Abstract
Aims
Left ventricular non‐compaction cardiomyopathy (LVNC) is a genetic heart disease, with heart failure, arrhythmias, and embolic events as main clinical manifestations. The goal of this study was to analyse a large set of echocardiographic (echo) and cardiac magnetic resonance imaging (CMRI) parameters using machine learning (ML) techniques to find imaging predictors of clinical outcomes in a long‐term follow‐up of LVNC patients.
Methods and results
Patients with echo and/or CMRI criteria of LVNC, followed from January 2011 to December 2017 in the heart failure section of a tertiary referral cardiologic hospital, were enrolled in a retrospective study. Two‐dimensional colour Doppler echocardiography and subsequent CMRI were carried out. Twenty‐four hour Holter monitoring was also performed in all patients. Death, cardiac transplantation, heart failure hospitalization, aborted sudden cardiac death, complex ventricular arrhythmias (sustained and non‐sustained ventricular tachycardia), and embolisms (i.e. stroke, pulmonary thromboembolism and/or peripheral arterial embolism) were registered and were referred to as major adverse cardiovascular events (MACEs) in this study. Recruited for the study were 108 LVNC patients, aged 38.3 ± 15.5 years, 48.1% men, diagnosed by echo and CMRI criteria. They were followed for 5.8 ± 3.9 years, and MACEs were registered. CMRI and echo parameters were analysed via a supervised ML methodology. Forty‐seven (43.5%) patients had at least one MACE. The best performance of imaging variables was achieved by combining four parameters: left ventricular (LV) ejection fraction (by CMRI), right ventricular (RV) end‐systolic volume (by CMRI), RV systolic dysfunction (by echo), and RV lower diameter (by CMRI) with accuracy, sensitivity, and specificity rates of 75.5%, 77%, 75%, respectively.
Conclusions
Our findings show the importance of biventricular assessment to detect the severity of this cardiomyopathy and to plan for early clinical intervention. In addition, this study shows that even patients with normal LV function and negative late gadolinium enhancement had MACE. ML is a promising tool for analysing a large set of parameters to stratify and predict prognosis in LVNC patients.
Keywords: Cardiomyopathy, Echocardiography, Follow‐up, Machine learning, Magnetic resonance imaging, Non‐compaction
Introduction
Left ventricular (LV) non‐compaction cardiomyopathy (LVNC) is considered a genetic heart disease, 1 related to intrauterine arrest of the process of myocardial compaction, leading to prominent ventricular trabeculations and deep intertrabecular recesses, 2 , 3 , 4 with a two‐layer myocardial structure: non‐compacted (NC) and a compacted (C) layer. This disease can lead to heterogeneous clinical manifestations ranging from completely asymptomatic to end‐stage heart failure, malignant arrhythmias, thromboembolic events, and death. Currently, the increase in awareness of this disease is due to the increased number of diagnoses owing to improvements in cardiovascular imaging techniques and familial screening. 5 , 6 , 7 Notwithstanding, there is a concern about overdiagnosis of this disease.
Many studies have demonstrated clinical and imaging prognostic indices in a long‐term follow‐up of LVNC patients to identify a subgroup with more severe cardiac impartment. 8 , 9 , 10
However, neither the prognostic value of the combination of echocardiographic and cardiac magnetic resonance imaging (CMRI) parameters nor parameters from both ventricles have clearly predicted major adverse cardiovascular events (MACEs). A recent meta‐analysis involving 2501 patients showed that those who had LV systolic dysfunction [LV ejection fraction (LVEF) less than 45%] had a poor prognosis. On the other hand, the burden of LV trabeculation is not a fefature related to MACE. Noteworthy, right ventricular (RV) evaluation was not included in the meta‐analysis paper. 11
The interest in diagnostic methods in cardiology with higher accuracy is growing, and machine learning (ML) is an emerging tool in this context. Previous studies showed its ability to identify interaction patterns among many variables, 12 , 13 with interesting findings in different subsets, mainly focusing on heart failure. 14 , 15 , 16 , 17 , 18 Thus, the objective of this study was to evaluate the predictive value of parameters from echo, CMRI, and both combined methods on outcomes in a long‐term follow‐up of a large subset of LVNC patients by using the ML algorithm.
Methods
Study population
Included in this retrospective study were 108 patients with echo and/or CMRI criteria of LVNC, who were followed from January 2011 to December 2017 in a heart failure outpatient clinic at a university cardiology centre. Baseline clinical data were obtained from electronic medical records of our institution—clinical and complementary cardiological exam records.
Only patients with a high pre‐test probability were included. 19 Two‐dimensional colour Doppler echocardiography was carried out, and all patients met Chin et al., 5 Jenni et al., 6 and Stöllberger et al. 7 echo criteria, whereas subsequent CMRI findings fulfilled Petersen et al. 20 criteria. Twenty‐four hour Holter monitoring was also performed for all the patients, who were treated according to current heart failure guidelines. 21 Events such as death, cardiac transplantation, heart failure hospitalization, aborted sudden cardiac death, complex ventricular arrhythmias (sustained and non‐sustained ventricular tachycardia that were included in the 24 h Holter Monitoring Protocol), and embolisms (i.e. stroke, pulmonary thromboembolism, and/or peripheral arterial embolism) were registered and are jointly referred to as MACEs in this study. Patients with other cardiac or systemic diseases were excluded.
