Abstract
Aims
Echocardiographic assessment of left ventricular filling pressures is performed using a multi‐parametric algorithm. Unselected sample of patients with heart failure with reduced ejection fraction (HFrEF) patients may demonstrate an indeterminate status of diastolic indices making interpretation challenging. We sought to test improvement in the diagnostic accuracy of standard and strain echocardiography of the left ventricle and left atrium (LA) to estimate a pulmonary capillary wedge pressure (PCWP) > 15 mmHg in patients with HFrEF.
Methods and results
Out of 82 consecutive patients, 78 patients were included in the final analysis and right heat catheterization, and echocardiogram was performed simultaneously. According to the univariable analysis, E wave velocity, the ratio between E‐wave/A‐wave (E/A, area under the curve [AUC] = 0.81, respectively), isovolumic relaxation time (AUC = 0.83), pulmonary vein D wave (AUC = 0.84), pulmonary vein S/D Ratio (AUC = 0.85), early pulmonary regurgitation velocity (AUC = 0.80), and accelerationa time at right ventricular out‐flow tract (RVOT AT, AUC = 0.84) identified with the highest accuracy PCWP > 15 mmHg. They were all tested in multivariate analysis, and they were not independently correlated with PCWP. Tricuspid regurgitation (TR) velocity was measurement with the highest predictive value in identifying PCWP > 15 mmHg (AUC = 0.89), compared with other established parameters such as the ratio between e‐wave velocity divided by mitral annular e' velocity (E/e'), deceleration time, or LA indexed volume (LAVi), which all reached a lower accuracy level (AUC = 0.75; 0.78; 0.76). Among strain measures, global longitudinal strain in four chamber view (GLS 4ch), the ratio between e‐wave velocity divided by mitral annular e' strain rate (E/e'sr), and LA longitudinal strain at the reservoir phase were helpful in estimating elevated PCWP (AUC = 0.77; 0.76; 0.75). According to multivariable analysis, the following two models had the greatest accuracy in detecting PCWP > 15 mmHg: (i) TR velocity, LAVi, and E wave velocity (receiver operating characteristic [ROC]‐AUC = 0.98), (ii) AT RVOT, LAVi and GLS 4ch (ROC‐AUC = 0.96). Neither E/A (ROC‐AUC = 0.81) nor E/e' (ROC‐AUC = 0.75) was an independent predictor when included in the model. The two MODELS were applicable to the entire population and demonstrated better agreement with the invasive reference (91% and 88%) than the guidelines algorithm (77%) regardless of the type of rhythm.
Conclusions
Our suggested echocardiographic approach could be used to potentially reduce the frequency of “doubtful” classification and increase the accuracy in predicting elevated left ventricular filling pressure leading to a decrease in the number of invasive assessment made by right heart catheterization.
Keywords: Echocardiography, Filling pressure, Heart failure, HFrEF, PCWP, Right heart catheterism
Introduction
Echocardiographic (ECHO) assessment of left ventricular (LV) diastolic function is an integral part of the routine evaluation of patients presenting with symptoms of dyspnoea or heart failure (HF). 1
Pulmonary capillary wedge pressure (PCWP) measured by right heart catheterization (RHC) has been widely used as a surrogate for LV filling pressure (FP) and is directly associated with functional capacity and prognosis in patients with HF. 2 , 3
In addition, RHC remains essential component in the evaluation of patients prior to heart and/or lung transplantation. 4
However, given the cost, potential complications, and lack of demonstrable benefits in routine use, hemodynamic assessment via RHC has decreased substantially over the last decade. 5 , 6 On the other hand, there has been a significant improvement in the diagnostic accuracy and availability of ECHO measures in predicting left ventricular filling pressure (LVFP), such as mitral inflow analysis, tissue Doppler annular velocities, tricuspid regurgitation (TR) velocity, and left atrial volume (LAV). While the 2016 algorithm (1) has been simplified from 2009 version, it still requires multiple parameters.
Moreover, a nonselected sample of patients with HFrEF (i.e. patients with atrial fibrillation or valvular disease) may present a spectrum of diastolic indices that do not clearly meet the strict definition of a particular diastolic dysfunction type, thereby making interpretation challenging as well.
Therefore, the primary goal of our study was to identify new algorithms involving standard as well as strain ECHO measurements in estimating PCWP pressure > 15 mmHg, validated by invasive hemodynamics, in an unselected consecutive sample of out‐patients with HFrEF referred to our centre to undergo heart transplantation.
Methods
Study population
We prospectively enrolled 82 consecutive out patients >18 years old with symptomatic HFrEF who underwent an RHC because of concerns about hemodynamic derangement and/or to assess heart transplantation candidacy at our institute, from 15 June 2016 through 15 June 2018.
Two patients were subsequently excluded because poor image quality and two patients because of a mechanical mitral valve. Finally, 78 patients were divided into two groups according to PCWP ≤ (Group I, N = 35) or > (Group II, N = 43) 15 mmHg. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (IRBB 20/16) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. “Informed consent was obtained from all individual participants included in the study.”
Inclusion criteria were as follows: LV ejection fraction <35% and New York Heart Association class III or IV. In order to assure generalizability of our observations, patients with cardiac resynchronization therapy–defibrillator (CRT‐D), atrial fibrillation, mild‐to‐moderate aortic valve stenosis and mitral valve insufficiency of any degree, previous aortic valve replacement, or mitral valve repair were not excluded. Patients were excluded if they had mechanical mitral valve in place, severe aortic and mitral stenosis or had poor ECHO image quality. Moreover, we decided to exclude these patients with acute on chronic HF or cardiogenic shock, because the high probability right catheterization and echocardiogram could not have been performed simultaneously.
The IRCCS‐ISMETT Institutional Research Review Board approved this research project, and informed consent was prospectively obtained from all patients.
