Abstract
Aims
Guideline‐directed medical therapy (GDMT) including beta‐blockers and renin–angiotensin system inhibitors is shown to reduce mortality risk in patients with heart failure (HF) and reduced left ventricular ejection fraction (LVEF). However, there is little evidence about the efficacy of additional administration of mineralocorticoid receptor antagonists (MRAs) with GDMT in patients ≥80 years presenting with HF. We aimed to investigate the prognostic impact of GDMT with MRA in relation to the age of patients with HF.
Methods and results
This observational study included patients admitted for HF with reduced LVEF who were discharged alive; among them, 224 patients were ≥80 years, and 661 patients were <80 years. Both populations were divided into three groups depending on whether they received GDMT with or without MRA or single/no GDMT drugs (GDMT+MRA+, GDMT+MRA−, or non‐GDMT, respectively). The primary endpoint was all‐cause mortality. In patients ≥80 years, all‐cause mortality was the lowest in the GDMT+MRA+ group (log‐rank trend, P = 0.034), and no significant differences were observed between the GDMT+MRA− and non‐GDMT groups. Multivariate Cox regression analysis revealed that GDMT+MRA+ was superior to GDMT+MRA−, even after adjusting for parameters at discharge (hazard ratio: 0.32, 95% confidence interval: 0.11–0.99). In patients <80 years, GDMT reduced all‐cause mortality; however, additional MRA was not associated with an improved outcome.
Conclusions
The results of this study suggest that additional MRA to GDMT at discharge is one of the therapeutic options for elderly HF patients with reduced LVEF. This finding is not well documented in previous clinical trials.
Keywords: Heart failure, Guideline‐directed medical therapy, Mineralocorticoid receptor antagonist, Octogenarian
Introduction
Heart failure (HF) is an increasing global public health problem that is associated with high mortality, and prolonged and frequent hospitalizations. This condition imposes a huge economic burden on health care resources. HF primarily occurs in older populations, as the age of patients hospitalized for HF is above 75 years in many developed countries. 1 A previous study from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE‐CARD) reported that nearly 30% of hospitalized patients with HF were ≥80 years. 2 A study that evaluated a large cohort of octogenarians with HF in Europe reported that octogenarians had a higher mortality rate than younger patients with HF. Moreover, medical management remains suboptimal in older populations. 3 Hence, therapeutic management of octogenarian HF patients is a worldwide concern.
Older patients are generally excluded from large clinical trials, mainly due to their high mortality rate and diverse co‐morbidities; thus, evidence on the utility of guideline‐directed medical therapy (GDMT) in octogenarian HF patients is insufficient. 4 , 5 , 6 GDMT consists of renin–angiotensin system inhibitors (RASi), which include angiotensin‐converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARBs), and beta‐blockers (BB). The prognostic effectiveness of these drugs have been validated in numerous clinical trials. 7 , 8 , 9 , 10 , 11 , 12 However, a report from Japan indicated that the prescription of RASi and BB does not affect the composite endpoint of cardiac death and re‐admission for patients ≥80 years with HF. 13
Clinical guidelines from the European Society of Cardiology recommend additional administration of mineralocorticoid receptor antagonist (MRA) in HF patients with reduced ejection fraction (HFrEF) if symptoms persist and the left ventricular ejection fraction (LVEF) remains under 35% when the maximum dose of GDMT, including RASi and BB, is given. 6 The use of MRA has been effective in reducing total mortality and cardiovascular (CV) death in patients with HFrEF. 14 , 15 , 16 However, the mean ages of patients in these trials were all young, which is not a realistic population for this condition. A meta‐analysis of studies on MRA administration in elderly patients with HF has shown the effect of MRA on all‐cause mortality in patients ≥75 years to be uncertain. 17 Taken together, the current literature indicates that the applicability of the suggestion of MRA for patients with reduced LVEF to be poorly understood in the case of octogenarian patients. Therefore, this study aimed to determine the differences in the prognostic effectiveness of the addition of MRA to GDMT, including RASi and BB, in young and octogenarian patients that were hospitalized for HF.
Methods
Study population and endpoints
This retrospective observational study initially included 1350 patients who were hospitalized for HF in a single CV centre between June 2013 and November 2017. Patients were diagnosed with HF using the Framingham HF diagnostic criteria. 18 After the exclusion of patients with preserved LVEF (≥50%), those who died in hospital, those lost to follow‐up after discharge, and those who remained in hospital at the time of investigation, the study population ultimately included 661 patients <80 years and 224 patients ≥80 years. Retrospective investigation of the oral medication at the time of discharge was carried out.
We defined GDMT as the administration of both RASi and BB. First, we divided both age groups according to whether GDMT was being administered at discharge or not. Patients who received BB, RASi, or neither drug were categorized into the non‐GDMT group, and those who received both BB and RASi were categorized into the GDMT group. The GDMT group was further subdivided into patients who received MRA in addition to GDMT and those who received GDMT without MRA [the GDMT+MRA+ (full medication) and GDMT+MRA− (GDMT only) groups, respectively] (Figure 1 ).
Figure 1.
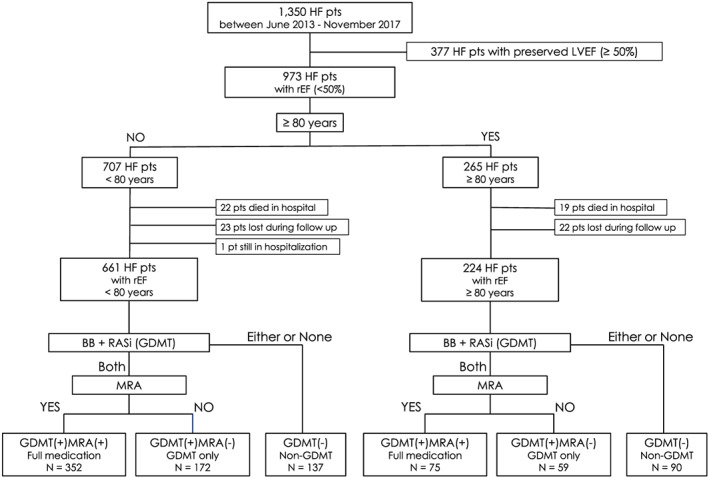
Flow chart of participant recruitment and categorization. BB, beta‐blockers; GDMT, guideline‐directed medical therapy; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; pt, patient; pts, patients; RASi, renin‐angiotensin system inhibitors; rEF, reduced left ventricular ejection fraction.
The primary clinical outcome evaluated in this study was death from any cause; CV death was also evaluated. The latter included death resulting from acute myocardial infarction, sudden cardiac death, death due to HF, death due to stroke, death due to CV procedures, death due to CV haemorrhage, and death due to other CV causes. This study and all its protocols were approved by the hospital's ethics committee, and patient enrolment was carried out according to the principles of the Declaration of Helsinki. Written informed consent for data use from medical records was obtained from all patients before enrolment.
Data collection and follow‐up
For each patient, we collected medical history data along with medication use and the following parameters at discharge: (i) vital signs; (ii) body weight, height, and body mass index; (iii) oral medications; and (iv) laboratory values including haemoglobin, albumin, total bilirubin, C‐reactive protein, renal functions [blood urea nitrogen, creatinine, and estimated glomerular filtration rate (eGFR)], serum sodium concentration, serum potassium concentration, brain natriuretic peptide, haemoglobin A1c, total cholesterol, low‐density lipoprotein cholesterol, and triglyceride. The average creatinine and eGFR were calculated for all patients, except those who underwent dialysis. Echocardiographic parameters including LVEF, left ventricular end‐diastolic diameter, the ratio of early mitral inflow velocity to early diastolic velocity of the lateral mitral annulus (E/e′ ratio), and right ventricular fractional area changes were evaluated during hospitalization. The parameters listed earlier were compared among subgroups. After discharge, most outpatient visits were scheduled at least once every 2 months, and patients were contacted by telephone if they missed a scheduled clinic visit. Information about events including adverse events, causes of death, causes of re‐admission, and date of events were collected by cardiologists from electronic medical records.
