ABSTRACT
Advances in the understanding of the pathogenesis of type 2 diabetes mellitus (T2D) have revealed a role for gut microbiota dysbiosis in driving this disease. This suggests the possibility that approaches to restore a healthy host–microbiota relationship might be a means of ameliorating T2D. Indeed, recent studies indicate that many currently used treatments for T2D are reported to impact gut microbiota composition. Such changes in gut microbiota may mediate and/or reflect the efficacy of these interventions. This article outlines the rationale for considering the microbiota as a central determent of development of T2D and, moreover, reviews evidence that impacting microbiota might be germane to amelioration of T2D, both in terms of understanding mechanisms that mediate efficacy of exiting T2D therapies and in developing novel treatments for this disorder.
KEYWORDS: Diabetes, inflammation, microbiota metformin, dietary fiber
Introduction: rationale for considering gut microbiota in diabetes
Type 2 diabetes (T2D) is characterized by loss of glycemic control resulting in hyperglycemia, especially post-prandially, due to hyporesponsiveness to insulin, i.e. insulin resistance. The notion that gut microbiota might play a role in this disorder stems largely from the general appreciation, originating from work of Jeff Gordon and colleagues, that gut microbiota contribute broadly to energy balance1,2 and the realization of Patrice Cani and colleagues that microbiota products such as LPS (lipopolysaccharide) can drive low-grade inflammation, 3 which had long been recognized as a potential cause of insulin resistance. Regarding the former, briefly, mice completely lacking microbiota (i.e. germ-free mice) exhibit reduced energy harvest from ingested food and increased energy expenditure, associated with increased activation of AMP-activated protein kinase (AMPK), which plays a central role in energy homeostasis. Such activation of AMPK has been suggested to protect germ-free mice from diet-induced diabetes.4 While germ-free mice can be considered an extreme state, interpolating based on their phenotype suggests that differences in microbiota composition can, more subtly but nonetheless broadly, influence metabolic phenotype and thereby be a determinant of diabetes and its inter-related metabolic diseases states, namely obesity and metabolic syndrome.5-7 In accord with this notion, obesity in mice and humans is associated with alterations in microbiota composition, and transfer of microbiota from obese hosts to germ-free mice leads to increased adiposity, relative to germ-free mice receiving microbiotas from lean hosts.2,8,9
The hypothesis that low-grade inflammation drives the insulin resistance that characterizes T2D originated from work of Hotamisligil and colleagues, who demonstrated that increases in adipose tissue characteristic of obesity is typically accompanied by increased expression of pro-inflammatory cytokines, which are produced by adipocytes themselves and macrophages that are recruited into adipose tissue as obesity develops.10,11 While Hotamisligil hypothesized that such pro-inflammatory gene expression resulted from intracellular stress of adipocytes being overloaded with lipids, Cani found that such inflammation and subsequently insulin resistance could result from translocation of lipopolysaccharide from the gut lumen into portal circulation resulting in activation of pro-inflammatory gene expression via toll-like receptor 4.3 This scenario suggests a variety of means by which alterations in microbiota composition could impact T2D, including altering abundance of species that produce LPS and/or other microbial products with strong pro-inflammatory potential. It also underscores a key role for epithelial barrier function in restricting microbial products to the gut lumen. In this context, gut barrier function includes not only intercellular junctions that directly impede passage of bacterial products but also host systems of mucus deployment and innate immunity that keep bacteria, themselves, at a safe distance from the epithelium and help maintain stable microbiota composition. These latter points are shown by our study of mice that with a discrete defect in innate immunity, namely absence of the flagellin receptor toll-like receptor 5 (TLR5). TLR5-deficient mice fail to manage their microbiota, resulting in altered composition, including elevated γ-Proteobacteria and, moreover, exhibit microbiota encroachment, which is defined as a decrease in bacterial-epithelial distance.12,13 Such alterations result in TLR5-deficient mice developing insulin resistance, which can be transferred to WT (wild type) germ-free mice via microbiota transplant. The general notion yielded by these studies, namely that altering microbiota can impact metabolic phenotype, provides a rational basis for targeting microbiota in order to treat and prevent T2D.
