Abstract
Aims
Heart rate reduction therapy using ivabradine, a selective inhibitor of the funny current of the sinoatrial node, is widely used in the systolic heart failure cohort. However, the optimal target of heart rate remains controversial. The association between heart rate and ‘overlap’ between E‐wave and A‐wave in the pulse wave transmitral flow Doppler echocardiography might be a key to find the ideal heart rate in each individual.
Methods and results
We performed transthoracic echocardiography in patients with systolic heart failure, and the association between heart rate, deceleration time, and overlap length between E‐wave and A‐wave was assessed. In total, 368 patients with systolic heart failure (median 76 years old, 190 men, median ejection fraction 40%) were included. The measured overlap length was 35 (−72, 115) ms. Given the results of multiple linear regression analyses, we constructed a formula: estimated overlap length (ms) = −589 + 6.2 × heart rate (bpm) + 0.81 × deceleration time (ms), which had a good agreement with actually measured one (r = 0.62). The ideal heart rate, at which the overlap length is ‘zero’ and probably cardiac output is maximized, is calculated as follows: ideal heart rate (bpm) = 93 – 0.13 × deceleration time (ms).
Conclusions
We proposed a novel formula using deceleration time to estimate ideal heart rate that achieves a zero overlap between E‐wave and A‐wave in patients with systolic heart failure. Prognostic impact of the formula‐guided heart rate optimization should be studied.
Keywords: Echocardiography, Deceleration time, Ivabradine
Background
Given cumulating results of large‐scale studies, up‐titration of beta‐blocker and heart rate reduction achievement are keys to reducing mortality in patients with heart failure with reduced ejection fraction (HFrEF). 1 However, medication titration is often insufficient in the real‐world practice mainly because of unstable haemodynamics with hypotension, particularly among advanced heart failure population. 2
Ivabradine is a recently developed selective inhibitor of If channels that purely reduces heart rate and improves prognosis in patients with HFrEF and higher heart rate with sinus rhythm (heart rate above 70 or 75 bpm is approved for insurance use). 3 , 4 However, the clinical benefit of heart rate reduction seems to become plateau as decreasing heart rate, and a target heart rate to maximally enjoy improved prognosis remains controversial. 5
In the pulse wave transmitral flow Doppler echocardiographic assessment, E‐wave and A‐wave merge at a higher heart rate. 6 On the contrary, a diastasis phase (i.e. a period between E‐wave and A‐wave) dominantly prolongs at sinus bradycardia. 7 At an ideal heart rate with maximized cardiac output, the ‘overlap’ between E‐wave and A‐wave might be ‘zero’, that is, E‐wave and A‐wave adjacent to each other without any overlap.
In this study, we investigated the association among heart rate, deceleration time, and overlap between E‐wave and A‐wave to construct a formula to calculate the ideal heart rate in each patient with HFrEF.
Methods
Patient selection
In this retrospective study, patients who were diagnosed as HFrEF (left ventricular ejection fraction < 50%) using transthoracic echocardiography between January 2019 and December 2019 were included. Patients with atrial fibrillation or atrioventricular block type I were excluded.
Echocardiographic assessment
Transthoracic echocardiography was performed following the current American Society of Echocardiography guidelines by the expert sonographers who were blinded to the protocol of this study. Heart rate was simultaneously measured by three‐lead electrocardiogram. Left ventricular ejection fraction was calculated by the modified Simpson method. The deceleration time of E‐wave was measured by the pulse Doppler echocardiography at transmitral flow in the apical four‐chamber view.
At the transmitral flow, the overlap between E‐wave and A‐wave was measured. If there was no overlap, the distance between the two waves was expressed as a negative value (Figure 1 ).
Figure 1.
