Abstract
Aims
End‐stage heart failure patients often present with severe kidney failure and have limited treatment options. We compared the clinical characteristics and outcomes among end‐stage heart and kidney failure patients who underwent combined heart and kidney transplant (HKTx) with those who underwent kidney transplant after heart transplant (KAH).
Methods and results
All patients from 2007–2016 who underwent combined HKTx (n = 715) and those who underwent KAH (n = 130) using the United Network for Organ Sharing database were included. Kaplan‑Meier curves and Cox models compared survivals and identified predictors of death. Number of combined HKTx performed annually in United States increased from 59 in 2007 to 146 in 2016 whereas KAH decreased from 34 in 2007 to 6 in 2016. Among KAH patients, average wait time for kidney transplant was 3.0 years, time to dialysis or to kidney transplant after heart transplant did not differ with varying severity of kidney disease at baseline (P for both >0.05). Upon follow‐up (mean 3.5 ± 2.7 years), 151 patients died. In multivariable models, patients who underwent combined HKTx had 4.7‐fold greater risk of death [95% confidence interval (CI) 2.4–9.4) than KAH patients upon follow up. A secondary analysis using calculation of survival only after kidney transplant for KAH patients still conferred higher risk for combined HKTx patients [hazard ratio (HR) 2.6 95% CI 1.33–5.15]. In subgroup analyses after excluding patients on dialysis (HR 3.99 95% CI 1.98–8.04) and analysis after propensity matching for age, gender, and glomerular filtration rate (HR 3.01 95% CI 1.40–6.43) showed similar and significantly higher risk for combined HKTx patients compared with KAH patients. Lastly, these results also remained unchanged after excluding transplant centres who performed only one type of procedure preferentially, i.e. HKTx or KAH (HR 4.70 95% CI 2.35–9.42).
Conclusions
National registry data show continual increase in combined HKTx performed annually in the United States but inferior survival compared with KAH patients. Differences in patient characteristics or level of kidney dysfunction at baseline do not explain these poor outcomes among HKTx patients compared with KAH patients. Consensus guidelines are greatly needed to identify patients who may benefit more from dual organ transplants.
Keywords: Heart transplant, Kidney transplant, Mortality
Introduction
Chronic kidney disease is highly prevalent among patients with end‐stage heart failure who are candidates for heart transplantation. After heart transplant, some patients develop de novo kidney disease, and some develop progressive kidney failure eventually requiring renal replacement therapies. The pathophysiology of kidney disease is multifactorial, and common causes include reduced kidney perfusion and venous congestion prior to transplant and calcineurin inhibitor‐mediated nephrotoxicity post‐transplant. 1 , 2 Established risk factors for developing chronic kidney disease post‐ transplantation are older recipient age, time after transplant, female gender, and pre‐transplant renal impairment. 3 , 4 , 5
Pre‐heart transplant and post‐heart transplant renal impairment has been associated with poor outcomes, especially in patients needing dialysis prior to heart transplant. 6 , 7 Combined heart and kidney transplantation (HKTx) is an option for patients suffering from combined heart and kidney failure. 8 In fact, International Society of Heart and Lung Transplant registry data show increase in number of combined HKTx performed every year in the last 5 years. 9 Several studies have indicated that certain patients would either benefit or have similar survival after combined HKTx compared with heart transplant alone. Those patients include heart re‐transplant, individuals with end‐stage renal disease (ESRD) on dialysis and individuals with non‐dialysis‐dependent renal impairment that is unlikely to resolve post‐heart transplant alone. 10 , 11 , 12 However, identifying patients who will not recover from the renal impairment after heart transplant is challenging and therefore no clear guidelines exist that recommend combined HKTx rather than heart transplant alone for any given level of kidney dysfunction.
Despite the efforts to increase and facilitate organ donation, the demand for organs for transplantation continues to increase. 13 Recent evidence suggests that patients receiving kidney transplant after heart transplant (KAH) have comparable long‐term survival with heart transplant recipients alone. 14 , 15 Additionally, it remains unclear whether heart transplant candidates with moderate kidney disease have superior outcomes with combined HKTx compared with heart transplant alone with an option to undergo kidney transplant later if their kidney failure were to get worse.
We therefore used the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) database to compare characteristics and survival of patients who received combined HKTx with KAH patients. Additionally, we examined important risk factors of higher mortality in each of these patient groups.
Methods
Study sample
We selected all patients aged ≥ 18 years who received combined HKTx (n = 822) and all patients who received KAH (n = 151) between 1 January 2007 to 31 December 2016 from de‐identified patient‐level data provided by the UNOS database using the STAR files. All combined HKTx procedures were performed either as simultaneous procedures or performed sequentially within 24 h of one another. All patients that underwent a combined HKTx with a history of prior heart transplant (re‐transplant) were excluded from the database (n = 120). Additionally, patients who required dialysis at the time of heart transplantation but only received a heart transplant were also excluded (n = 8). Therefore, our final sample was of 845 patients, 715 with combined HKTx and 130 with KAH transplants.