Alteration of gut microbiota composition in humans with T2D
The general rationale of impacting microbiota to treat T2D is supported by the notion that microbiota composition is altered in this disease state (Figure 1). Indeed, although much of our understanding of mechanisms whereby microbiota can impact glucose homeostasis comes from mouse studies, alterations in microbiota composition have also been observed in a range of human cohorts T2D.14-16 Larsen et al.15 observed differences at the phyla level, namely that ratios of Bacteroidetes:Firmicutes ratio and Bacteroides-Prevotella group to Clostridium coccoides–Eubacterium rectale group positively correlated with plasma glucose concentration. Other differences observed included decreased abundance of butyrate-producing bacteria, including Clostridiales spp. Eubacterium rectale, Faecalibacterium prausnitzii, Roseburia intestinalis and R. inulinivorans, and increased abundance of Lactobacillus species. Increased prevalence of Bacteroidetes and Proteobacteria phyla was also observed. Proteobacteria contain many pathobionts, which can be envisaged to have a role in inducing low-grade inflammation in diabetic patients through their LPS, flagella, and/or other surface components.15 Also fitting with the notion that altered microbiotas associated with T2D might promote LGI (low-grade inflammation) are findings in a cohort of Chinese T2D patients and healthy control subjects that observed increased abundance of opportunistic pathogens, including Bacteroides caccae, C. hathewayi, C. ramosum, C. symbiosum, Eggerthella lenta and Escherichia coli in patients with T2D.14 In a longitudinal cohort on monozygotic Korean twins, it is suggested that decreased Akkermansia muciniphila could be used as a biomarker for the early diagnosis of T2D.17 Moreover, Acidaminococcus, Aggregatibacter, Anaerostipes, Blautia, Desulfovibrio, Dorea, and Faecalibacterium have been identified as being associated with T2D in a Mendelian randomization study suggesting that these genera should be investigated more in future research.18 Collectively, these studies support the general notion that microbiota dysbiosis is a feature of T2D but yet the variety of specific alterations observed argues against a specific signature or alterations in this disorder. While, as mentioned above, one general theme of such differences is that many of the changes can be envisaged to reflect and/or promote inflammation, data are less clear regarding one of the more widely observed microbiome signature of inflammation, namely α-diversity, or species richness. Specifically, multiple studies have observed modest but yet not statistically significant reductions in this parameter.16,19 We speculate that such modest reduction in α-diversity may reflect that the level of inflammation in diabetes is modest. While the wide variety of specific differences can be envisioned to have a variety of functional consequences, we have observed that patients with TD2 exhibit microbiota encroachment.20 Thus, these human observational studies are in accord with the concept that targeting microbiota is a logical target to treat insulin resistance and, consequently T2D. Overall, these studies support the notion that changes in microbiota composition are a feature of T2D but beyond suggesting a role for LGI, the mechanisms underlying such differences and a true T2D microbiome signature in humans remains elusive.
Figure 1.
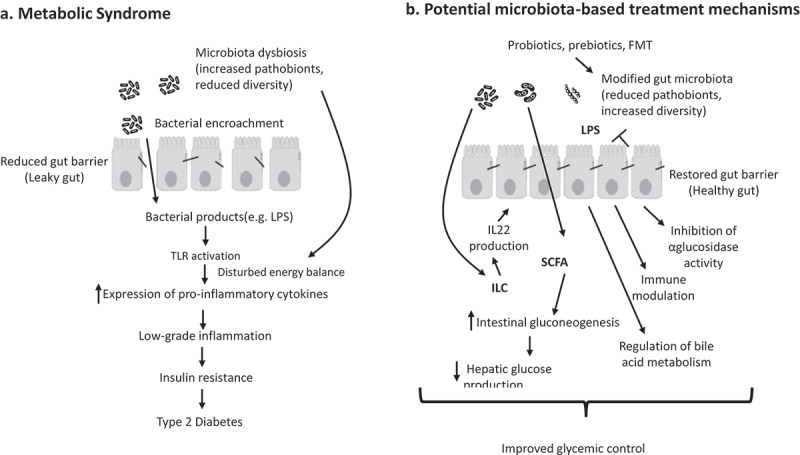
Overview of how a dysbiotic gut microbiota can promote type 2 diabetes (a) and how microbiota-based therapies might treat and/or prevent this disorder (b).
Interventions that deliberately target microbiota in T2D
Studies, some of which are outlined above, indicate that, caveats and unknowns notwithstanding, dysregulated or improperly managed microbiota may promote insulin resistance, leading to the suggestion that broadly suppressing levels of microbiota might be a means of ameliorating this disorder. While relatively short-term studies in mice support this concept, it is unlikely to prove a therapeutic option to manage this chronic disease in humans due to the general negative impacts of antibiotics on gut health, especially relating to risk of serious infection by antibiotic resistant bacteria. Moreover, numerous studies have associated frequent use of antibiotics with T2D, thus further supporting the role of a stable microbiota in preventing T2D but arguing against the notion that broad-based ablation of microbiota can be a practical approach to treat T2D in humans. Rather, direct deliberate attempts to influence microbiota composition to promote insulin sensitivity and thus ameliorate T2D have utilized fecal microbiota transplant (FMT), probiotics or prebiotics. The latter will be discussed below under dietary fiber, since such studies were often initiated prior to appreciation of the role of microbiota. Here, we discuss FMT and use of specific probiotics.
The logic of FMT is relatively straightforward, namely to replace a dysbiotic microbiota with a healthy one, which will have the needed diversity to stably persist in its new host. General proof of this concept comes from studies wherein transplanted communities persist in their new hosts for extended periods and the highly effective use of FMT to prevent recurrence of Clostridium difficile infection.21,22 Use of FMT to ameliorate insulin resistance in humans is largely the work of Nieuwdorp and colleagues, who have performed well-controlled randomized clinical trials5 studying this approach. Such trials have shown that, following colonoscopy in which the preparation of the colon removes a considerable portion of the total microbial mass in the intestine, FMT from healthy subjects can improve insulin sensitivity relative to FMT with one’s own feces (i.e. placebo control).23,24 However, the beneficial impacts from fecal transplants are transient as is the engraftment of the donor microbiota. Moreover, a variety of poorly understand factors in recipient’s microbiota influence both engraftment and any beneficial metabolic impacts.25 The transient nature of these effects may reflect that, unlike germ-free mice, the microbiota of a host with an established microbiota is more difficult to permanently replace and/or that whatever causes had led to an unhealthy microbiota in the first place, for an example, an unhealthy diet, have not changed and will result in failure to maintain the engraft microbiota. In any case, collectively, results from FMT studies support the notion that changing microbiota can positively impact diabetes but underscores that doing so in a lasting manner is not yet be easily achieved.