Statistical analysis
Continuous data were expressed as means ± standard deviation, and categorical data were presented as number (%). The Kruskal–Wallis test was used to compare continuous parameters across the three groups. The chi‐square test was used to compare categorical variables. All‐cause death and CV death were evaluated using a Kaplan–Meier analysis, and differences between groups were assessed using the log‐rank test. Univariate and multivariate Cox regression analyses were performed to evaluate the association between medications at discharge and prognosis. In the multivariate analysis, age, sex, brain natriuretic peptide, eGFR, C‐reactive protein, sodium concentration, blood urea nitrogen, and LVEF at discharge were used for adjustment. A two‐sided P‐value of <0.05 was considered statistically significant.
Results
Baseline characteristics
Of the 661 HF patients aged <80 years, 352 (53%) received full medication, 172 (26%) received GDMT without MRA, and 137 (21%) received one or no GDMT drugs at discharge. Of the 224 patients aged ≥80 years, 75 (33%) received full medication, 59 (26%) received GDMT without MRA, and 90 (40%) received one or no GDMT drugs at discharge. The percentage of patients who received full medication was significantly lower in the ≥80 years population than in the <80 years population (P < 0.001).
Table 1 displays the baseline characteristics and the echocardiographic results of the study participants. In the <80 years population, the GDMT+MRA+ group was significantly younger with a lower prevalence of hypertension and haemodialysis. Compared with the other groups, the GDMT+MRA+ group exhibited lower systolic and diastolic blood pressure, and reduced blood urea nitrogen and serum creatinine levels. Echocardiographic findings showed that LVEF was significantly lower and the left ventricular diastolic diameter was significantly larger in the GDMT+MRA+ group than in other groups. In the ≥80 years population, the GDMT+MRA+ group included significantly higher numbers of patients with dyslipidaemia and ischaemic heart disease. The patients in the GDMT+MRA+ group had lower systolic blood pressure, and reduced serum creatinine and brain natriuretic peptide levels. LVEF was significantly lower, and the left ventricular diastolic diameter was significantly larger in the GDMT+MRA+ group than in other groups. On comparison of the MRA‐related parameters at discharge between patients <80 and ≥80 years, the mean systolic blood pressure levels were 109.7 and 117.3 mmHg, the mean hazard ratios (HRs) were 71.2 and 70.5 beats/min, and the mean serum potassium levels were 4.4 and 4.3 mEq/L, respectively. Regarding medications, most patients did not receive maximum doses of GDMT and MRAs at discharge. The respective target dose percentages of BB, RASi, and MRA were 49.4%, 44.9%, and 68.7% in the <80 years population and 38.1%, 43.8%, and 58.0% in the ≥80 years population (Table 2 ). Additionally, the percentages of patients who received ≥50% of target doses of BB, RASi, and MRA were respectively 46.0%, 41.8%, and 93.8% in the <80 years population and 40.8%, 54.4%, and 90.1% in the ≥80 years population. Octogenarian patients tended to have a lower dose of BB. MRA seemed to easily be titrated to 50% of the target dose. No patients received sacubitril/valsartan, because its use was banned in Japan during the observational period.
Table 1.
Patient profiles at discharge
| <80 years old | ≥80 years old | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Full medication | GDMT only | Non‐GDMT | P value | All | Full medication | GDMT only | Non‐GDMT | P value | <80 vs. ≥80 | |
| n = 661 | n = 352 | n = 172 | n = 137 | n = 224 | n = 75 | n = 59 | n = 90 | P value | |||
| Age (years) | 62.5 ± 13.2 | 60.3 ± 14 | 63.3 ± 13 | 67.4 ± 9.6 | <0.001 | 84.7 ± 4 | 83.7 ± 3.7 | 84.7 ± 4.1 | 85.5 ± 4 | 0.008 | <0.001 |
| Male (%) | 483 (73.1) | 261 (74.1) | 134 (77.9) | 88 (64.2) | 0.02 | 151 (67.4) | 56 (74.7) | 40 (67.8) | 55 (61.1) | 0.18 | 0.11 |
| Hospital duration (days) | 25.4 ± 22.8 | 28.2 ± 24.8 | 22.7 ± 20.9 | 21.5 ± 18.3 | <0.001 | 23 ± 14.8 | 20.1 ± 10 | 25.4 ± 19.6 | 23.8 ± 14.2 | 0.38 | 0.997 |
| Emergency hospitalization (%) | 366 (55.4) | 191 (54.3) | 93 (54.1) | 82 (59.9) | 0.51 | 179 (79.9) | 61 (81.3) | 45 (76.3) | 73 (81.1) | 0.72 | <0.001 |
| Medical history | |||||||||||
| Hypertension (%) | 400 (60.5) | 194 (55.1) | 126 (73.3) | 80 (58.4) | <0.001 | 183 (81.7) | 62 (82.7) | 49 (83.1) | 72 (80) | 0.86 | <0.001 |
| Dyslipidaemia (%) | 361 (54.6) | 197 (56) | 95 (55.2) | 69 (50.4) | 0.53 | 121 (54) | 47 (62.7) | 30 (50.8) | 44 (48.9) | 0.18 | 0.88 |
| Diabetes (%) | 272 (41.1) | 143 (40.6) | 79 (45.9) | 50 (36.5) | 0.24 | 92 (41.1) | 36 (48) | 20 (33.9) | 36 (40) | 0.25 | 0.98 |
| Smoking (%) | 365 (55.2) | 186 (52.8) | 100 (58.1) | 79 (57.7) | 0.42 | 106 (47.3) | 43 (57.3) | 31 (52.5) | 32 (35.6) | 0.01 | 0.04 |
