ABSTRACT
Intestinal tissue has a specialized immune system that exhibits an exquisite balance between active and suppressive responses important for the maintenance of health. Intestinal immunity is functionally affected by both diet and gut commensal bacteria. Here, we review the effects of fatty acids on the regulation of intestinal immunity and immunological diseases, revealing that dietary fatty acids and their metabolites play an important role in the regulation of allergy, inflammation, and immunosurveillance in the intestine. Several lines of evidence have revealed that some dietary fatty acids are converted to biologically active metabolites by enzymes not only in the host but also in the commensal bacteria. Thus, biological interaction between diet and commensal bacteria could form the basis of a new era in the control of host immunity and its associated diseases.
KEYWORDS: Palm oil, palmitic acid, sphingolipid, IgA antibody, linseed oil, 178-epoxyeicosatetraenoic acid (178-EpETE), commensal bacteria
Introduction
The intestinal tract is a digestive organ that not only absorbs nutrients from dietary foods but also plays an important immunological role. Indeed, more than half of the immune cells in the body are present in the intestine. The intestinal immune system, including IgA antibody production, contributes to defense during the early phase of infection because pathogens – including viruses, bacteria, and toxins – invade the mucosal surface of the intestinal tract.1,2 Gut is exposed not only to pathogenic microorganisms but also to dietary foods and commensal microorganisms, which are beneficial or harmless to the body. For these beneficial components, intestinal immunity has a suppressive immune system, which shows tolerance and non-responsiveness. Thus, intestinal immunity is equipped with a system that maintains a balance between active and suppressive immune responses.3 In the event of collapse of the immunological homeostasis in the intestine, excessive immune responses lead to autoimmune diseases, inflammation, and allergy; in contrast, impaired immune functions are associated with an increase in the risk of infectious diseases.3 Therefore, disturbance in the homeostatic mechanism regulated by intestinal immunity has been related to various diseases, and the effect of various environmental factors related to the control has been clarified.
Nutrients are essential for the development, maintenance, and regulation of the host immune system including intestinal immunity.4,5 Deficient or inappropriate intake of nutrients is frequently associated with increased risk of infection, allergy, and inflammatory diseases. One of the three major nutrients essential for the human body is lipids, which act as components of energy sources, plasma membranes, etc. Fatty acids, which are a main component of lipids, undergo a variety of metabolic processes after absorption in the gut. These components are converted to fatty acid metabolites that have biological activities by endogenous enzymes, such as cytochrome P450 (CYP), cyclooxygenase, and lipoxygenase.6,7 For example, the fatty acids such as arachidonic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are metabolized to epoxy fatty acids and hydroxy fatty acids by CYP; to prostaglandin and thromboxane by cyclooxygenase; and to leukotriene, lipoxins, protectin, and resolvin by lipoxygenase. These metabolites have a variety of biological functions, including roles in various immune responses.
In addition, the gut microbiota also regulates the development of intestinal immunity and maintenance of immunological homeostasis.8 Peyer’s patches (PPs), a major gut-associated lymphoid tissue, are abnormally small in germ-free (GF) mice. Furthermore, the intestines of GF mice lack germinal centers, resulting in impaired immune responses such as IgA antibody production.9 Recent studies show the presence of several bacteria-specific immune regulation functions. For example, segmented filamentous bacteria promote IgA antibody production and are associated with induction and activation of Th17 cells in the intestine;10,11 Klebsiella is involved in the induction of Th1 immune response;12 and Alcaligenes, which is a symbiotic bacterium inside gut-associated lymphoid tissues such as PPs, promotes activation of dendritic cells and IgA antibody production.13,14 In contrast, lactic acid-producing bacteria such as Lactobacillus show anti-inflammatory properties, and some Clostridia species inhibit inflammation by inducing production of interleukin 10 and transforming growth factor β and promoting the differentiation and proliferation of Treg (regulatory T) cells.15-17
In addition to regulating intestinal immunity, the gut microbiota is involved in lipid metabolism. For example, gut commensal bacteria associate with bile acid metabolism in the enterohepatic circulation.18 Some of the bile acids generated in the liver (primary bile acids) and secreted into the intestine are metabolized by gut commensal bacteria to form secondary bile acids. Thus, the gut microbiota is involved in lipid metabolism including absorption of cholesterol through bile acid metabolism. Furthermore, recent studies revealed that the gut microbiota directly metabolizes fatty acids in the intestinal lumen. The fatty acid metabolic pathways of bacteria differ from those of mammals, and therefore bacteria produce unique metabolites from dietary fatty acids. In our previous studies, we consistently detected several unique fatty acid metabolites in the intestine and serum of specific pathogen-free (SPF) mice but not of GF mice.19 In this review, we explore the effects of fatty acids and their metabolites on intestinal immunity and diseases, focusing on host and bacterial metabolisms of dietary oils and the results of our recent studies.
