Abstract
Aims
Recently, B‐type natriuretic peptide (BNP) has been attracting attention as a predictor of stroke in patients with atrial fibrillation or those with prior stroke experience. However, the association between BNP and stroke has not been examined in patients with chronic heart failure (CHF). In the current study, we assessed whether BNP is associated with future occurrence of stroke in patients with CHF.
Methods and results
We prospectively studied 1803 consecutive patients who were admitted for decompensated HF and assessed the predictive value of circulating BNP levels for occurrence of post‐discharge stroke.
A total of 69 (3.8%) patients experienced a stroke (the stroke group) during the post‐discharge follow‐up period of a median of 1150 days. The stroke group showed a higher CHADS2 score. With respect to past medical history, the stroke group had a higher prevalence of arterial hypertension, atrial fibrillation, prior stroke, and chronic kidney disease. Echocardiographic parameters showed no significant differences between the two groups. In contrast, BNP levels were significantly higher in the stroke group than in the non‐stroke group (452.1 vs. 222.7 pg/mL, P < 0.001). Multivariate Cox proportional hazard analysis indicated that BNP levels were independently associated with post‐discharge stroke (hazard ratio 2.636, 95% confidence interval 1.595–4.357, P < 0.001). The survival classification and regression tree analysis revealed that the accurate cut‐off point of BNP in predicting post‐discharge stroke was 187.7 pg/mL. We added high BNP level (BNP ≥ 180 pg/mL) as one point to CHADS2 score. The BNP‐added CHADS2 score was compared with CHADS2 score alone by using c‐statistics. The areas under the curve of CHADS2 score, BNP, and BNP‐added CHADS2 score were 0.698, 0.616, and 0.723, respectively. The predictive value of BNP‐added CHADS2 score was higher compared with those of CHADS2 score (P = 0.026).
Conclusions
The assessment of BNP may predict the occurrence of stroke in CHF patients used alone or in combination with established CHADS2 score.
Keywords: Heart failure, B‐type natriuretic peptide, Stroke, CHADS2 score
Introduction
Currently, the mortality in patients with heart failure (HF) remains significantly high compared with those without HF. 1 Apart from cardiac causes, the high mortality rate of patients with HF is also related to non‐cardiac causes. 2 Stroke is considered one of the leading comorbidities among non‐cardiac diseases responsible for approximately 1% of death in this patient cohort. 2 The mortality rate from stroke has been unchanged in the last decade, and the prediction of stroke in this population remains an urgent clinical issue. 2 In the general population, atrial fibrillation (AF) is regarded as a leading cause of stroke, and CHADS2 score has been used to predict the occurrence of stroke in HF patients. 3 , 4 , 5 However, the predictive value of clinical criteria in the occurrence of stroke in these patients has not been sufficiently investigated.
B‐type natriuretic peptide (BNP) is a neurohormone secreted by cardiomyocytes of the ventricles in response to volume expansion and pressure overload. 6 , 7 , 8 Furthermore, BNP is a diagnostic marker of cardiac overload in patients with HF 8 , 9 , 10 and is generally used for diagnosis of HF and/or merkmal in HF management. BNP can be used as an independent predictor of functional status of the myocardium in patients with chronic HF (CHF). 8 Previous studies have identified BNP as a predictor of stroke in patients with AF or those with prior stroke experience. 11 , 12 , 13 However, the association between the levels of BNP and stroke has not been investigated in patients with CHF. Thus, in the current study, we assessed whether BNP is associated with future occurrence of stroke in patients with CHF.
Methods
Study population
We conducted a prospective observational study of 1960 consecutive decompensated HF patients who were both admitted to, and discharged from, Fukushima Medical University Hospital between 2010 and 2018. The diagnosis of decompensated HF was made by the patient's attending cardiologist based on the HF guidelines of the European Society of Cardiology (ESC). 14 We excluded 157 patients who were receiving dialysis. Consecutively, 1803 patients with decompensated HF were included in the study. During the follow‐up period, which was a median of 1150 days (ranging 2–3318 days), 69 patients experienced acute stroke (from 2–2945 days), 426 patients were re‐hospitalized from worsening HF (from 5–3039 days), and 458 experienced all‐cause mortality (from 12–3146 days). Stroke was diagnosed by experienced neurologists and defined as an acute episode of focal dysfunction of the brain, retina, or spinal cord lasting longer than 24 h or of any duration if computed tomography and/or magnetic resonance imaging or autopsy showed focal brain infarction or haemorrhage relevant to the patient's symptoms. 3 , 4 We divided the study population into two groups: patients who experienced a stroke after discharge (stroke group, n = 69, 3.8%) and those who did not (non‐stroke group, n = 1,734, 96.2%).
