ABSTRACT
Gaining a complete understanding of transmission risk factors will assist in efforts to reduce new HIV infections, especially within the disproportionally affected population of men who have sex with men (MSM). We recently reported that the fecal microbiota of MSM elevates immune activation in gnotobiotic mice and enhances HIV infection in vitro over that of fecal microbiota from men who have sex with women. We also demonstrated elevation of the gut homing marker CD103 (integrin αE) on CD4+ T cells by MSM-microbiota. Here we provide additional evidence that the gut microbiota is a risk factor for HIV transmission in MSM by showing elevated frequencies of the HIV co-receptor CCR5 on CD4+ T cells in human rectosigmoid colon biopsies. We discuss our interest in specific MSM-associated bacteria and propose the influx of CD103+ and CCR5+ CD4+ T cells into the colon as a potential link between the MSM microbiota and HIV transmission.
KEYWORDS: Colon, HIV transmission, microbiome, MSM, T-cell recruitment, CCR5
Introduction
Transmission of the human immunodeficiency virus (HIV) primarily occurs across mucosal surfaces. In particular, the rectum and colon mucosa are important1 as a portal of entry and during early disease progression, respectively. In the United States, 66% of all new HIV infections in 2017 were in men who have sex with men (MSM),2 with receptive anal intercourse being the main mode of transmission.3 Substantial gut microbiome compositional shifts have been previously described in HIV-infected populations; however, we now know the most prominent compositional changes are associated with sexual behavior.4,5 The MSM gut microbiome is dominated by Prevotella species compared with the Bacteroides-rich microbiome of culturally westernized men who have sex with women (MSW).4,5 Microbiome shifts associated with HIV infection are more subtle, typically require a large cohort to observe,1 and have been linked with low current and nadir CD4+ T cell counts and viremia.6–8 Our group investigated HIV-associated microbiome compositional effects on immune activation in vitro9 and recently published an evaluation of the effects of human fecal microbiota transplant on immune activation in a gnotobiotic mouse model.1 These studies revealed the fecal microbiota of MSM, regardless of HIV status, elevates immune activation over that seen with the fecal microbiota of MSW. Additionally, we observed that the MSM microbiota enhanced in vitro HIV infection.1 Here, the discussion will focus on the question, can the microbiota of MSM influence HIV transmission? We provide additional data that suggests the unique gut microbiota in MSM drives the influx of a population of CD4+ T cells expressing the HIV co-receptor CCR5 into the gut, supporting a link between the gut microbiota in HIV-negative MSM, the mucosal immune environment, and HIV transmission.
MSM-associated microbes of interest
The use of gnotobiotic mice colonized with fecal microbes from MSM and MSW allowed us to directly investigate microbiome effects on immune activation in vivo. Fecal pellet 16S rRNA gene sequencing from mouse recipients showed our model system captured compositional differences between MSW and MSM observed in donor fecal samples. Although the relative abundance of some microbes shifted when transplanted into mice, most notably MSM-associated Prevotellaceae, overall clustering was consistent with human donor cohorts. The observed differences in microbiome composition resulted in immunological differences in both the mice and human donors.
The concept of a healthy microbiome is not universal and the direct cause of the Prevotella dominance in the MSM gut is unclear.4,5 We know that microbiome composition is tissue-dependent, with factors such as geography/culture, diet, antibiotic use, and age being recognized considerations. A detailed analysis of the MSM gut microbiome has been previously published,4,5,10 as has a discussion on the subtle effects of HIV infection on the microbiome.5,9 Common MSM rectal hygiene practices (douching/enemas) can affect microbiome composition in some people11–13 and have been associated with increased infection by HIV and other STIs in MSM.14,15 Additionally, repeat use of hyperosmotic lubricants significantly decreased Bacteroides and trended toward increasing Prevotella, which could contribute to microbiome shifts.16 A further, little understood, factor that may influence the microbiome in MSM is the degree to which MSM-associated microbes are spread through intimate contact. Indeed, heterosexual couples who live together have more similar gut microbiome composition than unrelated individuals17 or than with their siblings,18 and this effect was especially strong in couples who self-reported to have very close relationships with their spouse.18 Anal intercourse and other sexual practices that increase gut microbiome exposures between partners may result in an even stronger effect in MSM. However, we found no relationship between frequency of receptive anal intercourse (RAI) and the MSM-associated microbiome, with MSM who never engaged in RAI still typically having a Prevotella-rich/Bacteroides-poor microbiome.5 The interplay between rectal hygiene, microbiome shifts, and HIV transmission risk has yet to be fully characterized. Furthermore, in the gut, increases in bacterial alpha diversity metrics are perceived as health promoting, and low alpha diversity leads to poorer health outcomes.19 The MSM microbiome is curious in that it has greater diversity when compared to non-MSM while simultaneously promoting gut-localized immune activation and T-cell homing to the gut.1,5,9 Additional studies are needed to elucidate how MSM-associated microbes came to flourish in the MSM gut environment.