All information on demographics, clinical variables such as history of hypertension or diabetes, type of cardiomyopathy, kidney function as defined by serum creatinine values and dialysis status, presence of ventricular assist device etc., are all self‐reported and entered by each individual transplant programme into the UNOS database. Information on waitlist times and UNOS waitlist status for both heart and kidney transplants were obtained from separate databases (STAR files) of heart and kidney transplant and linked using the common UNOS identification numbers. No separate informed consent was required because the data were de‐identified and used in compliance with the UNOS data use agreement. Data on mortality were also available from UNOS database, as reported by each centre. For the purpose of the present study, to estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease–Epidemiology Collaboration Equation. 16
Statistical analysis
We first examined the number of combined HKTx and KAH performed according to each calendar year from 1996–2016. However, note that data for rest of the analysis performed in the study were selected only from 2007–2016 as it represented the current clinical practices as opposed to previous era with different immunosuppressive regimes and the time period when practice changes were evolving. We then compared the demographics, waitlist times on heart (and kidney) transplant waiting lists, centre volume, serum creatinine levels at the time of transplant, cardiovascular risk factors, and other clinical parameters including presence of ventricular assist device or total artificial heart for combined HKTx patients with KAH transplants. Additionally, we also examined all information on demographics and clinical variables (as previously mentioned) according to eGFR levels prior to heart transplant. Next, for KAH transplant patients, we analysed and compared time period between the two transplants, time between heart transplant to the time first listed on kidney transplant list, time on kidney transplant waitlist, and time on dialysis after heart transplant according to baseline eGFR categories (<30, 30–45, 45–60, and >60 mL/min/1.73m2).
We then created Kaplan–Meier curves to examine the risk of death in patients who underwent combined HKTx (separately in groups if they were on dialysis or not) to those that underwent KAH.
We created the Cox proportional hazard regression models in a hierarchical fashion as follows:
-
(i)
Adjusting for age and sex.
-
(ii)
Adjusting for age, sex, gender mismatch, donor cytomegalovirus status, total bilirubin, calculated panel reactive antibody, diabetes mellitus, presence of total artificial heart or left ventricular assist device, presence of intra‐aortic balloon pump, and days on heart (or combined heart‑kidney) transplant waitlist.
-
(iii)
Stepwise regression model that began with age and sex and allowed the possibility of any of the covariates (as displayed in Table 1 ) to enter the model (P < 0.20) and eliminating any covariates if they were not significantly contributing to the model after entry of new covariates (P < 0.10).
Table 1.
Baseline characteristics comparing patients undergoing combined heart and kidney transplant to patients with and without the need for dialysis at time of heart transplant with initial heart transplant followed by kidney transplant
| Characteristics | HKTx–dialysis N = 243 | HKTx–no dialysis N = 472 | KAHN = 130 | P value |
|---|---|---|---|---|
| Recipient age, years | 50.6 (12.7) | 56.5 (10.4) | 51.3 (14.5) | <.001 |
| Donor age, years | 31.4 (12.4) | 31.6 (11.1) | 29.3 (11.5) | 0.14 |
| Female recipient, n (%) | 46 (19) | 90 (19) | 40 (31) | 0.010 |
| Male recipient, female donor, n (%) | 48 (24) | 62 (16) | 27 (30) | 0.004 |
| Female recipient, male donor, n (%) | 17 (37) | 41 (46) | 16 (40) | 0.60 |
| Race, n (%) | 0.001 | |||
| Asian | 15 (6) | 11 (2) | 1 (1) | |
| African‑American | 77 (32) | 154 (33) | 26 (20) | |
| Hispanic | 25 (11) | 33 (7) | 10 (8) | |
| Caucasian | 117 (49) | 265 (57) | 88 (69) | |
| Other | 4 (2) | 5 (1) | 3 (2) | |
| High school education, n (%) | 229 (94) | 460 (97) | 123 (95) | 0.07 |
| BMI, kg/m2 | 25.9 (5.2) | 27.0 (5.0) | 26.5 (5.1) | 0.024 |
| Waitlist status, n (%) | <.001 | |||
| 1A | 138 (57) | 313 (66) | 76 (58) | |
| 1B | 75 (31) | 143 (30) | 40 (31) | |
| 2 | 30 (12) | 16 (3) | 14 (11) | |
| Ischaemic time, h | 3.2 (1.1) | 3.0 | 3.4 (1.1) | 0.002 |
| Time on waitlist, days | 169.5 (244.5) | 250.0 (398.7) | 152 (260.8) | 0.001 |
| High‐volume centre (>200), n (%) | 170 (70) | 325 (69) | 88 (68) | 0.90 |
| Missing smoking status, n (%) | 1 (0) | 3 (1) | 1 (1) | 0.90 |
| Prior smoking, n (%) | 86 (36) | 208 (44) | 53 (41) | 0.08 |
| Diagnosis, aetiology for heart failure, n (%) | 0.048 | |||
| Ischaemic cardiomyopathy | 89 (37) | 171 (36) | 44 (34) | |
| Restrictive cardiomyopathy | 8 (3) | 24 (5) | 4 (3) | |
| Congenital | 5 (2) | 11 (2) | 10 (8) | |
| Other | 141 (58) | 266 (56) | 72 (55) | |
| Diabetes, n (%) | 0.09 | |||
| No | 140 (58) | 266 (56) | 89 (68) | |
| Type I | 18 (7) | 22 (5) | 8 (6) | |
| Type II | 78 (32) | 171 (36) | 30 (23) | |
| Other type | 1 (0) | 1 (0) | 0 (0) | |