Roles of dietary palmitic acid and its metabolite, sphingolipids, in intestinal immune responses
Fatty acids containing carbon chains of 16 or more are generally referred to as long-chain fatty acids. Long-chain fatty acids are divided into saturated fatty acids, which have no double bonds in their carbon chains, and unsaturated fatty acids, which have double bonds. Palmitic acid is the most common saturated fatty acid found in our body and is provided from endogenous synthesis and diet.20 As the name indicates, palmitic acid is contained in palm oil, but it is also found in meat, dairy products, breast milk, and other sources. Palmitic acid occurs in membrane phospholipids and adipose triacylglycerols and has multiple fundamental biological functions at the cellular and tissue levels. Therefore, disruption of palmitic acid homeostasis leads to several pathophysiological consequences, including tumor growth, metabolic disorder, and inflammation.20
Palmitic acid is metabolized to several metabolites by endogenous enzymatic processes. For example, palmitic acid is converted to palmitoleic acid, oleic acid, and stearic acid by desaturation and elongation reactions.20 Sphingolipids, which are endogenously produced from palmitic acid by serine-palmitoyl transferase, are involved in several cellular events including differentiation, proliferation, migration, and apoptosis.21 In addition, some kinds of gut commensal bacteria in the Bacteroidetes phylum also produce sphingolipids through bacterial serine-palmitoyl transferase. Bacteria require sphingolipids for stress resistance and survival; for instance, genetic knockout of serine-palmitoyl transferase reduces resistance to oxidative stress.22 Bacteria-derived sphingolipids can act as signaling molecules to modulate host immune responses in the gut. Glycosphingolipids produced by bacteria are recognized by invariant natural killer T (iNKT) cells through a non-polymorphic major histocompatibility complex class I-like molecule, CD1d, and therefore, play an important role in intestinal iNKT cell homeostasis.23 Bacteroides fragilis-derived glycosphingolipids suppress excessive proliferation of iNKT cells in the colon and exert protective effects against colitis.23 Thus, palmitic acid metabolism by host and bacteria plays important roles in several biological functions including maintenance of intestinal immunological homeostasis.
