Abstract
Aims
The focus on wild‐type transthyretin amyloid cardiomyopathy (ATTRwt‐CM) is increasing because of novel treatment options. There is currently no report on a large number of Japanese patients with ATTRwt‐CM. The study aimed to examine the characteristics and prognosis of ATTRwt‐CM in Japan.
Methods and results
Consecutive patients (78.5 ± 6.4 years old at diagnosis) with ATTRwt‐CM diagnosed at Kumamoto University Hospital between December 2002 and December 2019 were retrospectively reviewed. Data, including demographic characteristics, co‐morbidities, clinical manifestations at diagnosis, laboratory results, electrocardiographic and echocardiographic data, imaging and pathological findings, and treatment were obtained. Of 129 patients included in this study, 110 patients (85%) were male. The median period from initial symptom onset to diagnosis was 15.5 (2–75) months. Heart failure was the most common clinical manifestation leading to diagnosis (61%) and initial manifestations (49%). Of 106 patients, carpal tunnel syndrome was observed in 57 patients (54%), and the median period from initial symptom onset to diagnosis was 96 (48–120) months. Histopathological confirmation of transthyretin amyloid was achieved in 94 patients (73%), including 66 (51%) and 28 cases (22%) with endomyocardial and extracardiac biopsies. During the observation period (median 15.0 [inter‐quartile range, 5.4–33.2] months after diagnosis), 34 patients (26%) died. Of these, 27 patients (79%) had cardiovascular deaths (heart failure, 25; sudden death, two). The median survival duration was 58.9 months and the 5 years' survival rate was 48%. According to a multivariate Cox hazard analysis, age [hazard ratio (HR), 1.14; 95% confidence interval (CI), 1.05–1.23, P = 0.002] and low serum sodium levels (HR, 0.89; 95% CI, 0.79–0.996; P = 0.04) contributed to all‐cause mortality, and low serum sodium levels contributed to hospitalization for heart failure (HR, 0.86; 95% CI, 0.77–0.96; P = 0.005).
Conclusions
Clinical characteristics and prognosis of ATTRwt‐CM patients in Japan were examined. Carpal tunnel syndrome can be considered an indication for diagnosis of ATTRwt‐CM. Age and low serum sodium level were significant predictive factors of all survival outcomes. The clinical features of ATTRwt‐CM should be recognized to provide appropriate treatment.
Keywords: Cardiac amyloidosis, Transthyretin, Cardiomyopathy, Heart failure, Prognosis
Introduction
Amyloidosis is a systematic organ dysfunction due to the deposition of β‐sheet structured amyloid fibrils, and cardiac involvement is the factor that most affects patient outcomes. Cardiac amyloidosis is a progressive infiltrative cardiomyopathy characterized by restrictive cardiomyopathy and causes arrhythmias by infiltration of the conduction system. 1 There are mainly two types of cardiac amyloidosis, namely, amyloid light‐chain (AL) amyloidosis and transthyretin (TTR) amyloidosis (ATTR). ATTR is classified into two subtypes on the basis of the presence or absence of a genetic mutation: mutant ATTR (ATTRv) and wild‐type ATTR (ATTRwt). Compared with that of other types of cardiac amyloidosis, the onset of ATTRwt is delayed and is more frequent in patients aged >60 years. 2
Although transthyretin amyloid cardiomyopathy (ATTR‐CM) is considered a rare disease, recent studies have shown that a considerable number of elderly patients with heart failure is associated with wild‐type ATTR cardiomyopathy (ATTRwt‐CM). A previous autopsy study has revealed that at least 25% of individuals aged >80 years have histological evidence of amyloid deposit in their cardiac tissue. 3 Another study reported that 13% of patients with heart failure with preserved ejection fraction (HFpEF) were diagnosed with ATTRwt‐CM. 4 Given the advent of the super‐aging society in Japan, the number of elderly HFpEF patients is expected to increase 5 ; similarly, patients with ATTRwt‐CM are predicted to increase in the future.
Previous reports have described the clinical characteristics and prognosis of patients with ATTRwt‐CM; however, these studies were mainly conducted in Western countries. 6 , 7 , 8 , 9 Currently, there are no reports on a large number of Japanese patients with ATTRwt‐CM. Thus, this study aimed to examine the clinical characteristics, diagnostic approach, and prognosis in patients with ATTRwt‐CM in Japan.
Methods
Study population
We enrolled 129 consecutive patients diagnosed with ATTRwt‐CM at Kumamoto University Hospital between December 2002 and December 2019. Data including demographic characteristics, co‐morbidities, clinical manifestations at diagnosis, laboratory results, electrocardiographic and echocardiographic data, imaging and pathological findings, and treatment were obtained. Clinical examination was performed while the patient was in a clinically stable, non‐congested condition. Manifestation of onset of ATTRwt was defined as the date of the first clinical symptom related to ATTRwt [e.g. heart failure, arrhythmia, carpal tunnel syndrome (CTS)] from clinical history. Similarly, clinical signs that contributed to diagnosis of ATTRwt‐CM were also reviewed.