Cardiac catheterization
Readings of invasive cardiac pressure measurements were performed and interpreted by a senior expert investigator, which was kept blind to the ECHO results. The pressure transducers were balanced before data acquisition with the zero level at mid‐axillary line. Pulmonary artery (PA) catheters were used to measure PA pressure (P) systolic (s), mean (m), diastolic (d), mean central venous pressure and PCWP. A value of PCWP > 15 mmHg was used to define high LVFP as used before. 7 The wedge position was verified by fluoroscopy, phasic changes in pressure waveforms, and oxygen saturation. Cardiac output and cardiac index were derived by the thermodilution technique and by the Fick equation through sampling of a mixed central venous blood gas taken in the PA and of an arterial blood gas. The varying RR intervals in patients with atrial fibrillation data from 10 consecutive beats were averaged.
Echocardiography
Echocardiography was performed using commercially available ultrasound systems (portable VIVID I 2016; Vingmed, General Electric Healthcare; Milwaukee, Wisconsin). Patients underwent a comprehensive ECHO 2D Doppler and 2D speckle tracking strain examination within 30 min of the invasive LVFP assessment in the cath‐lab Standard measurements of LV and right ventricular chambers, LAV, LV end‐diastolic, and end systolic volumes (biplane Simpson method), ejection fraction (EF) and LAV (indexed for body surface area—LAVi) were obtained as reported elsewhere. 8 , 9 E and A wave velocities as well as E/A ratio were computed and collected as previously reported. 1 Likewise, pulsed wave‐tissue Doppler imaging analysis of mitral annulus was performed, and TR jet peak velocity was measured wherever feasible.
Acceleration time (AT) was measured at right ventricular outflow tract (RVOT) by placing a 1‐ to 2‐mm pulsed wave Doppler sample volume in the proximal RVOT just within the pulmonary valve. Three cardiac cycles (in sinus rhythm) or five (in atrial fibrillation) were included in digital cine‐loop or Doppler images and averaged. All ECHO and Doppler data were obtained in digital format and stored on optical disks for offline analysis.
Speckle tracking analysis
Two investigators (GR and SM), blind to the clinical and RHC evaluations, measured both LV and LA strain (LAS) imaging on recruited patients. Consecutive digital data from three cardiac cycles were analysed offline with dedicated software (EchoPAC version BT12; GE Healthcare). LV global longitudinal strain (GLS) was assessed. 10 Analysis was performed considering all 16 LV segments individually (data not shown) and also combining them in clusters by LV wall: strain values were averaged for the anterolateral, inferoseptal (GLS 4 ch), inferolateral, anteroseptal, inferior, and anterior walls.
High frame rate (50–90 frames per second) was used. Early and late diastolic strain rate (e'sr; a'sr) were measured. Global e'sr and a'sr were calculated from the mean values of all LV segments. The E/e'sr ratio was calculated as E velocity divided by global e'sr. 11
For strain analysis of the LA, the focus was set to the level of mid‐LA to optimize the image quality. Three consecutive heart cycles were recorded during a single breath hold using a frame rate of >80 frames per second for offline analysis. The endocardial border of LA was manually traced and a region of interest was manually adjusted to include the entire LA wall thickness. LAS was measured using a non‐foreshortened apical four chamber view (4 ch) of the left atrium, at the reservoir (r) phase, at the conduit (cd) phase, and contraction (ct) phase, as previously recommended LAS curves were measured at end‐diastole triggered on QRS (q) on sinus rhythm and on atrial fibrillation. 12
Statistical analysis
Statistical analyses were done with a commercially available software programme (stata v15.1). The Mann–Whitney U test, the unpaired t‐test, or the Fisher's exact test were used to compare Group I (Patients with Wedge pressure ≤ 15 mmHg by RHC) and Group II (Patients with wedge pressure > 25 mmHg, by RHC).
Univariable logistic regression was performed in order to identify significant predictors of high FP (i.e. wedge > 15 mmHg), and the derived receiver operating characteristics (ROCs) were plotted for demographic, clinical, standard and strain measures, to assess accuracy in discriminating Group I and Group II patients. Multivariable models were created for each group of variables sequentially (clinical and biomarkers, standard, and strain echocardiography) using a mixed (i.e. backward and then forward) approach. Candidate variables for the multivariable selection were those that had a univariable P value < 0.20. Nested model tests were performed to see if there was an incremental gain in information at each model step. Because TR velocity has extremely high accuracy in predicting patients with PCWP >15 mmHg, but is not always measurable (as not every patient has TR), we repeated the same approach including and then excluding TR velocity as a predictor of PCWP.
The final model was constructed with clinical, biochemical, standard echo parameters of LV, and RV function, in addition to LV and LA deformation analysis measures, and was intended to test whether strain imaging parameters of LA function add information to the model.
Data are presented as mean (SD), median (interquartile range), or number (percentage). A difference was considered statistically significant if the P value was less than .05 (Figure S1 and Data SI).
Inter‐observer variability
To examine inter‐observer variability, a sample of 8 ECHO examinations was randomly selected for masked review by a co‐investigator (GR) blinded to the clinical information and to the results of the first investigator (SM). Intraclass correlation coefficients were calculated using previously described formulae 13 for longitudinal systolic strain average in apical 4 chambers view.
Results
Patient characteristics
Demographic clinical and catheterization data of the study population are listed in Table 1. The two groups were comparable in terms of age, New York Heart Association class, and cardiovascular risk factors. Three patients underwent aortic valve replacement in Group II. Moderate to severe mitral regurgitation, atrial fibrillation, and chronic kidney disease were more frequent, and NT‐proBNP was higher in Group II. Concerning hemodynamic data, systolic arterial pressure, mean central venous pressure, PAPd, PAPm and PAPs, PCWP, and pulmonary vascular resistance were higher in Group II.
Table 1.