The recording of patient age, sex, body mass index, past medical history, prescribed medications, laboratory data, and echocardiographic data was performed prior to discharge. The presence of comorbidities and past medical history was defined in accordance with previous reports. 15 , 16 , 17 , 18 , 19 These assessments were performed during hospitalization. We evaluated CHADS2 score, which consists of clinical parameters, such as congestive HF, arterial hypertension (AH), age ≥ 75 years old, diabetes mellitus, and prior stroke. 5 Data regarding history of prior HF hospitalization, coronary artery disease, peripheral arterial disease, stroke, and malignant cancer were obtained from the patients' medical records. The presence of AF was determined via electrocardiogram performed upon hospital admission and/or from medical records. 15 , 16 , 17 , 18 , 19 Peripheral artery disease was diagnosed according to the guidelines using computed tomography, angiography, and/or ankle‐brachial index based on the peripheral arterial disease guidelines of American College of Cardiology/American Heart Association. 20 , 21 The patients were followed up until March 2019 for occurrence of stroke. The data on clinical status and mortality of study patients were obtained from the patients' medical records, attending physicians at the patient's referring hospitals, or by contacting patients by telephone. 15 , 16 , 17 , 18 , 19 Survival time was calculated from the date of discharge. The study protocol was approved by the ethical committee of Fukushima Medical University, the investigation conforms with the principles outlined in the Declaration of Helsinki, and reporting of the study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement. 15 , 16 , 17 , 18 , 19 The written informed consent was obtained from all study participants at discharge.
Blood samples
Blood samples were obtained with the patients in a stable condition without changes in medications prior to discharge. BNP levels were measured using a specific immunoradiometric assay (Shionoria BNP kit, Shionogi, Osaka, Japan).
Assessment of echocardiographic data
Echocardiography was blindly performed by experienced cardiologists using standard techniques within 1 week prior to discharge. The left ventricular ejection fraction (LVEF) was determined and measured from the four‐chamber view using Simpson's methods. 15 , 16 , 17 , 18 , 19 HF with preserved ejection fraction was defined as LVEF ≥ 50%, HF with mid‐range ejection fraction was defined as LVEF 40–49%, and HF with reduced ejection fraction LVEF <40%. 22 In the present study, there were 862 (47.8%) HF with preserved ejection fraction patients, 233 (12.3%) HFmrEF patients, and 390 (21.6%) HF with reduced ejection fraction patients. All measurements were performed using ultrasound systems (ACUSON Sequoia, Siemens Medical Solutions USA, Inc., Mountain View, CA, USA).
Statistical analysis
The Shapiro–Wilk test showed that all continuous variables were non‐parametric and were therefore presented as median (interquartile range). Categorical variables were expressed as numbers and percentages. Continuous variables were compared using the Mann–Whitney U test, and the chi‐square test was used for comparisons of categorical variables. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of each variable associated with stroke were calculated by the Cox proportional hazard model. The best cut‐off points of BNP for a new stroke were explored using survival classification and regression tree (CART) analysis. Kaplan–Meier analysis was used for presenting post‐discharge new stroke, and the log‐rank test was used for initial comparisons. To evaluate improvements of prognostic value by adding BNP, we also performed c‐statistics to compare conventional CHADS2 score and the BNP‐added CHADS2 score. We included high BNP level as one component to the CHADS2 score (high BNP level was determined via CART analysis). A P value of <0.05 was considered statistically significant for all comparisons. These analyses were performed using statistical software packages (SPSS ver. 24.0, IBM, Armonk, NY, USA; EZR ver. 1.37, Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
Of the 1803 hospitalized HF patients, stroke occurred in 68 (3.8%) after discharge. The clinical characteristics of the study patients are presented in Table 1 . The stroke group had a higher CHADS2 score compared with the non‐stroke group, which may be explained by higher prevalence of AH, AF, prior stroke, and/or chronic kidney disease in these patients. The number of stroke patients who received angiotensin‐converting enzyme inhibitors and/or angiotensin receptor blockers and calcium‐channel antagonists was higher than that of non‐stroke patients. In contrast, sex, body mass index, New York Heart Association (NYHA) functional class, the HF type, other co‐morbidities, and medications did not significantly differ between the two groups. BNP levels were significantly higher in the stroke group compared with the non‐stroke group (452.1 vs. 222.7 pg/mL, P < 0.001, respectively). Echocardiographic parameters showed no significant differences between the two groups.