By focusing on the composition of fatty acids, especially palmitic acid, in dietary oils and examining their effects on intestinal immunity, we have revealed that fatty acids and their metabolites affect intestinal immune functions, including IgA antibody production. For example, we discovered that IgA concentration in fecal samples and accumulation of IgA-producing cells in the large intestine are higher in mice fed palm oil than in those fed soybean oil (Figure 1). As mentioned earlier, palm oil contains a large amount of palmitic acid; we therefore focused on the effect of palmitic acid on immune cells. We found two pathways by which palmitic acid enhances IgA antibody production: a direct pathway in which palmitic acid directly affects IgA-producing cells and an indirect pathway where palmitic acid acts through its metabolites (Figure 1). When we co-cultured IgA-producing cells and palmitic acid in vitro, IgA antibody production was enhanced by the direct action of palmitic acid in a palmitic acid concentration-dependent manner.24 Palmitic acid is converted to sphingolipids by serine–palmitoyl transferase in vivo, and we found that the increased IgA antibody production in mice fed with palm oil was canceled by administration of a serine–palmitoyl transferase inhibitor.24 This finding suggests that there is an indirect pathway to enhance IgA antibody production through palmitic acid metabolites mediated by endogenous serine–palmitoyl transferase (Figure 1). In another study, we found that sphingosine 1 phosphate, which is a palmitic acid metabolite mediated by serine–palmitoyl transferase, is associated with (1) differentiation of naïve B cells to IgA+ cells in PPs, which are place for the induction of antigen-specific immune responses in gut and (b) migration of IgA+ cells into intestinal lamina propria.25-27 Collectively, these findings suggest that the metabolic pathway from palmitic acid to sphingolipids plays an important role in biological defense through IgA antibody production. As mentioned earlier, some commensal bacteria can produce sphingolipids, suggesting that they metabolize dietary palmitic acid to its metabolites including sphingolipids in gut. When considering the interaction between bacteria and host immunity in the intestine, gut commensal bacteria may contribute to host defense against pathogenic microorganisms through their role as a sphingolipids supplier.
Figure 1.
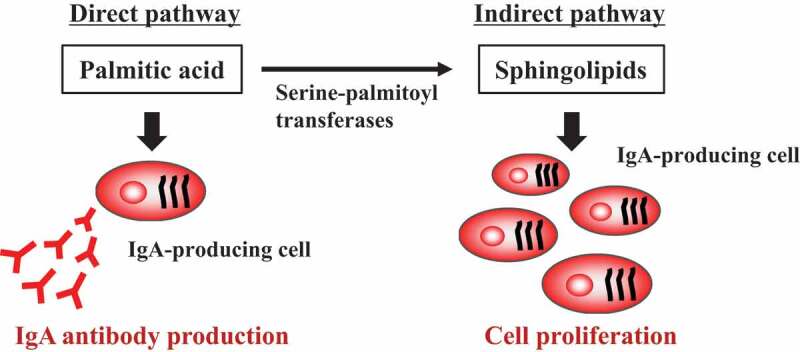
IgA antibody production enhanced by palmitic acid and its metabolite, sphingolipids.
Anti-inflammatory and anti-allergic effects of dietary omega 3 fatty acids
Omega 3 (ω3) and ω6 fatty acids are unsaturated fatty acids that are known as essential fatty acids because mammals (including humans) cannot synthesize them in the body and they must be obtained from the diet. The balance of dietary intake of ω3 and ω6 is involved in the maintenance of host immunological homeostasis; disturbance of the balance increases risk of allergic and inflammatory diseases.28 Among commonly consumed dietary oils, soybean oil, grape seed oil, corn oil, and cottonseed oil contain a large amount of linoleic acid, which is an ω6 fatty acid. Linoleic acid is endogenously metabolized to arachidonic acid, which is converted to fatty acid metabolites including prostaglandin and leukotriene.6,7 In contrast, α-linolenic acid, an ω3 fatty acid, is abundant in linseed oil and perilla oil and is endogenously metabolized to EPA and DHA. It is known that ω3 fatty acids have anti-inflammatory and cardiovascular protective effects.29,30 For example, the Inuit people, an aboriginal people who live in icy and snowy areas including northern Canada and consume fish and seals which contain many ω3 fatty acids, show a low mortality rate associated with heart disease compared to Danish people, who share the same genetic background with the Inuit.31 In comparison, Japanese people tend to overconsume ω6 fatty acids. Because excessive intake of linoleic acid (an ω6 fatty acid) has been suggested to increase the risk of allergy and inflammation, this fatty acid dietary habit is considered to be a potential cause of the recent increase in immune diseases, such as food allergy and pollinosis, in Japan.