Probiotics in diabetes
One potential approach to attaining a healthy microbiota is to directly administer beneficial bacteria, i.e. probiotics, which the International Scientific Association for Probiotics and Prebiotics defines as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”.26 One common general strategy to developing probiotics to benefit a particular condition is to administer bacteria from taxa whose reduced abundance is associated with disease. For example, in inflammatory bowel disease, probiotic approaches have generally sought to administer bacteria whose reduced abundance is associated with disease (i.e. replenish depleted taxa) such as F. prausnitzii, which is depleted in this disorder.27 While this strategy is used to some extent in metabolic syndrome, as discussed above, there is less consensus on what, if any, changes in specific taxa consistently associate with dysglycemia. Hence, strategies have generally focused on administering bacteria with anti-inflammatory properties and/or bacteria with seemingly beneficial metabolic properties such as propensity to produce short-chain fatty acids (SCFAs). Experimental studies and clinical trials support the hypothesis that the modulation of the intestinal microbiota in this manner might be effective in diabetes management,28 in which the most widely studied probiotics with respect to diabetes members of Bifidobacterium and Lactobacillus phyla. Specific strains of L. rhamnosus, L. acidophilus, L. gasseri, and L. casei have been demonstrated to exert anti-diabetic effects.29-33 Moreover, several strains of L. plantarum species have been reported to improve the glycemic control in obese and diabetic patients, likely via their carbohydrate-utilizing genes.34,35 Studies also indicate that administration of Bifidobacterium animalis, B. breve, and B. longum led to amelioration of glucose intolerance.28,36-38 While results of individual studies have been quite variable, a recent meta-analysis has shown that these probiotics improve glycemic control and significantly decrease the risk of gestational diabetes mellitus in pregnant women.39 The underlying mechanisms by which probiotics might have impacted host metabolism have not been well defined but may include favorable changes of the composition and/or activity of the microbiota, inhibition of α-glucosidase activity, production of anti-microbial lactic acid, improvement of intestinal barrier function, immune modulation, SCFA production, and regulation of bile acid metabolism.28,36,38,40 Other candidate probiotics include gut bacteria including A. muciniphila and F. prausnitzii, which are negatively associated with overweight and hyperglycemia, may be potential candidates for next-generation probiotics; further studies are needed in this field.41 A recent placebo-controlled trial supported this concept in that direct administration of A. muciniphilia improved glycemic control in persons with metabolic syndrome, although, unexpectedly, the impact of heat-killed Akkermansia appeared more significant than that of the live organism highlighting that further development is needed before deploying this strategy on a large scale.42
T2D pharmaceutical agents that impact microbiota
While deliberate targeting of the microbiota to ameliorate T2D is clearly in early stages of development, it is increasingly being appreciated that several drugs that have long been used to treat T2D result in impacts on gut microbiota in a manner that might contribute to their efficacy
Metformin
Metformin (dimethyldiguanide), discovered in 1922 based on study of the plant Galega officinalis (Goat’s Rue) to lower blood glucose, is a very common treatment for T2D, especially T2D associated with obesity. Metformin’s mechanism of action is unclear but may include inhibition of mitochondrial function via respiratory chain complex I or glycerophosphate dehydrogenase, activation of 5ʹ AMP-activated protein kinase (AMPK), and/or amelioration of glucagon-induced cAMP. Moreover, there is evidence to suggest a role for gut microbiota in mediating metformin’s ability to improve glycemic control. In contrast to oral metformin, intravenous administration of metformin lacks does not control hyperglycemia thus suggesting the intestine as an important site of metformin action.43 Furthermore, in both mice and humans, metformin alters microbiota composition to make it more resembling of microbiotas of healthy hosts.44-46 Some of these changes were observed amidst healthy persons who do not exhibit changes in glycemic control in response to this agent, thus suggesting the changes in the microbiota result from metformin itself rather than simply reflect improved glycemic control. Yet specific associations have been variable among different studies and different states of health in part reflecting the difficulty of dissociating impacts on microbiota due to drugs and/or disease. Overall, in healthy subjects, metformin impacted relative abundance of several phyla including a reduced abundance of Intestinibacter spp. and Clostridium spp., as well as an increased abundance of Escherichia/Shigella spp. and Bilophila wadsworthia.47 Some of these changes appear reminiscent of changes associated with disease and thus irrespective of potential impacts on glycemic control such change may contribute to gastrointestinal distress, which is the leading cause of metformin intolerance. Indeed, prevalent gastrointestinal side effects after metformin intake including diarrhea, nausea, vomiting, and bloating have been attributed to increased abundance of Escherichia.47
Regarding how such changes might impact glycemic control, metagenomic analysis of microbiota suggests a range of functional categories of microbial genes that are impacted, including those related to oxidative stress and metal transport. Additionally, metformin-induced changes in microbiota are proposed to impact production of butyrate and propionate activating intestinal gluconeogenesis.48,49 Stimulated gluconeogenesis in gut has beneficial effects on hepatic glucose production and also leads to appetite suppression, which can contribute to weight reduction and glycemic control.50 On the other hand, expression of microbial genes involved in the degradation of glycine and tryptophan was higher in the untreated diabetic patients compared to metformin-treated patients. That glycine has been reported to improved insulin sensitivity, suggesting this pathway might also contribute to metformin’s efficacy.51
An approach that suggests that the overall impact of metformin-induced changes in microbiota is beneficial is that transfer of microbiota from metformin-treated mice was observed to improve metabolic parameters in aged mice, suggesting that changes in microbiota play a functional role in its beneficial metabolic effects.45 However, this approach does not address the extent to which changes in microbiota are necessary for its impact. We recently investigated this question in mice. We found that the ability of metformin to beneficially impact metabolic syndrome in mice was not impacted by ablation of gut microbiota achieved by use of antibiotics or germ-free mice. Rather, while microbiota ablation itself suppressed diet-induced dysglycemia, other features of metabolic syndrome including obesity, hepatic steatosis, and low-grade inflammation were similarly suppressed by metformin in the presence or absence of gut microbiota.5 While this approach did not prove directly informative re the role of microbiota in metformin-induced improvement in glycemic control, it suggests a potential role for metformin’s anti-inflammatory activity, irrespective of gut microbiota, in driving some of this drug’s beneficial impacts.