TABLE 1.
Baseline patient characteristics
| Non‐stroke (n = 1,734) | Stroke (n = 69) | P value | |
|---|---|---|---|
| Demographic data | |||
| Age (years old) | 69.0 (59.0–77.0) | 72.0 (62.0–79.0) | 0.070 |
| Male sex (n, %) | 1,037 (59.8) | 38 (55.1) | 0.432 |
| Body mass index (kg/m2) | 22.8 (20.5–25.7) | 23.0 (20.7–25.8) | 0.674 |
| NYHA class | 68 (3.9) | 5 (7.2) | 0.144 |
| III or IV at discharge (n, %) | |||
| CHADS2 Score | 3 (2–3) | 4 (3–5) | <0.001 |
| Type of HF | 0.829 | ||
| HFrEF (n, %) | 377 (26.4) | 13 (23.6) | |
| HFmrEF (n, %) | 223 (15.6) | 10 (18.2) | |
| HFpEF (n, %) | 830 (58.0) | 32 (58.2) | |
| Past medical history | |||
| Smoking status (n, %) | 876 (51.7) | 36 (54.5) | 0.655 |
| Prior HF admission (n, %) | 543 (32.4) | 26 (38.2) | 0.313 |
| Arterial hypertension (n, %) | 1,164 (67.1) | 55 (79.7) | 0.029 |
| Diabetes mellitus (n, %) | 671 (38.7) | 26 (37.7) | 0.865 |
| Dyslipidemia (n, %) | 1,228 (70.8) | 45 (65.2) | 0.317 |
| Atrial fibrillation (n, %) | 688 (39.7) | 37 (53.6) | 0.021 |
| Coronary artery disease (n, %) | 499 (28.8) | 21 (30.4) | 0.766 |
| Peripheral arterial disease (n, %) | 174 (17.3) | 7 (14.3) | 0.579 |
| Prior stroke (n, %) | 278 (16.0) | 37 (53.6) | <0.001 |
| Chronic kidney disease (n, %) | 881 (50.8) | 44 (63.8) | 0.035 |
| Anaemia (n, %) | 835 (48.2) | 37 (53.6) | 0.373 |
| Malignant tumour (n, %) | 329 (19.7) | 18 (26.5) | 0.174 |
| Medication at discharge | |||
| β blocker (n, %) | 1,301 (75.0) | 55 (79.7) | 0.377 |
| ACEI/ARB (n, %) | 1,225 (70.6) | 60 (87.0) | 0.003 |
| MRA (n, %) | 717 (41.3) | 29 (42.0) | 0.911 |
| Calcium‐channel antagonist (n, %) | 568 (32.8) | 36 (52.2) | 0.001 |
| Loop diuretic (n, %) | 1,188 (68.5) | 52 (75.4) | 0.229 |
| Inotropic agent (n, %) | 186 (10.7) | 5 (7.2) | 0.357 |
| Anticoagulant (n, %) | 1,012 (58.4) | 47 (68.1) | 0.107 |
| Vitamin K antagonist (n, %) | 770 (44.4) | 35 (50.7) | 0.300 |
| Direct oral anticoagulants (n, %) | 318 (18.3) | 13 (18.8) | 0.916 |
| Antiplatelet agent (n, %) | 785 (45.3) | 33 (47.8) | 0.676 |
| Laboratory data | |||
| BNP (pg/mL) | 222.7 (77.5–552.2) | 452.1 (199.2–779.3) | <0.001 |
| Echocardiographic data | |||
| Left ventricular ejection fraction (%) | 54.1 (39.0–63.9) | 53.0 (40.2–63.4) | 0.823 |
NYHA, New York Heart Association; HF, heart failure; HFrEF, HF with reduced ejection fraction; HFmrEF, HF with mid‐range ejection fraction; HFpEF, HF with preserved ejection fraction; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; BNP, B‐type natriuretic peptide.
