Abstract
Aims
This study aims to investigate the prognostic impact of mineralocorticoid receptor antagonists (MRAs) on cardiovascular events in patients hospitalized for acute decompensated heart failure with preserved ejection fraction (HFpEF; defined as left ventricular ejection fraction ≥45%).
Methods and results
A prospective multicentre cohort study was conducted in Nagano prefecture, Japan, between July 2014 and December 2018 that contained 518 consecutive HFpEF patients hospitalized for acute decompensated heart failure (HF). The primary outcome was a composite of cardiovascular death and HF readmission. We compared the incidence of cardiovascular events between patients who were prescribed with MRAs and those who were not in a propensity score matched cohort using a Cox proportional hazards regression model with a propensity score derived from 23 baseline variables. For sensitivity analysis, we conducted Cox proportional hazards regression models for the primary outcome adjusting for 16 clinically relevant variables in the crude cohort. The median age was 83 years, and 53% were female. The median left ventricular ejection fraction was 61%. During a median follow‐up of 553 days, the primary outcome occurred in 192 (37%) patients. MRAs were used in 255 (49%) patients. After analysis, a matched cohort consisting of 370 patients was created. After propensity score matching, the baseline characteristics were well balanced between the two groups. The incidence of the primary outcome was significantly lower in MRA users than in non‐users [32% (59/185) vs. 49% (90/185); hazard ratio (HR) 0.669, 95% confidence interval (CI) 0.482–0.929, P = 0.016]. The incidence of cardiovascular death was also significantly lower in the MRA users [11% (21/185) vs. 22% (41/185); HR, 0.563; 95% CI, 0.333–0.953; P = 0.032]. The risk of HF readmission tended to be lower in the MRA users [29% (54/185) vs. 41% (75/185); HR, 0.738; 95% CI, 0.520–1.048; P = 0.089]. MRA use was also associated with a lower risk of the primary outcome after Cox proportional hazards analysis adjusting for 16 clinically relevant variables in the crude cohort (HR, 0.710; 95% CI 0.507–0.995; P = 0.047).
Conclusions
Mineralocorticoid receptor antagonist use was significantly associated with a lower risk of the primary composite outcome of cardiovascular death and HF readmission in patients hospitalized for acute decompensated HFpEF. The incidence of cardiovascular mortality was also significantly lower in these patients.
Keywords: Mineralocorticoid receptor antagonist, MRA, Spironolactone, Heart failure with preserved ejection fraction, HFpEF, Prognosis
Introduction
Approximately half of all patients with heart failure (HF) have a normal or near normal left ventricular ejection fraction (LVEF), a condition known as HF with preserved ejection fraction (HFpEF). 1 , 2 , 3 , 4 Although the prognosis of HF with reduced ejection fraction (HFrEF) has improved significantly in recent decades due to advances in HF therapy, the outlook of HFpEF has failed to progress over time 1 in that no therapies have shown a mortality reduction in afflicted patients 5 , 6 , 7 , 8 , 9 , 10 and current guidelines only recommend treatments to reduce symptoms from volume overload and manage coexisting conditions. 11 , 12 Recently, the angiotensin‐receptor‐neprilysin inhibitor sacubitril‐valsartan could not reduce the risk of cardiovascular events compared with valsartan monotherapy in HFpEF. 13 The effects of sodium‐glucose cotransporter 2 (SGLT‐2) inhibitors are now garnering attention in HFpEF after favourable results for dapagliflozin in patients with HFrEF. 14 With increase in the aging society, it is becoming urgent to identify effective treatments in patients with HFpEF.
Mineralocorticoid receptor antagonists (MRAs) have been demonstrated to reduce the risks of cardiovascular events in patients with HFrEF. 15 , 16 , 17 In terms of HFpEF, the TOPCAT trial showed that spironolactone could reduce the rate of hospitalization for HF but not that of cardiovascular death or the composite outcome in stable HFpEF. 5 Although several other studies have provided supportive evidence of MRAs in HFpEF on post hoc analyses, 18 , 19 , 20 the drug's precise effect remains inconsistent in the literature. Additionally, there are no studies investigating the impact of MRAs on cardiovascular mortality in acute decompensated HFpEF patients. Against this background, we aimed to identify the prognostic impact of MRAs on cardiovascular events in patients with acute decompensated HFpEF in a prospective cohort study.
