Abstract
Aims
Besides regulating calcium‐phosphate metabolism, fibroblast growth factor 23 (FGF‐23) has been associated with incident heart failure (HF) and left ventricular hypertrophy. However, data about FGF‐23 in HF and preserved ejection fraction (HFpEF) remain limited. The aim of this study was to assess the association between FGF‐23 levels, clinical and imaging characteristics, particularly diffuse myocardial fibrosis, and prognosis in HFpEF patients.
Methods and results
We prospectively included 143 consecutive HFpEF patients (78 ± 8 years, 61% female patients) and 31 controls of similar age and gender (75 ± 6 years, 61% female patients). All subjects underwent a complete two‐dimensional echocardiography and cardiac magnetic resonance with extracellular volume (ECV) assessment by T1 mapping. FGF‐23 was measured at baseline. Among the patients, differences in clinical and imaging characteristics across tertiles of FGF‐23 levels were analysed with a trend test across the ordered groups. Patients were followed over time for a primary endpoint of all‐cause mortality and first HF hospitalization and a secondary endpoint of all‐cause mortality. Median FGF‐23 was significantly higher in HFpEF patients compared with controls of similar age and gender (247 [115; 548] RU/mL vs. 61 [51; 68] RU/mL, P < 0.001). Among HFpEF patients, higher FGF‐23 levels were associated with female sex, higher incidence of atrial fibrillation, lower haemoglobin, worse renal function, and higher N terminal pro brain natriuretic peptide levels (P for trend < 0.05 for all). Regarding imaging characteristics, patients with higher FGF‐23 levels had greater left atrial volumes, worse right ventricular systolic function, and more fibrosis estimated by ECV (P for trend < 0.05 for all). FGF‐23 was moderately correlated with ECV (r = 0.46, P < 0.001). Over a mean follow‐up of 30 ± 8 months, 43 patients (31%) died and 69 patients (49%) were hospitalized for HF. A total of 87 patients (62%) reached the primary composite endpoint of all‐cause mortality and/or first HF hospitalization. In multivariate Cox regression analysis for the primary endpoint, FGF‐23 (HR: 3.44 [2.01; 5.90], P < 0.001) and E wave velocities (HR: 1.01 [1.00; 1.02], P = 0.034) were independent predictors of the primary composite endpoint. In multivariate Cox regression analysis for the secondary endpoint, ferritin (HR: 1.02 [1.01; 1.03], P < 0.001), FGF‐23 (HR: 2.85 [1.26; 6.44], P = 0.012), and ECV (HR: 1.26 [1.03; 1.23], P = 0.008) were independent predictors of all‐cause mortality.
Conclusions
Fibroblast growth factor 23 (FGF‐23) levels were significantly higher in HFpEF patients compared with controls of similar age and gender. FGF‐23 was correlated with fibrosis evaluated by ECV. High levels of FGF‐23 were significantly associated with signs of disease severity such as worse renal function, larger left atrial volumes, and right ventricular dysfunction. Moreover, FGF‐23 was a strong predictor of poor outcome (mortality and first HF hospitalization).
Keywords: FGF‐23, Biomarker, Troponin, Mortality, NT‐proBNP, Heart failure, Fibrosis, Prognosis
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is a major cause of cardiovascular morbidity and mortality, especially among the elderly. Over the last years, a new paradigm has been proposed for HFpEF. 1 Systemic proinflammatory state induced by multiple co‐morbidities has been identified as a potential cause of myocardial structural and functional alterations 1 and may play a key role in the genesis of vascular, skeletal muscle, and end‐organ damages observed in HFpEF. Furthermore, several studies using autopsies, myocardial biopsies, or extracellular volume (ECV) measured by cardiac magnetic resonance (cMR) T1 mapping have highlighted the role of myocardial structural abnormalities and interstitial fibrosis in HFpEF. 2 , 3 , 4 , 5
Fibroblast growth factor 23 (FGF‐23), a bone‐derived hormone regulating renal phosphate homeostasis and vitamin D metabolism, has a direct action on the cardiovascular system and has been recently related to adverse cardiovascular events in chronic HF and implicated in cardiac remodelling. 6 , 7 , 8 , 9 Several recent studies suggested that higher FGF‐23 levels were associated with left ventricular hypertrophy 10 and worse prognosis, particularly among patients with chronic kidney disease (CKD) 11 , 12 but also in stable ischaemic cardiomyopathy 13 and HF with reduced ejection fraction (HFrEF). 6 , 7 , 8 , 14 , 15 , 16 FGF‐23 has also been identified as a biomarker significantly related to atrial fibrillation. 17
However, little is known about the association of FGF‐23 with myocardial abnormalities prevalent in HFpEF, such as left ventricle (LV) diastolic dysfunction, LV hypertrophy, interstitial fibrosis, and severity of right ventricular (RV) dysfunction, nor about its role in the progression of myocardial dysfunction. In the present study, we sought to examine the relationship between FGF‐23 and baseline biomarkers, demographic, and imaging characteristics, particularly fibrosis estimated by ECV using T1 mapping by cMR, and its prognostic value in a control population and in HFpEF patients.
Methods
Study population
Between December 2014 and June 2017, consecutive patients with HFpEF were prospectively evaluated for inclusion in the study. 5
The following criteria had to be fulfilled for study inclusion: New York Heart Association functional class ≥II, typical signs of HF, N terminal pro brain natriuretic peptide (NT‐proBNP) >350 pg/mL and/or a hospitalization for HF in the previous 12 months, left ventricular ejection fraction ≥ 50%, and relevant structural heart disease [LV hypertrophy/left atrial (LA) enlargement] and/or diastolic dysfunction assessed by echocardiography. The exclusion criteria were severe valvular disease, infiltrative or hypertrophic cardiomyopathy, acute coronary syndrome in the previous 30 days, chronic obstructive pulmonary disease GOLD 3 or 4, congenital heart disease, pericardial disease, atrial fibrillation with a ventricular response > 140 bpm, and severe anaemia (haemoglobin < 8 g/dL).
Patients were compared with 31 controls of similar age and gender without history of cardiovascular disease. 18 All controls underwent a full clinical exam, electrocardiogram, echocardiography, and exercise stress test, which all had to be normal prior to inclusion.
All subjects underwent blood sampling, complete transthoracic echocardiography, and cMR.
The investigation conforms with the principles outlined in Declaration of Helsinki. The local ethics committee approved the study, and all patients gave written informed consent before study enrolment (Clinical trial NCT03197350).
Echocardiography
All subjects underwent a complete two‐dimensional transthoracic echocardiography at inclusion (iE33 system Philips) to assess LV and RV structure, systolic and diastolic function, and measurements of left and right atrial volumes, as well as a valvular evaluation. Pulmonary pressures were estimated using tricuspid regurgitation velocity. Strain analysis was performed on acquired images with acceptable quality in TOMTEC Software (Munich, Germany). All measurements were averaged over three beats in atrial fibrillation.
Cardiac magnetic resonance
Cardiac magnetic resonance (cMR) was performed using a three‐Tesla system (Ingenia, Philips Medical Systems, Best, The Netherlands). The different sequences have been previously described. 5
Pre‐contrast and post‐contrast MOLLI images were processed using the open‐source software MRmap v1.4 under IDL. Pre‐myocardial and post‐myocardial T1 times were measured in six regions of interest in the myocardium (anterior, anterolateral, inferolateral, inferior, inferoseptal, and anteroseptal). We calculated the average T1 time of the six different region of interests. Areas of ischaemic focal fibrosis identified by late gadolinium enhancement were excluded from the analysis. ECV was then computed according to the formula. 19
Biomarkers analysis
Blood samples were obtained by venipuncture at inclusion. After centrifugation at 3500 rpm for 10 min, aliquots of serum and plasma were stored at −80 °C. High‐sensitivity troponinT (hsTnT), NT‐proBNP, and parathyroid hormone (PTH) were measured with electrochemiluminescent two sites immunoassay on Cobas 8000 platform (Roche Diagnostics, Mannheim, Germany). C‐terminal FGF‐23 (referred to as FGF‐23 in the text) concentrations were determined with a second‐generation C‐terminal human enzyme‐linked immunosorbent assay enzyme‐linked immunosorbent assay (Immutopics, San Clemente, CA, USA). Soluble ST2 (sST2) was measured using the Presage® ST2 enzyme‐linked immunosorbent assay (Critical Diagnostics, CA, USA).
Follow‐up
Patients were prospectively followed by ambulatory visits and phone calls at 6‐month intervals. Clinical and survival status were obtained by follow‐up visits and by phone contact with the patients, their relatives, or their physician. The primary endpoint was a composite of all‐cause mortality or hospitalization for HF, whichever came first. HF hospitalization was defined as patients treated in the emergency room or admitted to a hospital, diagnosed with decompensated HF, and requiring IV diuretics. The secondary endpoint was all‐cause mortality.
