and (ii) lower baseline and stable serum hs‐TnT levels in contrast to increasing levels in those with persistent HFrEF. Taken together, these findings support the notion that significant improvement in LVEF may occur after 2 years and associate with lower and stable serum levels of cardiac damage.
Reverse remodelling and myocardial recovery
Reduction of LV volume with consequent improvement in LVEF, known as reverse remodelling (LVRR), has been described in 16–70% of patients with HFrEF, largely related to variable definitions according to absolute change in LVEF and duration of follow up. McNamara et al. 13 reported that a 6‐month improvement in LVEF from baseline of >10% occurred in 70% while 40% improved by >20%. Kalogeropoulos et al. 10 utilized a single cut point EF of 40% and reported only 16.5% of their cohort met this criteria while While Bhatt reported LV recovery in 263 of 568 patients using a single cut point EF of 35%. 16
Lupon used a single cut point of 45% in a sample of 1000 patients and found that 25% met criteria for improvement. 5 , 10 We have previously reported a much higher rate of LV improvement (37%) using a EF cut point of 40% in very large cohort (10 641, of whom 3124 have baseline LVEF < 40%). 6 These results compare with previous reviews of reverse remodelling, which suggest a 35% rate of reverse remodelling as defined by >10% improvement in LVEF at 24 months from baseline and with an initial mean improvement of 8–10%. 1 , 7 Thus, the prevalence of LVRR depends greatly upon both definition and disease duration. Importantly, no single definition of LVRR has been validated, which is perhaps not surprising given the continuous nature of this measure. Accordingly, we employed a less restrictive definition of LVRR for our analysis as a means of expanding the sample size of patients with recovered EF.
Predictors of late left ventricular reverse remodelling
Several correlates for LVRR have been reported in most studies, including younger age, female sex, non‐ischaemic aetiology, shorter duration of HF, higher baseline LV volume, and use of beta blockers. 1 , 6 , 7 Other factors reported to associate with LVRR, such as the presence of the minor allele of re777652, a hypocretin receptor‐2. 17 Recently, hs‐TnT has been reported to associate with outcomes in HF with a meta‐analysis of studies suggesting a level < 18 ng/L to predict lower risk of death and hospitalization, but this level was not tested for prediction of reverse remodelling. 18 Others have suggested superior predictive strength for LV recovery of circulating ST2 level, where a score has been developed and validated. 1 , 9 , 19 However, these results were derived from data at initial diagnosis (disease duration < 1 year) and its correlation with LVRR. In our cohort, the median time from diagnosis of HF to study baseline was 4 years. Thus, our results reflect processes occurring much later in the disease journey, a period that has not been studied. Upon availability of our multi‐marker data, we plan to assess these and other biomarkers for prediction of late improvement of LV function and of outcomes.
Characteristics that have been previously reported to be associated with recovery of LVEF were so associated in our cohort, although in multivariate analysis, we found that only duration from HF diagnosis remained predictive. Importantly, we noted patients with persistent HFrEF demonstrated a small but significant increase in hs‐TnT, a finding not previously reported. Although the between‐group differences in hs‐TnT levels were not ‘clinically’ elevated, serial measurement may provide clues to future clinical trajectory. Recent reports indicate subclinical but elevated levels of hs‐TnT associate with incident HF, irrespective of coronary artery disease, 20 , 21 and with worse clinical outcomes following HF decompensation. 18 Similarly, falling levels have been associated with improved prognosis, suggesting that progression of HF is preceded by ongoing myocyte injury. 20 , 22 , 23 In this report, we provide the link between late serial troponin levels and LV function. With upcoming availability of our multi‐marker data, we hope to examine other biomarker correlates of late reverse remodelling, including attempted validation of the ST2‐R2 score.
No differences in quality of life scores at baseline or were seen between our groups, reinforcing that assessment of LV remodelling cannot be ascertained on a clinical basis alone. However, scores in this study were higher than typically seen in patient registries, suggesting a relatively stable cohort with little potential for additional improvement in symptoms or quality of life.