Within the MSM microbiome, members of the Erysipelotrichaceae family, Catenibacterium mitsuokai and Holdemanella biformis have been identified by us and others as notable components.1,9,20–24 These bacteria are of particular interest over other MSM-associated bacteria, such as Prevotella, because they positively correlated with immune cell activation and T cell homing in both human donor blood and mouse recipients of human stool transfers.1 C. mitsuokai and H. biformis positively correlated with HIV-negative MSM donor blood CD8+CD38+HLADR+ activation and CD103+ gut homing, as well as, mouse recipient ileum CD8+CD69+ activation and CD8+ and CD4+ CD103+ expression.1 Peripheral blood mononuclear cells exposed in vitro to H. biformis (formerly E. biforme, reclassified in 201425) exhibited an elevated ratio of TNF-α to IL-10 production compared with other HIV-associated bacteria.26 The elevated cytokine data is, to our knowledge, the only record currently published investigating the potential for H. biformis to directly contribute to inflammation.
In the MSM cohorts from our parent paper, HIV-positive participants were anti-retroviral treatment (ART) naive and our HIV-negative MSM participants unlikely used pre-exposure prophylaxis (PrEP) due to sample collection occurring in 2014 before PrEP was commonly distributed.27 With this in mind, it is curious that C. mitsuokai was suggested as the driver of Erysipelotrichaeae family relative abundance enrichment increases after starting PrEP; H. biformis was also increased.20 Interpretation of these results is not straightforward because of the small sample size and because of the potential confounding of an increase in risky sexual behaviors that occurs following the start of PrEP.28–30 Additionally, the use of reverse transcriptase inhibitors, tenofovir disoproxil fumarate with emtricitabine in HIV-negative MSM is known to create enteric side effects and is cited as a reason for poor adherence to PrEP schedules.20 It is possible PrEP-associated enteropathy allows for select members of the Erysipelotrichaceae family to flourish as it is currently unknown if these bacteria contribute directly to localized gut inflammation, are suited to thriving in high inflammation environments, or function as a unique combination of both. To our knowledge, there has yet to be characterization of the growth rates of these gut microbes in the presence of human cytokines or inflammatory signals.
HIV transmission and dysbiosis
In the United States, most new HIV infections are the result of unprotected RAI with a risk rate of 138 per 10,000 exposures.31 The CDC lists in order of effectiveness (least to greatest) circumcision of adult males, male condom use, daily PrEP for HIV-negative individuals, and ART for HIV-positive individuals as strategies for the prevention of new HIV infections.32 Randomized clinical trials conducted among men in sub-Saharan Africa support male circumcision for reducing HIV transmission for the insertive partner during anal intercourse; however, as circumcision trials have not included a large enough number of MSM and many MSM practice both insertive and receptive anal intercourse, the CDC did not definitely conclude that male circumcision reduces risk of HIV acquisition in MSM practicing receptive anal sex.33
The most effective strategy for preventing HIV transmission is viral load suppression to less than 200 copies/mL (measured twice yearly) in people living with HIV (PLWH), through the use of ART.34 Viral load suppression, with or without the use of condoms, results in effectively zero new infections.34 The United States has recently described a plan for ending the HIV epidemic that is based on early detection of new infections and the use of PrEP for high-risk HIV-negative individuals.35,36 PrEP is the preferred strategy within the MSM community36 and is effective at reducing HIV acquisition by 90%.37 However, not all high-risk populations have awareness of or access to PrEP and ART treatments, especially the most at-risk black MSM population.38
Without pharmaceutical intervention, the presence of local inflammation at the exposure site dramatically increases HIV infection potential, a concept studied extensively in sub-Saharan African women. Bacterial vaginosis, an inflammatory state caused by bacterial overgrowth and dysbiosis in the vagina, increases the risk of vaginal HIV transmission.39 Indeed, in rhesus macaques, the introduction of tissue-localized chemokines is necessary in order to establish an infection with simian immunodeficiency virus (SIV).40 While the immune environments throughout the gastrointestinal tract are pertinent to HIV replication and disease progression as CD4+ T cell loss is more pronounced in the small intestine (duodenum, jejunum, and ileum) versus the large intestine (colon) of untreated PLWH; our work extends the conversation around tissue-localized bacteria-associated inflammation to the rectosigmoid colon, an important site of HIV transmission in the MSM population.