In light of this background information, we examined the effects of composition of fatty acids in dietary oils on host organisms by using animal models. In mice fed linseed oil, which contains abundant α-linolenic acid (an ω3 fatty acid), both α-linolenic acid itself and its metabolite, EPA, accumulated in the intestine32 (Figure 2). In contrast, in mice fed soybean oil, which contains abundant linolenic acid (an ω6 fatty acid), linolenic acid accumulated in the intestine.32 Thus, it was revealed that the composition of dietary essential fatty acids is directly reflected in the composition of fatty acids and their metabolites in living tissues.
Figure 2.
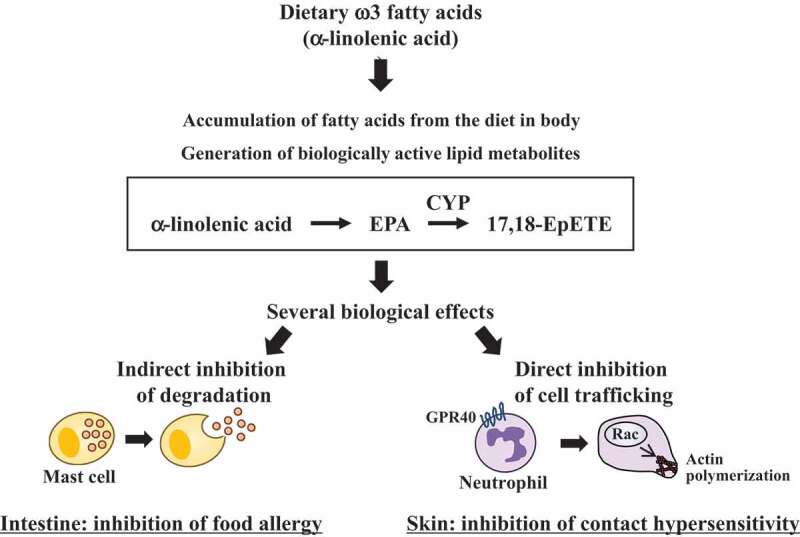
Anti-allergy and anti-inflammation effects of EPA metabolite, 17,18-EpETE.
Because, as mentioned earlier, ω3 fatty acids have anti-inflammatory properties, we next examined the effects of dietary fatty acids in linseed and soybean oil on food allergy in mice. Mice fed linseed oil showed a significantly lower rate of onset of allergic diarrhea compared with those fed soybean oil32 (Figure 2). Consistently, mast cell degranulation was inhibited in mice fed linseed oil.32 Thus, ω3 fatty acids ingested from dietary linseed oil accumulate in intestinal tissues and can prevent diseases through functional regulation of immune cells. Host immunity is associated with not only immune diseases such as inflammation and allergy but also lifestyle-related diseases including hypertension and biological functions of brain and liver. Therefore, a wide variety of physiological functions and diseases likely can be controlled by dietary ω3 fatty acids, and further research is needed.
Endogenous EPA and DHA metabolites showing anti-allergic and anti-inflammatory effects
As mentioned earlier, after absorption in the gut, fatty acids are converted by endogenous enzymatic processes to numerous fatty acid metabolites that display biological effects. Therefore, we used metabolome analysis to comprehensively measure the fatty acid metabolites in the intestinal tissue of mice fed linseed oil. We noted that a large amount of 17,18-epoxyeicosatetraenoic acid (17,18-EpETE), which is a fatty acid metabolite derived from EPA due to the endogenous enzyme CYP, was produced in the intestine of mice fed linseed oil32 (Figure 2). In addition, administration of chemically synthesized 17,18-EpETE prevented allergic diarrhea in mice fed soybean oil32 (Figure 2). 17,18-EpETE is considered to mediate the food allergy suppressive effects of dietary linseed oil. In addition, our recent studies showed that 17,18-EpETE could suppress contact hypersensitivity in mouse and nonhuman primate models.33 17,18-EpETE was recognized by G protein-coupled receptor (GPR) 40 (also known as free fatty acid receptor 1) and inhibited chemoattractant-induced Rac activation and pseudopod formation in neutrophils33 (Figure 2). Thus, 17,18-EpETE inhibits neutrophil mobility through the activation of GPR40, which is a potential therapeutic target for control of allergic inflammatory diseases.