| Unknown type | 6 (2) | 10 (2) | 1 (1) | |
| Diabetes status unknown | 0 (0) | 2 (0) | 2 (2) | |
| Dialysis, n (%) | <.001 | |||
| No dialysis | 0 (0) | 472 (100) | 130 (100) | |
| Haemodialysis | 214 (88) | 0 (0) | 0 (0) | |
| Peritoneal dialysis | 25 (10) | 0 (0) | 0 (0) | |
| Dialysis with unknown method | 4 (2) | 0 (0) | 0 (0) | |
| Cerebrovascular disease, n (%) | 11 (5) | 39 (8) | 8 (6) | 0.16 |
| Cardiac defibrillator, n (%) | 161 (66) | 265 (77) | 99 (76) | 0.005 |
| Serum creatinine at transplant, μmol/L | 486 (309) | 248 (141) | 141 (80) | <.001 |
| Bilirubin at listing, μmol/L | 24 (55) | 21 (53) | 22 (38) | 0.72 |
| Hospital stay, n (%) | 0.57 | |||
| Intensive care treatment | 86 (36) | 170 (36) | 43 (33) | |
| Hospitalized, not in the ICU | 56 (23) | 101 (22) | 23 (18) | |
| Not hospitalized | 100 (41) | 196 (42) | 64 (49) | |
| Ventilator support, n (%) | 5 (2) | 10 (2) | 4 (3) | 0.79 |
| ECMO, n (%) | 2 (1) | 1 (0) | 2 (2) | 0.19 |
| IABP, n (%) | 20 (8) | 29 (6) | 7 (5) | 0.47 |
| LVAD at listing, n (%) | 34 (14) | 61 (13) | 17 (13) | 0.92 |
| LVAD at transplant, n (%) | 10 (4) | 58 (12) | 17 (13) | 0.001 |
| RVAD at listing, n (%) | 9 (4) | 5 (1) | 3 (2) | 0.06 |
| RVAD at transplant, n (%) | 4 (2) | 5 (1) | 3 (2) | 0.53 |
| Total artificial heart, n (%) | 12 (5) | 21 (4) | 3 (2) | 0.47 |
| Inotrope, n (%) | 105 (43) | 217 (46) | 54 (42) | 0.59 |
| Cardiac output, L/min | 8.0 (2.7) | 7.9 (2.5) | 6.6 (2.4) | 0.06 |
| Systolic PAP, mmHg | 45.3 (13.9) | 45.1 (14.0) | 41.4 (14.0) | 0.025 |
| Mean PAP, mmHg | 30.5 (9.0) | 30.6 (9.8) | 28.1 (9.9) | 0.043 |
| PCWP, mmHg | 20.1 (8.6) | 20.5 (8.8) | 19.5 (9.0) | 0.56 |
| Donor CMV status, n (%) | <.001 | |||
| Negative | 83 (34) | 179 (38) | 59 (45) | |
| Not done/unknown | 1(0) | 8 (2) | 9 (7) | |
| Positive | 158 (65) | 280 (60) | 62 (48) | |
| Recipient CMV status, n (%) | 0.38 | |||
| Negative | 86 (35) | 182 (39) | 42 (32) | |
| Not done/unknown | 2 (1) | 1 (0) | 0 (0) | |
| Positive | 155 (65) | 289 (61) | 88 (68) | |
| PRA Class 1 > 10%, n (%) | 21 (12) | 56 (20) | 11 (9) | 0.009 |
| PRA Class 2 > 10%, n (%) | 15 (9) | 38 (14) | 9 (8) | 0.10 |
BMI, body mass index; CMV, cytomegalovirus; ECMO, extracorporeal membrane oxygenation; HKTx, combined heart and kidney transplantation; IABP, intra‐aortic balloon pump; ICU, intensive care unit; KAH, kidney transplant after initial heart transplantation; LVAD, left ventricular assist device; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RVAD, right ventricular assist device; PRA, panel reactive antibody.
Data are reported as mean ± standard deviation unless stated otherwise. P values are for comparisons using chi square test (or Fisher's exact test wherever applicable) for categorical variables and Kruskal‑Wallis test for continuous variables to assess any differences between categories.
Lastly, with the limited information available from UNOS database, we examined the cause of death for patients who died after receiving combined HKTx and compared them with causes of death among KAH patients.
Secondary analysis
Due to the design of our study, KAH patients had an advantage of survival up to their kidney transplant; therefore, we performed a secondary analysis where survival in the Cox models for KAH patients was measured starting from kidney transplant (instead of heart transplant). Hence, in this analysis, Cox models were examined where the survival was estimated after the combined heart and kidney transplant for the combined HKTx group and survival time was estimated starting at kidney transplant for the KAH group. All models were again adjusted for covariates in the similar hierarchical fashion as described above.
Subgroup analysis
Because one of the aims of our study was also to examine the survival differences specifically among patients with moderate kidney disease at baseline, hence in subgroup 1, we excluded all patients who were on dialysis at the time of their initial transplant. Cox models were constructed to examine the difference in survival between combined HKTx patients to the KAH transplant patients and adjusting for covariates in the same hierarchical fashion as described above.
In subgroup 2 analysis, we performed Cox models after propensity score matching based on age, sex, and eGFR with a 1:1 match. This subgroup included 130 patients in each group. Separate analyses were conducted to examine the risk of death for patients with combined HKTx compared with KAH patients after adjusting for covariates in the same hierarchical fashion as the main analyses previously mentioned.
Lastly, in subgroup 3 analysis, we examined the possibility of selection bias for transplant centre preferentially performing one type of procedure (HKTx or KAH) over the other and if such bias may have impacted our results. First, we identified each centre whose procedure allocation was not significantly different from the overall cohort via a binomial test and centres who had performed at least one procedure of each type. This resulted in a subcohort with 562 patients across 60 transplant centres. We then repeated the Cox models in this subgroup while adjusting for all variables in the similar hierarchical fashion as previously mentioned.