Methods
Study design
This prospective multicentre cohort study was conducted in Nagano prefecture, Japan. The cohort included patients hospitalized at 16 participating institutions with a primary diagnosis of acute decompensated HF. Acute coronary syndrome patients were excluded. Between July 2014 and December 2018, patients were enrolled after the approval of each hospital's Ethics Committee and the provision of informed consent. Data collected at the compensated state of HF before discharge included socio‐economic status, medical history, laboratory, electrocardiogram and echocardiography data, discharge medications and status, and post‐discharge follow‐up. All study procedures were performed in accordance with the Declaration of Helsinki. For the current analysis, we excluded 333 patients with HFrEF, 60 patients with valvular disease, and 23 patients with missing information of critical baseline variables or outcomes (Figure 1 ). The remaining patients were divided into two groups: those being treated with MRAs (MRA users) at discharge and those who were not (non‐MRA users). Clinical data and survival status were collected in August 2019 by chart review and telephone calls to patients. The primary outcome was a composite of cardiovascular death and HF readmission. The secondary outcomes were the components of the primary outcome.
Figure 1.
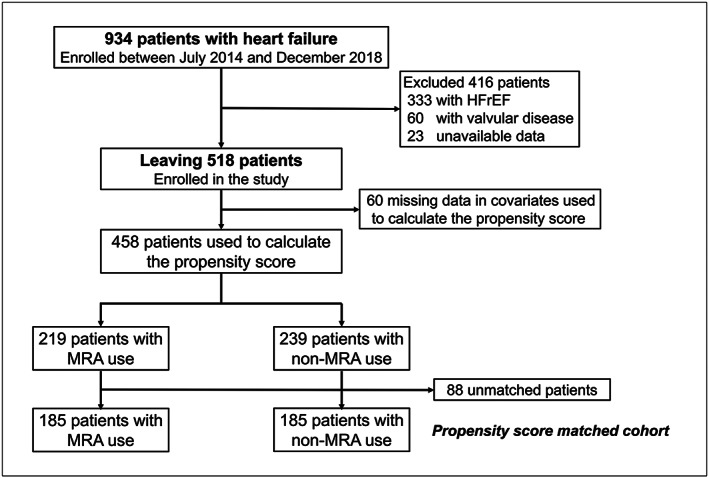
Patient flow chart.
Definitions
Acute decompensated HF was defined by the Framingham criteria. 21 The diagnosis of HF and acute coronary syndrome was made by the treating clinicians using all available symptom, laboratory, electrocardiogram, echocardiography, and coronary angiogram data. HF was classified according to the baseline LVEF at the compensated state before discharge as reduced EF (LVEF < 45%) or preserved EF (LVEF ≥ 45%). Cardiovascular death was defined as death related to HF, death from other cardiac causes, or cardiac sudden death. HF readmission was recorded as hospitalization during follow‐up due to worsening HF. MRA use was defined as newly or continuous prescription of spironolactone or eplerenone at discharge.
Statistical analysis
Continuous variables are summarized as the mean ± standard deviation if normally distributed and as the median and interquartile range if non‐normally distributed. Normality was assessed by the Shapiro–Wilk W‐test. Comparisons of baseline characteristics were made with a contingency table and the Pearson χ 2 test for categorical variables, the t‐test for normally distributed continuous variables, and either the Wilcoxon or Mann–Whitney U test for non‐normally distributed continuous variables. Propensity score adjustment was performed to reduce the confounding effects related to differences in background between MRA users and non‐users. For the calculation of propensity score, we adopted a logistic regression model in which the treatment status of MRA was regressed for the following 23 baseline characteristics: age, sex, blood pressure (systolic and diastolic), New York Heart Association (NYHA) class, previous HF admission, ischaemic heart disease, hypertension, diabetes mellitus, atrial fibrillation, antiplatelets, anti‐coagulants, angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers, beta‐blockers, loop diuretics, haemoglobin, albumin, serum creatine, serum sodium, serum potassium, B‐type natriuretic peptide, and LVEF; c‐statistic was calculated to examine the accuracy of the propensity score. The Hosmer Lemeshow test was used to assay the compatibility of the multiple logistic regression. In addition, patients with or without MRA use were matched based on the propensity score, using the greedy matching algorithm with a calliper width of 0.1. 22 To evaluate bias reduction, absolute standardized differences for all covariates after propensity matching were estimated. Absolute standardized differences of <10% indicated adequate matching. 23 Kaplan–Meier survival plots were calculated from baseline to time of events and compared using the log‐rank test in the crude and propensity score matched cohort. Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and confidence interval (CI) values. To evaluate the effect of MRA in patients with LVEF ≥50%, a similar propensity score matching analysis was repeated in a cohort without those with LVEF 45–49%. For sensitivity analysis, we conducted Cox proportional hazards regression models for the primary and secondary outcomes adjusting for 16 clinically relevant variables (age, sex, systolic blood pressure, previous HF admission, NYHA class, diabetes mellitus, atrial fibrillation, angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers, beta‐blockers, loop diuretics, haemoglobin, albumin, serum creatinine, B‐type natriuretic peptide, and LVEF) in the crude cohort.