| Atrial fibrillation (%) | 277 (41.9) | 151 (42.9) | 68 (39.5) | 58 (42.3) | 0.76 | 123 (54.9) | 39 (52) | 33 (55.9) | 51 (56.7) | 0.82 | <0.001 |
| Haemodialysis (%) | 74 (11.2) | 6 (1.7) | 39 (22.7) | 29 (21.2) | <0.001 | 10 (4.5) | 1 (1.3) | 4 (6.8) | 5 (5.6) | 0.26 | 0.002 |
| COPD (%) | 36 (5.4) | 12 (3.4) | 11 (6.4) | 13 (9.5) | 0.02 | 20 (8.9) | 7 (9.3) | 4 (6.8) | 9 (10) | 0.79 | 0.07 |
| PM (%) | 77 (11.6) | 35 (9.9) | 24 (14) | 18 (13.1) | 0.34 | 25 (11.2) | 9 (12) | 8 (13.6) | 8 (8.9) | 0.65 | 0.84 |
| ICD (%) | 143 (21.6) | 106 (30.1) | 16 (9.3) | 21 (15.3) | <0.001 | 24 (10.7) | 10 (13.3) | 5 (8.5) | 9 (10) | 0.64 | <0.001 |
| CRT (%) | 113 (17.1) | 84 (23.9) | 19 (11) | 10 (7.3) | <0.001 | 11 (4.9) | 2 (2.7) | 6 (10.2) | 3 (3.3) | 0.09 | <0.001 |
| Prior PCI (%) | 133 (20.1) | 62 (17.6) | 35 (20.3) | 36 (26.3) | 0.1 | 73 (32.6) | 23 (30.7) | 22 (37.3) | 28 (31.1) | 0.67 | <0.001 |
| Prior CABG (%) | 59 (8.9) | 33 (9.4) | 11 (6.4) | 15 (10.9) | 0.35 | 35 (15.6) | 13 (17.3) | 11 (18.6) | 11 (12.2) | 0.51 | 0.005 |
| Stroke (%) | 87 (13.2) | 43 (12.2) | 15 (8.7) | 29 (21.2) | 0.004 | 56 (25) | 16 (21.3) | 13 (22) | 27 (30) | 0.37 | <0.001 |
| Family history of cardiac event (%) | 225 (34) | 114 (32.4) | 62 (36) | 49 (35.8) | 0.63 | 42 (18.8) | 19 (25.3) | 11 (18.6) | 12 (13.3) | 0.15 | <0.001 |
| Cause of HF Ischaemic (%) | 131 (19.8) | 60 (17) | 42 (24.4) | 29 (21.2) | 0.13 | 79 (35.3) | 37 (49.3) | 20 (33.9) | 22 (24.4) | 0.004 | <0.001 |
| Cardiomyopathy (%) | 224 (33.9) | 150 (42.6) | 47 (27.3) | 27 (19.7) | <0.001 | 22 (9.8) | 8 (10.7) | 6 (10.2) | 8 (8.9) | 0.93 | <0.001 |
| Arrhythmogenic (%) | 114 (17.2) | 59 (16.8) | 34 (19.8) | 21 (15.3) | 0.56 | 51 (22.8) | 18 (24) | 12 (20.3) | 21 (23.3) | 0.87 | 0.07 |
| Valvular (%) | 102 (15.4) | 43 (12.2) | 22 (12.8) | 37 (27) | <0.001 | 45 (20.1) | 10 (13.3) | 13 (22) | 22 (24.4) | 0.19 | 0.11 |
| Others (%) | 16 (2.4) | 9 (2.6) | 3 (1.7) | 4 (2.9) | 0.78 | 5 (2.2) | 0 (0) | 1 (1.7) | 4 (4.4) | 0.15 | 0.87 |
| Clinical profile | |||||||||||
| Body mass index (dis) (kg/m2) | 22.4 ± 4.6 | 22.8 ± 4.7 | 22.3 ± 4.3 | 21.3 ± 4.6 | 0.006 | 20.8 ± 3.3 | 21 ± 3.6 | 21.1 ± 2.7 | 20.4 ± 3.3 | 0.33 | <0.001 |
| Systolic BP (dis) (mmHg) | 109.7 ± 18.9 | 104.2 ± 15.6 | 118.8 ± 21 | 112.4 ± 18.8 | <0.001 | 117.3 ± 16.2 | 113.2 ± 14.8 | 121.2 ± 17.6 | 118.2 ± 15.5 | 0.008 | <0.001 |
| Diastolic BP (dis) (mmHg) | 60.7 ± 10.2 | 59.3 ± 9.6 | 64.1 ± 10.9 | 60.3 ± 9.8 | <0.001 | 60.5 ± 8.4 | 59.6 ± 8.8 | 61.1 ± 7.8 | 60.9 ± 8.4 | 0.23 | 0.76 |
| Heart rate (dis) (beats/min) | 71.2 ± 12 | 70.3 ± 11.2 | 72.5 ± 12.6 | 71.7 ± 13 | 0.19 | 70.5 ± 12.1 | 70.3 ± 12.7 | 70.9 ± 9.6 | 70.5 ± 13.1 | 0.82 | 0.52 |
| Laboratory data at discharge | |||||||||||
| Haemoglobin (g/dL) | 12.5 ± 2.3 | 13 ± 2.1 | 12 ± 2.3 | 11.7 ± 2.3 | <0.001 | 11.5 ± 1.8 | 12 ± 1.9 | 11.3 ± 1.6 | 11.2 ± 1.8 | 0.02 | <0.001 |
| Albumin (mg/dL) | 3.7 ± 0.5 | 3.9 ± 0.5 | 3.6 ± 0.5 | 3.4 ± 0.6 | <0.001 | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.5 | 3.4 ± 0.5 | 0.24 | <0.001 |
| T‐Bil (mg/dL) | 0.9 ± 0.7 | 0.9 ± 0.5 | 0.8 ± 0.4 | 0.9 ± 1.1 | <0.001 | 0.8 ± 0.5 | 0.8 ± 0.4 | 0.7 ± 0.3 | 0.8 ± 0.7 | 0.01 | 0.01 |
| BUN (mg/dL) | 29.5 ± 17.4 | 25.4 ± 13.8 | 32.8 ± 18.6 | 35.9 ± 21.2 | <0.001 | 35 ± 17.5 | 33.9 ± 16.3 | 33.7 ± 16.4 | 36.8 ± 19.1 | 0.65 | <0.001 |
| Creatinine (mg/dL) | 1.3 ± 0.6 | 1.2 ± 0.5 | 1.4 ± 0.7 | 1.4 ± 0.7 | 0.008 | 1.5 ± 0.7 | 1.4 ± 0.6 | 1.5 ± 0.8 | 1.6 ± 0.8 | 0.16 | <0.001 |
| eGFR (mL/min/1.73 m2) | 48.7 ± 35.9 | 51.6 ± 40.8 | 43.8 ± 27.0 | 45.1 ± 25.1 | 0.007 | 37.9 ± 22.7 | 36.8 ± 16.1 | 37.8 ± 22.6 | 38.9 ± 27.1 | 0.44 | <0.001 |
| Sodium (mEq/L dis) | 137.8 ± 8 | 138.3 ± 3.4 | 137.9 ± 10.9 | 136.7 ± 11.5 | 0.03 | 138.7 ± 3.6 | 137.8 ± 4 | 139.2 ± 3.3 | 139.1 ± 3.1 | 0.05 | 0.03 |
| Potassium (mEq/L) | 4.4 ± 0.5 | 4.4 ± 0.4 | 4.4 ± 0.5 | 4.4 ± 0.5 | 0.48 | 4.3 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.3 ± 0.6 | 0.82 | 0.51 |
| CRP (mg/dL) | 0.9 ± 1.8 | 0.7 ± 1.8 | 1 ± 1.7 | 1.1 ± 1.9 | 0.004 | 0.9 ± 1.6 | 0.6 ± 0.8 | 0.8 ± 1.5 | 1.1 ± 2.1 | 0.42 | 0.67 |
| BNP (pg/mL) | 502 ± 692.6 | 346.5 ± 356.3 | 708.3 ± 981.2 | 692.1 ± 855.9 | <0.001 | 674.1 ± 788 | 414.3 ± 263.7 | 908 ± 944.4 | 723.5 ± 892 | 0.01 | <0.001 |
| HbA1c (%) | 6.5 ± 1.3 | 6.5 ± 1.2 | 6.4 ± 1.1 | 6.4 ± 1.6 | 0.13 | 6.3 ± 1 | 6.4 ± 0.8 | 6.3 ± 1 | 6.2 ± 1.1 | 0.45 | 0.45 |
| T‐cho (mg/dL) | 166.9 ± 44.9 | 167.6 ± 48.1 | 168.2 ± 41.7 | 162.9 ± 39.5 | 0.52 | 156.8 ± 39 | 160.1 ± 48.8 | 153.7 ± 29.6 | 155.9 ± 33.9 | 0.95 | 0.01 |
| LDL‐cho (mg/dL) | 95.7 ± 35.8 | 98 ± 38.6 | 94.9 ± 31.8 | 90.1 ± 31.9 | 0.09 | 86.7 ± 30.4 | 89 ± 36 | 82.3 ± 24.8 | 87.9 ± 27.9 | 0.52 | 0.002 |
| TG (mg/dL) | 114.6 ± 82.5 | 114.9 ± 85.3 | 121.5 ± 84.2 | 103.6 ± 69.3 | 0.29 | 80.7 ± 38.6 | 78.4 ± 34.5 | 84.5 ± 38.3 | 80 ± 42.3 | 0.56 | <0.001 |
| Echocardiographic parameters | |||||||||||
| LVEF (%) | 32.3 ± 9.3 | 29.7 ± 8.9 | 34.5 ± 8.6 | 36.1 ± 9.1 | <0.001 | 36.9 ± 8.3 | 35.8 ± 8 | 37.5 ± 9 | 37.5 ± 8 | 0.27 | <0.001 |