Statistical analysis
Statistical analyses were performed using SPSS version 22 (SPSS Corp., Somers, New York). All tests were two sided, and a P < 0.05 was considered statistically significant. Continuous variables were expressed as mean ± 1 SD if normally distributed or as median (25th and 75th percentiles) if not normally distributed. Categorical variables were expressed as counts and percentages. Biomarkers were log‐transformed to establish normality. Comparison between groups was performed using unpaired t‐tests or chi‐square test, when appropriate. The population of HFpEF patients was divided into three equal groups according to tertiles of FGF‐23 levels. Clinical, biological, and imaging parameters were compared among those groups using P for trend analysis.
Event‐free and overall survival of HFpEF patients was estimated using Kaplan–Meier method and Cox regression analysis. All baseline and imaging variables were initially proposed for inclusion in a univariate Cox proportional hazard model. Significant variables (P < 0.10) were entered into a stepwise forward multivariate Cox regression model. To avoid collinearity, the correlation coefficients between covariates were examined. In case of collinearity (r > 0.50), only the strongest of the two covariates was proposed for inclusion into the multivariate model. Kaplan‑Meier curves based on tertiles of FGF‐23 were used to illustrate event‐free and overall survival of HFpEF patients.
Results
Baseline characteristics
One hundred forty‐three consecutive HFpEF patients (78 ± 8 years, 61% female patients) and 31 controls of similar age and gender (75 ± 6 years, 61% female patients) were included in the study. The baseline demographic and imaging characteristics of HFpEF patients and controls of similar age and gender are presented in Table 1 . As expected, HFpEF patients had a higher incidence of cardiovascular risk factors and co‐morbidities compared with controls. They had lower haemoglobin and lower estimated glomerular filtration rate (eGFR). Median NT‐proBNP and FGF‐23 were significantly higher in HFpEF (NT‐proBNP: 1261 [589; 2663] pg/mL; and FGF‐23: 247 [115; 548] RU/mL, respectively) than in controls (NT‐proBNP: 117 [73; 158] pg/mL; and FGF‐23: 61 [51; 68] RU/mL) (all P < 0.001) (Figure 1 ). HFpEF patients had higher LA volumes, higher E wave velocities, higher E/e′ ratios, higher LV masses, higher pulmonary pressures, more signs of RV dysfunction, and higher ECV values, likely reflecting more diffuse myocardial fibrosis than controls. FGF‐23 levels were significantly correlated to ECV in the whole population (Figure 2 ).
Table 1.
Baseline characteristics of HFpEF patients and controls of similar age and gender
| Controls (n = 31) | HFpEF (n = 143) | P‐value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 75 ± 6 | 78 ± 8 | 0.06 |
| Female (n, %) | 19 (61%) | 87 (61%) | 0.96 |
| Body mass index (kg/m2) | 26 ± 4 | 29 ± 6 | 0.015 |
| Mean blood pressure (mmHg) | 113 ± 16 | 95 ± 14 | <0.001 |
| NYHA functional classes III and IV (n, %) | 0 (0%) | 62 (43%) | <0.001 |
| Medical history | |||
| Atrial fibrillation (n, %) | 0 (0%) | 88 (62%) | <0.001 |
| Ischaemic cardiomyopathy (n, %) | 0 (0%) | 50 (35%) | <0.001 |
| Previous heart failure episode (n, %) | 0 (0%) | 103 (72%) | <0.001 |
| Previous valvular surgery (n, %) | 0 (0%) | 16 (11%) | 0.051 |
| Chronic obstructive pulmonary disease (n, %) | 0 (0%) | 14 (10%) | 0.070 |
| Sleep apnoea (n, %) | 0 (0%) | 18 (13%) | 0.037 |
| Cardiovascular risk factors | |||
| Smoking (n, %) | 7 (23%) | 61 (43%) | 0.038 |
| Hypertension (n, %) | 19 (61%) | 134 (94%) | <0.001 |
| Diabetes (n, %) | 6 (19%) | 55 (38%) | 0.044 |
| Family history of CV disease (n, %) | 5 (16%) | 30 (21%) | 0.5 |
| Hypercholesterolemia (n, %) | 26 (84%) | 95 (66%) | 0,056 |
| Medication | |||
| Loop diuretic (n, %) | 0 (0%) | 101 (71%) | <0.001 |
| Thiazide (n, %) | 2 (6%) | 30 (21%) | 0.059 |
| Mineralocorticoid receptor antagonist (n, %) | 0 (0%) | 29 (20%) | 0.006 |
| Beta‐blocker (n, %) | 3 (10%) | 95 (66%) | <0.001 |
| ACEI or ARB (n, %) | 13 (42%) | 99 (69%) | 0.004 |
| Antiaggregant (n, %) | 8 (26%) | 59 (42%) | 0.10 |
| Oral anticoagulant (n, %) | 1 (3%) | 79 (55%) | <0.001 |
| Statins (n, %) | 8 (26%) | 64 (45%) | 0.053 |
| Biology | |||
| Haemoglobin (g/dL) | 13.9 ± 1.4 | 11.7 ± 2.0 | <0.001 |
| Total cholesterol (mg/dL) | 203 ± 45 | 154 ± 45 | <0.001 |
| GFR (mL/min/1.73 m2) by CK‐EPI | 63 ± 15 | 49 ± 19 | <0.001 |
| NT‐proBNP (pg/mL) | 117 (73; 158) | 1,261 (589; 2,663) | <0.001 |
| Hs TnT (pg/mL) | 7 (6; 11) | 26 (15; 38) | <0.001 |
| Iron (μg/dL) | 94 ± 33 | 77 ± 60 | 0.15 |
| Ferritin (μg/L) | 158 ± 167 | 231 ± 281 | 0.17 |
| Calcium (mmol/L) | 2.47 ± 0.14 | 2.39 ± 0.30 | 0.15 |
| Phosphorus (mmol/L) | 1.08 ± 0.17 | 1.19 ± 0.26 | 0.017 |
| Intact PTH (pg/mL) | 43 ± 23 | 68 ± 53 | 0.011 |
| 25OH‐Vitamin D (ng/mL) | 33 ± 15 | 25 ± 16 | 0.016 |
| Soluble ST2 (ng/mL) | 24 (21; 31) | 42 (31; 60) | <0.001 |
| FGF‐23 (RU/mL) | 61 (51; 68) | 247 (115; 548) | <0.001 |
| Echo study | |||
| LA volume index (mL/m2) | 20 ± 6 | 46 ± 19 | <0.001 |
| LV EDV index (mL/m2) | 59 ± 10 | 67 ± 18 | 0.024 |
| LV ejection fraction (%) | 65 ± 5 | 63 ± 7 | 0.055 |
| LV Endo GLS (%) | −21.0 ± 2.5 | −16.5 ± 3.2 | <0.001 |
| LV mass index (g/m2) | 71 ± 16 | 96 ± 26 | <0.001 |
| E wave velocity (m/s) | 55 ± 10 | 94 ± 31 | <0.001 |
| Septal e′ (m/s) | 7.3 ± 1.8 | 6.9 ± 2.2 | 0.003 |
| E/e′ septal ratio | 9.5 ± 1.8 | 19.4 ± 8.7 | <0.001 |
| RV/RA gradient (mmHg) | 18 ± 5 | 33 ± 11 | <0.001 |
| RV fractional area change (%) | 47 ± 7 | 41 ± 9 | 0.001 |
| TAPSE (mm) | 24 ± 4 | 19 ± 5 | <0.001 |
| cMR study | n = 31 | n = 121 | |
| LA volume index (mL/m2) | 31 ± 9 | 67 ± 29 | <0.001 |
| LV EDV index (mL/m2) | 64 ± 11 | 74 ± 19 | 0.009 |
| LV ejection fraction (%) | 66 ± 6 | 63 ± 8 | 0.030 |
| LV mass index (g/m2) | 57 ± 12 | 68 ± 15 | 0.001 |
| RV EDV index (mL/m2) | 68 ± 10 | 82 ± 27 | 0.005 |
| RV ejection fraction (%) | 61 ± 7 | 57 ± 9 | 0.001 |
| ECV (%) | 27.8 ± 2.4 | 32.7 ± 4.9 | 0.001 |
| Late gadolinium enhancement (%) | 0 (0%) | 1.5 ± 2.6 | 0.001 |
CK‐EPI, Chronic Kidney Disease Epidemiology Collaboration; ECV, extracellular volume; EDV, end‐diastolic volume; FGF‐23, fibroblast growth factor 23; GFR, glomerular filtration rate; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; hsTnT, high‐sensitivity troponinT; LA, left atrium; LV, left ventricle; NT‐proBNP, N terminal pro brain natriuretuic peptide; PTH, parathormon; RV, right ventricle; ST2, soluble suppression tumourigenicity 2; TAPSE, tricuspid annular plane systolic excursion.
Values are mean ± SD or median [IQR: 0.25; 0.75].
Figure 1.