The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. It was approved by the Institutional Review Board and the ethics committees of Kumamoto University (No. 1590). The requirement for informed consent was waived because of the low‐risk nature of this retrospective study and the inability to directly obtain consent from all subjects. Instead, the study protocol was extensively promoted at Kumamoto University Hospital and on the website (http://www.kumadai‐junnai.com); notably, patients were provided the opportunity to withdraw from the study.
Diagnosis of wild‐type transthyretin amyloid cardiomyopathy
The diagnosis of amyloid deposition was based on Congo red staining and apple‐green birefringence with cross‐polarized light microscopy. To confirm the presence of TTR in the amyloid, immunohistochemical staining was performed using antibodies that react with TTR. ATTRwt was diagnosed based on an absence of mutation in the TTR gene, which was revealed by genetic testing, or the absence of family history of amyloidosis in the elderly patients if genetic testing was not performed. Detailed diagnostic examination for ATTRwt‐CM including biopsy was performed based on the overall discretion of the attending physicians. Endomyocardial biopsy was considered to be standard; however, extracardiac biopsies were considered for patients at high risk of endomyocardial biopsy.
ATTR‐CM was diagnosed by (i) the presence of TTR deposition in the myocardium (Group 1); (ii) the presence of TTR deposition in extracardiac tissue, such as subcutaneous tissue or gastrointestinal tract with positive finding of 99mTc‐labelled pyrophosphate (99mTc‐PYP) scintigraphy (Group 2); or (iii) positive finding of 99mTc‐PYP scintigraphy without the confirmation of pathological TTR deposition and excluded AL amyloidosis by serum and urine protein electrophoresis by immunofixation electrophoresis (Group 3).
Clinical findings
Echocardiography was performed using a commercially available ultrasound equipment. Chamber size and wall thickness were measured in the transthoracic view. Left ventricular ejection fraction (LVEF) was calculated using a modified Simpson's method. Early and late atrial transmitral peak flow velocities were measured from mitral inflow velocities in the apical view. The peak early diastolic velocity (e′) was measured on the septal corner of mitral annulus, and the E/e′ ratio was calculated.
In addition, 12‐lead electrocardiograms (ECGs) were reviewed by two cardiologists (T. Y. and S. T.) blinded to the clinical data. The presence of a complete left or right bundle branch block was defined according to standard published criteria. Low voltage was defined as QRS amplitude < 0.5 mV in all limb leads or <1 mV in all precordial leads. The QS pattern was defined as the absence of R waves in leads V1–V3. 10 , 11
99mTc‐PYP scintigraphy was performed using a GE Discovery 670 dual‐headed single photon emission computed tomography/computed tomography camera with low‐energy, high‐resolution collimators (GE Healthcare, Waukesha, WI, USA). Anterior and lateral planar views of the heart were obtained 3 h following the administration of the radiotracer. 99mTc‐PYP scintigraphy was scored by board‐certified cardiovascular radiologists at the institution using the following grading system: grade 0, no cardiac uptake; grade 1, mild uptake less than bone; grade 2, moderate uptake equal to bone; and grade 3, high uptake greater than bone. 12 99mTc‐PYP positivity was defined as a visual score of 2 or 3.
Clinical follow‐up and prognosis
Mortality and hospitalization for heart failure were identified by a search of the medical records and were confirmed by a questionnaire and direct contact via a telephone interview of the patient, or if deceased, of a family member. All deaths were reviewed, and the mode of death was classified as cardiac or noncardiac death. Cardiac death was defined as a death due to worsening heart failure, cardiovascular event, or sudden death. On the contrary, noncardiac death was defined as a death that could be attributed to a noncardiac reason. These included subcategories such as renal, respiratory, cancer, trauma, and infection/sepsis. Hospitalization for heart failure was defined as unexpected hospitalization for heart failure.
Statistical analysis
The deadline date for data collection was December 2019. Normally distributed parameters were expressed as mean ± standard deviation (SD), whereas non‐normally distributed parameters were expressed as median with inter‐quartile range (IQR). Categorical values were presented as numbers (percentages). Univariate Cox hazard analyses were performed to identify parameters significantly related to survival from age, demographic or clinical characteristic, biomarker, or echocardiogram findings. On the contrary, a multivariate Cox hazard analysis was performed by the forced entry method. Survival and event‐free periods without heart failure were evaluated by Kaplan–Meier curves. B‐type natriuretic peptide (BNP) and high‐sensitivity cardiac troponin T (hs‐cTnT) were logarithmically transformed because they were not normally distributed. A two‐tailed value of P < 0.05 was considered statistically significant. Notably, all statistical analyses were performed using SPSS, version 25 (SPSS Inc., Chicago, IL, US).