Other drugs proven to benefit T2D
Acarbose, an α-glucosidase inhibitor, lowers postprandial blood glucose concentration via inhibiting conversion of oligosaccharides into mono- and di-saccharides and delaying intestinal glucose absorption. However, acarbose has also been recently appreciated to impact microbiota composition. For example, assessing gut microbiota alteration after acarbose treatment in patients with T2D showed increased abundance of B. longum and decreased concentration of lipopolysaccharides.52 In another clinical trial in patients with prediabetes, Butyricicoccus, Phascolarctobacterium, and Ruminococcus decreased while Lactobacillus, Faecalibacterium, and Dialister increased after acarbose intake.53 These compositional shifts of gut microbiota after acarbose intake suggested microbial mediation of the therapeutic effects of acarbose in part. The extent to which these changes may contribute to acarbose’s impacts on glycemic control is not known.
Stimulation of glucagon-like peptide-1 release has been declared as a potential mediating mechanism for the effects of SCFAs on glucose homeostasis. Glucagon-like peptide-1 as a gut hormone is involved in appetite control and gastric emptying. GLP-1 receptor agonists, such as liraglutide, slow gastric emptying, stimulate satiety, enhance insulin secretion, and suppress glucagon. In animal models of obesity, liraglutide induced a reduction of Proteobacteria and an increase of A. muciniphila in gut microbiota.54 Another study on diabetic male rats reported enhancement of SCFA-producing bacteria, including Bacteroides, Lachnospiraceae, and Bifidobacterium after injection of liraglutide.55 Liraglutide substantially altered the overall composition of the gut microbiota, consistent with its weight-lowering effect.56 Abundance of genera including Allobaculum, Turicibacter, Anaerostipes, Blautia, Lactobacillus, Butyricimonas and Desulfovibrio was enriched, while Clostridiales and Bacteroidales were diminished after intervention, consistent with changes reported in gut microbial composition after body weight control.56 Further investigations are needed to elucidate the role of microbial mediation in the therapeutic effects of GLP-1 receptor agonists.
Another agent used in treatment of T2D is Pioglitazone, which is a member of the thiazolidinedione class with hypoglycemic effects thought to result from stimulating activity of the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ). Such stimulation results in reducing insulin resistance and decreasing liver gluconeogenesis.57 In animal models of T2D, Pioglitazone decreases α-diversity of gut microbiota and shifts beta diversity in C57BL/6J mice. This suggests a possible involvement of the microbiota although human studies regarding how this drug impact microbiota in T2D and other disease states have not been reported.
It has been found that Sitagliptin and Vildagliptin, DPP-4 inhibitors, altered gut microbial composition in diabetic rats.58,59 These drugs reduced the diversity of microbiota, increased the abundances of SCFA-producing bacteria including Blautia, Roseburia, Clostridium, Baceroides, and Erysipelotrichaeae in gut microbiota and corrected the Bacteroidetes/Firmicutes ratio.58,59 Therefore, it has been suggested that these drugs may have beneficial effects on blood glucose partly through maintaining the gut barrier integrity and correcting the dysbiosis of intestinal microbiota in diabetes, although human studies are needed in this regard.