All analyses were performed using SAS version 9.4 (Cary, NC), and a two‐sided P value < 0.05 was considered statistically significant.
Results
Figure 1 displays the actual number of combined HKTx and KAH transplants performed annually since 1996 until 2016. Interestingly, over the last decade, the number of patients undergoing combined HKTx increased significantly from 59 annually in 2007 to 146 in 2016 whereas the annual number of KAH performed decreased, from 34 in 2007 to 6 in 2016. More specifically, we observed a significant drop in number of KAH transplants performed since year 2000.
Figure 1.
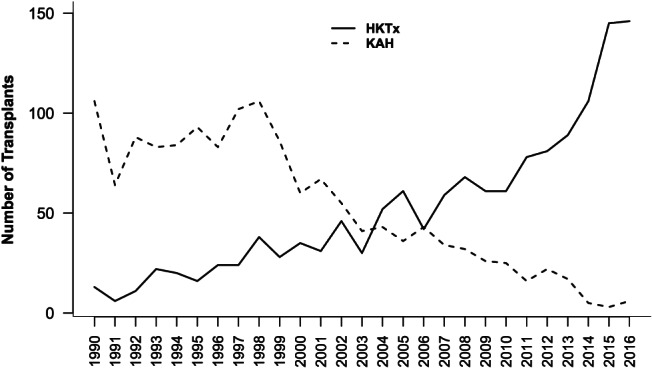
Number of patients undergoing combined heart and kidney transplant (HKTx) or kidney transplantation after initial heart transplantation (KAH) from 1 January 1990 until 31 December 2016.
Baseline characteristics
Demographics and baseline characteristics of patients as assessed at the time of heart transplant (or combined HKTx) are displayed in Table 1 . Patients undergoing combined HKTx (with or without dialysis) were older and more likely to be male patients. Combined HKTx patients who were not on dialysis were more likely on UNOS status 1A at the time of transplant and had the longest wait times on the transplant list and were less likely to have a defibrillator or left ventricular assist device compared with other patients. As expected, combined HKTx patients had higher serum creatinine concentrations at the time of transplant and demonstrated greater cardiac output with higher pulmonary artery pressures. Patients in combined HKTx group had higher prevalence of cytomegalovirus antibodies and higher class 1 panel reactive antibody levels. There were no differences between the groups regarding UNOS status 1A and 1B on the waitlist, whether the procedures were performed at a high‐volume centre (>200 transplants) or whether the patients were in the intensive care unit, hospitalized, or at home prior to the transplant. Finally, need for ventilator support, right ventricular assist device, or intra‐aortic balloon pump use at the time of transplant was similar between the two groups. Baseline characteristics and demographics of patients according to their eGFR (<30, 30–45, 45–60, and >60 mL/min/1.73m2) are displayed in Supporting Information, Table SS1 . Indeed, patients undergoing combined HKTx had lower eGFR and were far more likely to be on renal replacement therapy (33% vs. 0%) than KAH patients at the time of initial heart transplant. For patients with eGFR < 30 mL/min/1.73m2, vast majority (96%) of them underwent combined HKTx. Older age and prevalence of diabetes mellitus were associated with lower eGFR prior to heart transplantation. Furthermore, statistically significant differences existed by eGFR categories for their need for ventilator support, left ventricular device at listing, and whether the procedure was performed at a high‐volume centre. There was no difference regarding 1A and 1B status, time on the waiting list, panel reactive antibody class 1 > 10%, and panel reactive antibody class 2 > 10%. Finally, there was no difference in need for intensive care, general hospital care, or not requiring in‐patient care at time of transplantation according to different eGFR categories.
Kidney transplant after heart transplant
Figure 2 displays differences in waitlist times between heart transplant and kidney transplant according to baseline eGFR (in categories as previously mentioned) at the time of heart transplant. As expected, patients in the lowest eGFR category (eGFR < 30 mL/min/1.73m2) had significantly shorter time to be listed for kidney transplant. However, there was no difference in either time to kidney transplant after heart transplant or time to dialysis after heart transplant according to baseline eGFR categories. Less than half of the KAH patients received a kidney transplant from a living donor with no significant difference between the eGFR groups, and majority had these transplants performed at the same institution that performed the heart transplant.
Figure 2.
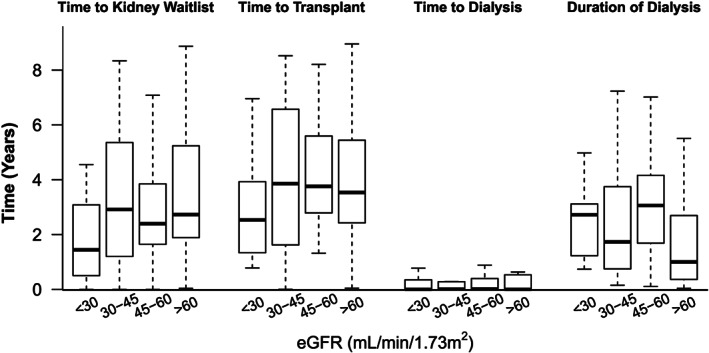
Time course between initial heart transplant and subsequent kidney transplant compared according to estimated glomerular filtration rate levels at the time of initial heart transplant for patients undergoing kidney transplant after initial heart transplant.