Clinical characteristics and catheterization data between patients with PCWP < 15 mmHg and PCWP > 15 mmHg
| Variables (M ± SD) | Group I (Wedge ≤15 mmHg) (N = 35) | Group II (Wedge ≥15 mmHg) (N = 43) | P value |
| Age | 54.26 ± 9.55 | 52.37 ± 11.88 | 0.813 |
| NYHA III | 25 (71) | 20 (58) | 0.26 |
| CAD | 31 (88) | 16 (37.2) | 0.521 |
| AF | 1 (3) | 8(19) | 0.05 |
| CKD | 15 (26.9) | 17 (39.5) | 0.004 |
| CRT | 14 (40) | 10 (23.3) | 0.101 |
| ICD | 28 (79.5) | 35 (81.4) | 0.499 |
| COPD | 7 (20) | 3 (7) | 0.405 |
| DM | 2 (6) | 4 (9) | 0. 561 |
| HTN | 20 (57) | 22 (51) | 0.218 |
| NT‐proBNP | 2457.62 ± 5367.17 | 4613.14 ± 4563.44 | <0.001 |
| HR | 67.11 ± 11.78 | 73.39 ± 13.95 | 0.052 |
| SBP | 116 ± 17.76 | 106.6 ± 14.61 | 0.018 |
| DPB | 69.85 ± 11.17 | 66.14 ± 9.37 | 0.285 |
| CVP | 4.6 ± 2.16 | 8.86 ± 4.37 | <0.001 |
| PAPd | 14.14 ± 4.84 | 27.07 ± 8.12 | <0.001 |
| PAPm | 17.79 ± 4.9 | 33.65 ± 7.58 | <0.001 |
| PAPs | 23.8 ± 5.88 | 43.16 ± 10.06 | <0.001 |
| PCWP | 9.57 ± 3.37 | 24.16 ± 5.09 | <0.001 |
| TPG cath | 7.91 ± 4.79 | 9.49 ± 4.36 | 0.249 |
| PVR (wood unit) | 1.8 ± 0.95 | 2.74 ± 2.53 | 0.039 |
AF, atrial fibrillation; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; CVP, central venous pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; HR, heart rate; HTN, hypertension; ICD, implatable cardioverter defibrillator; NT‐proBNP, NT‐type pro brain natriuretic peptide; NYHA, New York Heart Association functional class; PAPd, pulmonary artery diastolic pressure; PAPm, pulmonary artery mean pressure; PAPs, pulmonary artery systolic pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance on wood unit; SBP, systolic blood pressure; TPG, transpulmonary gradient.
Echocardiography
Two‐dimensional Doppler and strain measurements are reported in Table 2. LVEF, GLS, and GLS 4 ch were lower, and moderate to severe mitral regurgitation was prevalent in Group II compared with patients with lower PCWP. E/A ratio, E/e' ratio, LAV index, and the TR velocity were all greater in Group II. Isovolumic relaxation time (IVRT), DT, PV S wave, PV D wave, as well as S/D PV ratio, were also higher. The difference in time of PV Ar dur–Am dur was longer in patients in Group II.
Table 2.
Echocardiographic measurements in Group I (PCWP < 15 mmHg) and in Group II (PCWP > 15 mmHg), and univariable analysis in predicting PCWP >15 mmHg
| Variables (M ± SD) | Group I (Wedge ≤ 15 mmHg) (N = 35) | Group II (Wedge ≥ 15 mmHg) (N = 43) | P value | OR | AUC, [95%‐CI] | P value |
| LVEDD index | 33.96 ± 4.67 | 35.46 ± 5.67 | 0.2 | 1.06 | 0.58 [0.46–0.71] | 0.21 |
| EF | 29.74 ± 6.19 | 25.94 ± 6.97 | 0.015 | 0.92 | 0.66 [0.60–0.80] | 0.02 |
| GLS | −11.94 ± 15.03 | −7 ± 5.17 | 0.001 | 1.14 | 0.75 [0.61–0.89] | 0.11 |
| GLS 4ch | −9.68 ± 2.78 | −6.77 ± 2.82 | 0.005 | 1.43 | 0.77 [0.64‐0.90] | <0.001 |
| TAPSE | 14.16 ± 8.18 | 14.01 ± 7.57 | 0.696 | |||
| MR, n (%) | 1 (3) | 9 (21) | 0.002 | |||
| AVR or MVpl. | 1 (1) | 2 (2) | 0.338 | |||
| E | 0.66 ± 0.21 | 0.96 ± 0.28 | <0.001 | 3.46 | 0.81 [0.72–0.91] | <0.001 |
| E/A | 1.47 ± 1.4 | 3.1 ± 1.71 | <0.001 | 1.98 | 0.81 [0.70–0.92] | <0.001 |
| E/e' | 15.68 ± 9.33 | 25.48 ± 12.91 | <0.001 | 1.09 | 0.75 [0.64–0.86] | <0.001 |
| LAV | 91.7 ± 28.65 | 123.33 ± 36.17 | <0.001 | 1.03 | 0.75 [0.64–0.86] | <0.001 |
| LAVi | 49 ± 15 | 68 ± 19 | <0.001 | 1.07 | 0.78 [0.65–0.91] | <0.001 |
| TR velocity | 2.33 ± 0.36 | 3.13 ± 0.54 | <0.001 | 8.05 | 0.89 [0.81–0.98] | <0.001 |
| IVRT | 109.45 ± 26.4 | 78.67 ± 20.37 | <0.001 | 0.95 | 0.83 [0.72–0.93] | <0.001 |
| DT | 234.06 ± 106.42 | 160.19 ± 70.12 | <0.001 | 0.99 | 0.76 [0.64–0.89] | <0.001 |
| PV S wave | 0.44 ± 0.24 | 0.29 ± 0.28 | 0.001 | 0.07 | 0.73 [0.63–0.84] | 0.03 |
| PV D wave | 0.42 ± 0.16 | 0.66 ± 0.2 | <0.001 | 1 | 0.84 [0.74–0.94] | <0.001 |
| S/D Ratio | 1.06 ± 0.92 | 0.49 ± 0.44 | 0.002 | 1.17 | 0.85 [0.75–0.95] | 0.002 |
| PV Ar dur – Am dur | 9.47 ± 53.3 | 41.29 ± 37.6 | 0.03 | 0.98 | 0.69 [0.60–0.80] | 0.03 |
| AT RVOT | 112.68 ± 24.52 | 83.15 ± 18.42 | <0.001 | 0.94 | 0.84 [0.74–0.94] | <0.001 |
| ET RVOT | 318.97 ± 44.55 | 275.79 ± 46.09 | <0.001 | 0.98 | 0.75 [0.64–0.86] | <0.001 |
| E‐PULM velocity | 1.54 ± 0.49 | 2.14 ± 0.56 | 0.002 | 8.43 | 0.80 [0.62–0,91] | 0.01 |
| L‐PULM velocity | 1.27 ± 0.39 | 1.7 ± 0.51 | 0.005 | 8.05 | 0.76 [0.62–0.91] | 0.01 |