Herbal agents used to treat T2D and gut microbiota
Many societies have long treated T2D with a variety of plant-based products and extracts. In some cases, active ingredient(s) have been isolated with results suggesting a potential role for microbiota. Additionally, a range of herbal-derived products including berberine, resveratrol, alliin, capsaicin, betacyanins, and cranberry proanthocyanidins have bioactions and antidiabetic effects potentially mediated by modulation of gut microbiota.60-67 Galactomannan, pectin, capsaicin, and red pitaya betacyanins altered the proportion of Firmicutes to Bacteroidetes.62,65,66 Increased fecal butyrate concentration and Roseburia abundance and decreased Bacteroides and Parabacteroides abundances have been reported after intervention by capsaicin in obese diabetic ob/ob mice.65 Alliin from garlic caused a decrease in Lachnospiraceae abundance and an increase in Ruminococcaceae abundance, which enhanced glucose homeostasis and insulin sensitivity, but has no effect on adiposity.67 Berberine decreased the relative abundance of branched-chain amino acids-producing bacteria, including Streptococcus and Prevotella whereas it increased the relative abundance of SCFA-producing bacteria, including Blautia and Allobaculum.60,68 Direct comparison of berberine to metformin found that both agents exert comparable effects in altering the microbial diversity and overall structure of the gut microbiota in high-fat diet-induced obese rats.69
For some herbal extracts, active ingredients are not well defined, nor is their efficacy, nor mechanism of action, although there is increasing effort being applied to fill these gaps of knowledge by study of microbiota. Herbs common in traditional Chinese medicine for glycemic control of diabetic patients includes Folium Mori, Dendrobium candidum, Rhizoma Dioscoreae, Coptis chinensis, and Fructus MoriL.70,71 For example, a multicenter randomized clinical trial on patients with T2D revealed that both metformin and a traditional Chinese herbal formula significantly alleviated hyperglycemia and dyslipidemia.55 Such effects correlated with impacts on gut microbiota wherein the herbal mixture had larger effect on all parameters. Changes in microbiota induced by these herbal formulations include enrichment of Blautia and Faecalibacterium spp., which are thought to be beneficial herbal preparations impact gut microbiota composition, suggesting it as a possible contributor to their effects. A range of individual herbal extracts that have been used to treat T2D impact microbiota. Alpinia oxyphylla Miq. extract was found to improve glycemic control and renal function in diabetic mice in a manner that associated with increased abundance of Akkermansia and increasing the ratio of Bacteroidetes-to-Firmicutes.72 Increased Bacteroidetes to Firmicutes has also shown after intervention with Qijian mixture (Astragalus membranaceus, Ramulus euonymi, Coptis chinensis, and Pueraria lobata) and extract of D. loddigesii.73,74 Increased abundance of Akkermansia was also associated with glucose-lowering properties of polyphenol-rich extracts of cranberry.75 Water-ethanol extract of green macroalgae Enteromorpha prolifera and Oil tea (green tea and ginger) enriched Lachnospiraceae has been reported after intake of in animal studies,76,77 wherein it was suggested to underlie its beneficial impact on glycemic control. Extracts of cinnamon bark and grape pomace induced a decrease in Peptococcus, Desulfovibrio, Lactococcus abundances and an increase in Allobaculum and Roseburia abundances,78 which associated with decreased fat mass, reduced adipose inflammation, and improved glucose tolerance.78 The notion that beneficial impacts of herbal extracts are mediated by reduced inflammation is supported by a study that found D. loddigesii and Houttuynia cordata, which are traditional Chinese treatment for T2D results decreased abundance of Gram negative bacteria including E. coli and Bacteriodetes fragilis that was suggested to improve T2D by reducing exposure to LPS absorption and subsequently inflammatory.74,79 Moreover, D. loddigesii was reported to improve the gut barrier integrity, which can also reduce metabolic endotoxemia and insulin resistance.74,79 In summary, changes in gut microbiota in response to herbal extracts include increasing microbial diversity, reducing the Firmicutes/Bacteroidetes ratio, increasing the abundances of anti-inflammatory bacteria such as Bifidobacterium, Lactobacillus, Akkermansia, and Faecalibacterium, and decreasing pathogenic bacteria such as E. coli and Enterococcus, which, together, might alleviate low-grade inflammation subsequently improving glycemic control.
Microbiota-metabolizable carbohydrates (fermentable fiber)
In addition to specialized, plants and extracts, one major type of macronutrient that has long been recognized as important for metabolic health in general, and thus potentially promoting good glycemic control is dietary fiber. While mechanisms by which fiber might promote metabolic health are complex, it has recently been appreciated that such beneficial impacts are mediated, at least in part by gut microbiota.80 Briefly, complex carbohydrates that reach the colon that can be fermented by gut bacteria are the major fuel source of the microbiota and has such will have a major impact on total levels of bacteria, composition and its functional activity. Re the former, in mice, lack of fiber decimates microbiota density, which slows enterocyte proliferation, deteriorates mucus, and alters microbiota composition, which together result in microbiota encroachment that promotes low-grade inflammation and insulin resistance.22,80 Hence, enriching a “western-style” low-fiber high-fat diet with fermentable but not insoluble fiber restores gut health and prevents these consequences. The notion that changes in microbiota are pivotal to such impacts of fiber on glycemic control include lack of effects of such fiber in germ-free conditions and that addition of some of the specific bacteria enriched by fiber, namely bifidobacterial could provide beneficial metabolic impacts. A range of other fermentable fibers, including pectin and glucomannan, and resistant corn starches, which can be considered functionally fibers, also impact microbiota and improve glycemic control. The caveats of such studies include that it is difficult to disentangle impacts on glycemic control from other interrelated parameters of metabolic syndrome and that they are largely restricted to mice. In contrast, study of one highly fermentable fiber, inulin, has revealed that this fiber can increase levels of A. muciniphilia in both mice and humans.81 Levels of this microbe are reduced in T2D and direct administration of this microbe to mice improves glycemic control and has shown promise in a recent human trial. In terms of mechanisms, by far the most studied aspects of how nourishing microbiota might improve glycemic control involve the major product of fiber fermentation, SCFAs. SCFAs have a variety of direct metabolic benefits that can improve insulin-resistance irrespective and have a variety of anti-inflammatory actions that can be expected to indirectly. However, ability of fermentable fiber, while fully microbiota-dependent, does not absolutely require SCFA per se in that blocking fermentation only moderately reduced impact of such fibers. Rather, nourishing microbiota with fermentable fiber inulin led to host IL-22 production that was necessary to improve glycemic control and restore gut health, which is impaired by western-style diet. Yet, another means by which inulin improves glycemic control involves its enrichment of A. muciniphilia, which restores mucus robustness resulting in protection against low-grade inflammation.82 While the notion that a bacteria that feeds on mucus results in more robust mucus is somewhat counter-intuitive, it can be viewed as analogous to the notion that cutting a lawn of grass encourages dense growth. Thus, to some extent microbiota mediated approaches to treating preventing T2D can be viewed in terms of ameliorating inflammation.