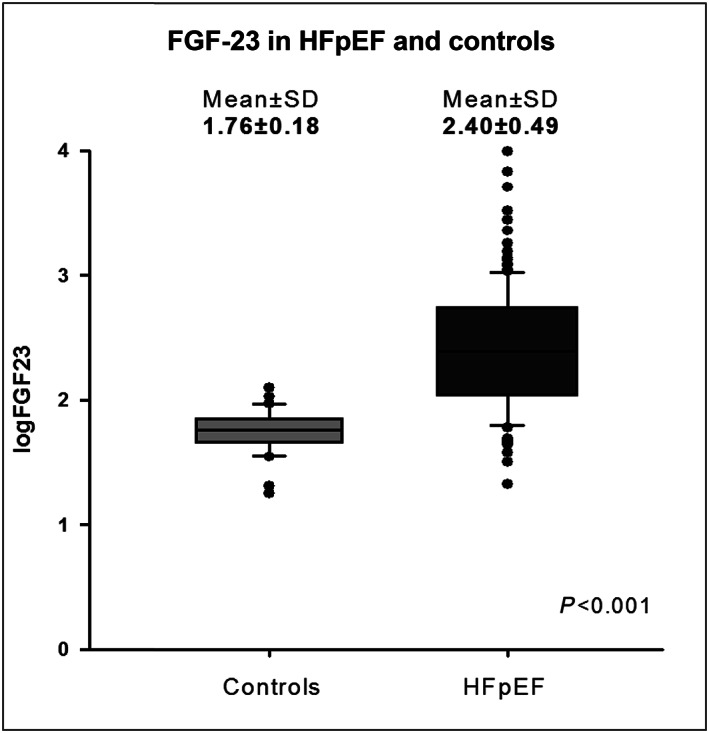
Box‐plot of FGF‐23 in heart failure with preserved ejection fraction (HFpEF) and controls of similar age and gender.
Figure 2.
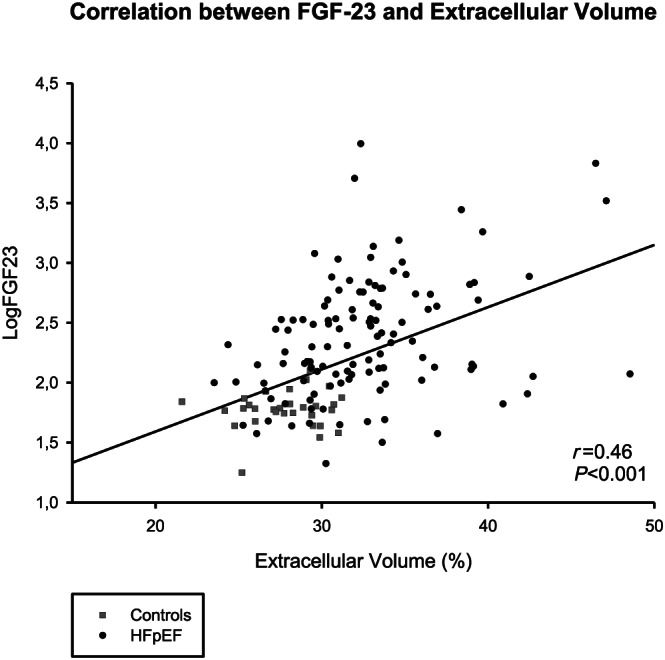
Correlation between fibroblast growth factor 23 (FGF‐23) and extracellular volume (ECV) in heart failure with preserved ejection fraction (HFpEF) and controls of similar age and gender.
Comparison of clinical and imaging parameters across tertiles of fibroblast growth factor 23 in heart failure with preserved ejection fraction patients
Heart failure and preserved ejection fraction (HFpEF) patients in the highest FGF‐23 tertile were more often female patients and had higher incidence of atrial fibrillation. They had lower haemoglobin, worse renal function, higher NT‐proBNP and hsTnT, higher PTH, and higher sST2. The rise of FGF‐23 was independent of the 25OH vitamin D status. Regarding imaging parameters, they had greater LA volumes with worse RV systolic function (lower RV FAC P = 0.027, lower TAPSE P = 0.030, and lower RVEF by cMR P = 0.003). Interestingly, ECV values significantly increased across tertiles of FGF‐23 (Table 2 ).
Table 2.
Clinical and imaging parameters according to tertiles of FGF‐23 in HFpEF
| FGF‐23 | ||||
|---|---|---|---|---|
|
Tertile 1 n = 47 (<134 RU/mL) |
Tertile 2 n = 47 (134–406 RU/mL) |
Tertile 3 n = 48 (>406 RU/mL) |
P‐for trend | |
| Baseline characteristics | ||||
| Age (years) | 78 ± 9 | 78 ± 8 | 79 ± 9 | 0.57 |
| Female (n, %) | 20 (43%) | 33 (69%) | 34 (71%) | 0.005 |
| Body mass index (kg/m2) | 28 ± 5 | 30 ± 7 | 27 ± 6 | 0.51 |
| Mean blood pressure (mmHg) | 97 ± 15 | 98 ± 11 | 90 ± 13 | 0.021 |
| NYHA functional classes III and IV (n, %) | 18 (38%) | 19 (40%) | 25 (52%) | 0.18 |
| Medical history | ||||
| Atrial fibrillation (n, %) | 22 (47%) | 30 (63%) | 36 (75%) | 0.005 |
| Ischaemic cardiomyopathy (n, %) | 18 (38%) | 14 (29%) | 18 (38%) | 0.94 |
| Previous heart failure episode (n, %) | 29 (62%) | 37 (77%) | 37 (77%) | 0.097 |
| Previous valvular surgery (n, %) | 5 (11%) | 4 (8%) | 7 (15%) | 0.54 |
| Chronic obstructive pulmonary disease (n, %) | 5 (11%) | 6 (13%) | 3 (6%) | 0.47 |
| Sleep apnoea (n, %) | 4 (9%) | 8 (17%) | 6 (13%) | 0.57 |
| Cardiovascular risk factors | ||||
| Smoking (n, %) | 24 (51%) | 17 (35%) | 20 (42%) | 0.36 |
| Hypertension (n, %) | 41 (87%) | 47 (98%) | 46 (96%) | 0.087 |
| Diabetes (n, %) | 15 (32%) | 17 (35%) | 23 (48%) | 0.11 |
| Family history of CV disease (n, %) | 12 (26%) | 8 (17%) | 10 (21%) | 0.58 |
| Hypercholesterolemia (n, %) | 29 (62%) | 32 (67%) | 34 (71%) | 0.35 |
| Medication | ||||
| Loop diuretic (n, %) | 27 (57%) | 35 (73%) | 40 (85%) | 0.011 |
| Thiazide (n, %) | 13 (27%) | 11(23%) | 6 (13%) | 0.25 |
| Mineralocorticoid receptor antagonist (n, %) | 5 (10%) | 10 (21%) | 14 (30%) | 0.06 |
| Beta‐blocker (n, %) | 31 (65%) | 33 (69%) | 32 (68%) | 0.90 |
| ACEI or ARB (n, %) | 33(69%) | 33 (69%) | 32 (68%) | 0.99 |
| Antiaggregant (n, %) | 23 (48%) | 22 (46%) | 14 (30%) | 0.15 |
| Oral anticoagulant (n, %) | 20 (42%) | 28 (58%) | 32 (68%) | 0.032 |
| Statins (n, %) | 24 (51%) | 23 (49%) | 17 (38%) | 0.40 |
| Biology | ||||
| Haemoglobin (g/dL) | 12.6 ± 2.1 | 11.9 ± 1.7 | 10.8 ± 1.6 | <0.001 |
| GFR (mL/min/1.73 m2) by CKD‐EPI | 60 ± 20 | 44 ± 17 | 44 ± 18 | <0.001 |
| NT‐proBNP (pg/mL) | 750 (360; 1289) | 1201 (628; 2177) | 2441 (1013; 4075) | <0.001 |
| Hs TnT (pg/mL) | 16 (12; 31) | 28 (18; 37) | 33 (18; 42) | <0.001 |
| Iron (μg/dL) | 84 ± 50 | 77 ± 49 | 71 ± 78 | 0.29 |
| Ferritin (μg/L) | 300 ± 360 | 230 ± 198 | 164 ± 254 | 0.021 |
| Calcium (mmol/L) | 2.35 ± 0.41 | 2.45 ± 0.23 | 2.37 ± 0.23 | 0.76 |
| Phosphorus (mmol/L) | 1.13 ± 0.15 | 1.23 ± 0.28 | 1.22 ± 0.31 | 0.078 |
| Intact PTH (pg/mL) | 50 ± 28 | 71 ± 39 | 84 ± 76 | 0.003 |
| 25OH‐Vitamin D (ng/mL) | 23 ± 15 | 27 ± 16 | 26 ± 18 | 0.39 |
| Soluble ST2 (ng/mL) | 35 (26; 56) | 42 (32; 57) | 47 (36; 69) | 0.027 |
| FGF‐23 (RU/mL) | 89 (60; 110) | 245 (160; 311) | 701 (549; 1131) | <0.001 |
| Echo study | ||||
| LA volume index (mL/m2) | 44 ± 21 | 41 ± 12 | 52 ± 20 | 0.030 |
| LV EDV index (mL/m2) | 69 ± 16 | 64 ± 16 | 68 ± 22 | 0.63 |
| LV ejection fraction (%) | 62 ± 7 | 63 ± 8 | 63 ± 8 | 0.58 |
| LV Endo GLS (%) | −16.3 ± 3.5 | −16.5 ± 3.1 | −16.8 ± 2.9 | 0.45 |
| LV mass index (g/m2) | 97 ± 26 | 92 ± 22 | 99 ± 31 | 0.79 |
| E wave velocity (m/s) | 89 ± 32 | 91 ± 29 | 101 ± 31 | 0.068 |
| Septal e′ (m/s) | 5.1 ± 1.4 | 5.2 ± 1.4 | 5.2 ± 1.3 | 0.93 |
| E/e′ septal ratio | 18.8 ± 9.2 | 18.5 ± 7.8 | 20.8 ± 9.2 | 0.27 |
| RV/RA gradient (mmHg) | 31 ± 9 | 32 ± 11 | 34 ± 12 | 0.17 |
| RV/RA gradient >37 mmHg | 14 (30%) | 14 (29%) | 18 (38%) | 0.42 |
| RV fractional area change (%) | 44 ± 7 | 40 ± 10 | 40 ± 9 | 0.027 |
| RV fractional area change<35% (n, %) | 4 (5%) | 12 (15%) | 29 (35%) | <0,001 |
| TAPSE (mm) | 20 ± 5 | 19 ± 5 | 17 ± 6 | 0.030 |
| TAPSE <17 mm (n, %) | 6 (7%) | 14 (17%) | 42 (51%) | <0.001 |
| cMR study | ||||
| LA volume index (mL/m2) | 62 ± 36 | 64 ± 21 | 76 ± 26 | 0.030 |
| LV EDV index (mL/m2) | 76 ± 20 | 70 ± 18 | 74 ± 19 | 0.62 |