Survival analysis
Upon follow up (mean 3.5 ± 2.7 years), 151 of the 845 patients died during the study period. Kaplan‑Meier curves (Figure 3 ) show higher risk of death for combined HKTx patients (with or without dialysis) compared with KAH patients (log‐rank P = 0.001). In age‐adjusted and sex‐adjusted models (Table 2 ), the risk of death was 4.7‐fold higher among combined HKTx recipients compared with KAH transplant patients on follow up. The risk of death on follow up remained unchanged with multivariate adjustment, especially when all the covariates as displayed in Table 1 are considered in the model. Lastly, presence of total artificial heart, intra‐aortic balloon pump, implantable cardioverter defibrillator, and being on dialysis prior to heart transplant were all associated with increased risk of death.
Figure 3.
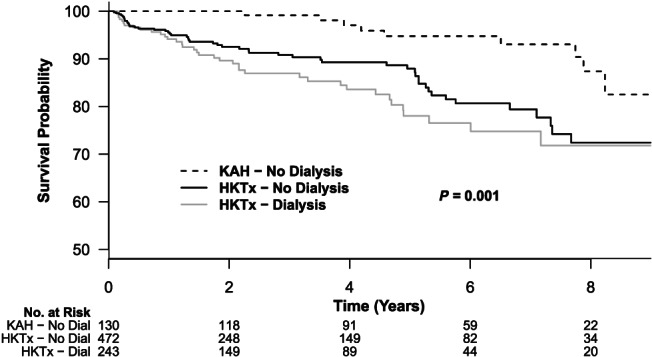
Kaplan‑Meier curves estimates of survival comparing patients with combined heart and kidney transplant (HKTx), stratified based on dialysis pre‐transplant or not, and patients undergoing kidney transplant after initial heart transplant (KAH).
Table 2.
Multivariable Cox regression models examining the risk of death in patients with combined heart and kidney transplant and in those with kidney transplant after heart transplant
| Models | Age and sex adjusted HR (95% CI) | Multivariable a HR (95% CI) | Multivariable with stepwise selection b HR (95% CI) |
|---|---|---|---|
| KAH | Referent | Referent | Referent |
| HKTx | 4.77 (2.42–9.40) | 4.77 (2.41–9.44) | 4.74 (2.40–9.35) |
| Subgroup 1 analyses (excluding patients on dialysis at the time of heart transplant) c | |||
| HKTx | 4.01 (2.00–8.07) | 4.07 (2.01–8.23) | 3.99 (1.98–8.04) |
| Subgroup 2 Analyses (Propensity matched for age, gender, and eGFR at baseline) d | |||
| HKTx | 6.35 (3.01–13.39) | 7.22 (3.36–15.55) | 3.01 (1.40–6.43) |
| Subgroup 3 analyses (after excluding centres with selection bias) e | |||
| HKTx | 4.20 (2.39–9.54) | 4.41 (2.76–12.42) | 4.70 (2.35–9.42) |
CI, confidence interval; HKTx, combined heart and kidney transplantation; HR, hazard ratio; KAH, kidney transplantation after initial heart transplant.
The model included age, female gender, gender mismatch between donor and recipient, total artificial heart, intra‐aortic balloon pump, T‐bilirubin, waitlist times in days, treatment with dialysis (when applicable), positive donor cytomegalovirus, and panel reactive antibody class 1 > 10%.
All variables shown in Table 1 had the chance to enter into the multivariable stepwise model. In the end, following variables contributed significantly to the model: recipient age, sex, presence of total artificial heart or intra‐aortic balloon pump, implantable cardioverter defibrillator, and total bilirubin levels prior to transplant.
Subgroup 1 includes 601 patients of them 91 died upon follow up.
Subgroup 2 includes 260 patients (130 in each group) of them 41 died upon follow up.
Subgroup 3 includes 562 patients of them 98 died upon follow up.
We compared the causes of death in combined HKTx with the KAH patients (Supporting Information, Table S2 ). Bacterial infection, malignancy, cardiac arrest, and multiple organ failure were some of the major causes of death among patients with combined HKTx whereas KAH patients primarily had kidney failure, malignancy, or unknown cause of death.
Secondary analysis
When we compared survival of HKTx patients to KAH patients but analysing KAH patients' survival from the time of kidney transplant (instead of heart transplant as in the models previously mentioned), combined HKTx patients still had a 2.6‐fold (95% CI 1.33–5.15) higher risk of death on follow up in the multivariable models.
Subgroup analysis
After excluding all patients who were on dialysis prior to transplant, the subgroup 1 had 601 patients and during follow up, 91 died. Risk of death for combined HKTx recipients compared with KAH patients was about four times higher in age‐adjusted and sex‐adjusted and multivariable models (Table 2 ; Subgroup 1). In fact, these results essentially remained unchanged with stepwise multivariable adjustment when all known covariates were considered in the model (Table 2 ).
In subgroup 2 analysis, after propensity matching the two groups for age, gender, and eGFR at baseline, there were a total of 260 patients (130 in each group). Upon follow up, 41 patients died. The risk of death for patients after combined HKTx was 6.3‐fold higher (Table 2 ; Subgroup 2) compared with KAH patients. Risk of death for combined HKTx patients compared with KAH patients remained robustly and statistically higher even after adjusting for multiple variables in the models (Table 2 , multivariable models).