Subgroup analyses were performed on pre‐specified clinical factors using Cox models in the matched cohort. Continuous variables were dichotomized by either clinical meaningful values or medians. We estimated the interactions between the subgroup factors and expressed the effect of MRA in the primary outcome as HRs with 95% CI values. A P‐value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics software for Windows Version 26 (IBM Corp., Armonk, NY, USA) and R statistical software (The ‘R’ Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Among the 518 patients enrolled in this study, 255 (49%) patients were treated with MRAs. Spironolactone was administered to 226 patients (88% of patients treated with MRAs), and eplerenone was given to 30 patients (12% of patients treated with MRAs). The baseline patient characteristics are shown in Table 1 and Supporting Information, Table S1 . The median age was 83 (interquartile range: 76–87) years, and 53% (n = 273) were female. The median LVEF was 61 (interquartile range: 53–67) per cent. Compared with non‐MRA users, MRA users had significantly lower systolic blood pressure, lower serum creatinine, lower LVEF, and higher haemoglobin. There were no differences in co‐morbidities between the two groups. While anti‐platelet drugs were administered more frequently to non‐MRA users, loop diuretics were prescribed more frequently to MRA users.
Table 1.
Baseline characteristics
| Variable | Before propensity score matching | P‐value | After propensity score matching | P‐value | ASD (%) | ||
|---|---|---|---|---|---|---|---|
| MRA use | MRA use | ||||||
| Yes (n = 255) | No (n = 263) | Yes (n = 185) | No (n = 185) | ||||
| Age (years) | 82 (75–87) | 83 (78–87) | 0.270 | 83 (76–88) | 84 (79–88) | 0.278 | 10.0 |
| Female, n (%) | 142 (56) | 131 (50) | 0.180 | 102 (55) | 97 (52) | 0.602 | 5.4 |
| BMI (kg/m2) | 21.1 (19.0–23.9) | 21.0 (19.0–24.6) | 0.618 | 21.0 (18.9–23.9) | 21.2 (19.0–24.3) | 0.514 | 1.4 |
| Systolic blood pressure (mmHg) | 114 (102–125) | 118 (106–130) | 0.022 | 114 (102–126) | 115 (103–128) | 0.593 | 3.8 |
| Diastolic blood pressure (mmHg) | 64 (55–73) | 64 (57–74) | 0.992 | 64 (55–71) | 64 (55–73) | 0.937 | 0.5 |
| NYHA Class III or IV, n (%) | 47 (18) | 42 (16) | 0.458 | 31 (17) | 31 (17) | 1 | 0.0 |
| Previous HF admission, n (%) | 72 (28) | 72 (27) | 0.827 | 55 (30) | 54 (29) | 0.909 | 1.1 |
| Ischaemic heart disease, n (%) | 47 (18) | 60 (23) | 0.218 | 36 (20) | 38 (21) | 0.795 | 2.5 |
| Hypertension, n (%) | 177 (69) | 185 (70) | 0.817 | 125 (68) | 128 (69) | 0.737 | 3.4 |
| Dyslipidaemia, n (%) | 66 (26) | 69 (26) | 0.927 | 47 (25) | 42 (23) | 0.543 | 6.3 |
| Diabetes mellitus, n (%) | 62 (24) | 81 (31) | 0.099 | 47 (25) | 54 (29) | 0.414 | 8.5 |
| Atrial fibrillation, n (%) | 157 (62) | 140 (53) | 0.061 | 114 (62) | 104 (56) | 0.291 | 11.0 |
| Medication | |||||||
| Anti‐platelet drugs, n (%) | 61 (24) | 91 (35) | 0.008 | 49 (27) | 53 (29) | 0.642 | 4.7 |
| Anti‐coagulants, n (%) | 166 (65) | 156 (59) | 0.175 | 123 (67) | 116 (63) | 0.447 | 8.0 |
| ACE‐Is/ARBs, n (%) | 168 (66) | 171 (65) | 0.836 | 117 (63) | 119 (64) | 0.829 | 2.3 |
| Beta‐blockers, n (%) | 174 (69) | 161 (61) | 0.083 | 119 (64) | 118 (64) | 0.914 | 1.0 |