Results
Clinical characteristics of study patients
Demographic characteristics for the study population are presented in Table 1. Mean age at diagnosis and onset of manifestation were 78.5 ± 6.4 and 74.3 ± 6.4 years, respectively. One hundred ten patients (85%) were male. In total, 60 patients (47%) experienced atrial fibrillation. Of 106 patients whose medical history of CTS were verified, 57 patients (54%) had a positive medical history of CTS. The majority of the study population was symptomatic (New York Heart Association functional class > II, 88%). In laboratory tests, the median hs‐cTnT level was 0.056 ng/mL (IQR, 0.040–0.089 ng/mL), and the BNP level was 288 pg/mL (181–464 pg/mL) at diagnosis. In echocardiography, mean intraventricular septal thickness and median LVEF were 15.7 ± 2.7 mm and 53.2% (44.5–59.7%), respectively. LVEF < 50% occurred in 53 patients (41%). ECG findings, including both low voltage and bundle branch block, were observed in 43 patients (36%). Regarding drug treatment, diuretics were used in 90 patients (70%) and were the most common class of therapeutic agent. This hospital participated in the transthyretin amyloid cardiomyopathy clinical trial for tafamidis 13 and enrolled seven patients. In this trial, there were two patients in the tafamidis 80 mg and tafamidis 20 mg groups and three patients in the placebo group.
TABLE 1.
Baseline clinical characteristics and clinical tests results of ATTRwt‐CM patients in this study
| Variables | All patients (n = 129) |
|---|---|
| Age at diagnosis, years | 78.5 ± 6.4 |
| Age at onset of symptom | 74.3 ± 6.4 |
| Male, n (%) | 110 (85%) |
| Past medical history | |
| Hypertension, n (%) | 69 (53%) |
| Diabetes mellitus, n (%) | 29 (22%) |
| Dyslipidaemia, n (%) | 43 (33%) |
| Atrial fibrillation, n (%) | 60 (47%) |
| Carpal tunnel syndrome, n (%) | 57 (n = 106, 54%) |
| Prior HF hospitalizations, n (%) | 53 (41%) |
| NYHA, n (%) | (n = 126) |
| I | 15 (12%) |
| II | 61 (48%) |
| III | 48 (38%) |
| IV | 2 (2%) |
| Blood testing | |
| hs‐cTnT, ng/mL | 0.056 (n = 123, 0.040–0.089) |
| BNP, pg/mL | 288 (181–464) |
| Sodium, mEq/L | 139.9 ± 3.0 |
| Potassium, mEq/L | 4.31 ± 0.42 |
| Creatinine, mg/dL | 1.09 (0.89–1.29) |
| eGFR, mL/min/1.73 m2 | 50.3 ± 14.9 |
| Haemoglobin, mg/dL | 13.4 (11.9–14.2) |
| Echocardiogram parameter | |
| LVDd mm | 42.4 ± 6.7 |
| LVDs, mm | 31.6 ± 6.7 |
| IVSd, mm | 15.7 ± 2.7 |
| LVPWd, mm | 15.7 ± 2.9 |
| LVEF, % | 53.2 (44.5–59.7) |
| LVEF < 50% | 53 (41%) |
| E/A ratio | 1.45 (n = 70, 0.76–2.50) |
| E/e′ ratio | 20.6 (16.1–25.7) |
| Electrocardiographic parameter | |
| Pacing rhythm, n (%) | 9 (7%) |
| V1–3 QS pattern, n (%) | 38 (n = 120, 32%) |
| Low voltage, n (%) | 43 (n = 120, 36%) |
| Bundle branch block, n (%) | 43 (n = 120, 36%) |
| CLBBB, n (%) | 14 (n = 120, 12%) |
| CRBBB, n (%) | 29 (n = 120, 24%) |
| Treatment | |
| RAS‐I, n (%) | 65 (50%) |
| MRA, n (%) | 42 (33%) |
| Beta‐blocker, n (%) | 38 (29%) |
| Diuretics, n (%) | 90 (70%) |
| PMI, n (%) | 15 (12%) |
| ICD, n (%) | 7 (5%) |
| CRT‐D/P, n (%) | 5 (4%) |
ATTRwt‐CM, wild‐type transthyretin amyloid cardiomyopathy; BNP, B‐type natriuretic peptide; CLBBB, complete left bundle branch block; CRBBB, complete right bundle branch block; CRT, cardiac resynchronization therapy; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; hs‐cTnT, high‐sensitivity cardiac troponin T; ICD, implantable cardioverter defibrillator; IVSd, interventricular septum diameter; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVPWd, left ventricular posterior wall diameter; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PMI, pacemaker implantation; RAS‐I, renin–angiotensin system inhibitor.
Data are expressed as median [inter‐quartile range], mean ± standard deviation, or n (%).
Clinical manifestations leading the diagnosis are shown in Figure 1 . Heart failure was the most common clinical manifestation (n = 79, 61%), followed by left ventricular hypertrophy without heart failure (n = 14, 11%). Eight patients (6%) were diagnosed with ATTRwt‐CM during evaluation of aortic stenosis.
FIGURE 1.

Fraction of manifestations leading to the diagnosis of wild‐type transthyretin amyloid (ATTR) cardiomyopathy.