| LAS cd | 7.67 ± 5.91 | 4.8 ± 3.65 | 0.023 | 0.85 | 0.69 [0.60–0.80] | 0.06 |
| LAS ct | 6.38 ± 4.59 | 3.63 ± 2.24 | 0.004 | 0.78 | 0.74 [0.63–0.85] | 0.02 |
| LAS r | 12.23 ± 6.76 | 7.49 ± 3.69 | <0.001 | 0.81 | 0.75 [0.64–0.86] | <0.001 |
| E/e'sr | 0.78 ± 0.26 | 1.19 ± 0.59 | <0.001 | 9.15 | 0.76 [0.64–0.89] | <0.001 |
AT RVOT, acceleration time at right ventricular outflow track; AUC, area under the curve; AVR: aortic valve replacement; CI, confidence interval; DT, deceleration time; E, peak mitral e‐wave velocity; E/A, peak e‐wave velocity/peak a‐wave velocity ratio; E/e' peak: e‐wave velocity divided by mitral annular e' velocity (average); E/e' sr, peak e‐wave velocity divided by strain rate derived mitral annular early diastolic velocity; EF, ejection fraction; E‐PULM velocity, early diast pulmonary regurgitation velocity; ET RVOT, ejection time AT RVOT; GLS, global longitudinal strain; GLS 4ch, global longitudinal strain in four chamber view; IVRT, isovolumic relaxation time; L‐PULM velocity, late diastolic pulmonary regurgitation velocity; LAS cd, left atrial strain at the counduit phase; LAS ct, left atrial strain at the contraction phase; LAS r, left atrial strain at the reservoir phase; LAV, left atrial volume; LAVi, left atrial volume indexed; LVEDDi, left ventricular end diastolic diameter indexed; MR, mitral regurgitation; MVpl, mitral valve plasty; PV Ar dur, pulmonary vein A wave duration; PV D wave, pulmonary vein D wave velocity; PV S wave, pulmonary vein S wave velocity; S/D ratio, pulmonary vein S wave velocity divided by pulmonary vein D wave; TAPSE, tricuspid annular plane systolic excursion; TR velocity, tricuspid regurgitation peak velocity.
Regarding RVOT Doppler measurements, acceleration time at right ventricular outflow track (AT RVOT) was shorter, and ET RVOT longer in group II. Both early and late diastolic pulmonary regurgitation velocities were higher in patients with PCWP >15 mmHg.
Concerning LA deformation analysis, LAS cd, LAS ct, and LAS r view showed reduced values in Group II.
Logistic regression/receiver operating characteristic analysis
According to the univariable analysis, E wave velocity, (AUC = 0.82), IVRT (AUC = 0.83), PV D wave (AUC = 0.84), S/D Ratio (AUC = 0.85), early pulmonary regurgitation velocity (E‐PULM velocity) (AUC = 0.80), and RVOT AT (AUC = 0.84) were the measurements with the highest accuracy in identifying patients with PCWP >15 mmHg (Table 2), Accordingly, they were all tested in multivariate analysis; nevertheless, they were not independently correlated with PCWP.
TR velocity was the single measurement with the highest predictive value in identifying PCWP >15 mmHg (AUC = 0.89), compared with other established parameters such as E/e' ratio, DT, or LAVi, which all reached a lower accuracy level. Among atrioventricular deformation measures, GLS 4 ch, E/e'sr, LAS r, and LAS ct (AUC = 0.77; 0.76; 0.75; and 0.74) were the only ones helpful in discriminating Groups I and II.
In patients with atrial fibrillation as special population, we used IVRT, septal E/e', and the slope of inflow from mitral valve plane into LV chamber during early diastole (the E/vp ratio) (P = 0.06; 0,002; 0.83; respectively) as ECHO recommended (1) measurements. Among these parameters, only septal E/e' was accurate in estimate PCWP >15 mmHg with an ROC‐AUC of 0.75.
According to multivariable logistic regression, two models with similar accuracy in detecting PCWP >15 mmHg were identified, (Table 3). Model 1 included TR velocity (cut‐off value ≥ 2.7 mt/sec, Sens 81% Spec 92%), LAVi (cut‐off value ≥ 51 mL/m2, Sens 88%, Spec 58%), and E wave velocity (cut‐off value ≥ 0.82, Sens 78%, Spec 74%), and had ROC‐AUC = 0.98, (95% confidence interval [CI 0.95, 1.00]).
Table 3.
Multivariable logistic regression
| Model 1 | OR [95% CI]; Beta | P value | AUC |
| TR velocity | 5.1 [0.96, 14]; 2.1 | <0.031 | 0.98; 95% CI [0.95, 1.00] |
| E | 7.89 [0.96, 14]; 2.2 | <0.026 | |
| LAV index | 0.07 [0.01, 0.14]; 1.9 | <0.047 | |
| Model 2 | |||
| AT RVOT | 0.05 [0.04, 0.019]; 3.1 | 0.031 | 0.96; 95% CI [0.91, 1.00] |
| LAV index | 0.05 [0.01, 0.1]; 2.4 | 0.021 | |
| GLS 4ch | 0.73 [0.11, 1.35]; 2.3 | 0.021 |
Models 1 and 2 in predicting PCWP > 15 mmHg.