| LVDd (mm) | 60.8 ± 10.9 | 63.8 ± 11.4 | 58.5 ± 8.5 | 55.4 ± 9.3 | <0.001 | 53.9 ± 9.9 | 54.6 ± 9.8 | 54.7 ± 9.9 | 52.6 ± 10 | 0.27 | <0.001 |
| E/e′ | 18.3 ± 8.7 | 18.1 ± 8.6 | 18.2 ± 8.7 | 18.9 ± 8.9 | 0.73 | 19.9 ± 8.9 | 19.6 ± 8.7 | 19.5 ± 9.1 | 20.3 ± 9 | 0.8 | 0.02 |
| RVSP (mmHg) | 40 ± 13.4 | 40.6 ± 14.1 | 39.2 ± 12.3 | 39.1 ± 12.6 | 0.68 | 43.5 ± 13.7 | 43 ± 12.8 | 44.1 ± 15.5 | 43.6 ± 13.5 | 0.99 | 0.001 |
| FAC (%) | 30.1 ± 11.2 | 30.6 ± 11.8 | 29.6 ± 9.8 | 28.8 ± 10.7 | 0.79 | 34.4 ± 8 | 32.4 ± 5.4 | 28.9 ± 4.9 | 38 ± 8.6 | 0.02 | 0.02 |
| TAPSE (mm) | 15.5 ± 4.8 | 14.8 ± 4.6 | 16 ± 4.5 | 16.7 ± 5.2 | 0.003 | 16 ± 4.6 | 14.9 ± 4.1 | 17.2 ± 3.9 | 16.1 ± 5.1 | 0.05 | 0.25 |
| Medication use at discharge | |||||||||||
| Beta‐blockers (%) | 570 (86.2) | 352 (100) | 172 (100) | 46 (33.6) | <0.001 | 147 (65.6) | 75 (100) | 59 (100) | 13 (14.4) | <0.001 | <0.001 |
| ACEi or ARBs (%) | 567 (85.8) | 352 (100) | 172 (100) | 43 (31.4) | <0.001 | 182 (81.3) | 75 (100) | 59 (100) | 47 (52.2) | <0.001 | 0.10 |
| ACEi (%) | 312 (47.2) | 217 (61.6) | 77 (44.8) | 18 (13.1) | <0.001 | 82 (36.6) | 41 (54.7) | 27 (45.8) | 14 (15.6) | <0.001 | <0.001 |
| ARBs (%) | 256 (38.7) | 135 (38.4) | 96 (55.8) | 25 (18.2) | <0.001 | 101 (45.1) | 34 (45.3) | 32 (54.2) | 34 (37.8) | <0.001 | <0.001 |
| MRAs (%) | 416 (62.9) | 352 (100) | 0 (0) | 64 (46.7) | <0.001 | 111 (49.6) | 75 (100) | 0 (0) | 36 (40.0) | <0.001 | <0.001 |
| Furosemide (%) | 530 (80.2) | 315 (89.5) | 117 (68) | 97 (70.8) | <0.001 | 193 (86.2) | 70 (93.3) | 48 (81.4) | 75 (83.3) | 0.08 | 0.05 |
| Furosemide dose (mg) | 28.9 ± 26.9 | 30.3 ± 21.4 | 25 ± 30.5 | 30.1 ± 33.6 | <0.001 | 31.8 ± 25.3 | 31.5 ± 20 | 31.3 ± 26.4 | 32.3 ± 28.3 | 0.77 | 0.06 |
| Thiazide (%) | 89 (13.5) | 41 (11.6) | 18 (10.5) | 31 (22.6) | 0.26 | 30 (13.4) | 11 (14.7) | 7 (11.9) | 12 (13.3) | 0.89 | 0.62 |
| Thienopyridine (%) | 92 (13.9) | 44 (12.5) | 31 (18) | 17 (12.4) | 0.2 | 55 (24.6) | 25 (33.3) | 13 (22) | 17 (18.9) | 0.09 | 0.008 |
| OAC (%) | 333 (50.4) | 190 (54) | 76 (44.2) | 67 (48.9) | 0.1 | 116 (51.8) | 44 (58.7) | 27 (45.8) | 45 (50) | 0.3 | 0.72 |
| Calcium channel blockers (%) | 114 (17.2) | 37 (10.5) | 44 (25.6) | 33 (24.1) | <0.001 | 70 (31.3) | 25 (33.3) | 16 (27.1) | 29 (32.2) | 0.72 | <0.001 |
| Inotrope (%) | 173 (26.2) | 111 (31.5) | 33 (19.2) | 30 (21.9) | 0.006 | 28 (12.5) | 9 (12) | 7 (11.9) | 12 (13.3) | 0.95 | <0.001 |
| Statins (%) | 291 (44) | 160 (45.5) | 75 (43.6) | 55 (40.1) | 0.53 | 106 (47.3) | 40 (53.3) | 31 (52.5) | 35 (38.9) | 0.19 | 0.39 |
ACEi, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BNP, brain natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; FAC, fractional area change; GDMT, guideline‐directed medical therapy; HbA1c, haemoglobin A1c; HF, heart failure; ICD, implantable cardioverter defibrillator; LDL‐cho, low‐density lipoprotein cholesterol; LVDd, left ventricular diastolic dimension; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; OAC, oral anticoagulant; PCI, percutaneous coronary intervention; PM, pacemaker; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; T‐Bil, total bilirubin; T‐cho, total cholesterol; TG, triglyceride.
Table 2.
Guideline‐directed medication dose at discharge
| <80 years old | ≥80 years old | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Full medication | GDMT only | Non‐GDMT | P value | All | Full medication | GDMT only | Non‐GDMT | P value | <80 vs. >80 | |
| N = 661 | N = 352 | N = 172 | N = 137 | N = 224 | N = 75 | N = 59 | N = 90 | P value | |||
| Beta‐blockers, n (%) | 570 (86.2) | 352 (100) | 172 (100) | 46 (33.6) | <0.001 | 147 (65.6) | 75 (100) | 59 (100) | 13 (14.4) | <0.001 | <0.001 |
| ≥50% of target dose, n (%) | 262 (46.0) | 182 (51.7) | 59 (34.3) | 21 (45.7) | <0.001 | 60 (40.8) | 31 (41.3) | 25 (42.4) | 4 (30.8) | 0.74 | 0.26 |
| % of target dose | 49.4 ± 38.0 | 52.6 ± 40.7 | 44.1 ± 37.5 | 41.4 ± 32.3 | 0.03 | 38.1 ± 27.3 | 38.5 ± 27.7 | 42.1 ± 28.6 | 26.8 ± 17.9 | 0.08 | <0.001 |
| Carvedilol dose (mg) | 9.1 ± 7 (n = 376) | 9.9 ± 7.6 (n = 258) | 7.9 ± 5.5 (n = 112) | 6.0 ± 4.2 (n = 29) | <0.001 | 6.5 ± 5.5 (n = 86) | 7.4 ± 6.1 (n = 53) | 5.2 ± 4.2 (n = 23) | 4.6 ± 3.5 (n = 12) | <0.001 | <0.001 |
| Bisoprolol dose (mg) | 3.0 ± 2.1 (n = 146) | 3.2 ± 2.3 (n = 92) | 2.7 ± 1.7 (n = 58) | 3.1 ± 1.9 (n = 17) | 0.002 | 2.3 ± 1.3 (n = 57) | 2.1 ± 0.9 (n = 20) | 2.6 ± 1.5 (n = 35) | 1.7 ± 0.8 (n = 9) | 0.16 | 0.14 |
| Propranolol dose (mg) | 60 ± 0 (n = 2) | 60 ± 0 (n = 2) | None | None | — | 120 ± 0 (n = 1) | 120 ± 0 (n = 1) | None | None | — | — |
| Metoprolol dose (mg) | 20 ± 0 (n = 2) | None | 20 ± 0 (n = 2) | None | — | 26.7 ± 9.4 (n = 3) | 40 ± 0 (n = 1) | 20 ± 0 (n = 1) | 20 ± 0 (n = 1) | 0.37 | 0.41 |
| ACEi or ARBs, n (%) | 567 (85.8) | 352 (100) | 172 (100) | 43 (31.4) | <0.001 | 182 (81.3) | 75 (100) | 59 (100) | 47 (52.2) | <0.001 | 0.10 |
| ≥50% of target dose, n (%) | 237 (41.8) | 154 (43.8) | 57 (33.1) | 26 (60.5) | 0.002 | 99 (54.4) | 44 (58.7) | 26 (44.1) | 29 (61.7) | 0.13 | 0.003 |