Heart failure was diagnosed based on clinical findings (signs and symptoms), 21 and it was supported by echo and CRMI exams (LVEF was less than 40%). In this study, LV diastolic dysfunction was characterized by echo criteria in moderate and significant patterns, according to the guidelines. 22 These patients were treated in accordance with the heart failure guidelines, 21 with drugs like angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, diuretics, spironolactone, and beta‐blockers. Hospitalization for heart failure was considered in those patients who had symptomatic heart failure (New York Heart Association III or IV), at any time during this follow‐up study, even with optimized therapy, also in accordance with the current heart failure guidelines. 21 The Ethics Committee approved the protocol, and all patients gave written informed consent.
Echocardiographic imaging protocol
Comprehensive echo studies were performed with a Sequoia 512 ultrasound machine (Acuson, Mountain View, CA, USA) with a 2.5 MHz harmonic imaging transducer. The following echo parameters were evaluated: aortic root and left atrium dimension (cm); left atrium enlargement; RV dimension (cm); LVEF (%) by Simpson biplane modified method and LV systolic dysfunction; LV fractional shortening (%); diastolic septum wall thickness (cm); diastolic posterior wall thickness (cm); LV end‐diastolic dimension (cm); LV end‐systolic dimension (cm); bidimensional LV end‐diastolic and end‐systolic volumes (mL); LV mass index by American Society of Echocardiography (ASE) (g/m2); relative wall thickness; RV systolic dysfunction; pulmonary hypertension (>35 mmHg); and LV diastolic dysfunction classified as normal, grades I to III. All these indices were acquired and calculated according to guidelines of chamber quantification 23 and diastolic dysfunction. 22 In this study, pulmonary hypertension was considered when pulmonary systolic artery pressure was higher than 35 mmHg, 24 and LV systolic dysfunction was classified as mild (40% to 50%), moderate (30% to 39%), or severe (<30%).
Cardiac magnetic resonance imaging imaging protocol
Cardiac magnetic resonance imaging studies were performed with two different scanners: 1.5 T CMR scanner (Philips Achieva, Best, The Netherlands) or 1.5 T GE CV/i CMR System (Wakeusha, Wisconsin, USA). A similar CMRI protocol was adopted in all studies according to the following parameters: slice thickness 10 mm, ACQ matrix 152 × 150, flip angle 40°, and inversion time increment 150 ms for the Philips Achieva scanner and slice thickness 8 mm, ACQ matrix 256 × 128/256 × 192, flip angle 45°/20°, and inversion time none/150 to 250 ms for the GE CV/i scanner. Ventricular function, volumes, and mass were obtained from at least 10 short‐axis ventricular slices, imaged with a steady‐state free precession pulse sequence, covering the entire LV. Late gadolinium enhancement (LGE) images were acquired 10–20 min after an intravenous bolus of 0.2 mmol/kg of gadolinium‐based contrast, with an inversion‐prepared gradient echo sequence.
All CMRI images were analysed using cvi42 software (Circle Cardiovascular Imaging Inc. Calgary, Canada) by a trained reader. End‐systolic and end‐diastolic LV volumes, LV mass, and LVEF were measured by standard methods. 25 The pattern of LGE was classified as subendocardial, midwall, subepicardial, or transmural.
The following CMRI parameters were studied: NC/C myocardial ratio; LGE; left atrium dimension (cm); left atrium enlargement; right atrium enlargement; septum thickness (cm); lateral wall thickness (cm); LV end‐diastolic dimension (cm); LV end‐systolic dimension (cm); LV end‐diastolic volume (mL); indexed LV end‐diastolic volume (mL/m2); LV end‐systolic volume (mL); indexed LV end‐systolic volume (mL/m2); LV mass (g); LVEF by the Simpson method; LV systolic dysfunction; RV major axis (cm); RV minor axis (cm); RV end‐diastolic volume (mL); indexed RV end‐diastolic volume (mL/m2); RV end‐systolic volume (mL); indexed RV end‐systolic volume (mL/m2); RV ejection fraction (%); RV systolic dysfunction; aortic root dimension (cm); ascending aorta dimension (cm); descending aorta dimension (cm); and pulmonary artery trunk dimension (cm).
Twenty‐four hour Holter monitoring protocol
Holter monitoring (Cardios®, Cardio Systems, São Paulo, Brazil) with three‐channel recording was used for 24 h electrocardiography monitoring of each patient. Each beat was automatically classified and labelled by the Cardios software using the template‐matching technique. Then, a single senior cardiologist performed a blinded evaluation over all exams and classified ventricular beats, non‐ventricular beats, and artefacts. Non‐sustained ventricular tachycardia and sustained ventricular tachycardia were reported.