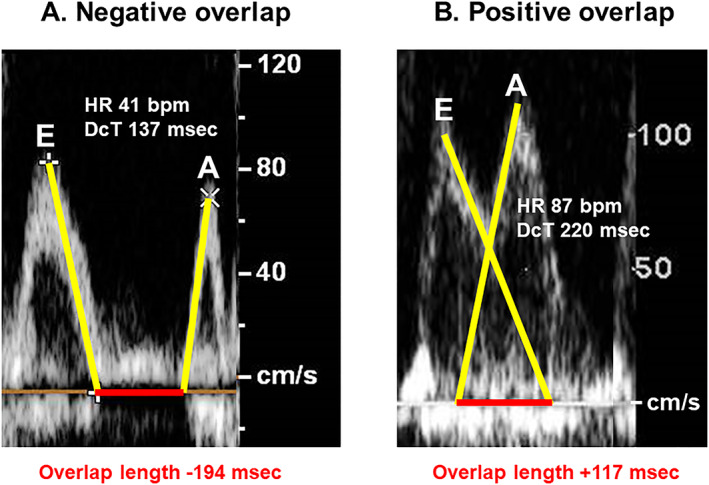
Examples of a case with negative overlap (A) and a case with positive overlap (B). In case A, a patient had a heart rate of 41 bpm and a deceleration time of 137 ms. E‐wave and A‐wave did not overlap. In case B, another patient had a heart rate of 87 bpm and a deceleration time of 220 ms. E‐wave and A‐wave considerably overlapped.
Statistical analyses
Statistical analyses were performed using SPSS Statistics 22 (SPSS Inc, Armonk, IL, USA). Two‐sided P values <0.05 were considered statistically significant. Continuous variables were expressed as median and interquartile.
The impact of heart rate and deceleration time on the measured overlap length was assessed by the multiple linear regression analyses. Considering the beta value of each variable, the formula to estimate overlap length was constructed.
The agreement between the estimated overlap length and measured one was assessed by Pearson's correlation coefficient. The ideal heart rate was calculated using the formula at the condition of ‘overlap length = 0’.
Results
Baseline characteristics
In total, 368 HFrEF patients (median 76 years old, 190 men) were included. The left ventricular ejection fraction was 40% (31%, 46%). The measured overlap length was 35 (−72, 115) ms and ranged between −457 and 364 ms. Heart rate was 67 (60, 77) bpm and deceleration time was 205 (152, 272) ms (Table 1 ). Examples of negative overlap case (Figure 1 A ) and positive overlap case (Figure 1 B ) are shown.
Table 1.
Baseline characteristics
| N = 368 | |
|---|---|
| Age (years) | 76 (68, 83) |
| Male sex | 190 (52%) |
| Left ventricular ejection fraction (%) | 40 (31, 46) |
| Heart rate (bpm) | 67 (60, 77) |
| Deceleration time (ms) | 205 (152, 272) |
| Overlap length (ms) | 35 (−72, 115) |
Variables are expressed as median and interquartile or number and percentage.
Estimation of the overlap length
Heart rate and deceleration time were both independent determinants of the overlap length (P < 0.05 for both). Considering the beta values of both variables, we constructed a formula: estimated overlap length (ms) = −589 + 6.2 × heart rate (bpm) + 0.81 × deceleration time (ms). The estimated overlap length had a good agreement with actually measured overlap length (r = 0.62, P < 0.001; Figure 2 ).
Figure 2.
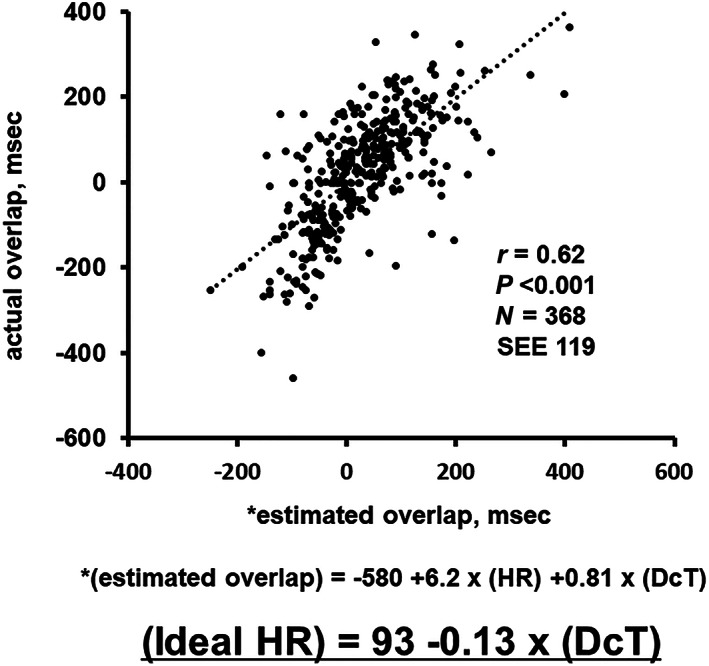
Agreement between the estimated overlap length and the actually measured overlap length. The estimated overlap was calculated from the formula using heart rate and deceleration time. DcT, deceleration time; HR, heart rate; SEE, standard error of estimate.