| % of target dose | 44.9 ± 32.2 | 45.6 ± 32.9 | 46.3 ± 32.2 | 34.4 ± 23.8 | 0.12 | 43.8 ± 27.0 | 45.4 ± 25.0 | 41.9 ± 25.1 | 42.8 ± 31.0 | 0.51 | 0.61 |
| ACEi, n (%) | 312 (47.2) | 217 (61.6) | 77 (44.8) | 18 (13.1) | <0.001 | 82 (36.6) | 41 (54.7) | 27 (45.8) | 14 (15.6) | <0.001 | <0.001 |
| Perindopril dose (mg) | 3.3 ± 2.3 (n = 210) | 3.4 ± 2.3 (n = 144) | 3.5 ± 2.2 (n = 54) | 2.5 ± 1.8 (n = 12) | 0.28 | 2.8 ± 1.7 (n = 61) | 3.0 ± 1.8 (n = 32) | 2.9 ± 1.8 (n = 19) | 1.4 ± 0.5 (n = 10) | 0.01 | 0.18 |
| Enalapril dose (mg) | 5.2 ± 3.1 (n = 95) | 5.4 ± 3 (n = 67) | 4.7 ± 3.2 (n = 23) | 5 ± 2.7 (n = 5) | 0.36 | 5.9 ± 3.1 (n = 18) | 7.5 ± 3.1 (n = 7) | 6.7 ± 2.4 (n = 8) | 3.8 ± 1.7 (n = 3) | 0.06 | 0.18 |
| Imidapril dose (mg) | 5.0 ± 0 (n = 1) | 5.0 ± 0 (n = 1) | None | None | — | 3.8 ± 1.3 (n = 3) | 2.5 ± 0 (n = 2) | None | 5.0 ± 0 (n = 1) | 0.32 | >0.99 |
| Trandolapril dose (mg) | 2.3 ± 1.1 (n = 4) | 2.7 ± 0.9 (n = 3) | None | 1 ± 0 (n = 1) | 0.16 | 0.9 ± 0.2 (n = 7) | 0.8 ± 0.2 (n = 5) | 1 ± 0 (n = 2) | None | 0.41 | 0.12 |
| Captopril dose (mg) | 37.5 ± 0 (n = 2) | 37. 5 ± 0 (n = 2) | None | None | — | None | None | None | None | — | — |
| ARBs, n (%) | 256 (38.7) | 135 (38.4) | 96 (55.8) | 25 (18.2) | <0.001 | 101 (45.1) | 34 (45.3) | 32 (54.2) | 34 (37.8) | <0.001 | <0.001 |
| Azilsartan dose (mg) | 23.6 ± 10.7 (n = 19) | 17.5 ± 4.3 (n = 5) | 27.1 ± 11.6 (n = 14) | None | 0.21 | 37.5 ± 4.3 (n = 6) | 4 0 ± 0 (n = 4) | 30 ± 0 (n = 1) | 40 ± 0 (n = 1) | 0.22 | 0.14 |
| Olmesartan dose (mg) | 23.1 ± 10.4 (n = 25) | 20 ± 8.7 (n = 10) | 26.7 ± 9.4 (n = 13) | 25 ± 15 (n = 2) | 0.46 | 29.5 ± 11.2 (n = 26) | 33.3 ± 9.4 (n = 8) | 27.1 ± 11.6 (n = 9) | 30 ± 11 (n = 9) | 0.73 | 0.11 |
| Candesartan dose (mg) | 5.2 ± 3.2 (n = 99) | 5.5 ± 3.3 (n = 55) | 5.1 ± 3.4 (n = 34) | 3.9 ± 1.9 (n = 10) | 0.44 | 4.1 ± 2.9 (n = 21) | 3.3 ± 0.9 (n = 5) | 3.1 ± 1.0 (n = 9) | 5.6 ± 4.2 (n = 7) | 0.71 | 0.07 |
| Valsartan dose (mg) | 70 ± 35.4 (n = 24) | 77.5 ± 32.3 (n = 9) | 68.6 ± 42.6 (n = 12) | 53.3 ± 9.4 (n = 3) | 0.43 | 60 ± 23.9 (n = 9) | None | 70 ± 17.3 (n = 6) | 46.7 ± 24.9 (n = 3) | 0.24 | 0.79 |
| Losartan dose (mg) | 25.7 ± 15 (n = 68) | 26.3 ± 15.7 (n = 48) | 27.1 ± 11.2 (n = 11) | 21.6 ± 12.4 (n = 9) | 0.58 | 52.1 ± 21.6 (n = 12) | 50 ± 25 (n = 4) | 75 ± 25 (n = 2) | 45.8 ± 9.3 (n = 6) | 0.43 | 0.005 |
| Telmisartan dose (mg) | 45.3 ± 22.5 (n = 21) | 54.3 ± 23.2 (n = 8) | 37.1 ± 19.8 (n = 12) | 40 ± 0 (n = 1) | 0.37 | 41.7 ± 20.1 (n = 20) | 40 ± 13.6 (n = 11) | 80 ± 0 (n = 2) | 35 ± 21.2 (n = 7) | 0.045 | 0.87 |
| Irbesartan dose (mg) | None | None | None | None | — | 200 ± 0 (n = 6) | 200 ± 0 (n = 2) | 200 ± 0 (n = 3) | 200 ± 0 (n = 1) | 1 | — |
| MRAs (%) | 416 (62.9) | 352 (100) | — | 64 (46.7) | <0.001 | 111 (49.6) | 75 (100) | — | 36 (40.0) | <0.001 | <0.001 |
| ≥50% of target dose, n (%) | 390 (93.8) | 332 (94.3) | — | 58 (90.6) | 0.26 | 100 (90.1) | 67 (89.3) | — | 33 (91.7) | 0.70 | 0.18 |
| % of target dose | 68.7 ± 33.5 | 69.9 ± 34.3 | — | 61.2 ± 26.7 | 0.08 | 58.0 ± 22.8 | 59.5 ± 24.2 | — | 54.9 ± 19.4 | 0.44 | 0.04 |
| Spironolactone dose (%) | 33.8 ± 16.5 (n = 340) | 34.3 ± 16.8 (n = 288) | — | 30.9 ± 13.9 (n = 51) | 0.18 | 27.1 ± 9.2 (n = 90) | 26.8 ± 8.7 (n = 55) | — | 27.5 ± 9.8 (n = 35) | 0.82 | <0.001 |
| Eplerenone dose (mg) | 37.7 ± 17.8 (n = 76) | 38.2 ± 18.4 (n = 64) | — | 27.5 ± 5 (n = 13) | 0.16 | 37.5 ± 15.8 (n = 21) | 38.2 ± 16 (n = 20) | — | 25 ± 0 (n = 1) | 0.43 | 0.83 |
ACEi, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; GDMT, guideline‐directed medical therapy; MRAs, mineralocorticoid receptor antagonists.
Prognoses
In terms of the primary endpoint, 130 deaths and 70 deaths were observed during the mean follow‐up period of 814 and 593 days in the <80 years population and the ≥80 years population, respectively. Only one patient, who was in the group aged ≥80 years, was hospitalized due to hyperkalaemia during follow‐up. Both all‐cause mortality and CV mortality of HF patients were significantly higher among those aged ≥80 years than those aged <80 years (log‐rank test: P < 0.001, P = 0.007, respectively; Figure 2 ).
Figure 2.
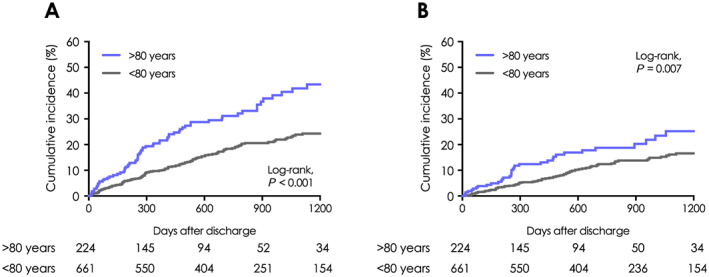
All‐cause and cardiovascular mortality of patients with heart failure in relation to age. Kaplan–Meier event curves for (A) all‐cause and (B) cardiovascular death in patients with heart failure aged <80 years compared with those aged ≥80 years.
In the entire test population, there were significant differences in all‐cause mortality among the three groups; GDMT+MRA+, GDMT+MRA−, and non‐GDMT (log‐rank trend test: P < 0.001, Figure 3 A ). All‐cause mortality was significantly lower in the GDMT+MRA− than in the non‐GDMT group (log‐rank test: P = 0.002, Figure 3 A ) and was lower in the GDMT+MRA+ group than in the GDMT+MRA− group although this was not statistically significant (log‐rank test: P = 0.06).
Figure 3.