Machine learning
The ML algorithm has two main phases: (i) a phase in which missing parameters are estimated and (ii) a phase in which predictive parameters are selected. The overall structure of the ML framework is illustrated in Figure 1 .
Figure 1.
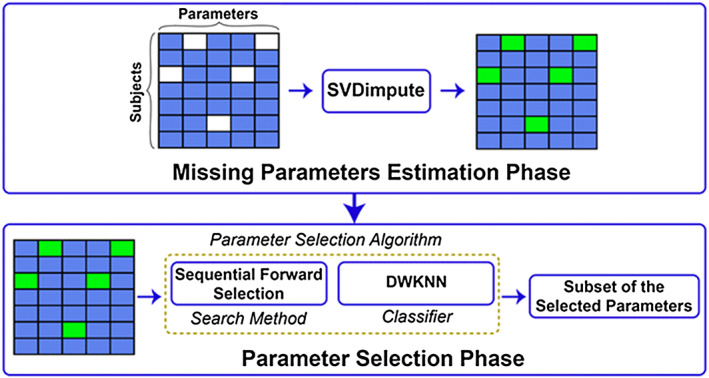
Overall structure of the proposed machine learning framework. The ‘SVDimpute’ method was first used to estimate the missing CMRI and echocardiographic parameters of the subjects (empty fields in the upper left panel). The complete data (the bottom left panel), with green fields representing the estimated missing parameters, were then fed into a parameter selection algorithm comprising sequential forward selection to explore the space of the parameters and DWKNN as a classifier to evaluate the predictive powers of the parameters. The outcome of the feature selection phase was a small subset of the parameters that enabled categorization of the control and patient groups with high accuracy. DWKNN, distance‐weighted k‐nearest neighbour.
Missing parameter estimation
Because not all CMRI and echo parameters were available for all the subjects studied, the ML framework included a ‘data imputation’ step during which the missing parameters (2% of the CMRI and 15% of the echo data) were estimated through a learning method called ‘SVDimpute’. 26 , 27
Parameter selection
As the CMRI and echo measurements resulted in a fairly large set of parameters (i.e. 47 parameters in this study), whereas not all of these parameters have added prognostic power, a parameter selection (also called feature selection) approach was used whereby a subset of the parameters was found to best predict MACE. 27 Hereto, we used a ‘wrapper parameter selection’ method 28 that was composed of a ‘sequential forward selection’ as a search method to explore the space of the parameters and of the ‘distance‐weighted k‐nearest neighbor’ 29 as a classifier to assess the predictive power of the parameters.
Performance evaluation
As is common in ML, the subjects were randomly split into a ‘training set’ for building the feature selection algorithm and a ‘testing set’ for evaluating the predictive power of different combinations of the selected parameters. The training set consisted of 74 subjects, where the same number of subjects was randomly selected from the patients with and without MACE during follow‐up, and the testing set contained the remaining 34 subjects. This process was repeated 50 times (i.e. cross‐validation), and a subset of the parameters that resulted in the best average classification performance was chosen. Using this process, we made sure that all subjects were used in the training and testing sets and that the selected parameters were not biased towards a subset of the subjects. All data analysis was performed in MATLAB 2015 (The MathWorks, Inc., Natick, Massachusetts, USA). 30
Statistical analysis
Initially, all variables were analysed in a descriptive form. For the quantitative variables, the analysis was done by observing the minimum and maximum values and calculating the means and standard deviations after assessment of normal data distribution. Absolute and relative frequencies were calculated for the qualitative variables. For the comparison of means between groups, a Student's t‐test was used. To evaluate the homogeneity between proportions, the χ2 test or Fisher's exact test was applied. 23 The software used for the calculations was SPSS v 17.0 (SPSS Inc, Chicago, IL). The level of significance used for the tests was 5%.
Results
Study population
A total of 108 patients from 1 to 69 years of age, 38.3 ± 15.5 years, 52 men (48.1%) were included in the study, as shown in Table 1 . The mean time of follow‐up was 5.8 ± 3.9 years during which 47 patients (43.5%) had at least one MACE (Table 2 ).
Table 1.
Baseline clinic parameters of both groups of patients
| Baseline parameters | Group with events (47), mean ± SD | Group without events (61), mean ± SD | P value |
|---|---|---|---|
| Age | 40.9 ± 15.0 | 34.0 ± 15.7 | 0.022 a |
| Male gender | Male: 51% | Male: 46% | 0.595 c |
| Body surface (m2) | 1.7 ± 0.3 | 1.8± 0.2 | 0.169 a |
| Follow‐up (months) | 69 ± 36.5 | 70 ± 50.9 | 0.752 b |
| LVNC family history | 15% | 62% | 0.001 c |
| Syncope | 23% | 13% | 0.164 c |
| Arterial hypertension | 40% | 28% | 0.170 c |
| Diabetes mellitus | 8% | 8% | 1.000 d |
| Dyslipidaemia | 27% | 28% | 0.981 c |
| Coronary artery disease | 0% | 0% | ‐ |
| Chronic renal disease | 12% | 2% | 0.041 d |
| Smoking | 38% | 18% | 0.019 c |
| Alcoholism | 8% | 3% | 0.400 d |
LVNC, left ventricular non‐compaction cardiomyopathy.