The univariate Cox proportional hazard analysis revealed that age, NYHA functional class of HF, CHADS2 score, AF, prior stroke, chronic kidney disease, the use of angiotensin‐converting enzyme inhibitors and/or angiotensin receptor blockers, calcium‐channel antagonists, as well as the levels of BNP were associated with post‐discharge stroke (Table 2 ). Furthermore, we performed a multivariate Cox proportional hazard analysis, which revealed that NYHA class, (HR 3.276, 95% CI 1.269–8.456, P = 0.014), prior stroke (HR 8.715, 95% CI 3.573–21.259, P < 0.001), the prescription of calcium‐channel antagonists (HR 2.058, 95% CI 1.241–3.414, P = 0.005), and levels of log‐BNP (HR 2.562, 95% CI 1.556–4.220, P < 0.001) were independent predictors of post‐discharge stroke. The survival CART analysis revealed the cut‐off point of BNP in predicting stroke (BNP of 187.7 pg/mL). Finally, this cut‐off point was evaluated by the Kaplan–Meier analysis (Figure 1 ). We have shown that patients with BNP of ≥187.7 pg/mL experienced a high incidence of stroke (log‐rank P < 0.001).
TABLE 2.
Cox proportional hazard analysis for predicting stroke
| Unadjusted | Multiple | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.027 | 1.008–1.047 | 0.006 | 1.011 | 0.987–1.035 | 0.363 |
| Male sex | 0.865 | 0.538–1.390 | 0.549 | – | – | – |
| Body mass index | 1.014 | 0.958–1.073 | 0.628 | – | – | – |
| NYHA class III or IV at discharge | 3.794 | 1.510–9.532 | 0.005 | 3.276 | 1.269–8.456 | 0.014 |
| CHADS2 Score | 1.690 | 1.422–2.009 | <0.001 | 0.734 | 0.512–1.052 | 0.092 |
| Type of HF | – | – | – | |||
| HFrEF | Reference | 0.666 | ||||
| HFmrEF | 1.199 | 0.526–2.735 | 0.830 | |||
| HFpEF | 1.073 | 0.563–2.045 | ||||
| Smoking history | 1.106 | 0.681–1.795 | 0.685 | – | – | – |
| Prior HF | 1.261 | 0.773–2.059 | 0.353 | – | – | – |
| Arterial hypertension | 1.619 | 0.899–2.916 | 0.108 | – | – | – |
| Diabetes mellitus | 0.951 | 0.584–1.547 | 0.838 | – | – | – |
| Dyslipidemia | 0.629 | 0.382–1.035 | 0.068 | – | – | – |
| Atrial fibrillation | 1.853 | 1.154–2.977 | 0.011 | 1.224 | 0.751–1.996 | 0.417 |
| Coronary artery disease | 1.090 | 0.653–1.820 | 0.742 | – | – | – |
| Peripheral arterial disease | 0.815 | 0.366–1.814 | 0.616 | – | – | – |
| Prior stroke | 5.673 | 3.534–9.107 | <0.001 | 8.715 | 3.573–21.259 | <0.001 |
| Chronic kidney disease | 1.885 | 1.153–3.083 | 0.011 | 1.249 | 0.739–2.108 | 0.406 |
| Anaemia | 1.289 | 0.803–2.069 | 0.293 | |||
| Malignant tumour | 1.656 | 0.965–2.839 | 0.067 | – | – | – |
| β blocker | 1.174 | 0.652–2.111 | 0.593 | – | – | – |
| ACEIs/ARB | 2.236 | 1.108–4.510 | 0.025 | 1.695 | 0.827–3.473 | 0.150 |
| MRA | 0.980 | 0.607–1.581 | 0.934 | – | – | – |
| Calcium‐channel antagonist | 2.108 | 1.314–3.381 | 0.002 | 2.058 | 1.241–3.414 | 0.005 |
| Loop diuretics | 1.473 | 0.851–2.547 | 0.166 | – | – | – |
| Inotropic agents | 0.778 | 0.313–1.933 | 0.588 | – | – | – |
| Anticoagulant | 1.400 | 0.843–2.324 | 0.193 | – | – | – |
| Antiplatelet agent | 0.958 | 0.597–1.537 | 0.858 | – | – | – |
| Log‐BNP | 2.862 | 1.812–4.523 | <0.001 | 2.562 | 1.556–4.220 | <0.001 |
| LVEF | 0.998 | 0.982–1.015 | 0.847 | – | – | – |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; HF, heart failure; HF with mid‐range ejection fraction; HFmrEF, HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazard ratio; log‐BNP, log‐transformed B‐type natriuretic peptide; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
FIGURE 1.