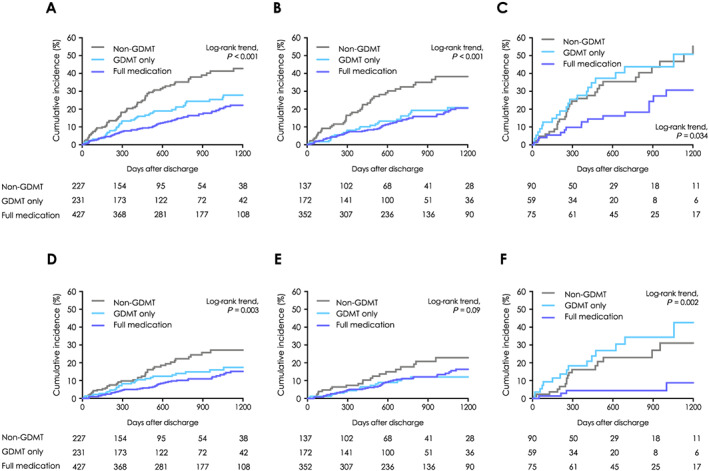
All‐cause and cardiovascular mortality of the three treatment groups among elderly and younger patients with heart failure. Kaplan–Meier event curves for any death of (A) the total study population, (B) patients <80 years, and (C) octogenarian patients with heart failure and cardiovascular death of (D) the total study population, (E) patients <80 years, and (F) octogenarian patients with heart failure. Guideline‐directed medical therapy (GDMT) indicates patients who received both angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker and beta‐blocker, full medication indicates patients who received GDMT plus mineralocorticoid receptor antagonist, and non‐GDMT indicates patients who received only one or none of the GDMT constituent drugs.
Among the <80 years population, all‐cause mortality was significantly lower in the GDMT+MRA− group than in the non‐GDMT group (log‐rank test: P < 0.001, Figure 3 B ). However, the GDMT+MRA+ and GDMT+MRA− groups were comparable in terms of all‐cause mortality (log‐rank test: P = 0.69). Conversely, among the ≥80 years population, there was no significant difference in all‐cause mortality between the GDMT+MRA− and non‐GDMT groups (log‐rank test: P = 0.80, Figure 3 C ). However, the GDMT+MRA+ group was associated with a better long‐term prognosis than the GDMT+MRA− group (log‐rank test: P = 0.02). Similar trends were observed when the analysis was limited to patients with LVEF <40% (Supporting Information, Figure S1 ). Regarding CV mortality, consistent trends were observed in all age categories with regard to all‐cause mortality (Figure 3 D–F ).
Multivariate analysis
Death from any cause
All‐cause mortality of <80 years patients in the GDMT+MRA+ group was not significantly different than the GDMT+MRA− group [unadjusted HR: 0.91, 95% confidence interval (CI): 0.58–1.44, P = 0.69]. After adjusting for covariates, the GDMT+MRA+ group still did not show superiority to the GDMT+MRA− group (adjusted HR: 0.66, 95% CI: 0.33–1.32, P = 0.24, Table 3 ). Additionally, the GDMT+MRA− group showed a reduced all‐cause mortality compared with the non‐GDMT group. The unadjusted HR of patients in the GDMT− group over those in the GDMT+MRA− group was 2.26 (95% CI: 1.41–3.63, P < 0.001, Table 3 ), and adjusted HR was 1.8 (95% CI: 0.92–3.51, P = 0.09, Table 3 ).
Table 3.
Cox proportional hazard model of any death
| <80 years old | HR | 95% CI | P value | ≥80 years old | HR | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| GDMT(+)/MRA(−) | Reference | GDMT(+)/MRA(−) | Reference | ||||
| GDMT(−) | GDMT(−) | ||||||
| Unadjusted | 2.26 | 1.41–3.63 | <0.001 | Unadjusted | 0.93 | 0.54–1.62 | 0.8 |
| Adjusted | 1.8 | 0.92–3.51 | 0.09 | Adjusted | 1.29 | 0.61–2.74 | 0.51 |
| GDMT(+)/MRA(+) | GDMT(+)/MRA(+) | ||||||
| Unadjusted | 0.91 | 0.58–1.44 | 0.69 | Unadjusted | 0.48 | 0.25–0.90 | 0.022 |
| Adjusted | 0.66 | 0.33–1.32 | 0.24 | Adjusted | 0.32 | 0.11–0.99 | 0.048 |
CI, confidence interval; GDMT, guideline‐directed medical therapy; HR, hazard ratio; MRA, mineral corticosteroid antagonist. Models were adjusted for age, sex, brain natriuretic peptide, estimated glomerular filtration rate, C‐reactive protein, sodium, blood urea nitrogen, and left ventricular ejection fraction at discharge.
Among the ≥80 years population, even after adjusting for covariates, the GDMT+MRA+ group showed superiority to the GDMT+MRA− group (HR 0.32, 95% CI: 0.11–0.99, P = 0.048, Table 3 ). Moreover, the risk for all‐cause mortality in the GDMT+MRA− and non‐GDMT groups was not significantly different after adjusting for covariates. The adjusted HR of patients in the GDMT− group over those in the GDMT+MRA− group was 1.29 (95% CI: 0.61–2.74, P = 0.51, Table 3 ).
Cardiovascular death
Among the <80 years population, CV mortality was not significantly different between the GDMT+MRA+ and GDMT+MRA− groups after adjusting for covariates (adjusted HR: 1.18, 95% CI: 0.45–3.09, P = 0.73, Table 4 ). However, CV mortality in the non‐GDMT group was significantly higher than the GDMT+MRA− group after adjusting for covariates. The adjusted HR of patients in the GDMT− group over those in the GDMT+MRA− group was 2.67 (95% CI: 1.01–7.03, P = 0.047, Table 4 ).
Table 4.
Cox proportional hazard model of cardiovascular death
| <80 years old | HR | 95% CI | P value | ≥80 years old | HR | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| GDMT(+)/MRA(−) | Reference | GDMT(+)/MRA(−) | Reference | ||||
| GDMT(−) | GDMT(−) | ||||||
| Unadjusted | 1.95 | 1.02–3.71 | 0.042 | Unadjusted | 0.68 | 0.34–1.38 | 0.29 |
| Adjusted | 2.67 | 1.01–7.03 | 0.047 | Adjusted | 0.55 | 0.22–1.40 | 0.21 |
| GDMT(+)/MRA(+) | GDMT(+)/MRA(+) | ||||||
| Unadjusted | 1.23 | 0.70–2.18 | 0.48 | Unadjusted | 0.19 | 0.069–0.53 | 0.001 |
| Adjusted | 1.18 | 0.45–3.09 | 0.73 | Adjusted | 0.05 | 0.007–0.33 | 0.002 |
CI, confidence interval; GDMT, guideline‐directed medical therapy; HR, hazard ratio; MRA, mineral corticosteroid antagonist. Models were adjusted for age, sex, brain natriuretic peptide, estimated glomerular filtration rate, C‐reactive protein, sodium, blood urea nitrogen, and left ventricular ejection fraction at discharge.
Among the ≥80 years population, the HR for CV death of patients in the GDMT+MRA+ group over those in the GDMT+MRA− group was 0.05 (95% CI: 0.007–0.33, P = 0.002, Table 4 ), even after adjusting for covariates. Conversely, the risk for CV mortality in the non‐GDMT group was not significantly different than the GDMT+MRA− group. The adjusted HR of patients in the GDMT− group over those in the GDMT+MRA− group was 0.55 (95% CI: 0.22–1.40, P = 0.21, Table 4 ).
Discussion
Principal findings of this study
The primary finding of this study is that the combination of MRA and first‐line GDMT, including RASi and BB, at discharge is associated with lower all‐cause mortality in HF patients aged ≥80 years with reduced LVEF. In patients <80 years, the combination of RASi and BB was supposed to be necessary to improve long‐term survival compared with an incomplete combination of GDMT. Conversely, the present study revealed that the combination of RASi and BB was not superior to GDMT only in patients ≥80 years; rather, the addition of MRA to full medication GDMT was required. This trend was consistent when CV mortality was considered. Even after taking into consideration that this is an observational study, the finding that additional MRA improves outcomes in extreme‐age HF patients with reduced LVEF may provide insight for this unsolved clinical problem. Particularly, we present important information regarding a high‐risk population that has previously been excluded from large clinical trials relating to therapeutic guidelines.