Study limitations
Our study had several limitations. This study enrolled participants from stable outpatient clinics from two tertiary care centres, limiting generalizability. The cohort of 360 patients, of which only 95 demonstrated LVEF recovery, is of modest size, limiting statistical power. While the study baseline and follow up data were interpreted and adjudicated centrally, the historical LVEF values were obtained from several different diagnostic centres, without standardized imaging frequencies and with baseline images were not available for interpretation to confirm the reported EF. This may have increased error in participant categorization and further selection bias. The threshold for improvement in LVEF in our study was 5%, in contrast to previous studies. However, EF is generally continuous measure and therefore no gold standard threshold to dichotomize recovery of EF has been established. Several reports have also included an absolute EF threshold value, such as 35%, 40%, 50%, above, or below which patients were categorized. While each level may have its own merit, EF is a generally continuous measure. We elected to include both an improvement threshold (5%) as well as an absolute level (35%) because this allowed for increased number of HFrecEF classifications and ability to align with previous reporting algorithms employed during historical measurements. The major concern with the use of a 5% absolute increase in EF would be lack of sensitivity for detection of differences between those with recovered vs. non‐recovered EF; however, our findings were consistent with previously reported studies and so we feel that 5% may be a reasonable threshold. Several unmeasured factors associated with reverse remodelling may not have been included in our logistic model for recovery for LVEF, such as medication adherence, family history of non‐ischaemic cardiomyopathy, or other reversible causes of HF such as tachycardia‐mediated cardiomyopathy or substance abuse. Further differences in LVEF or other biomarkers might have been evident with longer follow up. We defined HFrEF as less than 35%. We used this cut point primarily because earlier echocardiographic grading systems used 35% as the cut point in many labs to define severe HFrEF and many of the historical LVEF values were described in this manner. As such, we included patients with historical LVEF 36–40% as having HFpEF/HFmrEF. While we did not observe changes in quality of life scores, baseline levels were high and may limit opportunities for further improvement, particularly in the HFrecEF group.
Conclusions
In this Alberta HEART sub‐study, patients evaluated a median 4 years following diagnosis and with prior recovery of LVEF demonstrate, in addition to previously reported characteristics, lower and stable levels of circulating hs‐TnT and further improvement in LVEF. These findings help to provide insights into the nature of the LV remodelling process and support the importance of markers of low‐level myocyte injury and ongoing surveillance of LVEF, even without any change in symptoms.
Conflict of interest
None declared.
Funding
Funding was provided by an Alberta Innovates–Health Solutions Interdisciplinary Team Grant to Alberta Heart Failure Etiology and Analysis Research Team (Alberta HEART), grant no. AHFMR ITG 200801018.
Howlett, J. G. , Sharma, N. , Alemayehu, W. G. , Dyck, J. R. B. , Anderson, T. , Fine, N. , Becker, H. , White, J. A. , Paterson, D. I. , Thompson, R. B. , Oudit, G. Y. , Haykowsky, M. J. , and Ezekowitz, J. A. (2020) Circulating troponin and further left ventricular ejection fraction improvement in patients with previously recovered left ventricular ejection fraction. ESC Heart Failure, 7: 2725–2733. 10.1002/ehf2.12863.
References
- 1. Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail 2019; 7: 782–794. [DOI] [PubMed] [Google Scholar]
- 2. Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail 2011; 17: 527–532. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 4. Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, Giannetti N, Grzeslo A, Hamilton PG, Heckman GA, Howlett JG, Koshman SL, Lepage S, McKelvie RS, Moe GW, Rajda M, Swiggum E, Virani SA, Zieroth S, Al‐Hesayen A, Cohen‐Solal A, De D'Astous S, Estrella‐Holder E, Fremes S, Green L, Haddad H, Harkness K, Hernandez AF, Kouz S, LeBlanc M‐H, Masoudi FA, Ross HJ, Roussin A, Sussex B. 2017 comprehensive update of the Canadian Cardiovascular Society Guidelines for the management of heart failure. Can J Cardiol 2017; 33: 1342–1433. [DOI] [PubMed] [Google Scholar]