The MSM microbiome affects integrin and CCR5 expression
HIV preferentially infects activated CD4+ T cells that co-express CCR5/or CXCR4.41–44 Thus, factors affecting the recruitment or retention of these cell phenotypes in the colon/rectum/vagina can contribute to HIV sexual transmission risk. Cellular information to spur T cell movement into a tissue is regulated by, among other mechanisms, integrin and chemokine signals. Our study measured the mucosal homing marker CD103 (integrin αE of the αEβ7 pair) and CCR5 frequencies on human blood T cells. HIV-negative MSM had elevated CD103+CD4+ frequencies over MSW, with CD103+CD4+ frequency correlating with CCR5+CD4+ frequencies. However, there was no measurable difference in CCR5 expression in blood between MSW, MSM, or HIV+ MSM.1
Here, we have expanded our investigation of the immunological effects of the MSM microbiome by measuring CCR5 expression on lamina propria T cells from rectosigmoid colon biopsies in HIV-negative MSW and MSM. Lamina propria CD4+ T cell expression of CCR5 has been previously investigated in macaques45,46 and in humans while investigating CRAI; however, CCR5 expression has not previously been compared by sexual orientation through the use of mass cytometry (CyTOF).
In our MSW and MSM biopsies, we observed increased CCR5 frequencies on CD4+ T cells over that measured in the blood (Figure 1a) by CyTOF, which is consistent with traditional flow cytometry data in the literature.47 We measured a significant increase in CCR5 expression on MSM CD4+ T cells compared with MSW CD4+ T cells (Figure 1b), a difference not observed for rectal memory CD4+CCR5+ in MSM engaging in condom-less RAI.10 Furthermore, CCR5 frequencies on activated CD4+ T cells were increased in biopsies over that measured in the blood (Figure 1c), and MSM had increased CCR5 frequencies on activated CD38+HLADR+CD4+ T cells compared with MSW activated CD4+ T cells (Figure 1d). When considered together, our mouse experiments and observations in humans along with in vitro studies that demonstrate various HIV-associated bacteria can upregulate CCR5 expression,48 suggest that the MSM-associated microbiome can influence CCR5 expression and the influx of HIV target cells to the rectosigmoid colon through modulation of integrin and chemokine expression on T cells. These differences between MSW and MSM were likely not observed in the previous study investigating condom-less RAI because their control cohort did not explicitly exclude MSM, measured CCR5 expression on a different subset of CD4+ T cells using different technologies, and did not examine a cohort of high-risk MSM as we did.10
Figure 1.
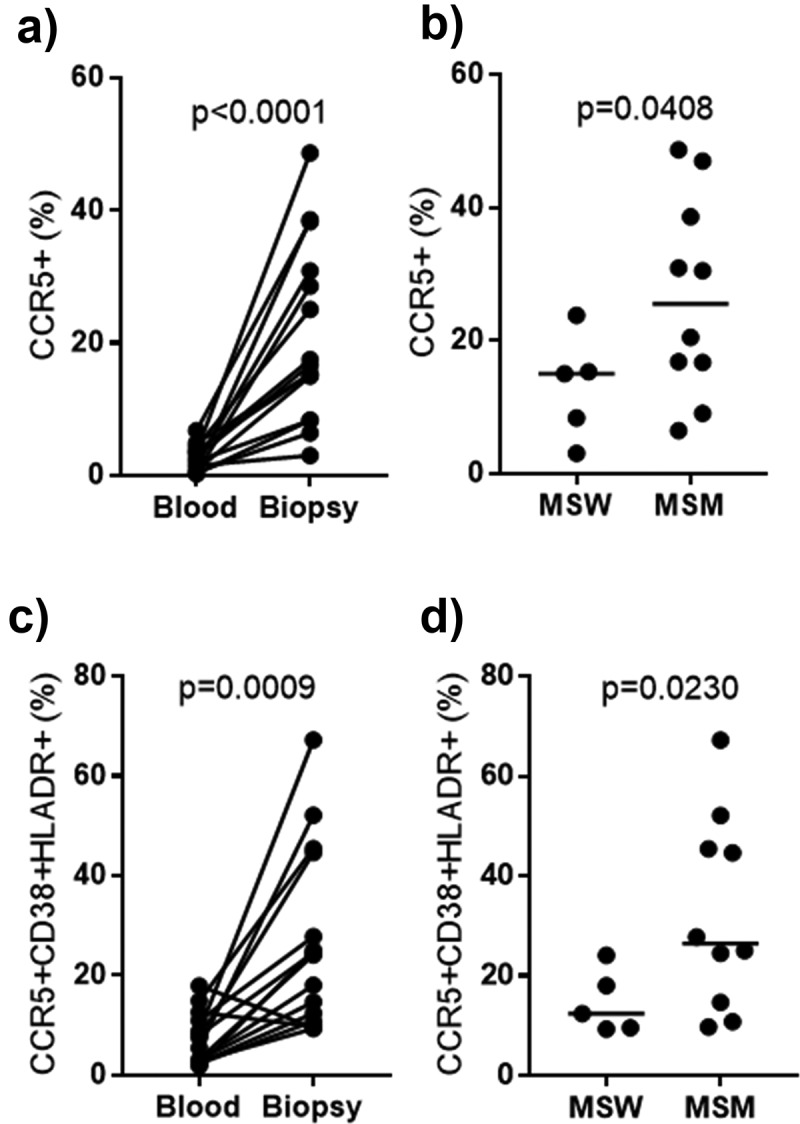
Elevated CCR5 frequencies on total and activated CD4+ T cells in the rectosigmoid colon of MSM.