| LV ejection fraction (%) | 63 ± 8 | 62 ± 9 | 63 ± 7 | 0.94 |
| LV mass index (g/m2) | 69 ± 15 | 65 ± 12 | 69 ± 18 | 0.82 |
| RV EDV index (mL/m2) | 75 ± 20 | 78 ± 22 | 93 ± 34 | 0.003 |
| RV ejection fraction (%) | 59 ± 7 | 57 ± 8 | 54 ± 9 | 0.003 |
| RV ejection fraction <45% (n, %) | 5 (6%) | 5 (6%) | 11 (17%) | 0.029 |
| ECV (%) | 31.7 ± 5.2 | 31.4 ± 3.3 | 35.5 ± 5.0 | <0.001 |
| Late gadolinium enhancement (%) | 1.1 ± 2.6 | 1.7 ± 2.3 | 1.8 ± 2.8 | 0.26 |
CK‐EPI, Chronic Kidney Disease Epidemiology Collaboration; ECV, extracellular volumeEDV, end‐diastolic volume; FGF‐23, fibroblast growth factor 23; GFR, glomerular filtration rate; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; hsTnT, high‐sensitivity troponinT; LA, left atrium; LV, left ventricle; NT‐proBNP, N terminal pro brain natriuretic peptide; PTH, parathormon; RV, right ventricle; ST2, soluble suppression tumourigenicity 2; TAPSE, tricuspid annular plane systolic excursion.
Values are mean ± SD or median [IQR 0.25; 0.75]. P‐value obtained by P for trend analysis.
Outcomes
Over a mean follow‐up of 30 ± 8 months, 43 patients (31%) died and 69 patients (49%) were hospitalized for HF. A total of 87 patients (62%) reached the primary composite endpoint of all‐cause mortality or HF hospitalization, whichever came first.
In univariate Cox regression analysis, New York Heart Association class, diabetes, haemoglobin, eGFR, HsTnT, phosphorus, intact PTH, sST2, FGF‐23, treatment with loop diuretics and thiazides, E wave velocity, and E/e′ ratio were predictors of the primary endpoint. Among cMR parameters, index RV volume and ECV were also significantly associated with the primary endpoint. In multivariate Cox regression analysis, only FGF‐23 (HR: 3.44 [2.01; 5.90], P < 0.001) and E wave velocity (HR: 1.01 [1.00; 1.02], P = 0.034) were independent predictors of the primary endpoint (Table 3 ).
Table 3.
Cox regression analysis for the primary endpoint
|
Cox regression analysis Combined events |
Univariate | Multivariate | ||
|---|---|---|---|---|
|
Hazard ratio 95% CI |
P‐value | |||
| Age | 1.01 [0.98; 1.04] | 0.58 | ||
| Female | 1.46 [0.94; 2.28] | 0.088 | ||
| Body mass index | 0.97 [0.94; 1.01] | 0.10 | ||
| Mean blood pressure | 0.99 [0.98; 1.01] | 0.31 | ||
| NYHA functional classes III and IV | 1.58 [1.03; 2.41] | 0.037 | ||
| Cardiovascular risk factors | ||||
| Diabetes | 1.65 [1.08; 2.52] | 0.022 | ||
| Medical history | ||||
| Atrial fibrillation | 1.25 [0.80; 1.95] | 0.33 | ||
| Ischaemic cardiomyopathy | 0.93 [0.60; 1.45] | 0.76 | ||
| Medication | ||||
| Loop diuretic | 2.16 [1.25; 3.72] | 0.005 | ||
| Thiazide | 0.55 [0.30; 0.99] | 0.048 | ||
| Mineralocorticoid receptor antagonist | 1.38 [0.85; 2.15] | 0.20 | ||
| Beta‐blocker | 0.97 [0.62; 1.52] | 0.90 | ||
| ACEI or ARB | 0.90 [0.57;1.40] | 0.63 | ||
| Oral anticoagulant | 1.46 [0.95; 2.27] | 0.088 | ||
| Biology | ||||
| Haemoglobin (g/dL) | 0.86 [0.76;0.97] | 0.013 | ||
| GFR (mL/min/1.73 m2) by CKD‐EPI | 0.98 [0.97; 0.99] | <0.001 | ||
| NT‐proBNP (pg/mL) | 1.19 [0.97; 1.46] | 0.10 | ||
| Hs TnT (pg/mL) | 1.70 [1.25; 2.30] | 0.001 | ||
| Iron (ug/dL) | 1.00 [1.00; 1.00] | 0.38 | ||
| Ferritin (ug/L) | 1.00 [1.00; 1.00] | 0.10 | ||
| Calcium (mmol/L) | 0.89 [0.47; 1.68] | 0.72 | ||
| Phosphorus (mmol/L) | 2.31 [1.04; 5.14] | 0.051 | ||
| Intact PTH (pf/mL) | 1.01 [1.00; 1.01] | 0.003 | ||
| 25OH‐Vitamin D (ng/mL) | 1.00 [0.99; 1.01] | 0.91 | ||
| Soluble ST2 (ng/mL) | 3.46 [1.23; 9.74] | 0.020 | ||
| FGF‐23 (RU/mL) | 2.21 [1.52; 3.22] | <0.001 | 3.15 [1.88; 5.31] | <0.001 |
| Echo study | ||||
| LA volume index (mL/m2) | 1.01 [1.00; 1.02] | 0.17 | ||
| LV ejection fraction (%) | 1.01 [0.98; 1.04] | 0.69 | ||
| LV Endo GLS (%) | 1.00 [0.93; 1.07] | 0.95 | ||
| E wave velocity (m/s) | 1.01 [1.00; 1.02] | 0.002 | 1.01 [1.00; 1.02] | 0.036 |
| E/e′ septal ratio | 1.03 [1.01; 1.05] | 0.018 | ||
| RV/RA gradient (mmHg) | 1.02 [1.00; 1.04] | 0.093 | ||
| RV fractional area change (%) | 0.27 [0.02; 3.11] | 0.29 | ||
| TAPSE | 0.97 [0.93; 1.01] | 0.13 | ||
| cMR study | ||||
| LA volume index (mL/m2) | 1.00 [1.00; 1.01] | 0.31 | ||
| LV EDV index (mL/m2) | 1.00 [0.99; 1.02] | 0.83 | ||
| LV ejection fraction (%) | 1.01 [0.98; 1.04] | 0.55 | ||
| LV mass index (g/m2) | 1.00 [0.99; 1.02] | 0.64 | ||
| RV EDV index (mL/m2) | 1.01 [1.00; 1.02] | 0.031 | ||
| RV ejection fraction (%) | 0.98 [0.95; 1.01] | 0.11 | ||
| Extracellular volume (%) | 1.10 [1.05; 1.15] | <0.001 | ||
| Late gadolinium enhancement | 1.09 [0.99; 1.20] | 0.11 | ||
ACEi, angiotensin‐converting‐enzyme inhibitor; ARB, Angiotensin II receptor blockers; ECV, extracellular volume; EDV, end‐diastolic volume; FGF‐23, fibroblast growth factor 23; GFR, glomerular filtration rate; GLS, global longitudinal strain; hsTnT, high‐sensitivity troponinT; LA, left atrium; LV, left ventricle; NT‐proBNP, N terminal pro brain natriuretuic peptide; PTH, parathormon; RV, right ventricle; ST2, soluble suppression tumourigenicity 2; TAPSE, tricuspid annular plane systolic excursion.