Diagnostic approach
The diagnostic approach is illustrated in Figure 2 . Sixty‐six cases (51%) were diagnosed with ATTRwt‐CM on the basis of the presence of TTR deposition as revealed by endomyocardial biopsy (Group 1). Twenty‐eight cases (22%) were pathologically diagnosed based on the extracardiac tissue (Group 2), in which TTR deposition was observed in the subcutaneous tissue (n = 11), gastrointestinal tract (n = 20), lip (n = 1), or urinary bladder (n = 1). The remaining 35 cases (27%) were diagnosed according to positive findings in 99mTc‐PYP scintigraphy without pathological confirmation of TTR deposition (Group 3). Average age at diagnosis was significantly higher among the cases in Group 3 than the other two groups (83.4 vs. 76.8 years old; P < 0.001).
FIGURE 2.

Annual number of wild‐type transthyretin amyloid cardiomyopathy (ATTRwt‐CM) diagnosis and method of diagnosis. The pie chart illustrates fraction of method of diagnosis. Group 1, presence of transthyretin (TTR) deposition in the myocardium; Group 2, presence of TTR deposition in extracardiac tissue with positive finding of 99mTc‐labelled pyrophosphate scintigraphy; Group 3, positive finding of 99mTc‐labelled pyrophosphate scintigraphy without confirmation of pathological TTR deposition.
Genetic testing was performed in 96 cases (74%) to confirm the absence of mutation in the TTR genes, all of which were negative. 99mTc‐PYP scintigraphy was performed in 125 cases (97%), with a total of 124 patients (99%) showing positive findings; however, only one case with negative finding of 99mTc‐PYP scintigraphy was observed despite amyloid deposition in the myocardium. The number of cases of ATTRwt‐CM diagnosis had been gradually increasing every year, especially since 2017, at our institute.
Initial manifestation of transthyretin amyloid
The initial manifestations of ATTR are shown in Figure 3 . The most common initial manifestation was heart failure (n = 64, 49%), followed by peripheral neuropathy (n = 40, 31%). Of the peripheral neuropathy cases, 39 cases were CTS. The median period from initial symptom onset to diagnosis was 15.5 (range, 2–75) months. For cases in which the initial manifestation was CTS, the median period from initial symptom onset to diagnosis was 96 (range, 48–120) months.
FIGURE 3.

Fraction of initial manifestation related to transthyretin amyloid (ATTR) before diagnosis.
Clinical outcomes
The median follow‐up period was 15.0 (range, 5.4–33.2) months, and 34 patients (26%) died during the observation period. Of these, 27 patients (79%) were cardiovascular deaths (heart failure, 25; sudden death, two). Noncardiac deaths occurred in seven patients (pneumonia, four; sepsis, two; and lung cancer, one). Thirty‐eight patients (29%) were hospitalized for heart failure, and five patients received cardiovascular implantable electronic device including pacemaker (n = 3), implantable cardioverter defibrillator (n = 1), and cardiac resynchronization therapy with a defibrillator (n = 1).
Kaplan–Meier analyses illustrate the period of overall survival (Figure 4A ) and period without hospitalization for heart failure (Figure 4B ). The median overall survival period was 58.9 months, with overall 3 and 5 years' survival rates of 68.7% and 48.0%, respectively. According to the multivariate Cox hazard analysis, age [hazard ratio (HR), 1.14; 95% confidence interval (CI), 1.05–1.23; P = 0.002] and low serum sodium level (HR, 0.89; 95% CI, 0.79–0.99; P = 0.04) contributed to all‐cause death (Table 2). Low sodium levels equally contributed to hospitalization for heart failure (HR, 0.86; 95% CI, 0.77–0.96; P = 0.005) (Table S1).
FIGURE 4.
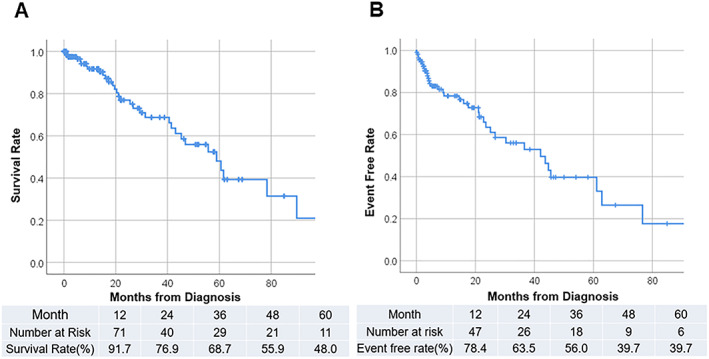
(A) Kaplan–Meier curve of all‐cause survival; table showing number at risk and survival rate by year. Median survival period was 58.9 months. (B) Kaplan–Meier curve of readmission for heart failure; table showing number at risk and event‐free ratio by year. Median event‐free period was 43.7 months.
TABLE 2.