Conclusions and perspective
TD2 is currently and seems likely to remain for some time, one of humanities major public health problems. As such humanity needs new approaches to treat and prevent this disorder. While most treatments currently in use, especially pharmaceutical agents with proven effects, have generally focused on agents designed to directly impact signaling pathways that directly regulate glucose, or work by unknown mechanisms, better understanding of root causes of T2D suggests that targeting the gut microbiota might be a logical approach to treating this disorder. As reviewed herein, studies, especially those in animal models, support this notion. Moreover, investigation of currently used pharmaceutical reagents suggests that their beneficial effects may be, in part, mediated by impacts on gut microbiota. Given that dysglycemia itself impacts microbiota, disentangling cause and effect is a major confounder this area of research. Yet, we submit that, even if such changes in microbiota are, initially, a consequence of improved glycemic control, they may still be part of maintaining good glycemic control. Thus, examining how, in humans. Current and future treatments of T2D are important to understanding impacts of these agents on health, regardless of whether these agents directly impact microbiota. Similar logic applies to diet-type approaches to ameliorate T2D. Indeed, approaches like caloric restriction to prevent T2D have long preceded appreciation of the microbiota but recent studies that this approach impacts microbiota suggest it as a possible mediator of its prevention of this disorder. In this regard, we suggest that further study and consideration of microbiota, in humans, in response to both pharmaceutical and dietary interventions should pave the way for better approaches to treat and prevent T2D.
Funding Statement
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [grant number DK099071].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI.. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 3.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 4.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104(3):979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adeshirlarijaney A, Zou J, Tran H, Chassaing B, Gewirtz AT. Amelioration of metabolic syndrome by metformin associates with reduced indices of low-grade inflammation independently of the gut microbiota. Am J Physiol Endocrinol Metab. 2019. doi: 10.1152/ajpendo.00245.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51(8):2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 7.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 8.Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2(1):ofv004. doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liou AP, Paziuk M, Luevano JM Jr., Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J Clin Endocrinol Metab. 2004;89(11):5577–5582. doi: 10.1210/jc.2004-0603. [DOI] [PubMed] [Google Scholar]
- 11.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters W, González A, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12(2):139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 15.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 17.Yassour M, Lim MY, Yun HS, Tickle TL, Sung J, Song Y-M, Lee K, Franzosa EA, Morgan XC, Gevers D, et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 2016;8(1):17. doi: 10.1186/s13073-016-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Lin SL, Kwok MK, Leung GM, Schooling CM. The roles of 27 genera of human gut microbiota in ischemic heart disease, Type 2 diabetes mellitus, and their risk factors: A Mendelian randomization study. Am J Epidemiol. 2018;187(9):1916–1922. doi: 10.1093/aje/kwy096. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8(8):e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chassaing B, Raja SM, Lewis JD, Srinivasan S, Gewirtz AT. Colonic microbiota encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol. 2017;4(2):205–221. doi: 10.1016/j.jcmgh.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuentes S, van Nood E, Tims S, Heikamp-de Jong I, Ter Braak CJ, Keller JJ, Zoetendal EG, de Vos WM. Reset of a critically disturbed microbial ecosystem: faecal transplant in recurrent Clostridium difficile infection. Isme J. 2014;8(8):1621–1633. doi: 10.1038/ismej.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe. 2018;23(1):41–53 e4. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga–Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6 e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 24.de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, Herrema H, Ackermans M, Serlie MJM, de Brauw M, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. pp.gutjnl-2019-318320. 2019. doi: 10.1136/gutjnl-2019-318320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kootte RS, Levin E, Salojarvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611–9 e6. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 27.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15(8):1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 28.Razmpoosh E, Javadi M, Ejtahed HS, Mirmiran P. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2016;32(2):143–168. doi: 10.1002/dmrr.2665. [DOI] [PubMed] [Google Scholar]
- 29.Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M, HOSONO A. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2003;67(6):1421–1424. doi: 10.1271/bbb.67.1421. [DOI] [PubMed] [Google Scholar]
- 30.Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107(5):1681–1686. doi: 10.1111/jam.2009.107.issue-5. [DOI] [PubMed] [Google Scholar]