For the secondary endpoint, BMI, eGFR, hsTnT, ferritin, sST2, FGF‐23, mineralocorticoid receptor antagonist medication, RV/RA gradient, and ECV were predictors of all‐cause mortality in univariate Cox regression. In multivariate Cox regression analysis, only ferritin (HR: 1.02 [1.01; 1.03], P < 0.001), FGF‐23 (HR: 2.85 [1.26; 6.44], P = 0.012), and ECV (HR: 1.26 [1.03; 1.23], P = 0.008) were independent predictors of all‐cause mortality (Table 4 ).
Table 4.
Cox regression analysis for the secondary endpoint
|
Cox regression analysis All‐cause mortality |
Univariate | Multivariate | ||
|---|---|---|---|---|
|
Hazard ratio 95% CI |
P‐value |
Hazard ratio 95% CI |
P‐value | |
| Age | 1.03 [0.99; 1.07] | 0.13 | ||
| Female | 1.18 [0.64; 2.20] | 0.60 | ||
| Body mass index | 0.91 [0.86; 0.96] | <0.001 | ||
| Mean blood pressure | 0.98 [0.96; 1.00] | 0.10 | ||
| NYHA functional classes III and IV | 1.12 [0.61; 2.06] | 0.72 | ||
| Cardiovascular risk factors | ||||
| Diabetes | 1.53 [0.84; 2.78] | 0.17 | ||
| Medical history | ||||
| Atrial fibrillation | 0.85 [0.46; 1.56] | 0.60 | ||
| Ischaemic cardiomyopathy | 0.67 [0.34; 1.31] | 0.23 | ||
| Medication | ||||
| Loop diuretic | 1.71 [0.79; 3.69] | 0.17 | ||
| Thiazide | 0.79 [0.35; 1.79] | 0.57 | ||
| Mineralocorticoid receptor antagonist | 2.12 [1.12; 4.02] | 0.021 | ||
| Beta‐blocker | 0.72 [0.39; 1.32] | 0.28 | ||
| ACEI or ARB | 0.62 [0.34; 1.15] | 0.13 | ||
| Oral anticoagulant | 1.19 [0.65; 2.20] | 0.58 | ||
| Biology | ||||
| Haemoglobin | 0.86 [0.73; 1.01] | 0.068 | ||
| GFR | 0.98 [0.97; 1.00] | 0.045 | ||
| NT‐proBNP | 1.30 [0.98; 1.73] | 0.078 | ||
| Hs TnT | 1.62 [1.12; 2.34] | 0.014 | ||
| Iron | 1.00 [1.00; 1.01] | 0.09 | ||
| Ferritin | 1.00 [1.00; 1.00] | 0.004 | 1.02 [1.01; 1.03] | <0.001 |
| Calcium | 0.85 [0.40; 1.78] | 0.67 | ||
| Phosphorus | 1.93 [0.64; 5.84] | 0.26 | ||
| Intact PTH | 1.00 [0.99; 1.01] | 0.79 | ||
| 25OH‐Vitamin D | 0.99 [0.97; 1.01] | 0.25 | ||
| Soluble ST2 | 20.24 [4.88; 84.03] | <0.001 | ||
| FGF‐23 | 2.12 [1.26; 3.67] | 0.010 | 2.85 [1.26; 6.44] | 0.012 |
| Echo study | ||||
| LA volume index | 1.01 [1.00; 1.02] | 0.20 | ||
| LV ejection fraction | 1.01 [0.97; 1.06] | 0.53 | ||
| LV Endo GLS | 1.04 [0.94; 1.15] | 0.42 | ||
| E wave velocity | 1.01 [1.00; 1.02] | 0.11 | ||
| E/e′ septal ratio | 1.03 [1.00; 1.06] | 0.084 | ||
| RV/RA gradient | 1.03 [1.01; 1.06] | 0.015 | ||
| RV fractional area change | 0.16 [0.01; 4.88] | 0.29 | ||
| TAPSE | 0.97 [0.91; 1.02] | 0.25 | ||
| cMR study | ||||
| LA volume index (mL/m2) | 1.00 [0.99; 1.01] | 0.71 | ||
| LV EDV index (mL/m2) | 1.01 [0.99; 1.03] | 0.26 | ||
| LV ejection fraction (%) | 0.99 [0.94; 1.03] | 0.60 | ||
| LV mass index (g/m2) | 1.02 [1.00; 1.04] | 0.15 | ||
| RV EDV index (mL/m2) | 1.00 [0.99; 1.02] | 0.66 | ||
| RV ejection fraction (%) | 0.98 [0.93; 1.03] | 0.39 | ||
| Extracellular volume (%) | 1.12 [1.05; 1.20] | 0.003 | 1.26 [1.03; 1.23] | 0.008 |
| Late gadolinium enhancement | 1.13 [1.00; 1.28] | 0.069 | ||
ACEi, angiotensin‐converting‐enzyme inhibitor; ARB, Angiotensin II receptor blockers; ECV, extracellular volume; EDV, end‐diastolic volume; FGF‐23, fibroblast growth factor 23; GFR, glomerular filtration rate; GLS, global longitudinal strain; hsTnT, high‐sensitivity troponinT; LA, left atrium; LV, left ventricle; NT‐proBNP, N terminal pro brain natriuretuic peptide; PTH, parathormon; RV, right ventricle; ST2, soluble suppression tumourigenicity 2; TAPSE, tricuspid annular plane systolic excursion.
Figure 3 A and 3 B shows the Kaplan‑Meier curves for the primary and secondary endpoint of HFpEF patients according to tertiles of FGF‐23, respectively, illustrating that patients with the highest FGF‐23 levels have the worse prognosis.
Figure 3.
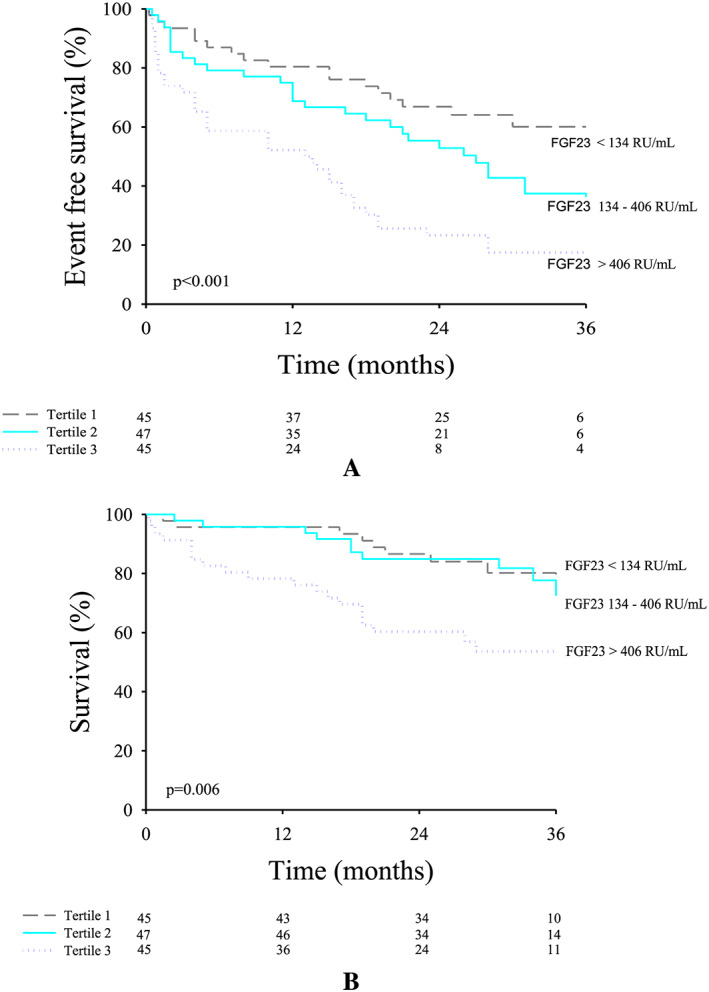
Kaplan‑Meier curves for the primary endpoint according to tertiles of fibroblast growth factor 23 (FGF‐23) in heart failure with preserved ejection fraction (HFpEF) patients (A) and Kaplan‑Meier curves for the secondary endpoint according to tertiles of FGF‐23 in HFpEF patients (B).