Univariate and multivariate Cox hazard analyses of predictors for all‐cause mortality
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (years) | 1.12 | 1.05–1.19 | 0.001 | 1.14 | 1.05–1.23 | 0.002 |
| Female (yes) | 1.78 | 0.52–6.05 | 0.36 | |||
| NYHA | 2.26 | 1.32–3.86 | 0.003 | 0.99 | 0.47–2.06 | 0.97 |
| Diabetes mellitus (yes) | 1.09 | 0.44–2.69 | 0.85 | |||
| Atrial fibrillation (yes) | 1.10 | 0.55–2.21 | 0.78 | |||
| Prior HF hospitalizations | 2.62 | 1.28–5.36 | 0.008 | 1.29 | 0.49–3.37 | 0.60 |
| Ln hs‐cTnT | 3.14 | 1.68–5.89 | <0.001 | 1.62 | 0.58–4.56 | 0.36 |
| Ln BNP | 2.59 | 1.50–4.46 | 0.001 | 1.95 | 0.93–4.09 | 0.077 |
| Sodium (mEq/L) | 0.83 | 0.75–0.92 | <0.001 | 0.89 | 0.79–0.996 | 0.04 |
| Potassium (mEq/L) | 0.65 | 0.28–1.51 | 0.32 | |||
| eGFR (mL/min/1.73 m2) | 0.94 | 0.92–0.97 | <0.001 | 0.99 | 0.95–1.03 | 0.68 |
| Haemoglobin (mg/dL) | 0.77 | 0.63–0.93 | 0.007 | 0.96 | 0.75–1.22 | 0.96 |
| LVDd (mm) | 0.97 | 0.93–1.02 | 0.27 | |||
| LVDs (mm) | 1.00 | 0.96–1.05 | 1.00 | |||
| IVSd (mm) | 1.00 | 0.90–1.12 | 0.96 | |||
| LVPWd (mm) | 0.95 | 0.84–1.07 | 0.36 | |||
| LVEF (%) | 0.98 | 0.95–1.02 | 0.27 | |||
| e′ | 0.82 | 0.59–1.13 | 0.22 | |||
| E/e′ ratio | 1.05 | 1.00–1.11 | 0.049 | 0.99 | 0.92–1.07 | 0.79 |
| Low voltage | 1.76 | 0.83–3.70 | 0.14 | |||
| V1–3 QS pattern (yes) | 1.00 | 0.48–2.09 | 1.00 | |||
| Pacing rhythm (yes) | 2.29 | 0.69–7.64 | 0.18 | |||
CI, confidence interval; HR, hazard ratio; Ln, log natural.
For other abbreviations, see Table 1 . Bold emphasis means significant difference in multivariate analysis.
In the identification of patients who benefitted from tafamidis, the prognostic factors associated with 18 month mortality were additionally evaluated in the study patients. According to multivariate logistic regression analysis, age (HR, 1.31; 95% CI, 1.08–1.59; P = 0.01) and reduced LVEF (HR, 0.90; 95% CI, 0.82–0.98; P = 0.02) were significant poor prognostic factors (Table S2). The patients were classified into three groups depending on age at diagnosis, as follows: <75 years (n = 34, low group), 75–80 years (n = 40, mid group), and >80 years (n = 55, high group). Kaplan–Meier analysis showed that the 18 month survival rates of each group were 95.8%, 87.4%, and 76.0% in the low, mid, and high groups, respectively (Figure 5 , log‐rank P = 0.06).
FIGURE 5.

Kaplan–Meier curve representing association between ages and all survival rate. Patients were classified into three groups based on age at diagnosis (low group, age < 70 years; mid group, age 75–80 years; high group, age > 80 years).
Discussion
This study revealed the characteristics, diagnostic approach, and prognosis for patients with ATTRwt‐CM in Japan. The major findings of the present study were as follows: (i) the diagnosis of ATTRwt‐CM dramatically increased in recent years, (ii) about half of the study patients had a medical history of CTS, (iii) the 5 year survival rate of patients with ATTRwt‐CM was 48.0%, and (iv) age and low serum sodium level were significant predictive factors of all survival outcome. To our knowledge, this is the first report to evaluate the patient's characteristics and the prognosis of patients with ATTRwt‐CM in the largest number of cases in Japan.
There were several reports about patient's characteristic and prognosis of ATTRwt‐CM mainly from Europe 7 , 8 and the USA. 6 In Japan, Sekijima et al. reported a nationwide survey on ATTRwt amyloidosis (n = 51). 14 Compared with these reports, several clinical features were consistent with the present study (Table 3). The most common age group was late 70s, with male predominance (≥80%) and symptomatic heart failure at diagnosis. Previous studies showed that the major manifestation leading to diagnosis was heart failure (54–86%), 6 , 7 , 8 , 14 which was confirmed in 61% of the study participants. Based on echocardiographic findings, 41% of study participants demonstrated LVEF < 50%, which was consistent with a previous report (37%). 8 It is generally recognized that ATTRwt‐CM is mainly observed in HFpEF patients; however, it should be recognized that ATTRwt‐CM was not limited to patients with HFpEF. Low voltage and bundle branch block were observed in 36% of the cases, which were similar to those of a previous study (low voltage, 13–33%; bundle branch block, 32–36%). 6 , 7 , 8 , 14
TABLE 3.