| Loop diuretics, n (%) | 226 (89) | 196 (75) | <0.001 | 160 (87) | 155 (84) | 0.465 | 7.6 |
| Laboratory data | |||||||
| Hb (g/dL) | 11.6 (10.4–13.5) | 11.0 (10.0–12.7) | 0.002 | 11.5 (10.5–13.4) | 11.4 (10.2–12.8) | 0.211 | 14.8 |
| ALB (g/dL) | 3.4 (3.1–3.7) | 3.4 (3.0–3.7) | 0.085 | 3.4 (3.1–3.7) | 3.4 (3.0–3.7) | 0.356 | 9.6 |
| Serum creatinine (mg/dL) | 1.00 (0.82–1.36) | 1.14 (0.91–1.53) | 0.001 | 1.08 (0.86–1.39) | 1.10 (0.90–1.42) | 0.523 | 7.5 |
| Serum sodium (mEq/L) | 139 (137–141) | 140 (138–142) | 0.132 | 139 (137–141) | 140 (138–141) | 0.179 | 0.9 |
| Serum potassium (mEq/L) | 4.3 (4.0–4.6) | 4.3 (4.0–4.7) | 0.664 | 4.3 ± 0.5 | 4.3 ± 0.6 | 0.784 | 2.7 |
| BNP (pg/mL) | 239 (105–438) | 203 (107–456) | 0.792 | 213 (97–436) | 206 (109–451) | 0.745 | 2.5 |
| Echocardiographic data | |||||||
| LVEF (%) | 59 (51–67) | 61 (55–67) | 0.049 | 60 (51–68) | 60 (53–66) | 0.941 | 0.3 |
| LVDd (mm) | 46 (41–51) | 47 (42–51) | 0.277 | 46 (41–51) | 45 (41–51) | 0.787 | 1.9 |
| LVDs (mm) | 30 (26–36) | 30 (27–35) | 0.899 | 30 (26–35) | 31 (27–35) | 0.304 | 5.7 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ALB, serum albumin; ARB, angiotensin‐receptor blocker; ASD, absolute standardized difference; BMI, body mass index; BNP, B‐type natriuretic peptide; Dd, diastolic dimension; Ds, systolic dimension; EF, ejection fraction; Hb, haemoglobin; HF, heart failure; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Values are presented as the mean ± SD, median (interquartile range), or n (%).
Propensity score matching resulted in the creation of 185 matched pairs of MRA and non‐MRA users. The multiple logistic regression model had a c‐statistic of 0.688. The Hosmer–Lemeshow test showed a P‐value of 0.290, and the compatibility of the multiple logistic regression was good. After propensity score matching, most of the baseline characteristics were well balanced between the groups, with absolute standardized differences of <10% apart from those of haemoglobin and the prevalence of atrial fibrillation, which were slightly over 10% (Table 1 ).
Clinical outcomes
During a median follow‐up of 553 (interquartile range: 275–944) days, 192 (37%) patients experienced the primary outcome (cardiovascular death, 71 patients; HF readmission, 168 patients). Event‐free survival curves of the primary and secondary outcomes after propensity score matching are shown in Figure 2 (Kaplan–Meier curves of the primary and secondary outcomes in the crude cohort are shown in Supporting Information, Figure S1 ). The HRs and 95% CI values of the primary and secondary outcomes before and after propensity score matching are presented in Table 2 . After propensity score matching, the incidence of the primary outcome was significantly lower in MRA users as compared with non‐users (32% vs. 49%; HR, 0.669; 95% CI, 0.482–0.929; P = 0.016). In terms of the secondary outcomes, the incidence of cardiovascular death was also significantly lower in the MRA users (11% vs. 22%; HR, 0.563; 95% CI, 0.333–0.953; P = 0.032). The risk of HF readmission tended to be lower in the MRA users (29% vs. 41%; HR, 0.738; 95% CI, 0.520–1.048; P = 0.089).
Figure 2.