P < 0.05.
Student's t‐test.
Wilcoxon test.
χ2 test.
Fisher's exact test.
Table 2.
Major adverse cardiovascular events frequency in non‐compaction cardiomyopathy patients
| Events | Number of patients (total: 47) | Frequency (%) |
|---|---|---|
| Death | 6 | 12.8 |
| Cardiac transplantation | 2 | 4.2 |
| Heart failure hospitalization | 26 | 55.3 |
| Aborted sudden cardiac death | 2 | 4.3 |
| Complex ventricular arrhythmias | 29 | 61.7 |
| Total embolic events | 8 | 17.0 |
| Stroke | 7 | 14.9 |
| Arterial embolism | 1 | 2.1 |
| Pulmonary thromboembolism | 0 | 0 |
Echocardiography results
Echo findings for patients with and without MACE during follow‐up are reported in Table 3 . Patients who experienced at least one event had significantly larger left atrial dimension; RV dimension; decreased LVEF and LV fractional shortening; increased LV end‐diastolic and end‐systolic dimension; volumes and indexed LV mass; RV systolic dysfunction; and impaired LV diastolic dysfunction (P < 0.05).
Table 3.
Echocardiographic parameters in non‐compaction cardiomyopathy patients with/without major adverse cardiovascular events
| Echocardiographic parameters | Group with events (47), mean ± SD | Group without events (61), mean ± SD | P value |
|---|---|---|---|
| Left atrium dimension (mm) | 43.2 ± 8.0 | 37.8 ± 8.1 | <0.001 |
| Aortic root dimension (mm) | 28.9 ± 5.1 | 28.7 ± 4.0 | 0.775 |
| RV dimension (mm) | 27.0 ± 7.1 | 24.3 ± 4.3 | 0.025 |
| LV ejection fraction (%) | 37.1 ± 13.9 | 51.7 ± 15.2 | <0.001 |
| LV fractional shortening (%) | 19.2 ± 7.5 | 27.4 ± 8.8 | <0.001 |
| Septum thickness (mm) | 9.2 ± 1.9 | 8.9 ± 1.4 | 0.320 |
| Posterior wall thickness (mm) | 9.1 ± 1.4 | 8.5 ± 1.4 | 0.048 |
| LV end‐diastolic dimension (mm) | 60.4 ± 10.9 | 53.9 ± 9.9 | 0.002 |
| LV end‐systolic dimension (mm) | 49.3 ± 12.5 | 39.2 ± 12.6 | <0.001 |
| LV end‐diastolic volume (mL) | 186.7 ± 80.3 | 142.6 ± 66.3 | 0.002 |
| LV end‐systolic volume (mL) | 126.2 ± 72.3 | 79.3 ± 62.3 | <0.001 |
| Indexed LV mass (g/m2) | 140.2 ± 59.5 | 102.3 ± 38.8 | <0.001 |
| Relative wall thickness | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.092 |
| Left atrium enlargement | 63.0% | 27.9% | 0.0019 |
| LV systolic dysfunction | 76.6% | 44.3% | 0.0005 |
| RV systolic dysfunction | 29.8% | 8.2% | 0.0206 |
| LV diastolic dysfunction | 61.7% | 31.1% | 0.0208 |
| Pulmonary hypertension | 25.5% | 11.5% | 0.0572 |
LV, left ventricle/ventricular; RV, right ventricle/ventricular.
Student's t‐test. P < 0.05.
Cardiac magnetic resonance imaging results
Cardiac magnetic resonance imaging data set was available in 107 patients (Table 4 ). In one patient, a pacemaker was implanted due to total atrioventricular block, and CMRI was not performed. Patients with events during follow‐up had increased left atrium dimension, LV end‐systolic and end‐diastolic dimension, volumes and mass, and decreased biventricular ejection fraction. The NC/C ratio did not show statistically significant differences between both groups. The presence of LGE was related to MACE (Table 4 ). In spite of 34% of patients in the MACE group having LGE, this represented only ~5.4% of all analysed segments (43 out of 799 segments), considering a 17‐segment model. 31
Table 4.