- 31.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Moller K, Svendsen KD, Jakobsen M, Pedersen BK. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104(12):1831–1838. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]
- 32.Honda K, Moto M, Uchida N, He F, Hashizume N. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J Clin Biochem Nutr. 2012;51(2):96–101. doi: 10.3164/jcbn.11-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P, Zhang Q, Dang H, Liu X, Tian F, Zhao J, Chen Y, Zhang H, Chen W. Antidiabetic effect of Lactobacillus casei CCFM0412 on mice with type 2 diabetes induced by a high-fat diet and streptozotocin. Nutrition. 2014;30(9):1061–1068. doi: 10.1016/j.nut.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Hariri M, Salehi R, Feizi A, Mirlohi M, Ghiasvand R, Habibi N. A randomized, double-blind, placebo-controlled, clinical trial on probiotic soy milk and soy milk: effects on epigenetics and oxidative stress in patients with type II diabetes. Genes Nutr. 2015;10(6):52. doi: 10.1007/s12263-015-0503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Wang N, Yin B, Fang D, Jiang T, Fang S, Zhao J, Zhang H, Wang G, Chen W, et al. Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J Appl Microbiol. 2016;121(6):1727–1736. doi: 10.1111/jam.2016.121.issue-6. [DOI] [PubMed] [Google Scholar]
- 36.Yin YN, Yu QF, Fu N, Liu XW, Lu FG. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol. 2010;16(27):3394–3401. doi: 10.3748/wjg.v16.i27.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenman LK, Waget A, Garret C, Klopp P, Burcelin R, Lahtinen S. Potential probiotic Bifidobacterium animalis spp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef Microbes. 2014;5(4):437–445. doi: 10.3920/BM2014.0014. [DOI] [PubMed] [Google Scholar]
- 38.Hampe CS, Roth CL. Probiotic strains and mechanistic insights for the treatment of type 2 diabetes. Endocrine. 2017;58(2):207–227. doi: 10.1007/s12020-017-1433-z. [DOI] [PubMed] [Google Scholar]
- 39.Han MM, Sun JF, Su XH, Peng YF, Goyal H, Wu CH, Zhu XY, Li L. Probiotics improve glucose and lipid metabolism in pregnant women: a meta-analysis. Ann Transl Med. 2019;7(5):99. doi: 10.21037/atm.2019.01.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerard C, Vidal H. Impact of gut microbiota on host glycemic control. Front Endocrinol (Lausanne). 2019;10:29. doi: 10.3389/fendo.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remely M, Hippe B, Zanner J, Aumueller E, Brath H, Haslberger AG. Gut microbiota of obese, Type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr Metab Immune Disord Drug Targets. 2016;16(2):99–106. doi: 10.2174/1871530316666160831093813. [DOI] [PubMed] [Google Scholar]
- 42.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 43.Bonora E, Cigolini M, Bosello O, Zancanaro C, Capretti L, Zavaroni I, Coscelli C, Butturini U. Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects. Curr Med Res Opin. 1984;9(1):47–51. doi: 10.1185/03007998409109558. [DOI] [PubMed] [Google Scholar]
- 44.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Krogh Pedersen H, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 46.Ejtahed HS, Tito RY, Siadat SD, Hasani-Ranjbar S, Hoseini-Tavassol Z, Rymenans L, Verbeke K, Soroush AR, Raes J, Larijani B. Metformin induces weight loss associated with gut microbiota alteration in non-diabetic obese women: a randomized double-blind clinical trial. Eur J Endocrinol. 2018;180:165–176. [DOI] [PubMed] [Google Scholar]
- 47.Rosario D, Benfeitas R, Bidkhori G, Zhang C, Uhlen M, Shoaie S, Mardinoglu A. Understanding the representative gut microbiota dysbiosis in metformin-treated Type 2 diabetes patients using genome-scale metabolic modeling. Front Physiol. 2018;9:775. doi: 10.3389/fphys.2018.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. 2014;80(19):5935–5943. doi: 10.1128/AEM.01357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de la Cuesta-zuluaga J, Mueller NT, Corrales-Agudelo V, Velasquez-Mejia EP, Carmona JA, Abad JM, Escobar JS. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40(1):54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 50.Ejtahed HS, Soroush AR, Angoorani P, Larijani B, Hasani-Ranjbar S. Gut microbiota as a target in the pathogenesis of metabolic disorders: a new approach to novel therapeutic agents. Horm Metab Res. 2016;48:349–358. [DOI] [PubMed] [Google Scholar]
- 51.Vallianou NG, Stratigou T, Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Athens). 2019;18(2):141–144. doi: 10.1007/s42000-019-00093-w. [DOI] [PubMed] [Google Scholar]
- 52.Su B, Liu H, Li J, Sunli Y, Liu B, Liu D, Zhang P, Meng X. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7(5):729–739. doi: 10.1111/1753-0407.12232. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Fang Z, Zhang C, Xia H, Jie Z, Han X, Chen Y, Ji L. Effects of acarbose on the gut microbiota of prediabetic patients: a randomized, double-blind, controlled crossover trial. Diabetes Ther. 2017;8(2):293–307. doi: 10.1007/s13300-017-0226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreira GV, Azevedo FF, Ribeiro LM, Santos A, Guadagnini D, Gama P, Liberti EA, Saad M, Carvalho C. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143–154. doi: 10.1016/j.jnutbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, Wang T, Wang X. Featured article: structure moderation of gut microbiota in liraglutide-treated diabetic male rats. Exp Biol Med (Maywood). 2018;243(1):34–44. doi: 10.1177/1535370217743765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Li P, Tang Z, Yan X, Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. 2016;6:33251. doi: 10.1038/srep33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem. 2006;281(13):8748–8755. doi: 10.1074/jbc.M505649200. [DOI] [PubMed] [Google Scholar]