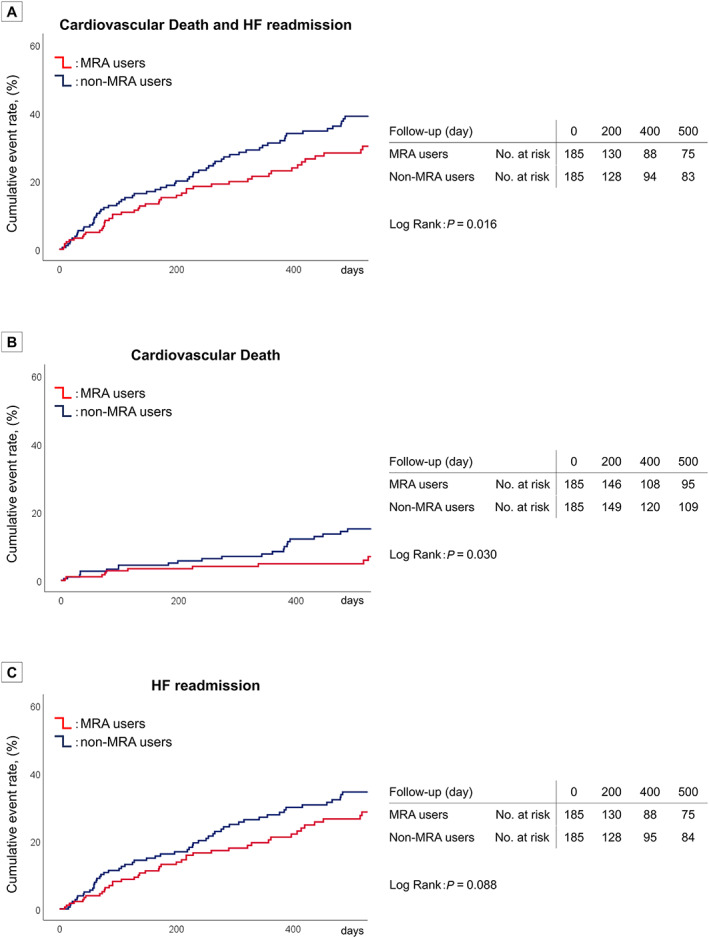
Kaplan–Meier plots of the primary and secondary outcomes after propensity score matching.
Table 2.
Risk of primary and secondary outcomes before and after propensity score matching
| Variable | Before propensity score matching | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|---|
| MRA use | MRA use | |||||||
| Yes (n = 255) | No (n = 263) | HR (95% CI) | P‐value | Yes (n = 185) | No (n = 185) | HR (95% CI) | P‐value | |
| Cardiovascular death and HF readmission | 79 (31%) | 113 (43%) | 0.737 (0.553–0.983) | 0.038 | 59 (32%) | 90 (49%) | 0.669 (0.482–0.929) | 0.016 |
| Cardiovascular death | 27 (11%) | 44 (17%) | 0.687 (0.425–1.109) | 0.124 | 21 (11%) | 41 (22%) | 0.563 (0.333–0.953) | 0.032 |
| HF readmission | 73 (28%) | 95 (36%) | 0.812 (0.598–1.102) | 0.181 | 54 (29%) | 75 (41%) | 0.738 (0.520–1.048) | 0.089 |
CI, confidence interval; HF, heart failure; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist.
Values are expressed as n (%).
In the crude cohort, risk of the primary outcome was significantly lower in MRA users as compared with non‐users (31% vs. 43%; HR, 0.737; 95% CI, 0.553–0.983; P = 0.038). After Cox proportional hazards analysis adjusting for 16 clinically relevant variables, MRA use was associated with a lower risk of the primary outcome (HR, 0.710; 95% CI 0.507–0.995; P = 0.047) (Supporting Information, Table S2 ). In terms of the similar analysis performed in a cohort with patients with LVEF ≥50%, propensity score matching resulted in the creation of 164 matched pairs of MRA and non‐MRA users. The primary outcome was significantly lower in MRA users [32% (52/164) vs. 46% (76/164); HR, 0.662; 95% CI, 0.465–0.943; P = 0.022] (Supporting Information, Figure S2 ).
Subgroup analysis
Subgroup analysis on clinically relevant factors are shown in Figure 3 . There were no significant interactions observed in the primary outcome associated with MRA use and clinical factors such as LVEF, body mass index, systolic blood pressure, ischaemic heart disease, diabetes mellitus, atrial fibrillation, serum creatinine, serum sodium, serum potassium, B‐type natriuretic peptide, use of angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers, and beta‐blockers. On the other hand, significant interactions of MRA effects were observed in sex difference. There was a significant association between MRA use and a lower risk of the primary outcome in patients with LVEF ≥50% (HR, 0.665; 95% CI, 0.465–0.952, P = 0.026).
Figure 3.