Cardiac magnetic resonance imaging parameters in non‐compaction cardiomyopathy patients with/without major adverse cardiovascular events
| CMRI parameters | Group with events (47), mean ± SD | Group without events (n = 61), mean ± SD | P value |
|---|---|---|---|
| NC/C ratio | 3.3 ± 1.1 | 3.0 ± 0.8 | 0.213 |
| Left atrium dimension (cm) | 3.6 ± 0.8 | 3.2 ± 0.8 | 0.006 |
| Septum thickness (cm) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.608 |
| Lateral wall thickness (cm) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.892 |
| LV end‐diastolic dimension (cm) | 6.3 ± 1.2 | 5.7 ± 0.9 | 0.001 |
| LV end‐systolic dimension (cm) | 5.3 ± 1.3 | 4.1 ± 1.2 | <0.001 |
| LV end‐diastolic volume (mL) | 218.9 ± 108.7 | 165.8 ± 59.7 | 0.004 |
| Indexed LV end‐diastolic volume (mL/m2) | 127.0 ± 58.6 | 91.2 ± 29.1 | <0.001 |
| LV end‐systolic volume (mL) | 142.5 ± 99.2 | 85.1 ± 56.1 | <0.001 |
| Indexed LV end‐systolic volume (mL/m2) | 89.4 ± 67.3 | 46.9 ± 29.2 | <0.001 |
| LV Mass (g) | 137.5 ± 55.8 | 114.4 ± 41.1 | 0.019 |
| LV ejection fraction (%) | 35.7 ± 15.6 | 52.4 ± 15.4 | <0.001 |
| RV major axis (cm) | 7.4 ± 1.4 | 7.7 ± 1.3 | 0.256 |
| RV minor axis (cm) | 4.0 ± 0.8 | 4.1 ± 0.6 | 0.677 |
| RV end‐diastolic volume (mL) | 138.5 ± 58.2 | 139.4 ± 40.8 | 0.929 |
| Indexed RV end‐diastolic volume (mL/m2) | 80.8 ± 29.9 | 76.6 ± 18.1 | 0.389 |
| RV end‐systolic volume (mL) | 78.1 ± 54.3 | 64.7 ± 27.6 | 0.129 |
| Indexed RV end‐systolic volume (mL/m2) | 45.5 ± 29.5 | 36.6 ± 13.6 | 0.0591 |
| RV ejection fraction (%) | 46.1 ± 15.6 | 52.0 ± 11.5 | 0.031 |
| Aortic root dimension (cm) | 2.7 ± 0.5 | 2.8 ± 0.5 | 0.877 |
| Ascending aorta dimension (cm) | 2.8 ± 0.6 | 2.6 ± 0.5 | 0.074 |
| Descending aorta dimension (cm) | 2.1 ± 0.5 | 2.02± 0.3 | 0.229 |
| Pulmonary artery trunk dimension (cm) | 2.4 ± 0.5 | 2.3 ± 0.5 | 0.247 |
| Left atrium enlargement | 57.4% | 35% | 0.0593 |
| Right atrium enlargement | 23.4% | 16.7% | 0.3827 |
| LV dysfunction | 83.0% | 41.7% | <0.0001 |
| RV dysfunction | 31.9% | 15% | 0.1122 |
| Presence of late gadolinium enhancement | 34.0% | 8.3% | 0.0009 |
| Late gadolinium enhancement prevalence in total cardiac segments | 5.4% | 0.7% | 0.0009 |
LV, left ventricle/ventricular; NC/C, non‐compacted/compacted ratio; RV, right ventricle/ventricular.
Echo and cardiac magnetic resonance imaging machine learning analysis
The algorithm selected the following combination of indices to best predict MACE by both methods: for echo parameters, (i) LVEF, (ii) RV systolic dysfunction, (iii) LV end‐diastolic volume, and (iv) LV mass index and for CMRI parameters, (i) LVEF, (ii) RV end‐systolic volume, (iii) LGE, and (iv) RV lower axis. Finally, when the algorithm analysed both methods, the best parameters were as follows: (i) LVEF (CMRI), (ii) RV end‐systolic volume (CMRI), (iii) RV systolic dysfunction (echo), and (iv) RV lower axis (CMRI), with accuracy, sensitivity, and specificity rates of 75.5%, 77%, and 75%, respectively (Figures 2 and 3 ).
Figure 2.
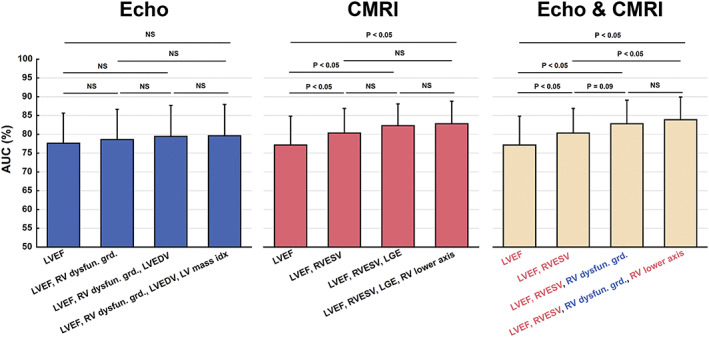
The results of receiver operating characteristic analyses on the classification outcomes obtained when using only echo or CMRI markers and a combination of both markers. For the latter case, the selected echo and CMRI markers are written in blue and red, respectively. The classification results at different steps of the parameter selection process were statistically compared. AUC, area under the curve; Echo, echocardiogram; CMRI, cardiac magnetic resonance imaging; NS, not significant; LGE, late gadolinium enhancement; LV, left vetricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; RV, right ventricular; Dysfun.grd, dysfunction grade; RVESV, right ventricular end‐systolic volume.