In subgroup 3 analyses, we examined the possibility of selection bias because of being at a transplant centre by excluding potentially biased centres who only performed one type of procedure (KAH vs. HKTx) and restricting our analyses to only 60 centres. There were 562 patients in this subgroup, and 98 died upon follow up. Patients who underwent combined HKTx still conferred a statistically significant higher risk of death (4.2‐fold to 4.7‐fold; Table 2 , Subgroup 3) compared with KAH patients.
Discussion
Principal findings
In the present study, we used the national registry database to compare clinical outcomes of patients undergoing combined HKTx with KAH patients during the period of 2007–2016. This analysis revealed several important findings. First, there is clear trend over the last decade in the United States that shows combined HKTx numbers are continuing to increase annually whereas the number of KAH procedures performed are declining. The ratio between the number of HKTx and KAH recipients has been steadily rising since 2003. The reason behind this trend is unknown but perhaps this can be partially explained by changes in immunosuppression post‐transplant, 14 better survival data from the past decade of patients requiring dialysis, and undergoing combined HKTx rather than heart transplant alone. 17 Vast majority that require dialysis at time of heart transplant receive combined HKTx instead of heart transplant alone in current era compared with two decades ago. Of interest, 17% of patients who underwent combined HKTx had an eGFR > 45 mL/min/1.73m2 whereas 38% of those who underwent KAH had an eGFR < 45 mL/min/1.73m2 at the time of initial heart transplant. It is also plausible that greater use of mTOR inhibitor and calcineurin inhibitor‐free immunosuppressive regimen in the current era have decreased incidence of kidney dysfunction after heart transplant. 14
Second, in multivariable‐adjusted Cox models, patients undergoing combined HKTx were at significantly higher risk of death compared with KAH patients upon follow up. Even after excluding patients that required dialysis at time of transplant, a known risk factor for death for these patients, 17 the risk of death was still fourfold higher with combined HKTx patients compared with KAH recipients. Independent predictors of death in the multivariable model included presence of total artificial heart, intra‐aortic balloon pump or implantable cardioverter defibrillator, and being on dialysis prior to the heart transplant. Notably, prior studies in heart transplant patients have also shown higher risk of death post‐transplant with total artificial heart, 9 intra‐aortic balloon pump, 18 and dialysis 7 prior to heart transplant. One note, there could be a selection bias for the KAH patients as they have already survived the initial heart transplant and have passed the preoperative kidney transplant evaluation. Therefore, to overcome that bias we, performed additional secondary analysis by giving KAH group an advantage of living up to their kidney transplant in the Cox models and still found similar results.
Third, for patients who underwent KAH, lower eGFR at the time of heart transplant was associated with shorter time to kidney transplant listing. However, time to reach the state of requiring dialysis after heart transplant or time between heart and kidney transplants did not differ according to eGFR at the time of heart transplant. Indeed, the aetiology of the nephropathy after heart transplant for KAH group is less likely to be from cardio‐renal syndrome and more likely from calcineurin inhibitor‐mediated nephrotoxicity post‐transplant. Unfortunately, accurate data regarding immunosuppression post‐heart transplantation are not available through registry database to associate those differences appropriately. These results are similar to previous reports comparing patients undergoing combined HKTx with heart transplant alone 8 , 10 and when combined HKTx patients were compared with kidney only recipients. 19
Prior literature
Several epidemiological studies have shown similar survival for patients undergoing combined HKTx (with or without pre‐transplant dialysis) compared with patients receiving heart transplant alone, demonstrating that the procedure appears to be safe and beneficial. 8 , 10 , 12 , 20 Understandably, patients with ESRD on dialysis 17 or with eGFR <30 mL/min have generally been considered potential candidates for combined HKTx. 7 , 21 Additionally, prior studies have noted that patients with combined HKTx have lower incidence of acute rejection compared with patients who received heart transplant alone. 10 , 22 At the same note, prior studies also show that the survival for individuals receiving KAH is similar compared with the patients that receive a heart transplant alone without ESRD, 14 and the kidney graft survival remains similar when comparing KAH with the combined HKTx patients. 23
The demand for organs for transplantation continues to grow at a higher pace despite efforts to increase and facilitate organ donation. 13 Currently, patients listed for dual organ transplants do not receive higher priority for heart transplant. 19 This concurs with our findings that the patients who underwent combined HKTx had longer time on the wait list compared with the KAH patients. Even with the newly introduced changes in the adult heart allocation system in the United States, patients listed for dual‐organ transplant did not get priority. 24 Patients listed for dual‐organ transplant may ascend to a higher acuity tier only if their cardiac status deteriorates, very similar to candidates for heart transplantation alone. 24 , 25 Unfortunately, patients with advanced heart failure and ESRD have limited treatment options that could lead to higher priority on the waiting list. Some centres will not consider implanting a mechanical circulatory assist device in patients on dialysis due to higher perioperative morbidity and mortality, increased risk of infection, and difficulty finding a dialysis centre willing to treat these patients. 26
Pros and cons of combined HKTx vs. KAH
One reported benefit of combined HKTx is the decreased incidence of T cell‐mediated rejection when compared with recipients of heart transplant alone especially if same donor is providing both heart and kidney. 22 , 27 However, combined HKTx does increase the time needed during the transplant procedure and requires more care coordination between heart and kidney transplant teams. The other potential benefits from combined HKTx are the ability to simultaneously correct the metabolic, haemodynamic, and symptomatic derangements associated with end‐stage cardiac and kidney disease. In contrast, patients receiving KAH have the options of getting a kidney transplant not only from a deceased donor but also from living donors. Kidney transplant recipients from living donors compared with deceased donors tend to have improved health‐related quality of life, better allograft and overall survival, and are likely to receive higher quality organ. 28 , 29 Due to limited availability of organs, resourceful utilization of organs is required. This calls for standardization and implementation of general rules in considering multi‐organ transplantation, specifically combined HKTx, the most commonly combined organ with heart transplant. Our findings demonstrate that KAH could be a more reasonable option for patients with end‐stage heart failure with evolving but not end‐stage kidney disease. Although, further research to identify the individuals who would benefit the most from each procedure is needed.