AT RVOT, acceleration time at right ventricular outflow track; AUC, area under the curve; AVR, aortic valve replacement; CI, confidence interval; DT, deceleration time; E, peak mitral e‐wave velocity; E/A, peak e‐wave velocity/peak a‐wave velocity ratio; E/e' peak: e‐wave velocity divided by mitral annular e' velocity (average); E/e' sr, peak e‐wave velocity divided by strain rate derived mitral annular early diastolic velocity; EF, ejection fraction; E‐PULM velocity, early diast pulmonary regurgitation velocity; ET RVOT, ejection time AT RVOT; GLS, global longitudinal strain; GLS 4ch, global longitudinal strain in four chamber view; IVRT, isovolumic relaxation time; L‐PULM velocity, late diastolic pulmonary regurgitation velocity; LAS cd, left atrial strain at the counduit phase; LAS ct, left atrial strain at the contraction phase; LAS r, left atrial strain at the reservoir phase; LAV, left atrial volume; LAVi, left atrial volume indexed; LVEDDi, left ventricular end diastolic diameter indexed; MR, mitral regurgitation; MVpl, mitral valve plasty; PV Ar dur, pulmonary vein A wave duration; PV D wave, pulmonary vein D wave velocity; PV S wave, pulmonary vein S wave velocity; S/D ratio, pulmonary vein S wave velocity divided by pulmonary vein D wave; TAPSE, tricuspid annular plane systolic excursion; TR velocity, tricuspid regurgitation peak velocity.
Because TR velocity was not samplable in 14 (18%) of patients, we tested an alternative model excluding TR velocity from the analysis and including all the variables that had shown a high predictive power in the univariate analysis. Finally, Model 2 included AT RVOT (cut‐off value ≤ 96 ms, Sens. 76%, Spec. 79%), LAVi and GLS 4ch (cut‐off value <7.5%, Sens. 77%, Spec. 71%), reaching a ROC‐AUC = 0.96, (95% CI [0.91, 1.00]), similar to Model 1 (P value of the ROC comparison = 0.46) (Figure 1).
Figure 1.
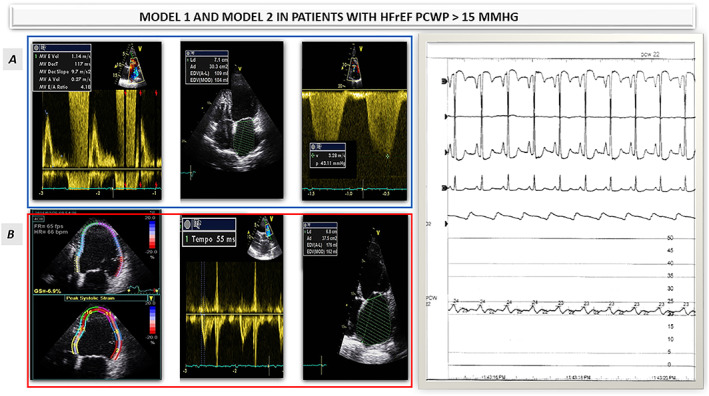
A) MODEL 1: E wave velocity, LAVi, and TR velocity were the most accurate predictors of PCWP >15 mmHg. B)MODEL 2:GLS4 ch, AT RVOT and LAVi. Model 1 and Model 2 were used in patient with HFrEF and predicted PCWP of 22 mmHg (see the hemodynamic data on the right side). HFrEF: Heart Failure and reducedejection fraction; PCWP: pulmonary capillary wedge pressure. AT RVOT, acceleration time at right ventricular outflow track; E/A, peak e‐wave velocity/peak a‐wave velocity ratio; E/e' peak, e‐wave velocity divided by mitral annular e' velocity (average); E wave, peak mitral e‐wave velocity; GLS 4ch, global longitudinal strain in four chamber view; HFrEF, heart failure and reduced ejection fraction; LAVi, left atrial volume indexed; PCWP, pulmonary capillary wedge pressure; TR velocity, tricuspid regurgitation peak velocity.
Of note neither E/4 nor E/e' ratio were independent predictors of PCWP >15 mmHg, being pulled‐off from the final model in favour of TR velocity, E wave velocity, AT RVOT, LAVi, and GLS 4ch (Figure 2).
Figure 2.
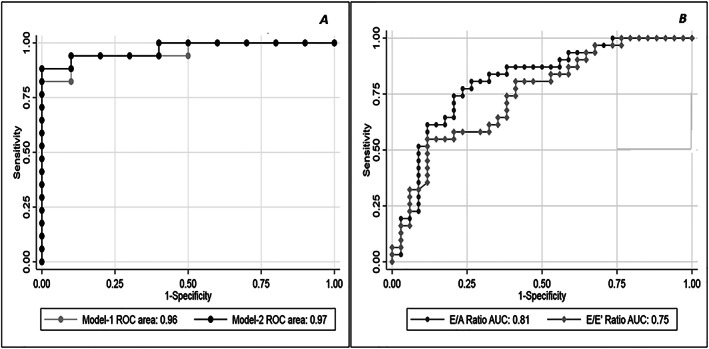
Multivariable logistic regression analysis. (A) Two models with similar accuracy in detecting PCWP >15 mmHg were identified Model 1 included TR velocity, LAVi, and E wave velocity, and had ROC‐AUC = 0.98; 95% CI [0.95, 1.00]. An alternative model excluding TR velocity from the analysis was tested: Model 2 included AT RVOT, LAVi, and GLS 4ch, reaching a ROC‐AUC = 0.96; 95% CI [0.91, 1.00], similar to Model 1 (P value of the ROC comparison = 0.46). (B) Multivariable logistic regression analysis: EA ratio and E/e' accuracy in detecting PCWP >15 mmHg. AT RVOT, acceleration time at right ventricular outflow track; CI, confidence intervals; E/A, peak e‐wave velocity/peak a‐wave velocity ratio; E/e' peak, e‐wave velocity divided by mitral annular e' velocity (average); E wave, peak mitral e‐wave velocity; GLS 4ch, global longitudinal strain in four chamber view; HFrEF, heart failure and reduced ejection fraction; LAVi, left atrial volume indexed; PCWP, pulmonary capillary wedge pressure; ROC‐AUC, receiver operating characteristic‐area under the curve; TR velocity, tricuspid regurgitation peak velocity.