Kaplan–Meier analysis for stroke in high [B‐type natriuretic peptide (BNP) ≥ 187.7 pg/mL] and low BNP (BNP ≤ 187.7 pg/mL) groups. Event rates were analysed by a log‐rank test.
We included a BNP level of ≥180 pg/mL as an additional parameter to CHADS2 score based on the optimal cut‐off points obtained by CART analysis. The BNP‐added CHADS2 score was compared with CHADS2 score alone for predicting future stroke by using a receiver operating characteristic curve (Figure 2). The areas under the curve of CHADS2 score, BNP, and BNP‐added CHADS2 score were 0.698, 0.616, and 0.723, respectively. The predictive value of BNP‐added CHADS2 score was higher compared with that of CHADS2 score alone (P = 0.026), suggesting that adding BNP may improve the predicting role of this score for future occurrence of stroke in patients with CHF.
FIGURE 2.
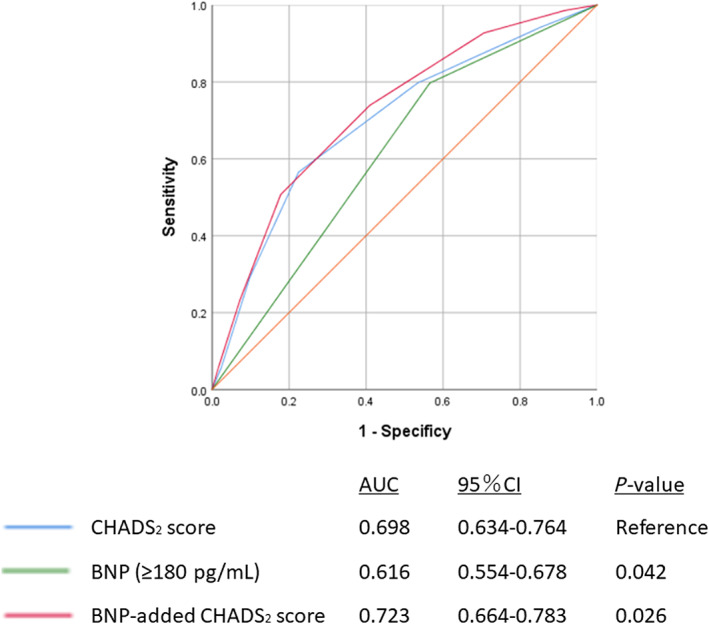
Receiver operating curves of CHADS2 score, B‐type natriuretic peptide (BNP), and BNP‐added CHADS2 score for predicting stroke. The BNP‐added CHADS2 score was compared with CHADS2 score for predicting future stroke by using c‐statistics. The predictive value of BNP‐added CHADS2 score was higher than that of CHADS2 score (P = 0.026). AUC, area under the curve; CI, confidence interval.
Discussion
To the best of our knowledge, the present study was the first to report that increased BNP level is an independent predictor of a high incidence of stroke in patients with CHF. The incidence of new stroke was significantly higher in CHF patients with high BNP levels compared with those with low levels of BNP. The optimal cut‐off point was more than 187.7 pg/mL as determined by CART analysis.
Patients with HF are at an increased risk of having a prothrombotic or hypercoagulable state because of abnormal blood flow, particularly in the left atrial appendage, and endothelial dysfunction secondary to dilatation and fibrotic changes in the left atrium. Such states can cause a stroke. Thus, HF is an independent risk factor for stroke and systemic embolism. 11 , 23 , 24 This is a reason why the CHADS2 score, one of the scoring systems to predict stroke risk in patients with AF, has congestive HF as one component. 5 , 25 Moreover, HF and AF are interrelated conditions. HF leads to structural and electrical atrial remodelling and can thus cause AF. On the other hand, AF can cause acute decompensation of CHF due to haemodynamic deterioration, which can also cause a stroke. 26 , 27 , 28 In the present study, we have shown that high levels of BNP are predictors of stroke in patients with CHF. This may be explained by the significant remodelling of the atrium and ventricle, a high occurrence of AF, and development of peripheral and/or pulmonary oedema in patients with severe HF, which consecutively increased the risk of stroke development in this patient cohort.