Integrins are a family of heterodimeric transmembrane proteins that direct cell traffic to and retain cells in various tissues.49 They consist of αβ pairs from combinations of 18α integrins and 8β subunits.50 CD103 (integrin αE) has in recent years been heavily investigated as an identifying marker for tumor antigen-specific T cells in cancers.51 CD103 (integrin αE) is not the only integrin of interest in gut T cell homing, α4β7 and α4β1 integrin have also been studied in the context of HIV transmission. Interestingly, α4β7 is expressed at similar frequencies to αEβ7 on CD4+ T cells in the rectum52 and monoclonal antibodies against α4β7 have been investigated as a strategy for reducing mucosal transmission of SIV in macaques.53 Though recent data from an open-label phase 1 clinical trial in humans did not show the anti-α4β7 monoclonal antibody, vedolizumab to be effective for inducing virological remission in HIV-infected individuals.54 This poor performance by an anti-α4β7 monoclonal antibody would be consistent with our data here that suggests an additional integrin could be involved in the recruitment of T cells to the gut.
We investigated CD103 (integrin αE), in particular, as it has been suggested to be particularly important at mucosal sites,52,55 and is a marker for tissue-resident memory cells,56 with memory CD4+ T cells being another phenotype preferentially infected by HIV.
CD4+CD103+ (integrin αE) T cells in the blood are relatively rare, with the majority of our cohorts having less than 1%;1 blood αEβ7 rarity was also observed in a cohort of low-risk Kenyan women.52 They saw greater αEβ7 frequencies on CD4+ T cells in the rectum, with a median of 5%. The increase in αEβ7 seen in rectum/gut mirrors the tissue differences observed with CCR5 expression. In ileum from gnotobiotic mice colonized with human MSM microbes, we reported CD4+CD103+ (integrin αE) T cell frequencies ranging from 3% to 50%, with median expression higher in MSM. These data suggest the MSM-associated microbiome can influence CD4+ T cells homing to and residing in the gut. This has implications for HIV transmission as Perciani et al. observed that the majority of cervical and rectal αE+β7+CD4+ T cells co-express CCR5 as well as CD69.52 Thus, an influx of CD103+ (integrin αE) into the rectosigmoid region would increase the frequencies of cell types susceptible to HIV infection and increase the risk of HIV transmission following an exposure event (Figure 2).
Figure 2.
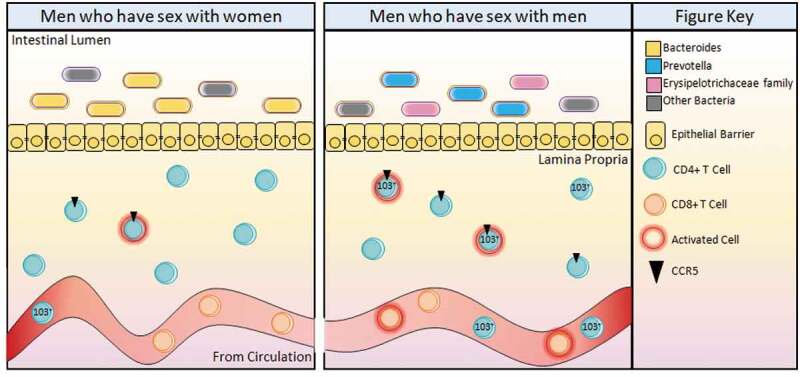
The MSM immune profile, shaped by the MSM-associated microbiome, enhances HIV infection upon exposure due to elevated frequencies of cell phenotypes preferentially infected by HIV in the rectosigmoid colon. MSM have a Prevotella-rich microbiome with the presence of Erysipelotrichaceae family members not commonly found in the Bacteroides-rich MSW microbiome. The lamina propria immune environment of MSM contains higher frequencies of CCR5 on total and activated CD4+ T cells. In the peripheral blood, MSM have more CD8+ T cell activation and increased frequencies of CD103+ T cell homing to the gut.