Discussion
In the present study, we sought to examine the relationship between FGF‐23 and baseline biomarkers, demographic, and imaging characteristics, particularly diffuse myocardial fibrosis, in controls and HFpEF patients and the prognostic value of FGF‐23 in HFpEF.
In this very well sub‐phenotyped cohort of controls and HFpEF patients, FGF‐23 was significantly higher in HFpEF patients compared with controls. FGF‐23 was associated with some proinflammatory co‐morbidities (i.e. renal dysfunction, diabetes, and atrial fibrillation), indices of cardiac dysfunction (i.e. LA dilatation and RV dysfunction), and biomarkers reflective of either ventricular stretch (NT‐proBNP), myocardial necrosis (hsTnT), fibrosis (sST2), or bone and mineral metabolism (PTH). Moreover, FGF‐23 was significantly correlated with myocardial fibrosis estimated by ECV. FGF‐23 was also a strong and independent predictor of the primary composite endpoint and the secondary endpoint of all‐cause mortality, even after adjusting for confounding factors such as renal function and other clinically relevant variables. Interestingly, both the interplay with the other biomarkers and with the prognosis value of FGF‐23 was independent of the vitamin D levels.
Mechanistic insight into the pathogenesis of disease
In HF, biomarkers are used primarily for diagnosis and risk stratification, natriuretic peptides playing a major role in this respect. 20 Nonetheless, biomarkers studies can also provide important insight into the pathophysiologic mechanisms leading to disease progression, which can then be targeted pharmacologically, such as the RAAS or the adrenergic system. However, this aspect of biomarker research is complicated by the fact that many biomarkers predictive of poor prognosis may reflect systemic organ failures rather than a specific mechanism underlying cardiac disease progression. Moreover, the plasma levels of most biomarkers are affected by multiple confounding factors such as age, sex, renal, and hepatic function. This complexity likely explains why progress has been slow in understanding the mechanisms responsible for the progression of HFpEF, as in this disease, LV systolic function remains by definition preserved to the very end while systemic organ failures inexorably progress.
Heart failure and preserved ejection fraction (HFpEF) is a clinical syndrome that has already been associated with changes in the extracellular matrix and in which fibrosis seems to be a crucial component of cardiac remodelling. 1 Among all the regulators involved in the pathophysiology of cardiac fibrosis, FGF‐23 seems to play an important role, probably still underestimated and understudied. Only few studies looked at the prognostic value of this biomarker in HFpEF, although experimental and in vivo data demonstrated the implication of FGF‐23 in pathophysiological mechanisms involved in the development of the disease such as cardiac hypertrophy, fibrosis, angiogenesis, and cardiac remodelling. 21 , 22 , 23 , 24 Indeed, in our study, we found a significant correlation between FGF‐23 levels and diffuse myocardial fibrosis estimated by ECV. Whether the association between FGF‐23 and ECV is causal cannot be concluded from our observational data, but previous studies demonstrated the implication of FGF‐23 in pathophysiological mechanisms involved cardiac remodelling. 22 , 23 , 24 It was demonstrated that FGF‐23 promotes hypertrophic growth of cardiac myocytes via FGF receptor 4 21 and that it contributes to myocardial fibrosis and diastolic dysfunction through the up‐regulation of active β‐catenin and TGF‐β. 25 It was also shown that FGF‐23 directly stimulates RAAS through the inhibition of ACE2 21 and that the interplay of FGF‐23 with PTH and RAAS might trigger adverse cardiovascular remodelling. 26 , 27 Those data taken together suggest that FGF‐23 is not only a marker of risk but might contribute to the progression of the disease. The availability of FGF‐23 antagonist or immunotherapy would allow testing that hypothesis. 25 , 28 Sodium glucose cotransporter‐2 inhibitors, the most recently approved class of drug for type 2 diabetes, has recently shown benefit in HF patients, independently from its effect on diabetes. 29 One hypothesis to explain this effect is that canagliflozin induces a prompt increase in serum phosphorus and triggers downstream changes in FGF‐23. 30 , 31 Studies (DELIVER and EMPEROR‐Preserved) are currently going on to determine whether sodium glucose cotransporter‐2 inhibitors will have a positive impact on the prognosis of HFpEF patients. 32
Association between fibroblast growth factor 23 levels and clinical characteristics
Although the mechanisms of sex differences in FGF‐23 remain unclear, the presence of higher FGF‐23 levels in female patients than in male patients has been described in previous studies, both in small children 33 and in adults with cardiovascular risk factors. 34 , 35 In the hypothesis that FGF‐23 actively plays a part in the development of the disease, this might contribute to the overrepresentation of women among patients with HFpEF.
Atrial fibrillation, a highly prevalent co‐morbidity in HFpEF, was also found to be associated with higher levels of FGF‐23. 36 , 37 , 38 Two possible mechanisms were described to explain this interaction. First, FGF‐23 could induce atrial fibrosis by increasing ROS production, subsequently activating STAT3 and SMAD3 signalling. 39 Second, FGF‐23 increases pulmonary vein arrythmogenesis through protein kinase C signalling and dysregulation of sodium and calcium homeostatis. 40
The association between elevated FGF‐23 and impaired renal function is largely described. Levels of FGF‐23 rise early in the course of CKD as part of the adaptive response to maintain neutral phosphate balance when renal excretory capacity declines. Chronic FGF‐23 elevation in CKD is independently associated with development of HF and death. 9 , 11 We also observed that haemoglobin decreased across increasing FGF‐23 levels. This is not only mediated through the interaction with renal function (where anaemia is mediated by decreased erythropoietin production, low serum active vitamin D levels, and high renin‐angiotensin‐aldosterone activities) but also through a direct inhibition of erythropoiesis by FGF‐23. 41
Recent data from PARAMOUNT 42 and RELAX 35 trials showed that sST2 levels were correlated with proinflammatory co‐morbidities and with the severity of HFpEF, indicated by higher levels of NT‐proBNP and signs of diastolic dysfunction. Authors from those trials concluded that patients with the more severe disease had a biomarker pattern associated with a more profibrotic milieu. 42 This is corroborated by our study, where FGF‐23 is associated both with the presence of myocardial fibrosis evaluated by ECV and with signs of disease severity (NT‐proBNP, renal function, and RV dysfunction). On the other hand, we did not find a significant association between FGF‐23 levels and E wave velocities or E/e′ ratio. This is consistent with a recent study by Okamoto et al. 24 showing that alpha‐klotho, a co‐receptor of FGF‐23 related to ageing suppression and organ protection, was significantly associated with E/e′ ratio, while FGF‐23 was not. However, in our study, higher FGF‐23 levels were associated with larger LA volumes, which is also an important marker of elevated filling pressures and diastolic dysfunction. 43
Prognostic and risk stratification
Beyond assessment of natriuretic peptides, other biomarkers of mechanisms contributing to the pathophysiology of HF, such as inflammation (GDF‐15 and sST2), LV hypertrophy (FGF‐23), and myocardial necrosis (hsTnT), could add important prognostic information and identify patients at higher risk of events. 44 , 45 , 46 , 47 Studies have already demonstrated the superiority of different biomarkers combinations, including NT‐proBNP, sST2, and Galectin‐3 for risk stratification of chronic HF patients. 48 , 49 In 2015, we showed the additive value of FGF‐23, Galectin 3, and sST2 compared with NT‐proBNP for prediction of cardiovascular death in HFrEF. 50 However, in the specific CORONA‐HF population, a multimarker approach using a panel of 20 inflammatory and extracellular matrix biomarkers (including sST2 and galectin‐3) was of limited clinical value for identifying the risk of adverse outcomes. 51
Although FGF‐23 has been associated with adverse cardiovascular outcomes in CKD and HFrEF, data are lacking in HFpEF. Koller et al. 14 demonstrated that FGF‐23 was independently associated with an increased risk of mortality in patients with HFrEF (n = 511) but not in those with HFpEF (n = 469). Only one large trial showed a significant prognostic value of FGF‐23 compared with NT‐proBNP for a combined outcome of all‐cause mortality and HF hospitalization in HFrEF and in HFpEF. 7 Our data corroborate the prognostic value of FGF‐23 in HFpEF, demonstrating the importance of exploring physiopathological pathways for a better understanding of this syndrome. Surprisingly, NTproBNP was not associated with the primary and the secondary endpoints in our study (borderline for the prediction of mortality HR: 1.30 [0.98; 1.73] P = 0.078), probably mainly due to the high levels of NT‐proBNP on average and the limited sample size.
Fibroblast growth factor 23 (FGF‐23) might increase fluid retention by stimulating RAAS, probably explaining the link between FGF‐23 and HF hospitalization (in univariate Cox regression analysis: HR 2.30 [1.51–3.49], P < 0.001) and might represent a potential therapeutic target. Even after adjustment for eGFR, FGF‐23 remained predictive in our population. However, the strong predictive value of FGF‐23 in our study might be related to the assay we used, and it might be hypothesized that assays targeting C‐terminal FGF‐23 fragments have stronger value for risk estimation than intact assays. 52
It could also be hypothesized that the rise of FGF‐23 precedes the development of HF in patients at high risk, such as diabetic and hypertensive patients. 53 , 54 , 55 The early sub‐phenotyping of these patients including measurement of FGF‐23 could represent an opportunity to precociously estimate their risk for developing HF and adjust their treatment accordingly. Further studies addressing the value of FGF‐23 for risk stratification in this specific population are needed to confirm this hypothesis.