Figure 3.
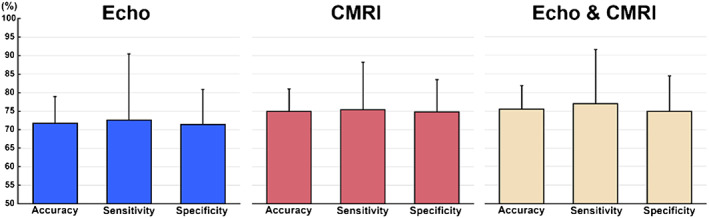
Accuracy, sensitivity, and specificity rates (%) (mean ± SD) obtained with the optimal cut‐off values of the receiver operating characteristic analyses presented in Figure 2. CMRI, cardiac magnetic resonance imaging.
Discussion
This is the first study to evaluate the combination of two imaging methods, echo and CMRI, in the prognostic stratification in a long‐term follow‐up evaluation of a large number of patients with LVNC using ML tool stratification. Thus, clinical, echo, and CMRI data were analysed in a combined manner, where the data in both imaging methods were evaluated for the diagnosis and prognostication of this disease. Surprisingly, we noticed the importance of biventricular assessment when applying both methods in prognostic stratification; we also found it significant that the RV dysfunction by echocardiogram showed additive value to the CMRI parameters in the severity of disease (Figure 2 ). LVEF was the most important parameter in this and in previous studies for detection of MACE. 2 , 9 Although adding RV dysfunction to LVEF did not substantially improve the results, the use of the combination of only 4 out of 46 parameters allowed a significant increase in accuracy, sensitivity, and specificity rates. Furthermore, the replacement of LGE (CMRI) by RV dysfunction (echo) also improved the performance. This form of assessment allows us to score the greatest impact markers in the prognosis of these patients, thus serving as a guide to determine which patient should have a more emphatic and early approach. More recent tools such as the use of myocardial deformation indices would also provide earlier and more robust data on the unfavourable evolution of patients with LVNC, and more studies are needed to clarify the role of RV involvement in LVNC as a primary impairment or secondary compromising to LV dysfunction.
The diagnosis of LVNC remains controversial because there is no gold standard method, as demonstrated when the three echocardiographic diagnostic criteria (Chin, Jeni, and Stöllberguer) were used in a single population, and only 29.8% of the patients fulfilled the three proposed criteria. 31 , 32 We used the three echocardiographic criteria as the first imaging method for LVNC diagnosis to improve the diagnostic specificity, and these patients all underwent CMRI to confirm the diagnosis; family screening was also performed.
Our results are in line with recent prospective studies 2 , 9 with 2.3 and 2.9 years of follow‐up and a meta‐analysis with 28 studies, 11 which demonstrated that functional class III/IV, reduction in LVEF, ventricular remodelling, pulmonary arterial hypertension, and the presence of LGE were markers of poor prognosis. Another odd fact is that the NC/C ratio did not have prognostic implications as described in previous publications, 11 which increases scepticism about the primary and single aetiology of LVNC. The NC/C ratio is important for the diagnosis, but not for LVNC prognosis.
In our study, 17.0% of patients with MACE had normal LVEF and 66.0% of this group did not have LGE. Among patients with LGE, this represented only 5.4% of the total analysed segments, showing that even patients with normal function and no LGE are at risk of MACE. Moreover, LGE was present in almost 28% of the patients with MACE and in only 9.7% of all patients. Similar findings are also shown by Andreini et al., who demonstrated that LGE has a prognostic impact. 10 Recently, the importance of extracellular volume quantification as a CMRI parameter in predicting ventricular arrhythmias in patients with LVNC has been demonstrated, showing the interstitial fibrosis as a predisposing factor in ventricular arrhythmias, even in patients with low LGE. 33
Another piece of interesting data in our sample is that almost 30% of patients with MACE had normal diastolic function patterns on echo. Thus, 14.8% of the LVNC patients had normal systolic and diastolic LV functions, no LGE, and still had adverse outcomes, which showed the severity of LVNC even in patients in the early disease. It is important that this health condition be considered by the physician when required to give permission for physical activity, professional or recreational. More prospective studies with a larger number of patients are needed to verify these findings.
This diagnostic and prognostic phenotypic heterogeneity results from a lack of knowledge about genetic mutations and their phenotypic expression. Van Waning et al. demonstrated the incremental value of the presence of genetic mutations in the risk stratification of LVNC patients. 34 The patients who had these mutations, in addition to ventricular remodelling and systolic dysfunction, had a worse prognosis than those without them.
Study limitations
Many different criteria have been suggested for LVNC diagnosis, and the use of quantification of LV non‐compacted myocardial mass is another acceptable criterion. 35 In this study, we used the Petersen 20 criteria associated with three current echocardiographic criteria 5 , 6 , 7 to judge the ML algorithm.