As mentioned, HIV infection requires co-receptors CCR5 or CXCR4 to be present on a cell; both of which are G-protein-coupled receptors. CCR5 interacts with cytokine family members CCL3/4/5 and CCR5 chemokine agonists have been an area of investigation for influencing the level of available CCR5 and thus reducing HIV utilization of the receptor for infection. Downregulation of peripheral blood chemokine agonist CCL3/4/5 gene expression was shown following acute infection with CCR5-tropic SHIV infection in rhesus macaques.57 For our MSM cohort, we demonstrated that CCR5 frequencies are increased in the rectosigmoid colon. Measurement of CCR5 agonists in the colon would be beneficial for determining the relative availability of the CCR5 receptor. It is not clear exactly how the microbiome influences integrin and chemokine receptor expression; however, with MSM-associated microbes influencing CCR5 expression (a G-protein-coupled receptor) it is interesting that the abundance of H. biformis (an MSM-associated bacteria) also associated with another G-protein-coupled receptor in HIV+ African children.58 Direct investigation of the effects of C. mitsuokai and H. biformis on T-cell activation and homing, as well as expression of CD103 (integrin αE) and CCR5 would help to fully explain MSM microbiome-associated inflammation and the risk of HIV transmission.
Conclusions
Through investigation of the MSM microbiome in vitro, in gnotobiotic mice, and with analysis of human MSM peripheral blood and rectosigmoid biopsies, our work confirmations MSM-specific compositional changes, with a keen interest in members of the Erysipelotrichaceae family, which influence both the systemic and colon-specific immune environments. We observed elevated T cell activation and gut homing markers in the peripheral blood and higher frequencies of the HIV co-receptor on total and activated T cells in the rectosigmoid colon. The MSM-associated microbiota may influence the risk of HIV transmission through integrin and chemokine receptor expression on T cells, thus determining the cell populations in the colon, providing greater opportunity for HIV infection upon exposure. As our understanding of the MSM microbiome influence on HIV transmission becomes clearer, there may be an opportunity for compositional manipulation through diet or pharmaceutical interventions, with a goal of reducing HIV transmission in MSM populations.
Methods
Rectosigmoid colon biopsy samples used in this analysis were collected from HIV-seronegative MSW (n = 5) and high-risk MSM (n = 10). Risk was assessed by frequency of unprotected anal intercourse, being in a relationship with an HIV-positive partner and number of partners in the 6 months prior to study entry. There was no significant difference between MSW and MSM in age (years: MSW 30.6 (19–44), MSM 41.8 (26–63), p = .0859), weight (kg: MSW 92.82 (74–118), MSM 79.44 (72.2–93.8), p = .1608), race (MSW: white = 5, other = 0; MSM: white = 9, other = 1, p = .4642) or ethnicity (Hispanic: MSW = 2, MSM = 2,p = .5604). Age and weight data expressed as mean (range) and compared with unpaired T-test with Welch’s correction. Race and ethnicity compared with Fisher’s Exact Test.
All participants were asked to prepare their bowel for biopsy using a Fleet Saline enema. Following the enema, 30 pinch biopsies were collected from the rectosigmoid region of the colon, approximately 3–10 cm from the anal verge. The pinches were digested for 1.5 h with DNase and collagenase then filtered through a 70μm nylon filter as previously described.1 Concurrently, peripheral blood mononuclear cells were isolated from heparinized peripheral blood from the same patient cohort as previously described.1 Both blood and biopsy were immediately stained with metal-labeled antibodies (Fluidigm) for mass cytometry. Data were analyzed with FlowJo software (BD Life Sciences). The protocols for biopsy and blood sample collection were approved by the Colorado Multiple Institutional Review Board (COMIRB No: 15–1692 and 17–1512) and all participants gave informed consent to participate in this study.
Acknowledgments
This work was supported by R01DK104047 (B.P. and C.L.), R01DK108366 (B.P., C.L., and T.C.), R01HL138639 (B.P., C.L., and T.C.) and by the Colorado Clinical and Translational Sciences Institute (UL1TR00005). We would particularly like to thank the study participants.
Funding Statement
This work was supported by the National Heart, Lung, and Blood Institute [R01HL138639]; National Institute of Diabetes and Digestive and Kidney Diseases [UL1TR00005]; National Institute of Diabetes and Digestive and Kidney Diseases [by R01DK104047]; National Institute of Diabetes and Digestive and Kidney Diseases [R01DK108366].