Octogenarian patients with heart failure
Compared with younger patients, octogenarian patients had a worse prognosis with regard to both all‐cause mortality and CV mortality in this study. This result was consistent with a large cohort of octogenarian individuals with HF in Europe. 3 In the real‐world clinical practice, 65.9% of outpatients with chronic HFrEF did not receive MRA without contraindication. On the contrary, the percentage of outpatients who did not received RASi or BB without contraindication was only 39.1% and 32.9%, respectively. 19 Although the prescription rates of MRA decrease with increasing age, 20 , 21 the OCTOCARDIO study reported that co‐morbidity did not influence the GDMT in octogenarian HF patients. 22 The results of these previous studies imply that the main reason MRA is underused in octogenarian HF patients is neither co‐morbidity nor contraindication, but age. Therefore, studies that provide evidence on treatment benefits for this population are meaningful.
There have been some large trials investigating the efficacy of MRA for patients with reduced LVEF. However, a systematic meta‐analysis of MRA in elderly patients with HF did not reveal a significant effect of MRA on mortality. 17 Randomized controlled trials targeting elderly patients with HF are required, but analysis is difficult because elderly patients are at high risk of mortality, and they have diverse co‐morbidities that often make prognoses unpredictable. The population of Japan, where the present study was carried out, is aging and has the highest percentage of individuals aged ≥65 years in the world. Japan is a representative country of the developed world, and so the problems of the aging society in Japan can be generalized to other developed countries. Additionally, Japan has a universal health coverage system that ensures that all elderly patients can receive the same quality of medical service. For these reasons, Japan is one of the most suitable countries to carry out studies on optimal medical therapy for elderly patients. In our study population, the combination of RASi and BB was not superior to GDMT only in patients ≥80 years; rather, the full combination of MRA and GDMT was associated with lower all‐cause mortality in patients aged ≥80 years.
Results from the West Tokyo Heart Failure (WET‐HF) registry have demonstrated the efficacy of the combination of RASi and BB, with a reduction in the composite endpoint of cardiac death and HF re‐admission observed among patients <80 years but not among patients ≥80 years, 13 which supports the results of the present study. Conversely, there were several studies that reported GDMT to be associated with improved CV mortality in patients ≥80 years. 23 , 24 The addition of MRA to the first‐line therapy, GDMT, may be the key to solve the controversy surrounding the efficacy of GDMT in octogenarian patients with HF.
Younger patients with heart failure
We found that co‐administering MRA with GDMT was not associated with better long‐term survival in patients <80 years. However, this does not imply that MRA is ineffective in patients <80 years. The EMPHASIS‐HF study revealed that eplerenone could reduce the risk of death in a younger population. 16 This study included HF patients with New York Heart Association class II, an LVEF of less than 35%, and mean age of 69 years, which was a different population from the current study, as the younger population in the current study was 7 years younger and had 6% higher LVEF compared with those in the EMPHASIS‐HF. This suggests that clinical guidelines from ESC for the recommendation of additional MRA use would be suitable for patients in realistic clinical settings. 6 The severity of disease could have influenced the attending physicians' decision to administer MRA. Indeed, clinical profiles were different between the full medication group and the GRMT‐only group, particularly in the <80 years population. Patients tended to have lower LVEF and higher percentages of non‐ischaemic aetiologies in the full medication group than in the GDMT‐only group, which could have led to the difference in results.
Possible mechanisms
The results of this study could be explained by the following hypotheses. One is related to the pharmacological action of MRA; RASi and BB block upstream of the renin–angiotensin–aldosterone system, whereas MRA blocks downstream. The mineral corticosteroid receptor is generally more activated in elderly than younger patients, 25 suggesting that it might be important to block both upstream and downstream of the renin–angiotensin–aldosterone system in HF patients aged ≥80 years. Another hypothesis is related to the ‘aldosterone breakthrough’ phenomenon. Although this mechanism is not completely understood, it has been reported that long‐term administration of ACEi or ARB results in higher serum aldosterone concentrations via the secondary pathway of the renin–angiotensin–aldosterone system in some patients. 26 Our observation that patients who received additional MRA to GDMT had better long‐term prognoses suggests that MRA inhibits the aldosterone breakthrough phenomenon in elderly patients receiving GDMT.
Another possible explanation is related to the effect of MRA on organs other than the heart. Although CV disorders were the leading cause of death, almost 50% of patients died from other causes (the details of causes of death during the follow‐up period are provided in Supporting Information, Table S1 ). Regardless of whether the patient has suffered HF, MRA can be used for blood pressure management, and the addition of MRA to ACEi or ARB reduces proteinuria in patients with chronic kidney disease (CKD) and diabetic nephropathy and can delay a progression of renal dysfunction. 27 , 28 , 29 , 30 This study demonstrates the efficacy of MRA additional to GDMT in all‐cause as well as CV mortality among patients ≥80 years; it is possible that these efficacies of MRA reduce the risk of not only CV but also all‐cause death. As Table 2 shows, patients ≥80 years in the GDMT+MRA+ group did not tend to receive lower doses of GDMT than in other groups. It suggested that additional MRA to GDMT was effective not only in patients with insufficient dose of GDMT.
Non‐suitable candidates for additional mineralocorticoid receptor antagonists
Patients with reduced LVEF and concomitant CKD are at an increased risk of CV death compared with those with preserved renal function; however, they are less likely to be treated with RASi or to receive the target dose of these agents due to the risks of hyperkalaemia or worsening renal function. 31 According to the Swedish Heart Failure Registry, MRA use decreases with impaired renal function, even in the creatinine clearance range of 30–59.9 mL/min where MRA is not contraindicated. 32 However, a sub‐study of the EMPHASIS‐HF reported that eplerenone was also effective in treating patients with eGFR <60 mL/min/1.73 m2. 31 In addition, a sub‐study of the RALES report revealed that the absolute benefit of spironolactone was highest among patients with reduced eGFR. 33 Considering these results, CKD and CKD‐induced hyperkalaemia might not necessitate underuse of MRA in patients with reduced LVEF.
Additional MRA administration was associated with better long‐term prognosis in elderly patients in this study, even though many patients with CKD were included. In fact, the average eGFR of patients ≥80 years in this study was very low (37.9 mL/min/1.73 m2). However, serum potassium levels at discharge were not significantly different among the three groups, and only one patient was hospitalized for hyperkalaemia during the follow‐up period. Although it is important to be aware of the possibility of hyperkalaemia, additional MRA may be effective in octogenarian patients, even in the case of CKD.
Patients with mid‐range left ventricular ejection fraction
Our study also included HF patients with mid‐range ejection fraction (40% ≤ LVEF <50%) (HFmrEF). We observed results similar to the main findings even when HFmrEF patients were excluded and cases were limited to patients with LVEF <40% (Supporting Information, Figure S1 ). Although medical management including GDMT and MRA has not been sufficiently studied in patients with HFmrEF, more than 70% of them received RASi and BB, and more than 40% received MRA. 20 There are some studies demonstrating the benefit of these medications in HFmrEF patients. 34 , 35 , 36 , 37 Considering the results from these studies and the current medical management practice for patients with HFmrEF, we included this population for analysis in the current study. However, we need to be careful in adapting this result to patients with HFmrEF.
Study limitations
The present study has several limitations, which should be acknowledged. First, this was a retrospective observational study performed at a single centre with a relatively small number of patients over a limited period of observation. This setting does not allow for robust statistical analyses and conclusions on the treatment effects in therapeutic subgroups. Second, we did not evaluate whether patients continued to receive medications or not during the follow‐up period. It is possible that in some cases, MRAs were discontinued due to hyperkalaemia or worsening renal function that was noted during outpatient assessment. Furthermore, cognitive impairment often occurs in elderly patients, and this could influence adherence to regimens. Third, we did not take it into account whether patients were naïve to medications at the time of hospitalization or had previously received the medications. Fourth, we did not consider dosages of medications at discharge when we categorized the patients into the three groups. Mean dosages of medications are given in Table 2 , and these were far from the mean doses utilized in prior clinical trials. 5 Fifth, although we performed covariate adjustment, unmeasured and unknown variables could have influenced the results. For example, the severity of HF, which could not be evaluated by covariates, might have influenced the prescription of GDMT with or without MRAs by the attending physicians. Despite these limitations, the results from this study provide important information that may inform clinical practice with regard to the management of elderly patients with HF.