We only included image parameters in the ML model, ruling out clinical data. The main goal of this study was to focus on determining findings from echo and CMRI that could be related in our established clinical outcome. Moreover, we did not compare it with other types of cardiomyopathy, which certainly would enrich our findings. This is an ongoing study.
In this study, deformation indices for ventricular analysis were not included given that they were not a routine tool in our department in the beginning of the follow‐up.
Conclusion
Our findings show the importance of biventricular assessment to detect high‐risk patient profiles to plan a more aggressive therapeutic approach. The combination of the following parameters is the best method to predict MACE: LVEF (CMRI), RVESV (CMRI), RV systolic dysfunction (echo), and RV lower axis (CMRI) with high accuracy, sensitivity, and specificity. In addition, this study shows that even patients with normal ventricular function and without LGE had cardiac events. ML is a promising tool for analysing a large set of parameters in order to stratify patients with LVNC and to predict prognosis.
Conflict of interest
None declared.
Funding
None.
Rocon, C. , Tabassian, M. , Tavares de Melo, M. D. , de Araujo Filho, J. A. , Grupi, C. J. , Parga Filho, J. R. , Bocchi, E. A. , D'hooge, J. , and Salemi, V. M. C. (2020) Biventricular imaging markers to predict outcomes in non‐compaction cardiomyopathy: a machine learning study. ESC Heart Failure, 7: 2431–2439. 10.1002/ehf2.12795.
References
- 1. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seldman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113: 1807–1816. [DOI] [PubMed] [Google Scholar]
- 2. Habib G, Charron P, Eincher JC, Giorgi R, Donai E, Laperche T, Boulmier D, Pascal C, Logeart D, Jondeau G, Gohen‐Solal A. Working Groups “Heart Failure and Cardiomyopathies” and “Echocardiography” of the French Society of Cardiology. Isolated left ventricular non‐compaction in adults: clinical and echocardiographic features in 105 patients. Results from a French registry. Eur J Heart Fail 2011; 13: 177–185. [DOI] [PubMed] [Google Scholar]
- 3. Carrilho‐Ferreira P, Almeida AG, Pinto FJ. Non‐compaction cardiomyopathy: prevalence, prognosis, pathoetiology, genetics, and risk of cardioembolism. Curr Heart Fail Rep 2014; 11: 393–403. [DOI] [PubMed] [Google Scholar]
- 4. Arbustini E, Weidemann F, Hall JL. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol 2014; 64: 1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990; 82: 507–513. [DOI] [PubMed] [Google Scholar]
- 6. Jenni R, Goebel N, Tartini R, Schneider J, Arbenz U, Oelz O. Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic, and pathologic anatomical findings. Cardiovasc Intervent Radiol 1986; 9: 127–131. [DOI] [PubMed] [Google Scholar]
- 7. Stöllberger C, Finsterer J. Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiogr 2004; 17: 91–100. [DOI] [PubMed] [Google Scholar]
- 8. Greutmann M, Mah ML, Silversides CK, Klaassen S, Attenhofer Jost CH, Jenni R, Oechsin EN. Predictors of adverse outcome in adolescents and adults with isolated left ventricular noncompaction. Am J Cardiol 2012; 109: 276–281. [DOI] [PubMed] [Google Scholar]
- 9. Tian T, Liu Y, Gao L, Wang J, Sun K, Zou Y, Wang L, Zhang L, Li Y, Xiao Y, Song L, Zhou X. Isolated left ventricular noncompaction: clinical profile and prognosis in 106 adult patients. Heart Vessels 2014; 29: 645–652. [DOI] [PubMed] [Google Scholar]
- 10. Andreini D, Pontone G, Bogaert J, Roghi A, Varison A, Schwitter J, Mushtaq S, Vovas G, Sormani P, Aquaro GD, Monney P, Segurini C, Guglielmo M, Conte E, Fusini L, Dello Russo A, Lombardi M, Gripari P, Baggiano A, Florentini C, Lombardi F, Bartorelli AL, Pepi M, Masci PG. Long‐term prognostic value of cardiac magnetic resonance in left ventricle noncompaction: a prospective multicenter study. J Am Coll Cardiol 2016; 68: 2166–2181. [DOI] [PubMed] [Google Scholar]
- 11. Aung N, Domino S, Ricci F, Sanghvi MM, Pedrosa C, Woodbridge SP, Al‐Balah A, Zemrak F, Khanjhi MY, Munroe PB, Naci H, Petersen SE. Prognostic significance of left ventricular noncompaction: systematic review and meta‐analysis of observational studies. Circ Cardiovasc Imaging 2020; 13: e009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narula S, Shameer K, Salem Omar AM, Dudley JT, Sengupta PP. Machine‐learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol 2016; 68: 2287–2295. [DOI] [PubMed] [Google Scholar]
- 13. Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol 2017; 69: 2657–2664. [DOI] [PubMed] [Google Scholar]
- 14. Ortiz J, Ghefter CG, Silva CE, Sabbatini RM. One‐year mortality prognosis in heart failure: a neural network approach based on echocardiographic data. J Am Coll Cardiol 1995; 26: 1586–1593. [DOI] [PubMed] [Google Scholar]