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Li SX, Sen S, Schneider JM, Xiong KN, Nusbacher NM, Moreno-Huizar N, Shaffer M, Armstrong AJS, Severs E, Kuhn K, et al. Gut microbiota from high-risk men who have sex with men drive immune activation in gnotobiotic mice and in vitro HIV infection. PLoS Pathog. 2019;15:e1007611. doi: 10.1371/journal.ppat.1007611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Division of HIV/AIDS Prevention, National Center for HIV/AIDS . HIV in the United States and dependent areas. Centers for Disease Control and Prevention, U.S. Department of Health & Human Services; 2019. https://www.cdc.gov/hiv/statistics/overview/ataglance.html
- 3.Sullivan PS, Salazar L, Buchbinder S, Sanchez TH.. Estimating the proportion of HIV transmissions from main sex partners among men who have sex with men in five US cities. AIDS. 2009;23:1153–1162. doi: 10.1097/QAD.0b013e32832baa34. [DOI] [PubMed] [Google Scholar]
- 4.Noguera-Julian M, Rocafort M, Guillen Y, Rivera J, Casadella M, Nowak P, Hildebrand F, Zeller G, Parera M, Bellido R, et al. Gut microbiota linked to sexual preference and HIV infection. Ebiomedicine. 2016;5:135–146. doi: 10.1016/j.ebiom.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong AJS, Shaffer M, Nusbacher NM, Griesmer C, Fiorillo S, Schneider JM, Preston Neff C, Li SX, Fontenot AP, Campbell T, et al. An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome. 2018;6:198. doi: 10.1186/s40168-018-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook RR, Fulcher JA, Tobin NH, Li F, Lee D, Javanbakht M, Brookmeyer R, Shoptaw S, Bolan R, Aldrovandi GM, et al. Effects of HIV viremia on the gastrointestinal microbiome of young MSM. AIDS. 2019;33:793–804. doi: 10.1097/qad.0000000000002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillén Y, Noguera-Julian M, Rivera J, Casadellà M, Zevin AS, Rocafort M, Parera M, Rodríguez C, Arumí M, Carrillo J, et al. Low nadir CD4+ T-cell counts predict gut dysbiosis in HIV-1 infection. Mucosal Immunol. 2019;12:232–246. doi: 10.1038/s41385-018-0083-7. [DOI] [PubMed] [Google Scholar]
- 8.Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, Lankowski A, Baldridge MT, Wilen CB, Flagg M, et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe. 2016;19. doi: 10.1016/j.chom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neff CP, Krueger O, Xiong K, Arif S, Nusbacher N, Schneider JM, Cunningham AW, Armstrong A, Li S, McCarter MD, et al. Fecal microbiota composition drives immune activation in HIV-infected individuals. EBioMedicine. 2018;30. doi: 10.1016/j.ebiom.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley CF, Kraft CS, de Man TJ, Duphare C, Lee HW, Yang J, Easley KA, Tharp GK, Mulligan MJ, Sullivan PS, et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal Immunol. 2017;10. doi: 10.1038/mi.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrell L, Wang Y, Antonopoulos D, Young V, Lichtenstein L, Huang Y, Hanauer S, Chang E. Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS One. 2012;7:e32545. doi: 10.1371/journal.pone.0032545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mai V, Greenwald B, Glenn Morris J, Raufman J-P, Stine OC. Effect of bowel preparation and colonoscopy on post-procedure intestinal microbiota composition. Gut. 2006;55:1822–1823. doi: 10.1136/gut.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien CL, Allison GE, Grimpen F, Pavli P. Impact of colonoscopy bowel preparation on intestinal microbiota. PLoS One. 2013;8:e62815. doi: 10.1371/journal.pone.0062815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan A, Blumenthal JS, Dube MP, Ellorin E, Corado K, Moore DJ, Morris SR. Effect of rectal douching/enema on rectal gonorrhoea and chlamydia among a cohort of men who have sex with men on HIV pre-exposure prophylaxis. Sex Transm Infect. 2018;94:508–514. doi: 10.1136/sextrans-2017-053484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Yuan T, Fitzpatrick T, Smith K, Zhao J, Wu G, Ouyang L, Wang Y, Zhang K, Zhou Y, et al. Association between rectal douching and HIV and other sexually transmitted infections among men who have sex with men: a systematic review and meta-analysis. Sex Transm Infect. 2019. doi: 10.1136/sextrans-2019-053964. [DOI] [PubMed] [Google Scholar]