- 5. Lupon J, Diez‐Lopez C, de Antonio M, Domingo M, Zamora E, Moliner P, González B, Santesmases J, Troya MI, Bayés‐Genís A. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail 2017; 19: 1615–1623. [DOI] [PubMed] [Google Scholar]
- 6. Ghimire A, Fine N, Ezekowitz JA, Howlett J, Youngson E, McAlister FA. Frequency, predictors, and prognosis of ejection fraction improvement in heart failure: an echocardiogram‐based registry study. Eur Heart J 2019; 40: 2110–2117. [DOI] [PubMed] [Google Scholar]
- 7. Lupon J, Gavidia‐Bovadilla G, Ferrer E, de Antonio M, Perera‐Lluna A, López‐Ayerbe J, Domingo M, Núñez J, Zamora E, Moliner P, Díaz‐Ruata P, Santesmases J, Bayés‐Genís A. Dynamic trajectories of left ventricular ejection fraction in heart failure. J Am Coll Cardiol 2018; 72: 591–601. [DOI] [PubMed] [Google Scholar]
- 8. Ezekowitz JA, Becher H, Belenkie I, Clark AM, Duff HJ, Friedrich MG, Haykowsky MJ, Howlett JG, Kassiri Z, Kaul P, Kim DH, Knudtson ML, Light PE, Lopaschuk GD, McAlister FA, Noga ML, Oudit GY, Paterson DI, Quan H, Schulz R, Thompson RB, Weeks SG, Anderson TJ, Dyck JRB. The Alberta Heart Failure Etiology and Analysis Research Team (HEART) study. BMC Cardiovasc Disord 2014; 14: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation 2014; 129: 2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol 2016; 1: 510–518. [DOI] [PubMed] [Google Scholar]
- 11. Savarese G, Vedin O, D'Amario D, Uijl A, Dahlström U, Rosano G, Lam CSP, Lund LH. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC Heart Fail 2019; 7: 306–317. [DOI] [PubMed] [Google Scholar]
- 12. Kubanek M, Sramko M, Maluskova J, Kautznerova D, Weichet J, Lupinek P, Vrbska J, Malek I, Kautzner J. Novel predictors of left ventricular reverse remodeling in individuals with recent‐onset dilated cardiomyopathy. J Am Coll Cardiol 2013; 61: 54–63. [DOI] [PubMed] [Google Scholar]
- 13. McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, Gorcsan J 3rd, Kip KE, Dec GW, IMAC Investigators . Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)‐2 study. J Am Coll Cardiol 2011; 58: 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merlo M, Caiffa T, Gobbo M, Adamo L, Sinagra G. Reverse remodeling in dilated cardiomyopathy: insights and future perspectives. Int J Cardiol Heart Vasc 2018; 18: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol 2011; 57: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 16. Bhat PK, Ashwath ML, Rosenbaum DS, Costantini O. Usefulness of left ventricular end‐systolic dimension by echocardiography to predict reverse remodeling in patients with newly diagnosed severe left ventricular systolic dysfunction. Am J Cardiol 2012; 110: 83–87. [DOI] [PubMed] [Google Scholar]
- 17. Perez MV, Pavlovic A, Shang C, Wheeler MT, Miller CL, Liu J, Dewey FE, Pan S, Thanaporn PK, Absher D, Brandimarto J, Salisbury H, Chan K, Mukherjee R, Konadhode RP, Myers RM, Sedehi D, Scammell TE, Quertermous T, Cappola T, Ashley EA. Systems genomics identifies a key role for hypocretin/orexin receptor‐2 in human heart failure. J Am Coll Cardiol 2015; 66: 2522–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aimo A, Januzzi JL, Vergaro G, Ripoli A, Latini R, Masson S, Magnoli M, Anand IS, Cohn JN, Tavazzi L, Tognoni G, Gravning J, Ueland T, Nymo SH, Brunner‐La Rocca H‐P, Genis AB, Lupón J, de Boer RA, Yoshihisa A, Takeishi Y, Egstrup M, Gustafsson I, Gaggin HK, Eggers KM, Huber K, Tentzeris I, Tang WHW, Grodin J, Passino C, Emdin M. Prognostic value of high‐sensitivity troponin T in chronic heart failure. Circulation 2018; 137: 286–297. [DOI] [PubMed] [Google Scholar]
- 19. Lupón J, Gaggin HK, de Antonio M, Domingo M, Galán A, Zamora E, Vila J, Peñafiel J, Urrutia A, Ferrer E, Vallejo N, Januzzi JL, Bayes‐Genis A. Biomarker‐assist score for reverse remodeling prediction in heart failure: the ST2‐R2 score. Int J Cardiol 2015; 184: 337–343. [DOI] [PubMed] [Google Scholar]
- 20. Evans JDW, Dobbin SJH, Pettit SJ, Di Angelantonio E, Willeit P. High‐sensitivity cardiac troponin and new‐onset heart failure: a systematic review and meta‐analysis of 67,063 patients with 4,165 incident heart failure events. JACC Heart failure 2018; 6: 187–197. [DOI] [PubMed] [Google Scholar]
- 21. Howlett JG. Dancing cats, heart failure, and circulating troponin. J Card Fail 2019; 25: 238–239. [DOI] [PubMed] [Google Scholar]
- 22. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets D, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 23. Welsh P. High sensitivity troponin T and incident heart failure in older men: British Regional Heart Study. In: Papacosta O, Ramsay S, McMurray J, Wannamethee G, Sattar N, editors. J Card Fail 2019; 25: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]