Other reports have similarly indicated that ω3 fatty acids are converted to a variety of metabolites with anti-allergic and anti-inflammatory properties. Protectin D1 produced from DHA ameliorates intestinal inflammation in a mouse colitis model.34 DHA-derived maresin 1 exerts protective actions in a mouse colitis model by inhibiting the NF-kB pathway and consequently multiple inflammatory mediators, as well as by enhancing the macrophage M2 phenotype.35,36 Furthermore, resolvins D1 and E1 exert protective effects on diverse immune diseases, including periodontitis, psoriatic dermatitis, and allergic airway inflammation.37-40
Bacteria-derived ω3 and ω6 fatty acid metabolites and their biological effects
Lipid metabolism by bacteria also generates several fatty acid metabolites, such as conjugated fatty acids and trans-fatty acids that are biologically active and affect host functions (Figure 3). For example, conjugated linoleic acid is now recognized as a beneficial fatty acid metabolite and is used widely as a functional food.41 Dietary intake of conjugated linoleic acid shows beneficial effects, including reduced body fat42,43 and prevention of diabetes,44 colitis,45 atherosclerosis,46 and cancer.47 As an underlying mechanism, conjugated linoleic acid is a potent agonist of peroxisome proliferator-activated receptor (PPAR)-α and increases catabolism of lipids in liver. In addition, conjugated linoleic acid modulates macrophage function and induces anti-inflammatory M2 macrophages in a PPAR-γ-dependent manner. Similarly, conjugated α-linolenic acid is produced in the intestine and possesses several bioactivities. For example, jacaric acid, an isomer of conjugated α-linolenic acid, shows antitumor48 and anti-obesity49 effects. Conversely, consumption of trans-fatty acids increases the risk of coronary heart disease by increasing low-density lipoprotein cholesterol and reducing high-density lipoprotein cholesterol levels.50 Therefore, trans-fatty acids are thought to be harmful for health.
Figure 3.
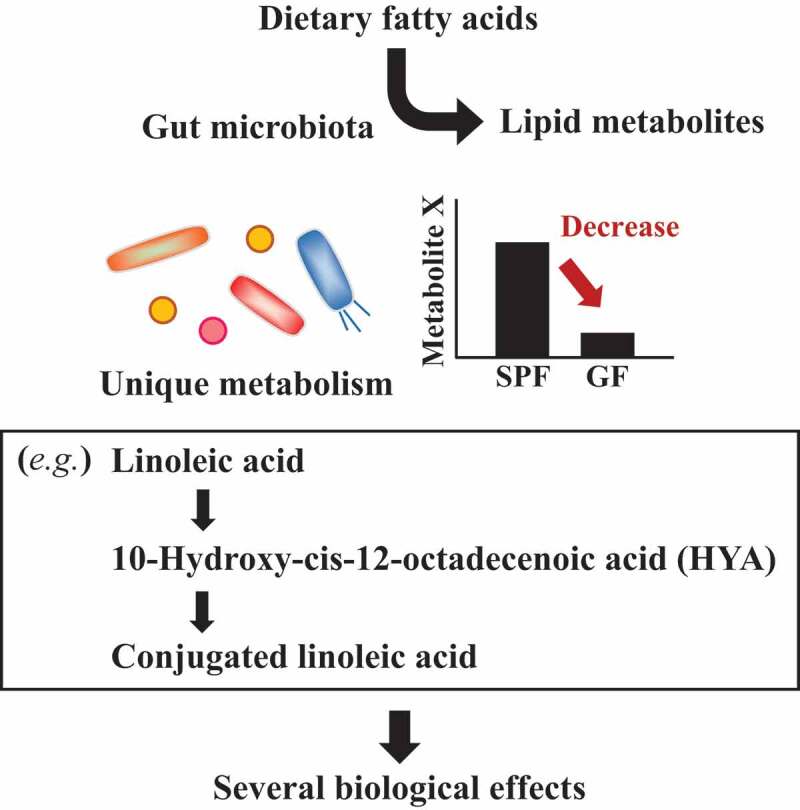
Fatty acid metabolism by commensal bacteria.