Clinical characteristics, medical history, and prognosis in the present study compared with previous studies in Western countries and in Japan
| Country sample size |
Present study, Japan n = 129 |
Sekijima Y., Japan 14 n = 51 |
Connors L. H., USA 6 n = 121 |
Gonzalez‐Lopez E., Spain/Italy 8 n = 108 |
|---|---|---|---|---|
| Age at diagnosis (years) | 78.5 ± 6.4 | 73.6 ± 9.2 | 75.1 (59.0–87.5) | 78.6 ± 8 |
| Male (%) | 85% | 80% | 98% | 81% |
| Past AF (%) | 47% | 18% | 67% | 56% |
| Past CTS (%) | 54% | 45% | 46% | 33% |
| NYHA III or IV (%) | 40% | — | 85% (II–IV) | 32% |
| Major manifestationleading diagnosis (%) | HF (61%) | HF (69%) | HF (86%) | HF (68%) |
| Cardiac troponin (ng/mL) | 0.056(0.040–0.089) a | 0.076 ± 0.042 a | 0.126(0.020–1.198) b | — |
| BNP (pg/mL) | 288 (181–464) | — | 482 ± 337 | — |
| NT‐proBNP (pg/mL) | — | 5872 ± 7584 | — | 2997 (1592–9621) |
| LVEF (%) | 53.2 (44.5–59.7) | 49.7 ± 13.8 | 48.1 ± 10.5 | 52 ± 14 |
| LVEF < 50% | 41% | 59% (EF < 55) | — | 37% |
| IVSTd | 15.7 ± 2.7 | 16.4 ± 4.1 | 16.3 ± 3.0 | 17.5 ± 3 |
| E/e′ | 20.6 (16.1–25.7) | 23.6 ± 11.5 | 20.5 ± 8.6 | — |
| Low voltage in ECG (%) | 36% | 33% | 33% | 22% |
| Median survival (month) | 58.9 | — | 46.7 | — |
| 3/5 years' survival rate | 69%/48% | — | —/36% | 74%/— |
Data are presented as median [inter‐quartile range], mean ± standard deviation, or n (%). For other abbreviations, see Table 1 .
ECG, electrocardiogram.
Measured by high‐sensitivity cardiac troponin T.
Measured by high‐sensitivity cardiac troponin I.
CTS is known as an early symptom in ATTRwt preceding cardiac involvement, and a history of bilateral CTS is a ‘red flag’ in ATTRwt‐CM diagnosis. 15 The incidence of CTS was reported to range between 33% and 48% in patients with ATTR‐CM. 6 , 7 , 8 , 14 If possible, the history of CTS was re‐examined to prevent any case being overlooked; consequently, the frequency was slightly higher in this study than in previous reports. Although CTS has been reported to be more common among women, 16 , 17 , 18 this study observed no sex differences in co‐morbidities between male (49%) and female (47%) patients. Nakagawa et al. reported that the CTS preceded cardiac symptoms by a mean of 6.1 years 19 ; this result was consistent with our finding (median 8 years). It has been reported that patients subjected to surgical treatment for CTS were associated with a higher risk of amyloidosis and future heart failure relative to matched control subjects. 20 Therefore, patients who undergo surgical treatment for CTS are to be considered a high‐risk group for ATTRwt‐CM. The presence of TTR amyloid deposition in flexor tenosynovium specimens was identified in 33% of the patients and the occurrence of amyloid deposits increased with age in patients with CTS. 21 It would be useful for an early diagnosis of cardiac amyloidosis to evaluate serial cardiac symptoms and structural future in CTS patients with amyloid deposition in the tenosynovial tissue.
In this study, eight patients were diagnosed with ATTRwt‐CM in the process of examination for aorticstenosis. Aortic stenosis is common among the elderly and causes left ventricular hypertrophy; therefore, aortic stenosis with ATTR‐CM is occasionally underdiagnosed. There is a report that ATTR‐CM was prevalent in 16% of patients with severe aortic stenosis who underwent transcatheter aortic valve implantation, 22 and the prognosis of aortic stenosis patients with ATTR‐CM is poor. 23 The clinical utility of the identification of occult ATTR‐CM of myocardial extracellular volume quantification using pre‐procedural contrast‐enhanced computed tomography for transcatheter aortic valve implantation has been previously reported. 24 This knowledge facilitates easier identification of aortic valve stenosis patients suspected of ATTR‐CM and to perform 99mTc‐PYP scintigraphy in our institution.
The median survival period of ATTRwt‐CM has been reported to range between 3.9 and 6.1 years, 6 , 7 , 8 which was equivalent to our study (4.9 years). In previous reports, several factors contributing to mortality were identified (serum uric acid, BNP, LVEF, and relative wall thickness) 6 ; in addition, the staging system with combinations NT‐proBNP and serum hs‐cTnT levels or estimated glomerular filtration rate has been proposed for predicting the mortality of ATTR‐CM patients. 25 , 26 Age and serum sodium level have been suggested as factors contributing to the mortality in this study. In addition, low sodium levels have been reported to be a classic poor prognostic factor in heart failure. 27 , 28
Recently, a TTR stabilizer (tafamidis) has been used clinically. It was reported that tafamidis reduced all‐cause mortality, cardiovascular‐related hospitalizations, declining functional capacity, and quality of life than did placebo in patients with ATTR‐CM. 12 This study reported that the effect of tafamidis on overall survival emerged after approximately 18 months of treatment; this implies that survival of 18 months or more is required to benefit from tafamidis. Thus, it is important to predict survival after 18 months of treatment. According to our data, elderly patients (>80 years old) with reduced ejection fraction obtained less effective results than patients (≤80 years old) with preserved ejection fraction.