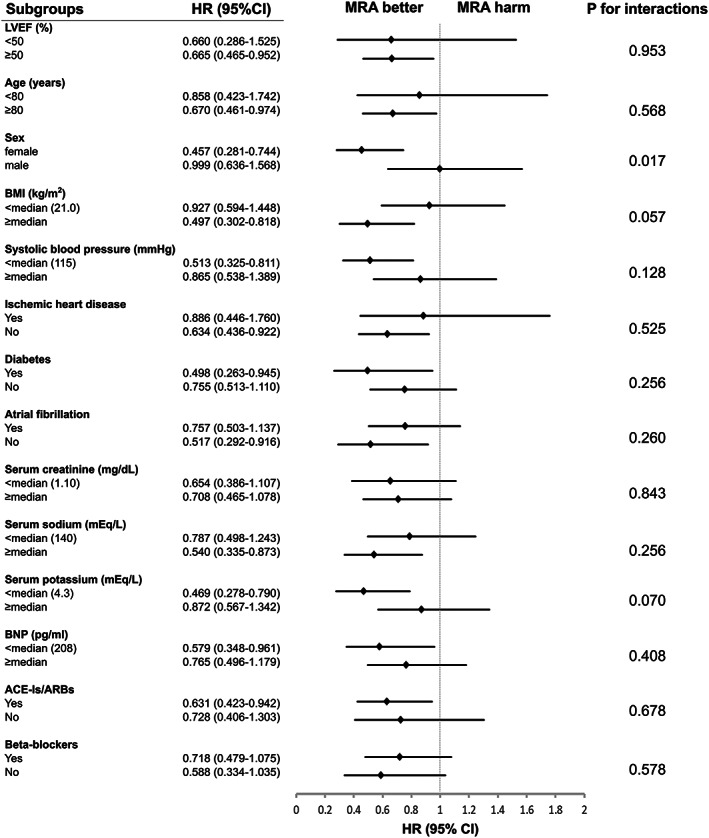
Subgroup analysis for the primary outcome after propensity score matching. ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist.
Discussion
The novel finding of the present study is that MRA administration at discharge in HFpEF patients hospitalized for acute decompensated HF was associated with a lower risk of the primary composite outcome of cardiovascular death and HF readmission. Moreover, importantly in the secondary outcomes, the incidence of cardiovascular mortality was also lower in these patients. The result was consistent in a similar analysis performed in a cohort of patients with LVEF ≥50%. The expected main reason to explain our study results was the enrolled patient cohort of acute decompensated HF with more severe conditions compared with previous studies.
The effect of MRAs on HFpEF is inconsistent in the literature. 24 In the TOPCAT trial, spironolactone did not reduce the rate of the primary composite outcome of cardiovascular death, aborted cardiac arrest, and HF readmission. 5 However, in the TOPCAT trial, there was a marked regional variation in event rates, with patients in the placebo group who were enrolled in Russia and Georgia having a much lower likelihood of outcome events than those enrolled in the Americas (patients enrolled from the USA, Canada, Brazil, and Argentina). 5 This discrepancy in event rates was unexpected and unexplained and could have affected the results. A post hoc analysis of the TOPCAT trial reported that spironolactone reduced the rate of all‐cause death, cardiovascular death, and HF hospitalization in the Americas with significantly higher event rates compared to patients enrolled from Russia and Georgia. 19 The fact that almost every important baseline characteristic and event rate were different between the two populations could lead to the hypothesis that the enrolled patient cohort were inconsistent between regions. Several prospective observational studies have shown an association of MRAs and improved prognosis in HFpEF but only in post hoc analyses. Yaku et al. investigated the beneficial effect of MRAs in patients with acute decompensated HF and additionally revealed the association between MRA use and a risk reduction of a composite outcome of all‐cause death and HF readmission in patients with LVEF ≥40%. 18 The Karolinska–Rennes study reported that MRA use independently predicted a lower risk of a similar composite outcome in acute decompensate HF patients with LVEF ≥45%. 20 Meanwhile, mechanistic studies have reported that MRAs improved diastolic function in HFpEF. 25 , 26
In the current study, we identified a significant association between MRA use and lower risks of cardiovascular events in elderly Japanese HFpEF patients hospitalized for acute decompensated HF. To the best of our knowledge, no other study has investigated the association of MRA and cardiovascular mortality improvement in these patients. The propensity score matching method was employed to reduce the confounding effects related to differences in background between MRA users and non‐users. The baseline characteristics were well balanced after analyses. In the subgroup analysis, there was a significant association between MRA use and a lower risk of the primary outcome in patients with LVEF ≥50%. Moreover, we investigated that the effect of MRA was consistent in a similar analysis performed in a cohort of patients with LVEF ≥50%. These results indicate that our study was not strongly affected by patients with a LVEF of 45–49%, which may have a different pathophysiology compared with patients with LVEF ≥50%. Our findings have important clinical implications that enable us to propose the administration of MRAs to improve prognosis in acute decompensated HFpEF patients.