Some intermediates generated during the production of conjugated fatty acid likewise exert beneficial effects on host health51 (Figure 3). Certain lactic acid-producing bacteria, such as Lactobacillus plantarum, metabolize linoleic acid and convert it to 10-hydroxy-cis-12-octadecenoic acid (HYA).19 HYA is detected in the feces of SPF mice but not GF mice, suggesting that it is mainly produced by gut commensal bacteria. Subsequent studies revealed that HYA acts as a GPR40 agonist and enhances the barrier function of intestinal epithelium by increasing the expression of tight junction-related molecules.52 In addition, HYA suppresses colitis in mice by inhibiting the NF-κB pathway.52 Like other bacteria-derived intermediates, 10-oxo-trans-11-octadecenoic acid, which is also generated from linoleic acid, shows anti-inflammatory effects on macrophages; it suppresses proinflammatory cytokine production by downregulating nuclear NF-κB p65 protein levels through GPR120.53,54 The α-linolenic acid metabolites 13-hydroxy-9(Z),15(Z)-octadecadienoic acid and 13-oxo-9(Z),15(Z)-octadecadienoic acid induce differentiation of anti-inflammatory M2 macrophages through GPR40.55 Thus, dietary fatty acids are metabolized by the gut microbiota before they are absorbed in the intestine. Elucidation of bacterial fatty acid metabolism will lead to a deeper understanding of the relationship between fatty acid intake and human health.
Conclusion
Intestinal immunity exhibits an exquisite balance between active and suppressive immune responses to prevent infectious diseases and suppress immune disorders, including food allergy. This balance is controlled by not only genomic influences but also a variety of environmental factors. Among them, we have focused on dietary fatty acid, paying particular attention to the results of our recent research. Given that the oil ingested daily is metabolized and the component fatty acids continuously used and replaced, we can easily imagine that daily diet influences immune function and the crisis of immunological disease. In the future, further accumulation of scientific evidence of immunoregulation through diet and nutrition and advances in our knowledge of fatty acid metabolism through the gut microbiota should lead to a comprehensive understanding of the diet–gut microbiota–host interaction and the development of individualized nutrition, which is expected to fundamentally alter health science.
Funding Statement
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Japan Society for the Promotion of Science under grant numbers 18H02150 (J.K.), 18H02674 (J.K.), 17K09604 (J.K.), and 18K17997 (K.H.); the Japan Agency for Medical Research and Development (AMED) under grant numbers 17fk0108223h0002, 17ek0410032s0102, 17fk0108207h0002, 17ek0210078h0002, 17ak0101068h0001, 17gm1010006s0101, 18ck0106243h0003, and 19ek0410062h0001 (J.K.); Cross-ministerial Strategic Innovation Promotion Program (J.K.); the Ministry of Health, Labour, and Welfare of Japan under grant numbers 19KA3001 (K.H.); Joint Research Project of the Institute of Medical Science, the University of Tokyo (J.K. and H.K.); the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (J.K.); the Terumo Foundation for Life Sciences and Arts (J.K.); the ONO Medical Research Foundation (J.K.); and the Canon Foundation (J.K.). None of these funding sources had a role in study design; in the collection, analysis, and interpretation of data; or in the writing of the report.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- 1.Kunisawa J, Kiyono H.. Immune regulation and monitoring at the epithelial surface of the intestine. Drug Discov Today. 2013;18:87–92. doi: 10.1016/j.drudis.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Nagatake T, Kunisawa J. Unique functions of mucosa-associated lymphoid tissues as targets of mucosal vaccines. Curr Top Pharmacol. 2013;17:13–23. [Google Scholar]