Previous studies have shown that levels of BNP were associated with occurrence of stroke in heterogeneous groups of patients (e.g. general population, patients with AH, AF, or acute ischaemic stroke). 11 , 12 , 13 , 29 , 30 However, the association of BNP levels with incidence of stroke has never been investigated in patients with CHF. BNP was reported to be a biomarker for predicting the incidence of cardioembolic stroke in the general population, although the detail of the general population such as comorbidities has not yet been fully examined. 13 A previous prospective observational study of AH patients showed that plasma BNP level (≥143 pg/mL) is a predictor for lacunar infarcts and ischaemic cerebral small vessel disease, which accounts for approximately 20% of all stroke cases. 31 BNP levels are affected by the presence of AH and/or stroke, and the simultaneous presence of AH and stroke results in a more significant increase in BNP levels, than the presence of either stroke or AH alone. 32 Additionally, a prospective study of patients with non‐valvular AF showed that high plasma BNP levels (≥170 pg/mL) were associated with thromboembolic events, and BNP may be a useful biomarker, which can either be used alone or in combination with scoring systems for stroke such as CHADS2 score. 11 Another retrospective study showed that high level of BNP (≥173 pg/mL) was the only independent predictor of left atrial appendage thrombus in anticoagulated AF patients. 33 The cut‐off values of BNP in these studies were similar to that in the present study for CHF patients. Thus, the present study is first to show that BNP is an independent predictor of post‐discharge stroke in hospitalized HF patients. The assessment of BNP levels seems to be useful for predicting prognosis and incidence of stroke in patients with CHF. In the established scoring systems for predicting stroke (e.g. CHADS2 score), only the presence of CHF itself has been considered. The results of the present study suggest that the predictive value of CHADS2 score alone for stroke seems to be insufficient in patients with CHF, and that adding the BNP levels (BNP ≥ 180 pg/mL) might improve prediction of stroke in patients with CHF. Not only the presence but also severity of HF, as manifested by BNP levels, seems to be associated with occurrence of future stroke. BNP is a neurohormone secreted by the cardiac ventricles in response to cardiac wall stress such as volume or pressure overload to act in the body's various tissues and induce vasodilation and natriuresis and is used to monitor the progression of cardiac congestion. 8 , 34 , 35 The increased wall stress may act directly or indirectly via cellular mediators to adjust a variety of molecular and cellular remodelling events determining the structural and functional properties of the myocardium. 7 Haemodynamic changes therefore occur in the cardiac atrium or ventricle, which results in a prothrombotic or hypercoagulable state. 11 , 23 Additionally, higher BNP levels may indicate asymptomatic or non‐recorded AF, which is one of the causes of stroke. The levels of BNP increase depend on the states of haemodynamic stress or neurohumoral activation in HF patients, 34 and might be associated with stroke, as well as poor cardiac prognosis. In conclusion, the BNP levels can predict the incidence of stroke in patients with CHF either alone or in combination with established CHADS2 score.
Study limitations
The present study has several limitations. First, the study included a relatively small number of patients from a single centre with low occurrence of stroke (3.8%); therefore, the results do not represent the general HF population. Second, because the present study included variables during hospitalization for decompensated HF, without taking into consideration changes in medical parameters and post‐discharge treatment, we should take care when extrapolating our findings to patients with stable CHF. Third, because of the study's observational design, we could not explain the causal relationship between BNP levels and stroke. Fourth, the percentage of patients who developed decompensated HF due to AF was not investigated and will be addressed in future studies. Therefore, the present results should be viewed as preliminary, and further studies with a larger population are needed.
Conflict of interest
None declared.
Hotsuki, Y. , Sato, Y. , Yoshihisa, A. , Watanabe, K. , Kimishima, Y. , Kiko, T. , Yokokawa, T. , Abe, S. , Misaka, T. , Sato, T. , Oikawa, M. , Kobayashi, A. , Yamaki, T. , Kunii, H. , Nakazato, K. , and Takeishi, Y. (2020) B‐type natriuretic peptide is associated with post‐discharge stroke in hospitalized patients with heart failure. ESC Heart Failure, 7: 2508–2515. 10.1002/ehf2.12818.