When the overlap length was ‘zero’ in the formula, the ideal heart rate to achieve zero overlap was calculated as follows: ideal heart rate (bpm) = 93–0.13 × deceleration time (ms). For example, when a deceleration time is 200 ms, the ideal heart rate, that is, a target heart rate for the heart rate reduction therapy, is calculated as 67 bpm.
Discussion
Heart rate reduction and acute haemodynamic change
In patients dependent on atrial pacing, heart rate reduction was associated with increased end‐diastolic volume and systolic volume, which preserved cardiac output by compensating reduced stroke time. 8 In another study, left ventricular contractility was preserved irrespective of heart rate reduction. As a result, the J‐SHIFT trial showed that systolic blood pressure remained unchanged when heart rate was reduced by ivabradine therapy. 4 Such a preserved haemodynamics during heart rate reduction would be essential to achieve future cardiac reverse remodelling and improvement in survival, probably by reducing cardiac potential energy per minute. 8
How to calculate the ideal heart rate
An increase in end‐diastolic volume during heart rate reduction is required to maintain haemodynamics and enjoy the prognostic effect of heart rate reduction therapy. However, given the result of SHIFT trial, too low heart rate might not have an advantage in increasing end‐diastolic volume. 5
Pulse wave transmitral flow Doppler echocardiography might be a good tool to assess the relationship between heart rate and left ventricular filling. At sinus tachycardia, E‐wave and A‐wave merge. 6 In a healthy cohort, a merged A‐wave gets higher as heart rate increases probably to compensate reduced left ventricular filling. 9 However, such compensation would not work in patients with HFrEF due to impaired atrial function. During heart rate reduction, the widths of E‐wave and A‐wave remain unchanged, whereas only a diastasis phase would prolong, 9 which might not increase end‐diastolic volume. In other words, there might be an optimal heart rate that has neither a merge between E‐wave and A‐wave nor a diastasis phase, that is, zero overlap. Deceleration time would be another dominant determinant of overlap between E‐wave and A‐wave. Given these hypotheses, we constructed a formula to estimate the ideal heart rate consisting of deceleration time, at which the overlap between E‐wave and A‐wave is zero.
In patients with HFrEF and sinus tachycardia, we would initiate ivabradine therapy with a target heart rate calculated by the novel formula. For example, if a patient has a heart rate of 80 bpm and a deceleration time of 200 ms, a target heart rate is calculated as 67 bpm so that heart rate reduction therapy is reasonable. On the contrary, if another patient has a similar heart rate but a deceleration time of 80 ms, a target heart rate is calculated as 83 bpm and heart rate reduction therapy is not required.
It is believed that a higher heart rate is appropriate for those with restrictive physiology, whereas a lower heart rate is appropriate for those with mitral stenosis. The ideal heart rate might vary depending on a variety of deceleration time in each individual with a specific clinical situation and each haemodynamic situation.
Future concern and limitations
We should state that there are several potential limitations in this study. This derivation study is a moderate size cohort, and the formula requires validation in another external large cohort. Our findings base on the hypothesis that the optimal heart rate would have neither merge between E‐wave and A‐wave nor diastasis phases to enjoy the maximal cardiac output. This should be demonstrated by the right heart catheterization test (or any other non‐invasive devices to estimate cardiac output) by precise control of heart rate using pacemaker. This study lacks longitudinal observation. The deceleration time (and calculated ideal heart rate) would remain unchanged at clinically stable conditions during short‐term follow‐up (data not shown). However, the deceleration time would vary during long‐term follow‐up, and we should repeat calculation using the formula to update the target heart rate.