The 2016 guidelines based algorithm (1) for estimation of LVFP and grading LV diastolic function in patients with depressed LVEF was applicable in 69 (88%) patients on sinus rhythm and correctly classified 52 (77%) patients with PCWP >15 mmHg.
Model 1 was applicable in 64 (82%)patients (with TR velocity samplable) and correctly classified 58 patients (91%, P = 0.01 compared with guideline based algorithm); furthermore, Model 2 correctly classified 69 (88.4%, P = 0.02 compared with algorithm guideline based) patients regardless the heart rhythm (applicable in 100% of patients) (Figure 3).
Figure 3.
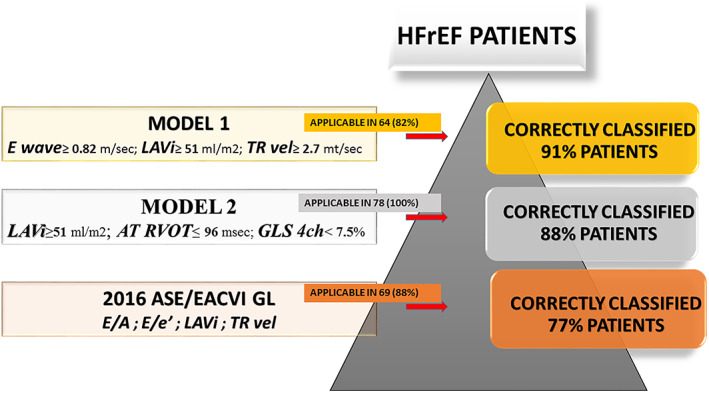
Comparison Models 1 and 2 to the 2016 ASE/EACVI guidelines based algorithm to correctly classified HFrEF patients with PCWP >15 mmHg. The 2016 guidelines based algorithm (1) for estimation of LVFP and grading LV diastolic function in patients with depressed LVEF was applicable in 69 (88%) patients on sinus rhythm and correctly classified 52 (77%) patients with PCWP >15 mmHg. Model 1 was applicable in 64 (82%) patients (with TR velocity samplable) and correctly classified 58 patients (91%, P = 0.01 compared with guideline based algorithm); furthermore, Model 2 correctly classified 69 (88%, P = 0.02 compared with algorithm guideline based) patients regardless the heart rhythm (applicable in 78,100% of patients) HFrEF, heart failure and reduced ejection fraction; LVFP; left ventricular filling pressure; PCWP, pulmonary capillary wedge pressure; TR velocity, tricuspid regurgitation peak velocity.
Intra‐observer and inter‐observer variability
The intraclass correlation coefficient for inter‐reader reproducibility was more than acceptable for LV Longitudinal strain average in Apical 4 Chambers (0.83 [95% CI 0.74, 1.00]).
Discussion
In the present study, we searched for a combination of ECHO measurements that could yield an accurate estimation of FP in patients with advanced HFrEF, testing LV and LAS parameters, assessing agreement with the invasively measured PCWP and comparing our conclusive models to the gold‐standard guidelines based algorithm (1). Our findings confirm TR velocity as an extremely accurate measure of high wedge pressure, in particular when associated to LAVi and E wave velocity (Model 1).
Should TR velocity not be available, the combination of AT RVOT, LAVi, and GLS 4ch (Model 2) has a comparable high accuracy in detecting elevated LVFP in HFrEF patients.
Furthermore, proposed models (both 1 and 2) outperformed E/A and E/e' ratios in identifying high FP and were also superior to guidelines based algorithm in this specific subset of patients. As an added benefit, the models heretofore proposed are measurable even in patients with atrial fibrillation, a subgroup where diastolic evaluation has been traditionally difficult to assess. Finally, the higher accuracy of the MODELS could lead to potentially reduce the frequency of “doubtful” classification and increase the accuracy in predicting elevated LVFP leading to a decrease in invasive assessment made by RHC.
Based on the cornerstone parameters (TR velocity, E wave velocity, and LAVi) the incremental predictive value of Model 1 in estimating elevated LVFP might seem more intuitive than expected.
Indeed, E wave velocity reflect the LA–LV pressure gradient during early diastole and in patients with dilated cardiomyopathy correlate better with LVFP. 1 Although E wave assessment is slightly challenging during arrhythmias and age dependent, univariate logistic regression has shown that such simple measure was as accurate as the E/A and better than E/e' ratio in identifying patients with high FP.
Although LAV changes very little with changes in wedge pressure and is not a suitable index for tracking changes in pressure, LAVi is, in our opinion, extremely useful for several reasons: first of all, assessment is possible in virtually 100% of patients; secondly, it increases with worsening LV diastolic dysfunction, reflecting the magnitude and chronicity of increased LVFP. 1
In addition, LA volume parameters are affected by acute hemodynamic changes, can identify LVFP >15 mmHg, offering better performance compared with transmitral Doppler parameters. 14
Although a significant limitation of this marker is if HF therapy has been resulted in normalization of pressure with persistent LA dilatation. 1 In addition, the value of LAVi >51 mL/m2 corresponds to severely enlarged left atrium, which makes it not practical in day to day application. However, it works very well in this specific subsample of patients with advanced HFrEF.
We have already said about the definitive usefulness of TR velocity in discriminating between patients with normal or high LVFP. However, adequate sampling of a full envelope is not always possible. 1 Consistently with previous reports, we could not sample TR jet in 14 (18%) of our patients, affecting the diagnostic usefulness of such measure. This main limitation justified our search for alternative and accurate parameters of elevated PCWP. In facts, the proposed Model 2 is a valid alternative when TR velocity is missing.
Model 2 was built encompassing two interesting parameters other than LAVi: AT RVOT and GLS 4ch.