- 3.Kayama H, Takeda K. Regulation of intestinal homeostasis by innate and adaptive immunity. Int Immunol. 2012;24:673–680. doi: 10.1093/intimm/dxs094. [DOI] [PubMed] [Google Scholar]
- 4.Hosomi K, Kunisawa J. The specific roles of vitamins in the regulation of immunosurveillance and maintenance of immunologic homeostasis in the gut. Immune Netw. 2017;17:13–19. doi: 10.4110/in.2017.17.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamichhane A, Kiyono H, Kunisawa J. Nutritional components regulate the gut immune system and its association with intestinal immune disease development. J Gastroenterol Hepatol. 2013;28(Suppl 4):18–24. doi: 10.1111/jgh.2013.28.issue-s4. [DOI] [PubMed] [Google Scholar]
- 6.Murakami M. Lipid mediators in life science. Exp Anim. 2011;60:7–20. doi: 10.1538/expanim.60.7. [DOI] [PubMed] [Google Scholar]
- 7.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr Bethesda Md. 2015;6:513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 9.Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012;64:523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evrard B, Balestrino D, Dosgilbert A, J-Lj B-G, Charbonnel N, Forestier C, Tridon A. Roles of capsule and lipopolysaccharide O antigen in interactions of human monocyte-derived dendritic cells and Klebsiella pneumoniae. Infect Immun. 2010;78:210–219. doi: 10.1128/IAI.00864-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunisawa J, Kiyono H. Alcaligenes is commensal bacteria habituating in the gut-associated lymphoid tissue for the regulation of intestinal IgA responses. Front Immunol. 2012;3:65. doi: 10.3389/fimmu.2012.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata N, Kunisawa J, Hosomi K, Fujimoto Y, Mizote K, Kitayama N, Shimoyama A, Mimuro H, Sato S, Kishishita N, et al. Lymphoid tissue-resident Alcaligenes LPS induces IgA production without excessive inflammatory responses via weak TLR4 agonist activity. Mucosal Immunol. 2018;11:693–702. doi: 10.1038/mi.2017.103. [DOI] [PubMed] [Google Scholar]
- 15.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 16.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Wahlström A, Sayin SI, Marschall H-U BF. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Kishino S, Takeuchi M, Park S-B, Hirata A, Kitamura N, Kunisawa J, Kiyono H, Iwamoto R, Isobe Y, Arita M, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci U S A. 2013;110:17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carta G, Murru E, Banni S, Manca C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol. 2017;8:902. doi: 10.3389/fphys.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moye ZD, Valiuskyte K, Dewhirst FE, Nichols FC, Davey ME. Synthesis of sphingolipids impacts survival of porphyromonas gingivalis and the presentation of surface polysaccharides. Front Microbiol. Internet 2016. cited 2019 February15; 7. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5126122/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, Kasper DL. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunisawa J, Hashimoto E, Inoue A, Nagasawa R, Suzuki Y, Ishikawa I, Shikata S, Arita M, Aoki J, Kiyono H. Regulation of intestinal IgA responses by dietary palmitic acid and its metabolism. J Immunol Baltim Md. 1950[2014];193:1666–1671. [DOI] [PubMed] [Google Scholar]
- 25.Kunisawa J, Kurashima Y, Gohda M, Higuchi M, Ishikawa I, Miura F, Ogahara I, Sphingosine KH. 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood. 2007;109:3749–3756. doi: 10.1182/blood-2006-02-004234. [DOI] [PubMed] [Google Scholar]