The main reason to explain our study results could be the cohort of enrolled patients with acute decompensated HF, including 144 (28%) patients with previous HF admission and 89 (17%) patients with NYHA class III or IV at discharge. The renin‐angiotensin‐aldosterone system and the sympathetic nerve system are excessively activated in acute decompensated HF, 27 and the neurohormonal balance differs from that in stable HF. There are several hypotheses against why MRAs may have better effects in severe HF than in mild or stable conditions. First, loop diuretics are more frequently used in severe HF patients, possibly inducing hypokalaemia. The potassium sparing effect of MRAs can reduce the risk of hypokalaemia, which is associated with an increased risk of death in HF. 28 Second, advanced heart disease causes cardiac fibrosis. MRA decreases extracellular matrix turnover and myocardial collagen content in patients with HF. 29 Patients who experienced acute decompensated HF may have higher levels of serum collagen type I aminoterminal peptide and procollagen type III aminoterminal peptide, markers of collagen turnover. Previous studies have reported that the use of MRAs in patients with higher levels of collagen turnover markers was associated with a better response in MRAs 30 and reduced mortality. 31 Considering these facts, there is a possibility that patients with severe HFpEF may be better responders to MRAs than those with stable HF. Third, higher event rates in the current study compared with that in stable HFpEF could be another reason to explain our results (as low event rates would be difficult to reduce further). The event rate in TOPCAT (a study population of stable HFpEF) was clearly lower than that of patients hospitalized for acute decompensated HFpEF. The incidences of the primary and secondary outcomes in our study were similar to or slightly higher than the rates observed in other observational studies, including those in Japan, 1 , 18 , 20 , 32 possibly due to a higher median age than in prior trials. The event rate in this study was also higher than in other randomized control trials for HFpEF. 5 , 8 , 9 , 10 However, the prognosis in randomized control trials tends to be better than in observational studies, particularly for HFpEF. 20 Randomized control trials may have included patients that were healthier, younger, or selected. Regional variation could be another reason to explain this discrepancy, as evidenced by a significant difference in the event rate between patients from the Americas and from Russia/Georgia in the post hoc analysis of the TOPCAT trial. 19
Based on our findings, we hypothesize that MRAs may reduce cardiovascular mortality in acute decompensated HFpEF patients. Importantly, this study's results uncovered a significant association between MRAs and a risk reduction of a composite outcome of cardiovascular mortality and HF readmission, which could provide significant benefits in afflicted patients. Further randomized control trials, observational studies including larger numbers of patients hospitalized for acute decompensated HFpEF, and basic studies are needed to verify our hypotheses.
Our study has several limitations. First, it had a relatively small sample size and short median follow‐up period. Second, we had no prescription data after discharge; thus, the possible changes in HF treatment during follow‐up were not considered. Third, in our study, we defined HFpEF as LVEF ≥45% which differs from the definition mentioned in current guidelines. 11 , 12 To compare and discuss our results with previous studies, we used the same LVEF definition as the TOPCAT trial (i.e. LVEF ≥45%). However, patients with a LVEF of 45–49% could have a different pathophysiology compared with those with LVEF ≥50%, with possible stronger association with ischaemic heart disease. Although we performed additional analyses, we still could not exclude the possibility that our study results were affected by these patients. Further studies including large numbers of patients are necessary to verify the possible opinion that patients with a LVEF of 45–49% associated with ischaemic heart disease would emphasize the beneficial effect of MRAs against HFpEF. Finally, although we performed adequate propensity score matching using 23 baseline characteristics to reduce confounding effects, residual unmeasured confounding factors could have affected the results.
Conclusions
Mineralocorticoid receptor antagonist use was significantly associated with a lower risk of the primary composite outcome of cardiovascular death and HF readmission in patients hospitalized for acute decompensated HFpEF. Moreover, importantly in the secondary outcomes, the incidence of cardiovascular mortality was also significantly lower in these patients. The administration of MRAs at discharge may therefore improve the prognosis of patients with HFpEF.
Conflict of interest
None declared.
Funding
This research received no grants from any funding agency in the public, commercial, or not‐for‐profit sector.
Supporting information
Figure S1. Kaplan Meier plots of the primary and secondary outcomes in the crude cohort.
Figure S2. Kaplan Meier plots of the primary outcome in the crude and propensity matched cohort in patients with LVEF ≥50%.
Table S1. Baseline characteristics before and after propensity score matching
Table S2. Multivariate Cox proportional hazards analyses
Acknowledgements
We thank all of the following 14 hospitals that participated in this study: Shinshu Ueda Medical Center, Nagano Red Cross Hospital, Matsumoto Medical Center, Nagano Municipal Hospital, Iida Municipal Hospital, Aizawa Hospital, Ina Central Hospital, Okaya City Hospital, Saku Central Hospital, Hokushin General Hospital, Suwa Red Cross Hospital, Matsushiro General Hospital, Shinshu Medical Center, and Asama Nanroku Komoro Medical Center. The authors also acknowledge the secretarial assistance of Minako Aono.