In the advent of disease‐specific treatment to prevent disease progression, early diagnosis and treatment could become even more important than ever. In recent years, the number of ATTRwt‐CM diagnosed has increased in our institution. First, it can be attributed to more awareness of physicians regarding this disease. This study similarly included a quarter of patients diagnosed by non‐invasive diagnostic criteria. This may have contributed to an increased number of diagnoses, especially in these several years; however, histologically diagnosed groups equally increased during this period. Previously, we reported that hs‐cTnT could be a clue in early diagnosis 29 and suggested the application of the Kumamoto criteria, 30 a minimally invasive approach for predicting the probability of 99mTc‐PYP positivity with a combination of electrophysiological (QRS width), structural (left ventricular posterior wall thickness), and biochemistry (hs‐cTnT level) parameters. These practices equally contributed to increasing the number of diagnoses in our institution.
Limitations
This study was a retrospective single‐centre study and comprised a smaller number of cases compared to previous cohort studies in Western countries. Nonetheless, this research remains to date the only large‐scale study on ATTRwt‐CM in Asia. There were some incomplete data including history CTS and level of serum hs‐cTnT. Further, not all patients underwent genetic testing; therefore, the sample may have included sporadically aged ATTRv patients.
Conflict of Interest
None declared.
Funding
This work was supported by Grants‐in‐Aid for Young Scientists B from the Japan Society for the Promotion of Science (S.T., 26860574).
Supporting information
Table S1. Univariate and multivariate Cox hazard analyses of predictors for hospitalization for heart failure.
Table S2. Univariate and multivariate Cox hazard analyses of predictors for 1.5 years all‐cause mortality.
Acknowledgement
We would like to thank Editage (www.editage.com) for the English‐language editing.
Yamada, T. , Takashio, S. , Arima, Y. , Nishi, M. , Morioka, M. , Hirakawa, K. , Hanatani, S. , Fujisue, K. , Yamanaga, K. , Kanazawa, H. , Sueta, D. , Araki, S. , Usuku, H. , Nakamura, T. , Suzuki, S. , Yamamoto, E. , Ueda, M. , Kaikita, K. , and Tsujita, K. (2020) Clinical characteristics and natural history of wild‐type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Failure, 7: 2829–2837. 10.1002/ehf2.12884.
References
- 1. Izumiya Y, Takashio S, Oda S, Yamashita Y, Tsujita K. Recent advances in diagnosis and treatment of cardiac amyloidosis. J Cardiol 2018; 71: 135–143. [DOI] [PubMed] [Google Scholar]
- 2. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019; 73: 2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014; 2: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzalez‐Lopez E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐Del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, Garcia‐Pavia P. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015; 36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- 5. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015; 17: 884–892. [DOI] [PubMed] [Google Scholar]
- 6. Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, Berk JL, Seldin DC. Heart failure resulting from age‐related cardiac amyloid disease associated with wild‐type transthyretin: a prospective. Observ Cohort Stud Circ 2016; 133: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, Wechalekar A, Gibbs SD, Venner CP, Wassef N, McCarthy CA, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD, Lachmann HJ. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2013; 2: e000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez‐Lopez E, Gagliardi C, Dominguez F, Quarta CC, de Haro‐Del Moral FJ, Milandri A, Salas C, Cinelli M, Cobo‐Marcos M, Lorenzini M, Lara‐Pezzi E, Foffi S, Alonso‐Pulpon L, Rapezzi C, Garcia‐Pavia P. Clinical characteristics of wild‐type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J 2017; 38: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 9. Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Plante‐Bordeneuve V, Coelho T, Mundayat R, Suhr OB, Waddington Cruz M, Rapezzi C, Investigators T. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 2016; 68: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Willems JL, de Medina EO, Bernard R, Coumel P, Fisch C, Krikler D, Mazur NA, Meijler FL, Mogensen L, Moret P, Pisa Z. Criteria for intraventricular conduction disturbances and pre‐excitation. J Am Coll Cardiol 1985; 5: 1261–1275. [DOI] [PubMed] [Google Scholar]