- 58.Yan X, Feng B, Li P, Tang Z, Wang L. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an animal study. J Diabetes Res. 2016;2016:2093171. doi: 10.1155/2016/2093171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q, Xiao X, Li M, Yu M, Ping F, Zheng J, Wang T, Wang X. Vildagliptin increases butyrate-producing bacteria in the gut of diabetic rats. PLoS One. 2017;12(10):e0184735. doi: 10.1371/journal.pone.0184735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit. 2011;17(7):RA164–7. doi: 10.12659/MSM.881842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anhe FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64(6):872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 62.Song H, Chu Q, Yan F, Yang Y, Han W, Zheng X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J Gastroenterol Hepatol. 2016;31(8):1462–1469. doi: 10.1111/jgh.2016.31.issue-8. [DOI] [PubMed] [Google Scholar]
- 63.Abbasi Oshaghi E, Goodarzi MT, Higgins V, Adeli K. Role of resveratrol in the management of insulin resistance and related conditions: mechanism of action. Crit Rev Clin Lab Sci. 2017;54(4):267–293. doi: 10.1080/10408363.2017.1343274. [DOI] [PubMed] [Google Scholar]
- 64.Huang G, Xu J, Lefever DE, Glenn TC, Nagy T, Guo TL. Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicol Appl Pharmacol. 2017;332:138–148. doi: 10.1016/j.taap.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song JX, Ren H, Gao YF, Lee CY, Li SF, Zhang F, Li L, Chen H. Dietary capsaicin improves glucose homeostasis and alters the gut microbiota in obese diabetic ob/ob mice. Front Physiol. 2017;8:602. doi: 10.3389/fphys.2017.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shtriker MG, Hahn M, Taieb E, Nyska A, Moallem U, Tirosh O, Madar Z. Fenugreek galactomannan and citrus pectin improve several parameters associated with glucose metabolism and modulate gut microbiota in mice. Nutrition. 2018;46:134–42 e3. doi: 10.1016/j.nut.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 67.Zhai B, Zhang C, Sheng Y, Zhao C, He X, Xu W, Huang K, Luo Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci Rep. 2018;8(1):3527. doi: 10.1038/s41598-018-21421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, Li M, Zhang C, Zhang Z, Zhang Y, et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7(8):e42529. doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, Zhang X, Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang B, Yue R, Chen Y, Yang M, Huang X, Shui J, Peng Y, Chin J. Gut microbiota, a potential new target for Chinese herbal medicines in treating diabetes mellitus. Evid Based Complement Alternat Med. 2019;2019:2634898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheng Y, Zheng S, Ma T, Zhang C, Ou X, He X, Xu W, Huang K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci Rep. 2017;7(1):12041. doi: 10.1038/s41598-017-12245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie Y, Xiao M, Ni Y, Jiang S, Feng G, Sang S, Du G. Alpinia oxyphylla Miq. extract prevents diabetes in mice by modulating gut microbiota. J Diabetes Res. 2018;2018:4230590. doi: 10.1155/2018/4230590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao K, Yang R, Zhang J, Wang Z, Jia C, Zhang F, Li S, Wang J, Murtaza G, Xie H, et al. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol Res. 2018;130:93–109. doi: 10.1016/j.phrs.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Li XW, Chen HP, He YY, Chen WL, Chen JW, Gao L, Hu H-Y, Wang J. Effects of rich-polyphenols extract of Dendrobium loddigesii on anti-diabetic, anti-inflammatory, anti-oxidant, and gut microbiota modulation in db/db mice. Molecules. 2018;23(12):3245. doi: 10.3390/molecules23123245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anhe FF, Nachbar RT, Varin TV, Vilela V, Dudonne S, Pilon G, Fournier M, Lecours MA, Desjardins Y, Roy D, et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab. 2017;6(12):1563–1573. doi: 10.1016/j.molmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan X, Yang C, Lin G, Chen Y, Miao S, Liu B, Zhao C. Antidiabetic potential of green seaweed Enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in Type 2 diabetic mice. J Food Sci. 2019;84(1):165–173. doi: 10.1111/1750-3841.14415. [DOI] [PubMed] [Google Scholar]
- 77.Lin R, He X, Chen H, He Q, Yao Z, Li Y, Yang H, Simpson S. Oil tea improves glucose and lipid levels and alters gut microbiota in type 2 diabetic mice. Nutr Res. 2018;57:67–77. doi: 10.1016/j.nutres.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 78.Van Hul M, Geurts L, Plovier H, Druart C, Everard A, Stahlman M, Rhimi M, Chira K, Teissedre P-L, Delzenne NM, et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Endocrinol Metab. 2018;314(4):E334–E52. doi: 10.1152/ajpendo.00107.2017. [DOI] [PubMed] [Google Scholar]
- 79.Wang JH, Bose S, Shin NR, Chin YW, Choi YH, Kim H, Lin R, He X, Chen H, He Q, et al. Pharmaceutical impact of Houttuynia cordata and metformin combination on high-fat-diet-induced metabolic disorders: link to intestinal microbiota and metabolic endotoxemia. Front Endocrinol (Lausanne). 2018;9:620. doi: 10.3389/fendo.2018.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Cani PD. Gut microbiota and obesity: lessons from the microbiome. Brief Funct Genomics. 2013;12(4):381–387. doi: 10.1093/bfgp/elt014. [DOI] [PubMed] [Google Scholar]
- 82.Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]