Strengths and limitations
Strengths of our study include the use of national database that provides a common platform for both heart and kidney transplant recipients and ability to account for many clinical and demographic variables that commonly influence transplant outcomes and the robust results of our subgroup analysis. We do acknowledge that given the retrospective design and despite our robust statistical analyses, all potential clinical variables may not be accounted accurately, leaving some possibility for bias (e.g. differences in immunosuppressive drug therapies or differences based on follow ups at each transplant centre); however, randomized trial design could be too difficult. Additionally, despite our secondary and subgroup analyses for patients with KAH, there may remain an unaccounted selection bias that makes KAH patients live longer than combined HKTx recipients (e.g. better social support, having tolerated the higher levels of immunosuppressive therapy after heart transplant, and selection criteria prior to kidney transplant). However, our retrospective design of our study makes it difficult to ascertain this bias in models.
Conclusions
There has been a significant increase in the number of patients receiving combined HKTx over the past two decades whereas the number of patients receiving KAH is diminishing. Our retrospective analysis of national database shows a significant survival disadvantage for patients receiving combined HKTx compared with KAH patients. Further research is needed to better define the indications for combined HKTx and policies to prioritize dual organ transplants on the waiting list.
Conflict of interest
None declared.
Funding
This work was supported in part by Health Resources and Services Administration contract 234‐2005‐370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Supporting information
Table S1. Baseline Characteristics of the Patients according to glomerular filtration rate at time of heart transplantation.
Table 2. Overview of the cause of death for the patients undergoing combined heart and kidney transplant (HKTx) and kidney transplant after the initial heart transplant (KAH).
Melvinsdottir, I. , Foley, D. P. , Hess, T. , Gunnarsson, S. I. , Kohmoto, T. , Hermsen, J. , Johnson, M. R. , Murray, D. , and Dhingra, R. (2020) Heart and kidney transplant: should they be combined or subsequent?. ESC Heart Failure, 7: 2734–2743. 10.1002/ehf2.12864.
References
- 1. Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015; 36: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stratta P, Canavese C, Quaglia M, Balzola F, Bobbio M, Busca A, Franchello A, Libertucci D, Mazzucco G. Posttransplantation chronic renal damage in nonrenal transplant recipients. Kidney Int 2005; 68: 1453–1463. [DOI] [PubMed] [Google Scholar]
- 3. Lachance K, White M, de Denus S. Risk factors for chronic renal insufficiency following cardiac transplantation. Ann Transplant 2015; 20: 576–587. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez‐Vilchez F, Arizon JM, Segovia J, Almenar L, Crespo‐Leiro MG, Palomo J, Delgado JF, Mirabet S, Rabago G, Perez‐Villa F, Diaz B, Sanz ML, Pascual D, de la Fuente L, Guinea G, Group IS . Chronic renal dysfunction in maintenance heart transplant patients: the ICEBERG study. Transplant Proc 2014; 46: 14–20. [DOI] [PubMed] [Google Scholar]
- 5. Thomas HL, Banner NR, Murphy CL, Steenkamp R, Birch R, Fogarty DG, Bonser AR, Steering Group of the UKCTA . Incidence, determinants, and outcome of chronic kidney disease after adult heart transplantation in the United Kingdom. Transplantation 2012; 93: 1151–1157. [DOI] [PubMed] [Google Scholar]
- 6. Alam A, Badovinac K, Ivis F, Trpeski L, Cantarovich M. The outcome of heart transplant recipients following the development of end‐stage renal disease: analysis of the Canadian Organ Replacement Register (CORR). Am J Transplant 2007; 7: 461–465. [DOI] [PubMed] [Google Scholar]
- 7. Habib PJ, Patel PC, Hodge D, Chimato N, Yip DS, Hosenpud JD, Wadei HM. Pre‐orthotopic heart transplant estimated glomerular filtration rate predicts post‐transplant mortality and renal outcomes: An analysis of the UNOS database. J Heart Lung Transplant 2016; 35: 1471–1479. [DOI] [PubMed] [Google Scholar]
- 8. Karamlou T, Welke KF, McMullan DM, Cohen GA, Gelow J, Tibayan FA, Mudd JM, Slater MS, Song HK. Combined heart‐kidney transplant improves post‐transplant survival compared with isolated heart transplant in recipients with reduced glomerular filtration rate: analysis of 593 combined heart‐kidney transplants from the United Network Organ Sharing Database. J Thorac Cardiovasc Surg 2014; 147: 456–461 e1. [DOI] [PubMed] [Google Scholar]