Strengths and limitations
To our knowledge, this is one of the first prospective study investigating the role of FGF‐23 in a cohort of HFpEF patients and in a control group well characterized by echocardiography and cMR. Our cohort is comparable with PARAMOUNT 42 and RELAX 35 biomarker analysis in terms of number of patients and demographic characteristics. However, this should be considered as exploratory and hypothesis generating and should be tested in larger mechanistic HFpEF studies.
Conclusions
Heart failure with preserved ejection fraction (HFpEF) patients showed significantly higher levels of circulating FGF‐23 than controls. High FGF‐23 levels were significantly associated with LA volume (a marker of diastolic dysfunction), RV dysfunction, and with diffuse myocardial fibrosis evaluated by cMR. FGF‐23 was also a strong predictor of poor outcome, even after adjusting for usual confounding factors. Sub‐phenotyping of HFpEF patients with FGF‐23 might therefore participate in a more personalized care.
Conflict of interest
The Cliniques St. Luc UCL has a master clinical research agreement with Philips Medical Instruments, and the MOLLI patch was supplied by Philips Medical under the terms of this agreement. The authors declare that they have no competing interests.
Funding
This work was funded by a grant of the Fondation Nationale de la Recherche Scientifique of the Belgian Government (FRMS CDR 23597851). Dr Pouleur is and Dr Beauloye was supported by a Post‐doctorate Clinical Master Specialist of the Fondation Nationale de la Recherche Scientifique of the Belgian Government (FRSM: SPD 10844948). Dr Horman is a researcher associated of FNRS. Dr Roy is supported by Fondation Damman and Fondation Saint Luc for her fellowship. This work was funded by an unrestricted grant from AstraZeneca.
Roy, C. , Lejeune, S. , Slimani, A. , de Meester, C. , Ahn AS, S. A. , Rousseau, M. F. , Mihaela, A. , Ginion, A. , Ferracin, B. , Pasquet, A. , Vancraeynest, D. , Beauloye, C. , Vanoverschelde, J.‐L. , Horman, S. , Gruson, D. , Gerber, B. L. , and Pouleur, A.‐C. (2020) Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Failure, 7: 2494–2507. 10.1002/ehf2.12816.
Clotilde Roy and Sibille Lejeune contributed equally to this work.
References
- 1. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 2. Duca F, Kammerlander AA, Zotter‐Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, Bonderman D, Mascherbauer J. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging 2016; 9e005277 [DOI] [PubMed] [Google Scholar]
- 3. Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Taguri M, Kimura K, Umemura S. Prognostic significance of quantitative assessment of focal myocardial fibrosis in patients with heart failure with preserved ejection fraction. Int J Cardiol 2015; 191: 314–319. [DOI] [PubMed] [Google Scholar]
- 4. Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015; 131: 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde JL, Gerber BL, Pouleur AC. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson 2018; 20: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gruson D, Lepoutre T, Ketelslegers JM, Cumps J, Ahn SA, Rousseau MF. C‐terminal FGF23 is a strong predictor of survival in systolic heart failure. Peptides 2012; 37: 258–262. [DOI] [PubMed] [Google Scholar]
- 7. Ter Maaten JM, Voors AA, Damman K, van der Meer P, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC. Fibroblast growth factor 23 is related to profiles indicating volume overload, poor therapy optimization and prognosis in patients with new‐onset and worsening heart failure. Int J Cardiol 2018; 253: 84–90. [DOI] [PubMed] [Google Scholar]
- 8. Poelzl G, Trenkler C, Kliebhan J, Wuertinger P, Seger C, Kaser S, Mayer G, Pirklbauer M, Ulmer H, Griesmacher A. FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur J Clin Invest 2014; 44: 1150–1158. [DOI] [PubMed] [Google Scholar]
- 9. Niizuma S, Iwanaga Y, Yahata T, Miyazaki S. Renocardiovascular biomarkers: from the perspective of managing chronic kidney disease and cardiovascular disease. Frontiers in cardiovascular medicine 2017; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon‐Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St. John Sutton M, Ojo A, Gadegbeku C, di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro‐o M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group . Fibroblast growth factor 23 and risks of mortality and end‐stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M, the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . Fibroblast growth factor‐23 and cardiovascular events in CKD. Journal of the American Society of Nephrology: JASN 2014; 25: 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, Loehr LR, Eckfeldt JH, Coresh J. Fibroblast growth factor‐23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the atherosclerosis risk in communities study. J Am Heart Assoc 2014; 3: e000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koller L, Kleber ME, Brandenburg VM, Goliasch G, Richter B, Sulzgruber P, Scharnagl H, Silbernagel G, Grammer TB, Delgado G, Tomaschitz A, Pilz S, Berger R, Mörtl D, Hülsmann M, Pacher R, März W, Niessner A. Fibroblast growth factor 23 is an independent and specific predictor of mortality in patients with heart failure and reduced ejection fraction. Circ Heart Fail 2015; 8: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 15. Plischke M, Neuhold S, Adlbrecht C, Bielesz B, Shayganfar S, Bieglmayer C, Szekeres T, Hörl WH, Strunk G, Vavken P, Pacher R, Hülsmann M. Inorganic phosphate and FGF‐23 predict outcome in stable systolic heart failure. Eur J Clin Invest 2012; 42: 649–656. [DOI] [PubMed] [Google Scholar]
- 16. von Jeinsen B, Sopova K, Palapies L, Leistner DM, Fichtlscherer S, Seeger FH, Honold J, Dimmeler S, Aßmus B, Zeiher AM, Keller T. Bone marrow and plasma FGF‐23 in heart failure patients: novel insights into the heart‐bone axis. ESC heart failure 2019; 6: 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, Thomas MR, Kotecha D, Lip GYH, Kirchhof P, Fabritz L. Data‐driven discovery and validation of circulating blood‐based biomarkers associated with prevalent atrial fibrillation. Eur Heart J 2019; 40: 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Vanoverschelde JL, Pouleur AC, Gerber BL. Age and sex corrected normal reference values of T1, T2 T2* and ECV in healthy subjects at 3T CMR. J Cardiovasc Magn Reson 2017; 19: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson 2012; 14: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kristensen SL, Jhund PS, Kober L, McKelvie RS, Zile MR, Anand IS, Komajda M, Cleland JG, Carson PE, McMurray JJ. Relative importance of history of heart failure hospitalization and N‐terminal pro‐b‐type natriuretic peptide level as predictors of outcomes in patients with heart failure and preserved ejection fraction. JACC Heart failure 2015; 3: 478–486. [DOI] [PubMed] [Google Scholar]
- 21. Leifheit‐Nestler M, Haffner D. Paracrine effects of FGF23 on the heart. Front Endocrinol 2018; 9: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hao H, Li X, Li Q, Lin H, Chen Z, Xie J, Xuan W, Liao W, Bin J, Huang X, Kitakaze M, Liao Y. FGF23 promotes myocardial fibrosis in mice through activation of beta‐catenin. Oncotarget 2016; 7: 64649–64664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faul C. Cardiac actions of fibroblast growth factor 23. Bone 2016; 100: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okamoto Y, Fujita S, Morita H, Kizawa S, Ito T, Sakane K, Sohmiya K, Hoshiga M, Ishizaka N. Association between circulating FGF23, alpha‐klotho, and left ventricular diastolic dysfunction among patients with preserved ejection fraction. Heart Vessels 2016; 31: 66–73. [DOI] [PubMed] [Google Scholar]
- 25. Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, Czaya B, Singh S, Wolf M, Hermann S, Stypmann J, di Marco GS, Brand M, Wacker MJ, Faul C. FGF23/FGFR4‐mediated left ventricular hypertrophy is reversible. Sci Rep 2017; 7: 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hassan M, Qureshi W, Sroujieh LS, Albashaireh D, BouMalham S, Liroff M, Amjad W, Khalid F, Hadid H, Alirhayim Z. Interplay of parathyroid hormone and aldosterone antagonist in prevention of heart failure hospitalizations in chronic kidney disease. Journal of the renin‐angiotensin‐aldosterone system: JRAAS 2014; 15: 278–285. [DOI] [PubMed] [Google Scholar]