- 16.Haaland RE, Fountain J, Hu Y, Holder A, Dinh C, Hall L, Pescatore NA, Heeke S, Hart CE, Xu J, et al. Repeated rectal application of a hyperosmolar lubricant is associated with microbiota shifts but does not affect PrEP drug concentrations: results from a randomized trial in men who have sex with men. J Int AIDS Soc. 2018;21:e25199. doi: 10.1002/jia2.25199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dill-McFarland KA, Tang ZZ, Kemis JH, Kerby RL, Chen G, Palloni A, Sorenson T, Rey FE, Herd P. Close social relationships correlate with human gut microbiota composition. Sci Rep. 2019;9:703. doi: 10.1038/s41598-018-37298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol. 2018;44:34–40. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubé MP, Park SY, Ross H, Love TMT, Morris SR, Lee HY. Daily HIV pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate-emtricitabine reduced Streptococcus and increased Erysipelotrichaceae in rectal microbiota. Sci Rep. 2018;8:15212. doi: 10.1038/s41598-018-33524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Wilson JE, Koenigsknecht MJ, Chou WC, Montgomery SA, Truax AD, Brickey WJ, Packey CD, Maharshak N, Matsushima GK, et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat Immunol. 2017;18:541–551. doi: 10.1038/ni.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vujkovic-Cvijin I, Somsouk M. HIV and the gut microbiota: composition, Consequences, and Avenues for Amelioration. Curr HIV/AIDS Rep. 2019;16:204–213. doi: 10.1007/s11904-019-00441-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaakoush NO. Insights into the role of erysipelotrichaceae in the human host. Front Cell Infect Microbiol. 2015;5:84. doi: 10.3389/fcimb.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truax AD, Chen L, Tam JW, Cheng N, Guo H, Koblansky AA, Chou WC, Wilson JE, Brickey WJ, Petrucelli A, et al. The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating gut microbiota homeostasis. Cell Host Microbe. 2018;24:364–78.e6. doi: 10.1016/j.chom.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Maesschalck C, Van Immerseel F, Eeckhaut V, De Baere S, Cnockaert M, Croubels S, Haesebrouck F, Ducatelle R, Vandamme P. Faecalicoccus acidiformans gen. nov., sp. nov., isolated from the chicken caecum, and reclassification of streptococcus pleomorphus, eubacterium biforme and eubacterium cylindroides within the family erysipelotrichaceae. Int J Syst Evol Micr. 2014;64:3877–3884. doi: 10.1099/ijs.0.064626-0. [DOI] [PubMed] [Google Scholar]
- 26.Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14:329–339. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss G, Smith DK, Newman S, Wiener J, Kitlas A, Hoover KW. PrEP implementation by local health departments in US cities and counties: findings from a 2015 assessment of local health departments. PLoS One. 2018;13:e0200338. doi: 10.1371/journal.pone.0200338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenness SM, Weiss KM, Goodreau SM, Gift T, Chesson H, Hoover KW, Smith DK, Liu AY, Sullivan PS, Rosenberg ES. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis. 2017;65:712–718. doi: 10.1093/cid/cix439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zablotska IB, Vaccher SJ, Bloch M, Carr A, Foster R, Grulich AE, Guy R, McNulty A, Ooi C, Pell C, et al. High adherence to HIV pre-exposure prophylaxis and no HIV seroconversions despite high levels of risk behaviour and STIs: the Australian demonstration study PrELUDE. AIDS Behav. 2019;23:1780–1789. doi: 10.1007/s10461-018-2290-3. [DOI] [PubMed] [Google Scholar]
- 30.Holt M, Newman CE, Lancaster K, Smith AK, Hughes S, Truong -H-HM. HIV pre-exposure prophylaxis and the ‘problems’ of reduced condom use and sexually transmitted infections in Australia: a critical analysis from an evidence-making intervention perspective. Sociol Health Ill. 2019:1–14. doi: 10.1111/1467-9566.12967. [DOI] [PubMed] [Google Scholar]
- 31.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Division of HIV/AIDS Prevention, National Center for HIV/AIDS . Effectiveness of prevention strategies to reduce the risk of acquiring or transmitting HIV. Centers for Disease Control and Prevention, U.S. Department of Health & Human Services; 2019. https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html [Google Scholar]
- 33.Division of HIV/AIDS Prevention, National Center for HIV/AIDS . Information for providers counseling male patients and parents regarding male circumcision and the prevention of HIV infection, STIs, and other health outcomes. Centers for Disease Control and Prevention, U.S. Department of Health & Human Services; 2018. https://stacks.cdc.gov/view/cdc/58456 [Google Scholar]
- 34.LeMessurier J, Traversy G, Varsaneux O, Weekes M, Avey MT, Niragira O, Gervais R, Guyatt G, Rodin R. Risk of sexual transmission of human immunodeficiency virus with antiretroviral therapy, suppressed viral load and condom use: a systematic review. CMAJ. 2018;190:E1350–E60. doi: 10.1503/cmaj.180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United StatesEnding the HIV epidemicEditorial. JAMA. 2019;321:844–845. doi: 10.1001/jama.2019.1343. [DOI] [PubMed] [Google Scholar]