References
- 1. Taylor CJ, Ordonez‐Mena JM, Roalfe AK, Lay‐Flurrie S, Jones NR, Marshall T, Hobbs FDR. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000‐2017: population based cohort study. BMJ 2019; 364: l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ushigome R, Sakata Y, Nochioka K, Miyata S, Miura M, Tadaki S, Yamauchi T, Sato K, Onose T, Tsuji K, Abe R, Oikawa T, Kasahara S, Takahashi J, Shimokawa H, Investigators C . Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan—report from the CHART studies. Circ J 2015; 79: 2396–2407. [DOI] [PubMed] [Google Scholar]
- 3. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council CoCS, Anesthesia , Council on Cardiovascular R , Intervention, Council on C, Stroke N, Council on E, Prevention , Council on Peripheral Vascular D , Council on Nutrition PA, Metabolism . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hankey GJ. Stroke. Lancet 2017; 389: 641–654. [DOI] [PubMed] [Google Scholar]
- 5. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 6. Tokola H, Hautala N, Marttila M, Magga J, Pikkarainen S, Kerkela R, Vuolteenaho O, Ruskoaho H. Mechanical load‐induced alterations in B‐type natriuretic peptide gene expression. Can J Physiol Pharmacol 2001; 79: 646–653. [PubMed] [Google Scholar]
- 7. Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B‐type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006; 47: 742–748. [DOI] [PubMed] [Google Scholar]
- 8. Kruger S, Hoffmann R, Graf J, Janssens U, Hanrath P. Brain natriuretic Peptide. Diagnostic and prognostic value in chronic heart failure. Med Klin (Munich) 2003; 98: 562–567. [DOI] [PubMed] [Google Scholar]
- 9. McCullough PA. B‐type natriuretic peptides. A diagnostic breakthrough in heart failure. Minerva Cardioangiol 2003; 51: 121–129. [PubMed] [Google Scholar]
- 10. Hill SA, Booth RA, Santaguida PL, Don‐Wauchope A, Brown JA, Oremus M, Ali U, Bustamam A, Sohel N, McKelvie R, Balion C, Raina P. Use of BNP and NT‐proBNP for the diagnosis of heart failure in the emergency department: a systematic review of the evidence. Heart Fail Rev 2014; 19: 421–438. [DOI] [PubMed] [Google Scholar]
- 11. Hayashi K, Tsuda T, Nomura A, Fujino N, Nohara A, Sakata K, Konno T, Nakanishi C, Tada H, Nagata Y, Teramoto R, Tanaka Y, Kawashiri MA, Yamagishi M, Hokuriku‐Plus AFRI. Impact of B‐type natriuretic peptide level on risk stratification of thromboembolism and death in patients with nonvalvular atrial fibrillation‐the Hokuriku‐plus AF registry. Circ J 2018; 82: 1271–1278. [DOI] [PubMed] [Google Scholar]
- 12. Gupta HV, Finlay CW, Jacob S, Raina SK, Lee RW, Hinduja A. Can admission BNP level predict outcome after intravenous thrombolysis in acute ischemic stroke? Neurologist 2019; 24: 6–9. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura M, Ishibashi Y, Tanaka F, Omama S, Onoda T, Takahashi T, Takahashi S, Tanno K, Ohsawa M, Sakata K, Koshiyama M, Ogasawara K, Okayama A, Iwate KSG. Ability of B‐type natriuretic peptide testing to predict cardioembolic stroke in the general population‐comparisons with C‐reactive protein and urinary albumin. Circ J 2018; 82: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 15. Sato Y, Yoshihisa A, Kimishima Y, Kiko T, Kanno Y, Yokokawa T, Abe S, Misaka T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Takeishi Y. Low T3 syndrome is associated with high mortality in hospitalized patients with heart failure. J Card Fail 2019; 25: 195–203. [DOI] [PubMed] [Google Scholar]
- 16. Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Ishida T, Takeishi Y. Impact of nutritional indices on mortality in patients with heart failure. Open Heart 2018; 5: e000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshihisa A, Yokokawa T, Misaka T, Oikawa M, Kobayashi A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Takeishi Y. Soluble neprilysin does not correlate with prognosis in pulmonary hypertension. ESC Heart Fail 2019; 6: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suzuki S, Yoshihisa A, Sato Y, Watanabe S, Yokokawa T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Ishida T, Takeishi Y. Association between sleep‐disordered breathing and arterial stiffness in heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail 2018; 5: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshihisa A, Watanabe S, Yokokawa T, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Takeishi Y. Associations between acylcarnitine to free carnitine ratio and adverse prognosis in heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail 2017; 4: 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakamura Y, Kunii H, Yoshihisa A, Takiguchi M, Shimizu T, Yamauchi H, Iwaya S, Owada T, Abe S, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Sugimoto K, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Impact of peripheral artery disease on prognosis in hospitalized heart failure patients. Circ J 2015; 79: 785–793. [DOI] [PubMed] [Google Scholar]