Conclusions
Our retrospective observational study indicates that the administration of MRA in addition to RASi and BB improves all‐cause and CV mortality in patients aged ≥80 years with HF and reduced LVEF, although the effectiveness of the combination of RASi and BB is limited in this population. Further studies are needed to confirm the optimal use and safety of GDMT with MRA in this population.
Conflict of interest
None declared.
Funding
None declared.
Supporting information
Table S1. Cause of death
Figure S1. Long‐term mortality in HF patients with LVEF <40%. Kaplan–Meier event curves for any death in (A) the total population, (B) patients aged under 80 y/o and (C) octogenarian patients with heart failure and left ventricular ejection fraction <40%. GDMT = guideline directed medical therapy; HF = heart failure; LVEF = left ventricular ejection fraction.
Acknowledgement
We thank Editage (www.editage.jp) for English language editing.
Abe, T. , Jujo, K. , Kametani, M. , Minami, Y. , Fukushima, N. , Saito, K. , and Hagiwara, N. (2020) Prognostic impact of additional mineralocorticoid receptor antagonists in octogenarian heart failure patients. ESC Heart Failure, 7: 2711–2724. 10.1002/ehf2.12862.
This study has been registered at the University Hospital Information Network Clinical Trials Registry (UMIN‐CTR) (UMIN identifier: UMIN000034883, http://www.umin.ac.jp/ctr/index.htm).
References
- 1. Dharmarajan K, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin 2017; 13: 417–426. [DOI] [PubMed] [Google Scholar]
- 2. Hamaguchi S, Kinugawa S, Goto D, Tsuchihashi‐Makaya M, Yokota T, Yamada S, Yokoshiki H, Takeshita A, Tsutsui H, Investigators J‐C. Predictors of long‐term adverse outcomes in elderly patients over 80 years hospitalized with heart failure. Circ J 2011; 75: 2403–2410. [DOI] [PubMed] [Google Scholar]
- 3. Komajda M, Hanon O, Hochadel M, Lopez‐Sendon JL, Follath F, Ponikowski P, Harjola VP, Drexler H, Dickstein K, Tavazzi L, Nieminen M. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J 2009; 30: 478–486. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 7. Dargie HJ, Lechat P, Erdmann E, Follath F, Höglund C, Lechat P, Lopez Sendon JL, Mareyev V, Remme WJ, Sadowski Z, Seabra‐Gomes RJ, Zannad F, Wehrlen‐Grandjean M, Funck‐Brentano C, Hansen S, Hohnloser S, Vanoli E, Jaillon P, De Baker G, Dahlstrˆm U, Hill C, Leizorovicz A, Bugnard F, Rolland C, Wiemann H, Verkenne P, Arab T, Cussac N, Dussous V, Haise S, Funck‐Brentano C. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999; 353: 9–13.10023943 [Google Scholar]
- 8. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357: 1385–1390. [DOI] [PubMed] [Google Scholar]
- 9. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325: 293–302. [DOI] [PubMed] [Google Scholar]
- 10. Kleber FX, Niemoller L, Doering W. Impact of converting enzyme inhibition on progression of chronic heart failure: results of the Munich Mild Heart Failure Trial. Br Heart J 1992; 67: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB, Chang PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet 1997; 349: 747–752. [DOI] [PubMed] [Google Scholar]
- 12. Ferrari R. Effects of angiotensin‐converting enzyme inhibition with perindopril on left ventricular remodeling and clinical outcome: results of the randomized Perindopril and Remodeling in Elderly with Acute Myocardial Infarction (PREAMI) Study. Arch Intern Med 2006; 166: 659–666. [DOI] [PubMed] [Google Scholar]
- 13. Akita K, Kohno T, Kohsaka S, Shiraishi Y, Nagatomo Y, Izumi Y, Goda A, Mizuno A, Sawano M, Inohara T, Fukuda K, Yoshikawa T, West Tokyo Heart Failure Registry I. Current use of guideline‐based medical therapy in elderly patients admitted with acute heart failure with reduced ejection fraction and its impact on event‐free survival. Int J Cardiol 2017; 235: 162–168. [DOI] [PubMed] [Google Scholar]
- 14. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 15. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 16. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21.21073363 [Google Scholar]
- 17. Japp D, Shah A, Fisken S, Denvir M, Shenkin S, Japp A. Mineralocorticoid receptor antagonists in elderly patients with heart failure: a systematic review and meta‐analysis. Age Ageing 2017; 46: 18–25. [DOI] [PubMed] [Google Scholar]
- 18. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 19. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 20. Brunner‐La Rocca HP, Linssen GC, Smeele FJ, van Drimmelen AA, Schaafsma HJ, Westendorp PH, Rademaker PC, van de Kamp HJ, Hoes AW, Brugts JJ. Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK‐HF registry. JACC Heart fail 2019; 7: 13–21. [DOI] [PubMed] [Google Scholar]
- 21. Vorilhon C, Chenaf C, Mulliez A, Pereira B, Clerfond G, Authier N, Jean F, Motreff P, Citron B, Eschalier A, Lusson JR, Eschalier R. Heart failure prognosis and management in over‐80‐year‐old patients: data from a French national observational retrospective cohort. Eur J Clin Pharmacol 2015; 71: 251–260. [DOI] [PubMed] [Google Scholar]
- 22. Moubarak G, Ernande L, Godin M, Cazeau S, Vicaut E, Hanon O, Zuily S, Tournoux F, Danchin N, Derumeaux G, Mechulan A. Impact of comorbidity on medication use in elderly patients with cardiovascular diseases: the OCTOCARDIO study. Eur J Prev Cardiol 2013; 20: 524–530. [DOI] [PubMed] [Google Scholar]
- 23. Savarese G, Dahlstrom U, Vasko P, Pitt B, Lund LH. Association between renin‐angiotensin system inhibitor use and mortality/morbidity in elderly patients with heart failure with reduced ejection fraction: a prospective propensity score‐matched cohort study. Eur Heart J 2018; 39: 4257–4265. [DOI] [PubMed] [Google Scholar]
- 24. Stolfo D, Uijl A, Benson L, Schrage B, Fudim M, Asselbergs FW, Koudstaal S, Sinagra G, Dahlstrom U, Rosano G, Savarese G. Association between beta‐blocker use and mortality/morbidity in older patients with heart failure with reduced ejection fraction. A propensity score‐matched analysis from the Swedish Heart Failure Registry. Eur J Heart Fail 2020; 22: 103–112. [DOI] [PubMed] [Google Scholar]
- 25. Pitt B. The role of mineralocorticoid receptor antagonists (MRAs) in very old patients with heart failure. Heart Fail Rev 2012; 17: 573–579. [DOI] [PubMed] [Google Scholar]
- 26. Navaneethan SD, Bravo EL. Aldosterone breakthrough during angiotensin receptor blocker use: more questions than answers? Clin J Am Soc Nephrol 2013; 8: 1637–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chrysostomou A, Becker G. Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N Engl J Med 2001; 345: 925–926. [DOI] [PubMed] [Google Scholar]
- 28. Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 2006; 1: 940–951. [DOI] [PubMed] [Google Scholar]
- 29. Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis 2008; 51: 199–211. [DOI] [PubMed] [Google Scholar]
- 30. Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2014; 4: CD007004. [DOI] [PubMed] [Google Scholar]
- 31. Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, Shi H, Vincent J, Rossignol P, Zannad F, Pitt B. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS‐HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol 2013; 62: 1585–1593. [DOI] [PubMed] [Google Scholar]
- 32. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlstrom U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018; 20: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 33. Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 2012; 60: 2082–2089. [DOI] [PubMed] [Google Scholar]
- 34. Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid‐range (borderline) ejection fraction: clinical implications and future directions. JACC Heart fail 2017; 5: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 36. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 37. Ferreira JP, Rossello X, Pitt B, Rossignol P, Zannad F. Eplerenone in patients with myocardial infarction and “mid‐range” ejection fraction: an analysis from the EPHESUS trial. Clin Cardiol 2019; 42: 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cause of death
Figure S1. Long‐term mortality in HF patients with LVEF <40%. Kaplan–Meier event curves for any death in (A) the total population, (B) patients aged under 80 y/o and (C) octogenarian patients with heart failure and left ventricular ejection fraction <40%. GDMT = guideline directed medical therapy; HF = heart failure; LVEF = left ventricular ejection fraction.