- 36.Cohen MS. Successful treatment of HIV eliminates sexual transmission. Lancet. 2019;393:2366–2367. doi: 10.1016/S0140-6736(19)30701-9. [DOI] [PubMed] [Google Scholar]
- 37.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnett M, Hirsch-Moverman Y, Franks J, Hayes-Larson E, El-Sadr WM, Mannheimer S. Limited awareness of pre-exposure prophylaxis among black men who have sex with men and transgender women in New York city. AIDS Care. 2018;30:9–17. doi: 10.1080/09540121.2017.1363364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anahtar Melis N, Byrne Elizabeth H, Doherty Kathleen E, Bowman Brittany A, Yamamoto Hidemi S, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini Mary E, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meditz AL, Haas MK, Folkvord JM, Melander K, Young R, McCarter M, MaWhinney S, Campbell TB, Lie Y, Coakley E, et al. HLA-DR+CD38+CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-Tropic HIV-1 RNA In Vivo. J Virol. 2011;85:10189–10200. doi: 10.1128/jvi.02529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Phetsouphanh C, Suzuki K, Aggrawal A, Graff-Dubois S, Roche M, Bailey M, Alcantara S, Cashin K, Sivasubramaniam R, et al. HIV-1 and SIV predominantly use CCR5 expressed on a precursor population to establish infection in T follicular helper cells. Front Immunol. 2017;8. doi: 10.3389/fimmu.2017.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dragic T, Litwin V, Allaway GP, Martin SR, Huang YX, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, et al. HIV-1 entry into CD4(+) cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 44.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T Cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 45.Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, Lackner AA. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000;74:11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anton PA, Elliott J, Poles MA, McGowan IM, Matud J, Hultin LE, Grovit-Ferbas K, Mackay CR, Chen ISY, Giorgi JV. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000;14:1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 48.Dillon SM, Lee EJ, Donovan AM, Guo K, Harper MS, Frank DN, McCarter MD, Santiago ML, Wilson CC. Enhancement of HIV-1 infection and intestinal CD4+ T cell depletion ex vivo by gut microbes altered during chronic HIV-1 infection. Retrovirology. 2016;13:5. doi: 10.1186/s12977-016-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertoni A, Alabiso O, Galetto AS, Baldanzi G. Integrins in T cell physiology. Int J Mol Sci. 2018;19:485. doi: 10.3390/ijms19020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9:2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perciani CT, Jaoko W, Farah B, Ostrowski MA, Anzala O, MacDonald KS; for the K-ICRT . αEβ7, α4β7 and α4β1 integrin contributions to T cell distribution in blood, cervix and rectal tissues: potential implications for HIV transmission. PLoS One. 2018;13:e0192482. doi: 10.1371/journal.pone.0192482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, Kersh EN, McNicholl JM, Hanson D, Reimann KA, et al. Targeting alpha4beta7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med. 2014;20:1397–1400. doi: 10.1038/nm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sneller MC, Clarridge KE, Seamon C, Shi V, Zorawski MD, Justement JS, Blazkova J, Huiting ED, Proschan MA, Mora JR, et al. An open-label phase 1 clinical trial of the anti-alpha4beta7 monoclonal antibody vedolizumab in HIV-infected individuals. Sci Transl Med. 2019;11. doi: 10.1126/scitranslmed.aax3447. [DOI] [PubMed] [Google Scholar]
- 55.Kiravu A, Gumbi P, Mkhize NN, Olivier A, Denny L, Passmore J-A. Evaluation of CD103 (αEβ7) integrin expression by CD8 T cells in blood as a surrogate marker to predict cervical T cell responses in the female genital tract during HIV infection. Clin Immunol. 2011;141:143–151. doi: 10.1016/j.clim.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21:688. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao W, Pahar B, Borda JT, Alvarez X, Sestak K. A decline in CCL3-5 chemokine gene expression during primary simian-human immunodeficiency virus infection. PLoS One. 2007;2:e726. doi: 10.1371/journal.pone.0000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourke CD, Gough EK, Pimundu G, Shonhai A, Berejena C, Terry L, Baumard L, Choudhry N, Karmali Y, Bwakura-Dangarembizi M, et al. Cotrimoxazole reduces systemic inflammation in HIV infection by altering the gut microbiome and immune activation. Sci Transl Med. 2019;11:eaav0537. doi: 10.1126/scitranslmed.aav0537. [DOI] [PMC free article] [PubMed] [Google Scholar]