- 9. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Chambers DC, Yusen RD, Stehlik J, International Society for H, Lung T . The Registry of the International Society for Heart and Lung Transplantation: thirty‐fourth adult heart transplantation report‐2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017; 36: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 10. Schaffer JM, Chiu P, Singh SK, Oyer PE, Reitz BA, Mallidi HR. Heart and combined heart‐kidney transplantation in patients with concomitant renal insufficiency and end‐stage heart failure. Am J Transplant 2014; 14: 384–396. [DOI] [PubMed] [Google Scholar]
- 11. Savla J, Lin KY, Pradhan M, Ruebner RL, Rogers RS, Haskins SS, Owens AT, Abt P, Gaynor JW, Shaddy RE, Rossano JW. Heart retransplant recipients have better survival with concurrent kidney transplant than with heart retransplant alone. J Am Heart Assoc 2015; 4: e002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narula J, Bennett LE, DiSalvo T, Hosenpud JD, Semigran MJ, Dec GW. Outcomes in recipients of combined heart‐kidney transplantation: multiorgan, same‐donor transplant study of the International Society of Heart and Lung Transplantation/United Network for Organ Sharing Scientific Registry. Transplantation 1997; 63: 861–867. [DOI] [PubMed] [Google Scholar]
- 13. Wynn JJ, Alexander CE. Increasing organ donation and transplantation: the U.S. experience over the past decade. Transpl Int 2011; 24: 324–332. [DOI] [PubMed] [Google Scholar]
- 14. Grupper A, Grupper A, Daly RC, Pereira NL, Hathcock MA, Kremers WK, Cosio FG, Edwards BS, Kushwaha SS. Kidney transplantation as a therapeutic option for end‐stage renal disease developing after heart transplantation. J Heart Lung Transplant 2017; 36: 297–304. [DOI] [PubMed] [Google Scholar]
- 15. Lonze BE, Warren DS, Stewart ZA, Dagher NN, Singer AL, Shah AS, Montgomery RA, Segev DL. Kidney transplantation in previous heart or lung recipients. Am J Transplant 2009; 9: 578–585. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gill J, Shah T, Hristea I, Chavalitdhamrong D, Anastasi B, Takemoto SK, Bunnapradist S. Outcomes of simultaneous heart‐kidney transplant in the US: a retrospective analysis using OPTN/UNOS data. Am J Transplant 2009; 9: 844–852. [DOI] [PubMed] [Google Scholar]
- 18. Kilic A, Weiss ES, George TJ, Arnaoutakis GJ, Yuh DD, Shah AS, Conte JV. What predicts long‐term survival after heart transplantation? An analysis of 9,400 ten‐year survivors. Ann Thorac Surg 2012; 93: 699–704. [DOI] [PubMed] [Google Scholar]
- 19. Organ Procurement and Transplantation Network Policies.
- 20. Vermes E, Grimbert P, Sebbag L, Barrou B, Pouteil‐Noble C, Pavie A, Obadia JF, Loisance D, Lang P, Kirsch M. Long‐term results of combined heart and kidney transplantation: a French multicenter study. J Heart Lung Transplant 2009; 28: 440–445. [DOI] [PubMed] [Google Scholar]
- 21. Russo MJ, Davies RR, Hong KN, Iribarne A, Kawut S, Bacchetta M, D'Ovidio F, Arcasoy S, Sonett JR. Who is the high‐risk recipient? Predicting mortality after lung transplantation using pretransplant risk factors. J Thorac Cardiovasc Surg 2009; 138: 1234–1238 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raichlin E, Kushwaha SS, Daly RC, Kremers WK, Frantz RP, Clavell AL, Rodeheffer RJ, Larson TS, Stegall MD, McGregor C, Pereira NL, Edwards BS. Combined heart and kidney transplantation provides an excellent survival and decreases risk of cardiac cellular rejection and coronary allograft vasculopathy. Transplant Proc 2011; 43: 1871–1876. [DOI] [PubMed] [Google Scholar]
- 23. Cassuto JR, Reese PP, Bloom RD, Doyle A, Goral S, Naji A, Abt PL. Kidney transplantation in patients with a prior heart transplant. Transplantation 2010; 89: 427–433. [DOI] [PubMed] [Google Scholar]
- 24. Kobashigawa J, Teuteberg J, Colvin M, Edwards L, Daun T, Luu M, Patel J, Vega JD, Meyer D, forum participants . Proceedings of the AST heart allocation meeting at the American Transplant Congress, Philadelphia, Pennsylvania, May 4, 2015. Clin Transplant 2016; 30: 641–648. [DOI] [PubMed] [Google Scholar]
- 25. Meyer DM, Rogers JG, Edwards LB, Callahan ER, Webber SA, Johnson MR, Vega JD, Zucker MJ, Cleveland JC Jr. The future direction of the adult heart allocation system in the United States. Am J Transplant 2015; 15: 44–54. [DOI] [PubMed] [Google Scholar]
- 26. Miller LW, Guglin M. Patient selection for ventricular assist devices: a moving target. J Am Coll Cardiol 2013; 61: 1209–1221. [DOI] [PubMed] [Google Scholar]
- 27. Trachiotis GD, Vega JD, Johnston TS, Berg A, Whelchel J, Smith AL, Lutz J, Kanter KR. Ten‐year follow‐up in patients with combined heart and kidney transplantation. J Thorac Cardiovasc Surg 2003; 126: 2065–2071. [DOI] [PubMed] [Google Scholar]
- 28. Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med 1995; 333: 333–336. [DOI] [PubMed] [Google Scholar]
- 29. Reese PP, Boudville N, Garg AX. Living kidney donation: outcomes, ethics, and uncertainty. Lancet 2015; 385: 2003–2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of the Patients according to glomerular filtration rate at time of heart transplantation.
Table 2. Overview of the cause of death for the patients undergoing combined heart and kidney transplant (HKTx) and kidney transplant after the initial heart transplant (KAH).