Acceleration time at right ventricular outflow track is traditionally used to estimate mean PA pressure. 15 Generally, the shorter the AT RVOT, the higher the PVR and, hence, the PA pressure.
To the best of our knowledge, there are no data on the predictive value of AT RVOT in detecting elevated LVFP. Surprisingly, in our study AT RVOT was also a very good independent predictor of elevated PCWP, which could represent a new tool in estimating LVFP and definitely a valuable surrogate in patients with no detectable TR velocity. In consideration of the reduced number of patients (5, 6%) suffering from mixed forms of pulmonary hypertension, we did not perform any analysis in order to assess the value of AT RVOT in this subset of patients. Anyway, further studies focus on this issue are encouraged.
As guidelines (1) stated: patients with reduced LVEFs also have impaired diastolic function. We have explored the potential added value of GLS 4ch in estimating elevated FP and for the first time, our study revealed that GLS 4ch could be an important and independent predictors of PCWP >15 mmHg. In general, patients with HF usually have abnormally depressed LV EF and GLS such that LVFP varies directly with LV GLS. 16
In addition, some investigators have reported that GLS is associated with LV relaxation such as seen in E and E', and that reduced GLS can coexist with LV diastolic dysfunction in HF patients with preserved LVEF. 17
However, standard GLS analysis consist of an average of the systolic strain measured across the 16 LV segments, requiring more time for image acquisition and analysis, which places a small additional time burden on already busy echocardiography laboratories.
For this reason, we have used an alternative approach considering not just the GLS but also strain measured on the 6 LV segments. Such analysis strategy let us pinpoint the most useful cluster of segments for the problem at hand (i.e. identification of high FP), that is, the average of the six LV segments obtained by a simple apical four chambers view. Our proposal, although perhaps not totally orthodox, has the undeniable benefit of being effective, maximizing the yield provided by strain analysis, at the same time containing the required effort.
The value of GLS 4ch identified <7.5%, that is very low and unusual, is expression of extremely advanced HF disease and could help to better stratify this group of patient and indicate even worse prognosis. 18
Finally, the routine use of strain echocardiography requires adequate training for physicians and sonographers. In contrast to older tissue Doppler imaging methods, newer speckle‐tracking imaging has increased the ease of assessment, improved inter‐observer variability, and decreased analysis times. 19 Training is an important step toward quality control as the clinical use of GLS is becoming widespread.
Following the multi‐parametric strategy recommended by the most recent guidelines, 1 we have explored the usefulness of several diastolic strain as well as atrial strain measures in assessing wedge pressure in our study population.
We tested diagnostic accuracy not only of e'sr but also a'sr and their combination with E velocity (E/e'sr) or with LV GLS, though these measures failed to provide better estimates of LVFP compared with the heretofore mentioned standard echo parameters.
LAS is a new ECHO method, which allows determining contractile, conduit, and reservoir functions separately.
Although, Cameli et al 20 demonstrated that LAS provided a better estimation of LV FPs than the E/e' ratio in patients with HFrEF, it seems this method could be particularly useful when changes are subtle and not easily determined by recommended parameters. 21 Consistently, LASr could accurately estimate LV FPs, showing better agreement with invasively determined pressures when compared with the 2016 guidelines in patients with preserved EF, without atrial fibrillation or valve disease. 22
Therefore, our data suggest LAS may not have any additional value in improving the estimation of higher FP than 2016 guidelines algorithm if applied in the presence of more severe LV remodelling, irreversible diastolic and systolic dysfunction.
According to our analysis, established ECHO measurements traditionally associated with elevated LVFP, such as transmitral E/A and E/e' ratios, did not reach, as single parameter, the expected (high) accuracy in our population of advanced HFrEF patients.
These findings confirm the current ASE/EACVI guidelines which recommends a multi‐parametric approach (1) incorporating a variety of parameters each of which has potential limitations or may provide inconsistent results.
Limitations
Although present study is the largest report on a cohort of nonselected patients with advanced HFrEF comparing standard recommended and strain echocardiography, validated by invasive hemodynamic assessment, sample is still relatively small, notably for subgroup analyses.
However, sample size was sufficient to run simple as well as multivariable analysis, reaching clinically significant conclusions.
Moreover, because severe valvular disease, atrial fibrillation, pacemaker implantation, previous surgical, or percutaneous valvular intervention were not excluded in the study, our observations can be reliably extended to most patients with advanced HFrEF.
Among patients with HFrEF, we considered only those needing an invasive hemodynamic study, mostly because of heart transplantation. This means that the study population includes the sickest HFrEF patients. It is unclear whether findings of this study also apply to less symptomatic HFrEF patients and in patients with HFpEF. Our data do not imply in any way that recommended echo‐assessment evaluation is not useful in HFrEF patients, but merely support the potential application of our stepwise approach, incorporating all available ECHO data (standard and strain) in estimating elevated FP in this particular population.
Finally, we had no chance to test proposed algorithm (including Models 1 and 2) in an external and independent sample of HFrEF acting as validation cohort, so our accuracy estimates are likely optimistic and must therefore be considered with caution.
In conclusion, our data do not imply in any way that recommended evaluation is not useful in HFrEF patients, but merely support the potential application of our two ECHO models which represent an alternative to the more validated guidelines based algorithm, and particularly useful to potentially reduce the frequency of doubtful classification increasing the accuracy in predicting elevated LVFP.
Conflict of interest
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Author contributions
GR carried out the conceptualization; SM, VA, and DB for the data curation; VA and DB for the formal analysis; CM and GD for the investigation; GR and DB for the methodology; CG and GN for the project administration; CMHB and SP for the resources; SP and GR for the software; GN and CG for the supervision; CF and CM for the validation; SM and GDG for the visualization; GR and FC for writing – original draft; and GR and DB for writing – review & editing of the manuscript.
Supporting information
Figure S1. Supporting Information
Data S1. Supporting Information
Acknowledgement
We thank Ms Rosaria Segreto for continual clinical support, patient care, and coordination.