- 11. Di Bella G, Minutoli F, Piaggi P, Casale M, Mazzeo A, Zito C, Oreto G, Baldari S, Vita G, Pingitore A, Khandheria BK, Carerj S. Usefulness of combining electrocardiographic and echocardiographic findings and brain natriuretic peptide in early detection of cardiac amyloidosis in subjects with transthyretin gene mutation. Am J Cardiol 2015; 116: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 12. Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi‐Reggiani L, Fallani F, Branzi A, Rapezzi C. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005; 46: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 13. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, ATTR‐ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 14. Sekijima Y, Yazaki M, Ueda M, Koike H, Yamada M, Ando Y. First nationwide survey on systemic wild‐type ATTR amyloidosis in Japan. Amyloid 2018; 25: 8–10. [DOI] [PubMed] [Google Scholar]
- 15. Witteles RM, Bokhari S, Damy T, Elliott PM, Falk RH, Fine NM, Gospodinova M, Obici L, Rapezzi C, Garcia‐Pavia P. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail 2019; 7: 709–716. [DOI] [PubMed] [Google Scholar]
- 16. Mondelli M, Giannini F, Giacchi M. Carpal tunnel syndrome incidence in a general population. Neurology 2002; 58: 289–294. [DOI] [PubMed] [Google Scholar]
- 17. Mattioli S, Baldasseroni A, Curti S, Cooke RM, Bena A, de Giacomi G, dell'Omo M, Fateh‐Moghadam P, Melani C, Biocca M, Buiatti E, Campo G, Zanardi F, Violante FS. Incidence rates of in‐hospital carpal tunnel syndrome in the general population and possible associations with marital status. BMC Public Health 2008; 8: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roquelaure Y, Chazelle E, Gautier L, Plaine J, Descatha A, Evanoff B, Bodin J, Fouquet N, Catherine B. Time trends in incidence and prevalence of carpal tunnel syndrome over eight years according to multiple data sources: Pays de la Loire study. Scand J Work Environ Health 2017; 43: 75–85. [DOI] [PubMed] [Google Scholar]
- 19. Nakagawa M, Sekijima Y, Yazaki M, Tojo K, Yoshinaga T, Doden T, Koyama J, Yanagisawa S, Ikeda S. Carpal tunnel syndrome: a common initial symptom of systemic wild‐type ATTR (ATTRwt) amyloidosis. Amyloid 2016; 23: 58–63. [DOI] [PubMed] [Google Scholar]
- 20. Fosbol EL, Rorth R, Leicht BP, Schou M, Maurer MS, Kristensen SL, Kober L, Gustafsson F. Association of carpal tunnel syndrome with amyloidosis, heart failure, and adverse cardiovascular outcomes. J Am Coll Cardiol 2019; 74: 15–23. [DOI] [PubMed] [Google Scholar]
- 21. Sueyoshi T, Ueda M, Jono H, Irie H, Sei A, Ide J, Ando Y, Mizuta H. Wild‐type transthyretin‐derived amyloidosis in various ligaments and tendons. Hum Pathol 2011; 42: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 22. Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, George I, Kodali S, Leon MB, Hahn R, Bokhari S, Maurer MS. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017; 38: 2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Treibel TA, Fontana M, Gilbertson JA, Castelletti S, White SK, Scully PR, Roberts N, Hutt DF, Rowczenio DM, Whelan CJ, Ashworth MA, Gillmore JD, Hawkins PN, Moon JC. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging 2016; 9. [DOI] [PubMed] [Google Scholar]
- 24. Oda S, Takashio S, Nagamatsu S, Yamashita T, Uchimura R, Kidoh M, Utsunomiya D, Nakaura T, Tsujita K, Yamashita Y. Myocardial extracellular volume quantification using CT for the identification of occult cardiac amyloidosis in patients with severe aortic stenosis referred for transcatheter aortic valve replacement. Amyloid 2019; 26: 97–98. [DOI] [PubMed] [Google Scholar]
- 25. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild‐type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016; 68: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 26. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018; 39: 2799–2806. [DOI] [PubMed] [Google Scholar]
- 27. Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC, OPTIMIZE‐HF Investigators and Coordinators . Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry. Eur Heart J 2007; 28: 980–988. [DOI] [PubMed] [Google Scholar]
- 28. Kusaka H, Sugiyama S, Yamamoto E, Akiyama E, Matsuzawa Y, Hirata Y, Fujisue K, Kurokawa H, Matsubara J, Sugamura K, Maeda H, Jinnouchi H, Matsui K, Ogawa H. Low‐normal serum sodium and heart failure‐related events in patients with heart failure with preserved left ventricular ejection fraction. Circ J 2016; 80: 411–417. [DOI] [PubMed] [Google Scholar]
- 29. Takashio S, Yamamuro M, Izumiya Y, Hirakawa K, Marume K, Yamamoto M, Ueda M, Yamashita T, Ishibashi‐Ueda H, Yasuda S, Ogawa H, Ando Y, Anzai T, Tsujita K. Diagnostic utility of cardiac troponin T level in patients with cardiac amyloidosis. ESC Heart Fail 2018; 5: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marume K, Takashio S, Nishi M, Hirakawa K, Yamamoto M, Hanatani S, Oda S, Utsunomiya D, Shiraishi S, Ueda M, Yamashita T, Sakamoto K, Yamamoto E, Kaikita K, Izumiya Y, Yamashita Y, Ando Y, Tsujita K. Combination of commonly examined parameters is a useful predictor of positive 99mTc‐labeled pyrophosphate scintigraphy findings in elderly patients with suspected transthyretin cardiac amyloidosis. Circ J 2019; 83: 1698–1708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate and multivariate Cox hazard analyses of predictors for hospitalization for heart failure.
Table S2. Univariate and multivariate Cox hazard analyses of predictors for 1.5 years all‐cause mortality.