- 21. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American Association for Vascular S, Society for Vascular S, Society for Cardiovascular A, Interventions, Society for Vascular M, Biology, Society of Interventional R, Disease AATFoPGWCtDGftMoPWPA, American Association of C, Pulmonary R, National Heart L, Blood I, Society for Vascular N, TransAtlantic Inter‐Society C, Vascular Disease F . ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113: e463–e654. [DOI] [PubMed] [Google Scholar]
- 22. Yoshihisa A, Ichijo Y, Sato Y, Kanno Y, Takiguchi M, Yokokawa T, Abe S, Misaka T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Takeishi Y. Comprehensive clinical characteristics of hospitalized patients with mid‐range left ventricular ejection fraction. Eur J Prev Cardiol 2019: 10.1177/2047487319859689. [DOI] [PubMed] [Google Scholar]
- 23. Abraham JM, Connolly SJ. Atrial fibrillation in heart failure: stroke risk stratification and anticoagulation. Heart Fail Rev 2014; 19: 305–313. [DOI] [PubMed] [Google Scholar]
- 24. Agarwal M, Apostolakis S, Lane DA, Lip GY. The impact of heart failure and left ventricular dysfunction in predicting stroke, thromboembolism, and mortality in atrial fibrillation patients: a systematic review. Clin Ther 2014; 36: 1135–1144. [DOI] [PubMed] [Google Scholar]
- 25. Yoshihisa A, Watanabe S, Kanno Y, Takiguchi M, Sato A, Yokokawa T, Miura S, Shimizu T, Abe S, Sato T, Suzuki S, Oikawa M, Sakamoto N, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Takeishi Y. The CHA2DS2‐VASc score as a predictor of high mortality in hospitalized heart failure patients. ESC Heart Fail 2016; 3: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seiler J, Stevenson WG. Atrial fibrillation in congestive heart failure. Cardiol Rev 2010; 18: 38–50. [DOI] [PubMed] [Google Scholar]
- 27. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke 2016; 47: 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kang SH, Kim J, Park JJ, Oh IY, Yoon CH, Kim HJ, Kim K, Choi DJ. Risk of stroke in congestive heart failure with and without atrial fibrillation. Int J Cardiol 2017; 248: 182–187. [DOI] [PubMed] [Google Scholar]
- 29. Sakamoto Y, Nito C, Nishiyama Y, Suda S, Matsumoto N, Aoki J, Shimoyama T, Kanamaru T, Suzuki K, Go Y, Mishina M, Kimura K. Accurate etiology diagnosis in patients with stroke and atrial fibrillation: a role for brain natriuretic peptide. J Neurol Sci 2019; 400: 153–157. [DOI] [PubMed] [Google Scholar]
- 30. Maruyama K, Uchiyama S, Shiga T, Iijima M, Ishizuka K, Hoshino T, Kitagawa K. Brain natriuretic peptide is a powerful predictor of outcome in stroke patients with atrial fibrillation. Cerebrovasc Dis Extra 2017; 7: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei W, Chen Y, Lei D, Zhang Y, Weng X, Zhou Y, Zhang L. Plasma brain natriuretic peptide is a biomarker for screening ischemic cerebral small vessel disease in patients with hypertension. Medicine (Baltimore) 2018; 97: e12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cakir Z, Saritas A, Emet M, Aslan S, Akoz A, Gundogdu F. A prospective study of brain natriuretic peptide levels in three subgroups: stroke with hypertension, stroke without hypertension, and hypertension alone. Ann Indian Acad Neurol 2010; 13: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawabata M, Goya M, Sasaki T, Maeda S, Shirai Y, Nishimura T, Yoshitake T, Shiohira S, Isobe M, Hirao K. Left atrial appendage thrombi formation in Japanese non‐valvular atrial fibrillation patients during anticoagulation therapy‐warfarin vs. Direct Oral Anticoagulants Circ J 2017; 81: 645–651. [DOI] [PubMed] [Google Scholar]
- 34. Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J 2013; 34: 1475–1480. [DOI] [PubMed] [Google Scholar]
- 35. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007; 50: 2357–2368. [DOI] [PubMed] [Google Scholar]