Romano, G. , Magro, S. , Agnese, V. , Mina, C. , Di Gesaro, G. , Falletta, C. , Pasta, S. , Raffa, G. , Baravoglia, C. M. H. , Novo, G. , Gandolfo, C. , Clemenza, F. , and Bellavia, D. (2020) Echocardiography to estimate high filling pressure in patients with heart failure and reduced ejection fraction. ESC Heart Failure, 7: 2268–2277. 10.1002/ehf2.12748.
Giuseppe Romano and Serena Magro equal contributor as first author.
References
- 1. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 4: 277–314. [DOI] [PubMed] [Google Scholar]
- 2. Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA, Tillisch JH. Improving survival for patients with advanced heart failure: a study of 737 consecutive patients. J Am Coll Cardiol 1995; 26: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 3. Haskell RJ, French WJ. Accuracy of left atrial and pulmonary artery wedge pressure in pure mitral regurgitation in predicting left ventricular end‐diastolic pressure. Am J Cardiol 1988; 1: 136–141. [DOI] [PubMed] [Google Scholar]
- 4. Keogh AM, Baron DW, Hickie JB. Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy assessed for cardiac transplantation. Am J Cardiol 1990; 13: 903–908. [DOI] [PubMed] [Google Scholar]
- 5. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW, ESCAPE Investigators and ESCAPE Study Coordinators . Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 6. Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993‐2004. JAMA 2007; 4: 423–429. [DOI] [PubMed] [Google Scholar]
- 7. Lancellotti P, Galderisi M, Edvardsen T, Donal E, Goliasch G, Cardim N, Magne J, Laginha S, Hagendorff A, Haland TF, Aaberge L, Martinez C, Rapacciuolo A, Santoro C, Ilardi F, Postolache A, Dulgheru R, Mateescu AD, Beladan CC, Deleanu D, Marchetta S, Auffret V, Schwammenthal E, Habib G, Popescu BA. Echo‐Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro‐Filling study. Eur Heart J Cardiovasc Imaging 2017; 18: 961–968. [DOI] [PubMed] [Google Scholar]
- 8. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 1: 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 9. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU, Industry representatives , Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee . Industry representatives; Reviewers: this document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using twodimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry TaskForce to standardize deformationimaging. Eur Heart J Cardiovasc Imaging 2018; 19: 591–600.29596561 [Google Scholar]
- 10. Mor‐Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL, From the University of Chicago, Chicago, Illinois (V.M.‐A., R.M.L.); the University of Padua, Padua, Italy (L.P.B.); Mayo Clinic, Scottsdale, Arizona (M.B.); Hospital da Luz, Lisbon, Portugal (N.M.C.); Universite Claude Bernard Lyon 1, Lyon, France (G.D.) . Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ase/eae consensus statement on methodology and indications endorsed by the Japanese society of echocardiography. Eur J Echocardiogr 2011; 12: 167–205. [DOI] [PubMed] [Google Scholar]
- 11. Chan YH, Lee HF, Wu LS, Wang CL, Wu CT, Yeh YH, Ho YWJ, Hsu LA, Chu PH, Kuo CT. Ratio of transmitral early filling velocity to early diastolic strain rate predicts outcomes in patients with systolic heart failure. Eur Heart J Cardiovasc Imaging 2017; 18: 79–85. [DOI] [PubMed] [Google Scholar]
- 12. Vieira MJ, Teixeira R, Gonçalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr 2014; 5: 463–478. [DOI] [PubMed] [Google Scholar]
- 13. Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ 1992; 304: 1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsiao SH, Lin KL, Chiou KR. Comparison of left atrial volume parameters in detecting left ventricular diastolic dysfunction versus tissue Doppler recordings. Am J Cardiol 2012; 5: 748–755. [DOI] [PubMed] [Google Scholar]
- 15. Dabestani A, Mahan G, Gardin JM, Takenaka K, Burn C, Allfie A, Henry WL. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol 1987; 59: 662–668. [DOI] [PubMed] [Google Scholar]
- 16. Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart 2002; 2: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vinereanu D, Lim PO, Frenneaux MP, Fraser AG. Reduced myocardial velocities of left ventricular long‐axis contraction identify both systolic and diastolic heart failure‐a comparison with brain natriuretic peptide. Eur J Heart Fail 2005; 7: 512–519. [DOI] [PubMed] [Google Scholar]
- 18. Alenezi F, Ambrosy AP, Phelan M, Chiswell K, Abudaqa L, Alajmi H, Kisslo J, Velazquez EJ. Left ventricular global longitudinal strain can reliably be measured from a single apical four‐chamber view in patients with heart failure. J Am Soc Echocardiogr 2019; 32: 317–318. [DOI] [PubMed] [Google Scholar]
- 19. Yamada A, Luis SA, Sathianathan D, Khandheira BK, Cafaro J, Hamilton‐Craig CR, Platts DG, Haseler L, Burstow D, Chan J. Reproducibility of regional and global longitudinal strains derived from twodimensional speckle‐tracking and doppler tissue imaging between expert and novice readers during quantitative dobutamine stress echocardiography. J Am Soc Echocardiogr 2014; 27: 880–887. [DOI] [PubMed] [Google Scholar]
- 20. Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, D'Ascenzi F, Focardi M, Favilli R, Pierli C, Fineschi M, Mondillo S. Correlation of left atrial strain and doppler measurements with invasive measurement of left ventricular end‐diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography 2016; 33: 398–405. [DOI] [PubMed] [Google Scholar]
- 21. Jarasunas J, Aidietis A, Aidietiene S. Left atrial strain—an early marker of left ventricular diastolic dysfunction in patients with hypertension and paroxysmal atrial fibrillation. Cardiovasc Ultrasound 2018; 1: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh A, Medvedofsky D, Mediratta A, Balaney B, Kruse E, Ciszek B, Shah AP, Blair JE, Maffessanti F, Addetia K, Mor‐Avi V, Lang RM. Peak left atrial strain as a single measure for the non‐invasive assessment of left ventricular filling pressures. Int J Cardiovasc Imaging 2019; 35: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Supporting Information
Data S1. Supporting Information