- 15. Mahmoud A, Bansal M, Sengupta PP. New cardiac imaging algorithms to diagnose constrictive pericarditis versus restrictive cardiomyopathy. Curr Cardiol Rep 2017; 19: 43. [DOI] [PubMed] [Google Scholar]
- 16. Sengupta PP, Huang YM, Bansai M, Ashrafi A, Fisher M, Shameer K, Gall W, Dudley JT. Cognitive machine‐learning algorithm for cardiac imaging: a pilot study for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ Cardiovasc Imaging 2016; 9: e004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asl BM, Setarehdan SK, Mohebbi M. Support vector machine‐based arrhythmia classification using reduced features of heart rate variability signal. Artif Intell Med 2008; 44: 51–64. [DOI] [PubMed] [Google Scholar]
- 18. Tabassian M, Sunderji I, Erdel T, Sanchez‐Martinez S, Degiovanni A, Marino P, Fraser AG, D'hooge J. Diagnosis of heart failure with preserved ejection fraction: machine learning of spatiotemporal variations in left ventricular deformation. J Am Soc Echocardiogr 2018; 31: 1272–1284. [DOI] [PubMed] [Google Scholar]
- 19. Petersen SE, Neubauer S. Excessive trabeculations and prognosis: the plot thickens. Circ Cardiovasc Imaging 2017; 10: E006908. [DOI] [PubMed] [Google Scholar]
- 20. Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005; 46: 101–105. [DOI] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Jarjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruliope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members . Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 22. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pelikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2008; 10: 165–193. [DOI] [PubMed] [Google Scholar]
- 23. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pelilkka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St. John Sutton M, Stewart W, American Society of Echocardiography's Nomenclature and Standards Committee , Task Force on Chamber Quantification , American College of Cardiology Echocardiography Committee , American heart Association , European Association of Echocardiography , European Society of Cardiology . Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108. [DOI] [PubMed] [Google Scholar]
- 24. Hardegree EL, Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Kushwaha SS, Hsiao JF, McCully RB, Oh JK, Pellikka PA, Kane GC. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am J Cardiol 2013; 111: 143–148. [DOI] [PubMed] [Google Scholar]
- 25. Schulz‐Menger J. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson 2013; 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosner B. Fundamentals of Biostatistics. (1986).
- 27. Troyanskaya O, Cantor M, Sherlock G, Grown P, Hastle T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics 2001; 17: 520–525. [DOI] [PubMed] [Google Scholar]
- 28. Guyon I, Elisseeff A. An introduction to variable and feature selection. J Mach Learn Res 2003; 3: 1157–1182. [Google Scholar]
- 29. Dudani SA. The distance‐weighted k‐nearest‐neighbor rule. IEEE Trans Syst Man Cybern 1976; SMC‐6: 325–327. [Google Scholar]
- 30. MATLAB 2015. (The MathWorks, Inc., Natick, Massachusetts, USA).
- 31. Kawel‐Boehm N, Maceira A, Valsangiacomo‐Buechel ER, Vogel‐Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluenke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015; 17: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, Sharma S, Elliott PM. Diagnosis of left‐ventricular non‐compaction in patients with left‐ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J 2008; 29: 89–95. [DOI] [PubMed] [Google Scholar]
- 33. Araujo‐Filho JAB, Assuncao AN Jr, Tavares de Melo MD, Biere L, Lima CR, Dantas RN Jr, Nomura CH, Salemi VMC, Jerosch‐Herold M, Parga JR. Myocardial T1 mapping and extracellular volume quantification in patients with left ventricular non‐compaction cardiomyopathy. Eur Heart J Cardiovasc Imaging 2018; 19: 888–895. [DOI] [PubMed] [Google Scholar]
- 34. van Waning JI, Caliskan K, Hoedemaekers YM, van Spaenmdonck‐Zwarts KY, Baas AF, Boekholdt SM, van Mellie JP, Teske AJ, Asselbergs FW, Backx APCM, du Marchie Sarvaas GJ, Dalinghaus M, Breur JMPJ, Linschoten MPM, Verlooij LA, Kardys I, Doojies D, Lekanne Deprez RH, LJpma AS, van den Berg MP, Hofstra RMW, van Siegtenhorst MA, Jongbloed JDH, Majoor‐Krakauer D. Genetics, clinical features, and long‐term outcome of noncompaction cardiomyopathy. J Am Coll Cardiol 2018; 71: 711–722. [DOI] [PubMed] [Google Scholar]
- 35. Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert JY, Vidal V, Bartoli JM, Habib G, Moulin G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non‐compaction. Eur Heart J 2010; 31: 1098–1104. [DOI] [PubMed] [Google Scholar]