This study is a proof of concept, and a prospective randomized control trial is warranted to investigate the prognostic implication of optimized heart rate, which is calculated by our formula, by heart rate reduction therapy using ivabradine. The target heart rate is calculated theoretically and the actual heart rate control might range around the target. Clinically acceptable ‘range’ would also be a future concern. Our strategy would be expanded into other rate control therapies including mechanical pacing.
Conclusions
We proposed a novel formula using deceleration time to estimate ideal heart rate that achieves a zero overlap between E‐wave and A‐wave in patients with HFrEF. The clinical implication of this formula as a target heart rate in heart rate reduction therapy remains the next concern.
Conflict of interest
None.
Funding
None.
Izumida, T. , Imamura, T. , Nakamura, M. , Fukuda, N. , and Kinugawa, K. (2020) How to consider target heart rate in patients with systolic heart failure. ESC Heart Failure, 7: 3231–3234. 10.1002/ehf2.12814.
The first two authors contributed equally to the study.
References
- 1. Hori M, Sasayama S, Kitabatake A, Toyo‐oka T, Handa S, Yokoyama M, Matsuzaki M, Takeshita A, Origasa H, Matsui K, Hosoda S, Investigators M. Low‐dose carvedilol improves left ventricular function and reduces cardiovascular hospitalization in Japanese patients with chronic heart failure: the Multicenter Carvedilol Heart Failure Dose Assessment (MUCHA) trial. Am Heart J 2004; 147: 324–330. [DOI] [PubMed] [Google Scholar]
- 2. Kato N, Kinugawa K, Imamura T, Muraoka H, Minatsuki S, Inaba T, Maki H, Shiga T, Hatano M, Yao A, Komuro I, Nagai R. Trend of clinical outcome and surrogate markers during titration of beta‐blocker in heart failure patients with reduced ejection fraction: relevance of achieved heart rate and beta‐blocker dose. Circ J 2013; 77: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 3. Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L, Investigators S. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 4. Tsutsui H, Momomura SI, Yamashina A, Shimokawa H, Kihara Y, Saito Y, Hagiwara N, Ito H, Yano M, Yamamoto K, Ako J, Inomata T, Sakata Y, Tanaka T, Kawasaki Y, Investigators JSS. Efficacy and safety of ivabradine in Japanese patients with chronic heart failure—J‐SHIFT study. Circ J 2019; 83: 2049–2060. [DOI] [PubMed] [Google Scholar]
- 5. Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L, Investigators S. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet 2010; 376: 886–894. [DOI] [PubMed] [Google Scholar]
- 6. Chung CS, Afonso L. Heart rate is an important consideration for cardiac imaging of diastolic function. JACC Cardiovasc Imaging 2016; 9: 756–758. [DOI] [PubMed] [Google Scholar]
- 7. Salvi P, Palombo C, Salvi GM, Labat C, Parati G, Benetos A. Left ventricular ejection time, not heart rate, is an independent correlate of aortic pulse wave velocity. J Appl Physiol (1985) 2013; 115: 1610–1617. [DOI] [PubMed] [Google Scholar]
- 8. Yamanaka T, Onishi K, Tanabe M, Dohi K, Funabiki‐Yamanaka K, Fujimoto N, Kurita T, Tanigawa T, Kitamura T, Ito M, Nobori T, Nakano T. Force‐ and relaxation‐frequency relations in patients with diastolic heart failure. Am Heart J 2006; 152: 966 e1–966 e7. [DOI] [PubMed] [Google Scholar]
- 9. Chung CS, Kovacs SJ. Consequences of increasing heart rate on deceleration time, the velocity‐time integral, and E/A. Am J Cardiol 2006; 97: 130–136. [DOI] [PubMed] [Google Scholar]