- 27. Tomaschitz A, Pilz S, Rus‐Machan J, Meinitzer A, Brandenburg VM, Scharnagl H, Kapl M, Grammer T, Ritz E, Horina JH, Kleber ME, Pieske B, Kraigher‐Krainer E, Hartaigh B, Toplak H, van Ballegooijen AJ, Amrein K, Fahrleitner‐Pammer A, März W. Interrelated aldosterone and parathyroid hormone mutually modify cardiovascular mortality risk. Int J Cardiol 2015; 184: 710–716. [DOI] [PubMed] [Google Scholar]
- 28. von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, Atar D, Krum H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 2015; 8: 71–78. [DOI] [PubMed] [Google Scholar]
- 29. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 30. Huang T, Lin X, Li Q, Luo W, Song L, Tan X, Wang W, Li X, Wu X. Selection of a novel FGF23‐binding peptide antagonizing the inhibitory effect of FGF23 on phosphate uptake. Appl Microbiol Biotechnol 2015; 99: 3169–3177. [DOI] [PubMed] [Google Scholar]
- 31. Blau JE, Bauman V, Conway EM, Piaggi P, Walter MF, Wright EC, Bernstein S, Courville AB, Collins MT, Rother KI, Taylor SI. Canagliflozin triggers the FGF23/1,25‐dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI insight 2018; 3e99123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M, Packer M, Anker SD, Butler J, Filippatos GS, Zannad F, George J, Brueckmann M, Perrone S, Nicholls S, Janssens S, Bocchi E, Giannetti N, Verma S, Jian Z, Gomez Mesa JE, Spinar J, Böhm M, Merkely B, Chopra V, Senni M, Taddi S, Tsutsui H, Chuquiure E, la Rocca HPB, Ponikowski P, Vinereanu D, Sim D, Choi DJ, Juanatey JRG, Squire I, Butler J, Januzzi J, Pina I, Pocock SJ, Carson P, Doehner W, Miller A, Haas M, Pehrson S, Komajda M, Anand I, Teerlink J, Rabinstein A, Steiner T, Kamel H, Tsivgoulis G, Lewis J, Freston J, Kaplowitz N, Mann J, Petrie M, Bernstein R, Cheung A, Green J, Januzzi J, Kaul S, Ping CLS, Lip G, Marx N, McCullough P, Mehta C, Ponikowski P, Rosenstock J, Sattar N, Scirica B, Tsutsui H, Verma S, Wanner C, Welty FK, Parhofer KG, Clayton T, Pedersen TR, Lees KR, Konstam MA, Greenberg B, Palmer M. Evaluation of the effects of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved Trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 33. Holmlund‐Suila E, Enlund‐Cerullo M, Valkama S, Hauta‐Alus H, Rosendahl J, Helve O, Hytinantti T, Viljakainen H, Andersson S, Mäkitie O. Sex and iron modify fibroblast growth factor 23 concentration in 1‐year‐old children. J Clin Endocrinol Metab 2017; 102: 4526–4533. [DOI] [PubMed] [Google Scholar]
- 34. di Giuseppe R, Kuhn T, Hirche F, Buijsse B, Dierkes J, Fritsche A, Kaaks R, Boeing H, Stangl GI, Weikert C. Potential predictors of plasma fibroblast growth factor 23 concentrations: cross‐sectional analysis in the EPIC‐Germany study. PLoS ONE 2015; 10: e0133580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. AbouEzzeddine OF , McKie PM, Dunlay SM, Stevens SR, Felker GM, Borlaug BA, Chen HH, Tracy RP, Braunwald E, Redfield MM. Suppression of tumorigenicity 2 in heart failure with preserved ejection fraction. J Am Heart Assoc 2017; 6e004382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyamura M, Fujita S, Morita H, Sakane K, Okamoto Y, Sohmiya K, Hoshiga M, Ishizaka N. Circulating fibroblast growth factor 23 has a U‐shaped association with atrial fibrillation prevalence. Circulation journal: official journal of the Japanese Circulation Society 2015; 79: 1742–1748. [DOI] [PubMed] [Google Scholar]
- 37. Mehta R, Cai X, Lee J, Scialla JJ, Bansal N, Sondheimer JH, Chen J, Hamm LL, Ricardo AC, Navaneethan SD, Deo R, Rahman M, Feldman HI, Go AS, Isakova T, Wolf M. Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the chronic renal insufficiency cohort study. JAMA Cardiol 2016; 1: 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mizia‐Stec K, Wieczorek J, Polak M, Wybraniec MT, Wozniak‐Skowerska I, Hoffmann A, Nowak S, Wikarek M, Wnuk‐Wojnar A, Chudek J, Więcek A. Lower soluble klotho and higher fibroblast growth factor 23 serum levels are associated with episodes of atrial fibrillation. Cytokine 2018; 111: 106–111. [DOI] [PubMed] [Google Scholar]
- 39. Dong Q, Li S, Wang W, Han L, Xia Z, Wu Y, Tang Y, Li J, Cheng X. FGF23 regulates atrial fibrosis in atrial fibrillation by mediating the STAT3 and SMAD3 pathways. J Cell Physiol 2019; 234: 19502–19510. [DOI] [PubMed] [Google Scholar]
- 40. Huang SY, Chen YC, Kao YH, Hsieh MH, Lin YK, Chung CC, Lee TI, Tsai WC, Chen SA, Chen YJ. Fibroblast growth factor 23 dysregulates late sodium current and calcium homeostasis with enhanced arrhythmogenesis in pulmonary vein cardiomyocytes. Oncotarget 2016; 7: 69231–69242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Vuren AJ, Gaillard C, Eisenga MF, van Wijk R, van Beers EJ. The EPO‐FGF23 signaling pathway in erythroid progenitor cells: opening a new area of research. Front Physiol 2019; 10: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, Prescott MF, Shi V, Lefkowitz M, McMurray J, Solomon SD, Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) Investigators . Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: data from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction study. Circ Heart Fail 2016; 9e002551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 44. Bartunek J, Delrue L, Van Durme F, Muller O, Casselman F, De Wiest B, Croes R, Verstreken S, Goethals M, de Raedt H, Sarma J. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol 2008; 52: 2166–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation 2011; 123: 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsutamoto T, Kawahara C, Yamaji M, Nishiyama K, Fujii M, Yamamoto T, Horie M. Relationship between renal function and serum cardiac troponin T in patients with chronic heart failure. Eur J Heart Fail 2009; 11: 653–658. [DOI] [PubMed] [Google Scholar]
- 47. Smith K, de Filippi C, Isakova T, Gutierrez OM, Laliberte K, Seliger S, Kelley W, Duh S‐H, Hise M, Christenson R, Wolf M, Januzzi J. Fibroblast growth factor 23, high‐sensitivity cardiac troponin, and left ventricular hypertrophy in CKD. Am J Kidney Dis 2013; 61: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grande D, Leone M, Rizzo C, Terlizzese P, Parisi G, Gioia MI, Leopizzi T, Segreto A, Guida P, Romito R, Ciccone M, Serio F, Iacoviello M. A multiparametric approach based on NT‐proBNP, ST2, and Galectin3 for stratifying one year prognosis of chronic heart failure outpatients. Journal of cardiovascular development and disease 2017; 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lupon J, Sanders‐van Wijk S, Januzzi JL, de Antonio M, Gaggin HK, Pfisterer M, Galán A, Shah R, Brunner‐La Rocca HP, Bayes‐Genis A. Prediction of survival and magnitude of reverse remodeling using the ST2‐R2 score in heart failure: a multicenter study. Int J Cardiol 2016; 204: 242–247. [DOI] [PubMed] [Google Scholar]
- 50. Gruson D, Ferracin B, Ahn SA, Rousseau MF. Comparison of fibroblast growth factor 23, soluble ST2 and Galectin‐3 for prognostication of cardiovascular death in heart failure patients. Int J Cardiol 2015; 189: 185–187. [DOI] [PubMed] [Google Scholar]
- 51. Nymo SH, Aukrust P, Kjekshus J, McMurray JJ, Cleland JG, Wikstrand J, Muntendam P, Wienhues‐Thelen U, Latini R, Askevold ET, Gravning J, Dahl CP, Broch K, Yndestad A, Gullestad L, Ueland T, CORONA Study Group . Limited added value of circulating inflammatory biomarkers in chronic heart failure. JACC Heart failure 2017; 5: 256–264. [DOI] [PubMed] [Google Scholar]
- 52. Fauconnier C, Roy T, Gillerot G, Roy C, Pouleur AC, Gruson D. FGF23: clinical usefulness and analytical evolution. Clin Biochem 2019; 66: 1–12. [DOI] [PubMed] [Google Scholar]
- 53. Berezin AE, Berezin AA. Impaired function of fibroblast growth factor 23/klotho protein axis in prediabetes and diabetes mellitus: promising predictor of cardiovascular risk. Diabetes & metabolic syndrome 2019; 13: 2549–2556. [DOI] [PubMed] [Google Scholar]
- 54. Frimodt‐Moller M, von Scholten BJ, Reinhard H, Jacobsen PK, Hansen TW, Persson FI, Parving HH, Rossing P. Growth differentiation factor‐15 and fibroblast growth factor‐23 are associated with mortality in type 2 diabetes—an observational follow‐up study. PLoS ONE 2018; 13: e0196634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119: 2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]