Suzuki, S. , Motoki, H. , Kanzaki, Y. , Maruyama, T. , Hashizume, N. , Kozuka, A. , Yahikozawa, K. , and Kuwahara, K. (2020) Prognostic impact of mineralocorticoid receptor antagonist in patients with heart failure with preserved ejection fraction. ESC Heart Failure, 7: 2752–2761. 10.1002/ehf2.12867.
References
- 1. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 2. Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail 2012; 5: 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med 2016; 375: 1868–1877. [DOI] [PubMed] [Google Scholar]
- 4. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 5. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J‐DHF). Eur J Heart Fail 2013; 15: 110–118. [DOI] [PubMed] [Google Scholar]
- 7. Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei Cas L. Effects of the long‐term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail 2012; 14: 219–225. [DOI] [PubMed] [Google Scholar]
- 8. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 9. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP‐CHF) study. Eur Heart J 2006; 27: 2338–2345. [DOI] [PubMed] [Google Scholar]
- 10. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 11. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 15. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 16. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 17. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21.21073363 [Google Scholar]
- 18. Yaku H, Kato T, Morimoto T, Inuzuka Y, Tamaki Y, Ozasa N, Yamamoto E, Yoshikawa Y, Kitai T, Taniguchi R, Iguchi M, Kato M, Takahashi M, Jinnai T, Ikeda T, Nagao K, Kawai T, Komasa A, Nishikawa R, Kawase Y, Morinaga T, Toyofuku M, Seko Y, Furukawa Y, Nakagawa Y, Ando K, Kadota K, Shizuta S, Ono K, Sato Y, Kuwahara K, Kimura T. Association of mineralocorticoid receptor antagonist use with all‐cause mortality and hospital readmission in older adults with acute decompensated heart failure. JAMA Netw Open 2019; 2: e195892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131: 34–42. [DOI] [PubMed] [Google Scholar]
- 20. Lund LH, Donal E, Oger E, Hage C, Persson H, Haugen‐Lofman I, Ennezat PV, Sportouch‐Dukhan C, Drouet E, Daubert JC, Linde C. Association between cardiovascular vs. non‐cardiovascular co‐morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16: 992–1001. [DOI] [PubMed] [Google Scholar]
- 21. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 22. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001; 54: 387–398. [DOI] [PubMed] [Google Scholar]
- 24. Capuano A, Scavone C, Vitale C, Sportiello L, Rossi F, Rosano GM, Coats AJ. Mineralocorticoid receptor antagonists in heart failure with preserved ejection fraction (HFpEF). Int J Cardiol 2015; 200: 15–19. [DOI] [PubMed] [Google Scholar]
- 25. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA 2013; 309: 781–791. [DOI] [PubMed] [Google Scholar]
- 26. Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation 2004; 110: 558–565. [DOI] [PubMed] [Google Scholar]
- 27. Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009; 53: 557–573. [DOI] [PubMed] [Google Scholar]
- 28. Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, Pitt B. A propensity‐matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J 2007; 28: 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lacolley P, Safar ME, Lucet B, Ledudal K, Labat C, Benetos A. Prevention of aortic and cardiac fibrosis by spironolactone in old normotensive rats. J Am Coll Cardiol 2001; 37: 662–667. [DOI] [PubMed] [Google Scholar]
- 30. Stienen S, Rossignol P, Barros A, Girerd N, Pitt B, Zannad F, Ferreira JP. Determinants of anti‐fibrotic response to mineralocorticoid receptor antagonist therapy: insights from the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) and Early Eplerenone Treatment in Patients with Acute ST‐elevation Myocardial Infarction without Heart Failure (REMINDER) trials. Clin Res Cardiol 2020; 109: 194–204. [DOI] [PubMed] [Google Scholar]
- 31. Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000; 102: 2700–2706. [DOI] [PubMed] [Google Scholar]
- 32. Tsuchihashi‐Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, Takeshita A, Tsutsui H. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE‐CARD). Circ J 2009; 73: 1893–1900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan Meier plots of the primary and secondary outcomes in the crude cohort.
Figure S2. Kaplan Meier plots of the primary outcome in the crude and propensity matched cohort in patients with LVEF ≥50%.
Table S1. Baseline characteristics before and after propensity score matching
Table S2. Multivariate Cox proportional hazards analyses