- 26.Gohda M, Kunisawa J, Miura F, Kagiyama Y, Kurashima Y, Higuchi M, Ishikawa I, Ogahara I, Kiyono H. Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer’s patches for intestinal IgA responses. J Immunol Baltim Md. 1950[2008];180:5335–5343. [DOI] [PubMed] [Google Scholar]
- 27.Kunisawa J, Gohda M, Kurashima Y, Ishikawa I, Higuchi M, Sphingosine KH. 1-phosphate-dependent trafficking of peritoneal B cells requires functional NFkappaB-inducing kinase in stromal cells. Blood. 2008;111:4646–4652. doi: 10.1182/blood-2007-10-120071. [DOI] [PubMed] [Google Scholar]
- 28.Chilton FH, Rudel LL, Parks JS, Arm JP, Seeds MC. Mechanisms by which botanical lipids affect inflammatory disorders. Am J Clin Nutr. 2008;87:498S–503S. doi: 10.1093/ajcn/87.2.498S. [DOI] [PubMed] [Google Scholar]
- 29.Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J Off Publ Fed Am Soc Exp Biol. 2017;31:1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr Bethesda Md. 2012;3:1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet Lond Engl. 1978;2:117–119. doi: 10.1016/S0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 32.Kunisawa J, Arita M, Hayasaka T, Harada T, Iwamoto R, Nagasawa R, Shikata S, Nagatake T, Suzuki H, Hashimoto E, et al. Dietary ω3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci Rep. 2015;5:9750. doi: 10.1038/srep09750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagatake T, Shiogama Y, Inoue A, Kikuta J, Honda T, Tiwari P, Kishi T, Yanagisawa A, Isobe Y, Matsumoto N, et al. The 17,18-epoxyeicosatetraenoic acid-G protein-coupled receptor 40 axis ameliorates contact hypersensitivity by inhibiting neutrophil mobility in mice and cynomolgus macaques. J Allergy Clin Immunol. 2018;142:470–484.e12. doi: 10.1016/j.jaci.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 34.Hamabata T, Nakamura T, Masuko S, Maeda S, Murata T. Production of lipid mediators across different disease stages of dextran sodium sulfate-induced colitis in mice. J Lipid Res. 2018;59:586–595. doi: 10.1194/jlr.M079095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DFP, Calixto JB. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J Immunol Baltim Md. 1950[2013];191:4288–4298. [DOI] [PubMed] [Google Scholar]
- 36.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J Immunol Baltim Md. 1950[2011];186:6129–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol Baltim Md. 1950[2012];189:1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawada Y, Honda T, Hanakawa S, Nakamizo S, Murata T, Ueharaguchi-Tanada Y, Ono S, Amano W, Nakajima S, Egawa G, et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J Exp Med. 2015;212:1921–1930. doi: 10.1084/jem.20150381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Den Hartigh LJ. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients. 2019;11(2). pii: E370. doi: 10.3390/nu11020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaullier J-M, Halse J, Høye K, Kristiansen K, Fagertun H, Vik H, Gudmundsen O. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr. 2005;135:778–784. doi: 10.1093/jn/135.4.778. [DOI] [PubMed] [Google Scholar]
- 43.Gaullier J-M, Halse J, Høye K, Kristiansen K, Fagertun H, Vik H, Gudmundsen O. Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am J Clin Nutr. 2004;79:1118–1125. doi: 10.1093/ajcn/79.6.1118. [DOI] [PubMed] [Google Scholar]
- 44.Ryder JW, Portocarrero CP, Song XM, Cui L, Yu M, Combatsiaris T, Galuska D, Bauman DE, Barbano DM, Charron MJ, et al. Isomer-specific antidiabetic properties of conjugated linoleic acid. Improved glucose tolerance, skeletal muscle insulin action, and UCP-2 gene expression. Diabetes. 2001;50:1149–1157. doi: 10.2337/diabetes.50.5.1149. [DOI] [PubMed] [Google Scholar]
- 45.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Carbo A, Shaykhutdinov R, Jobin C, Arthur JC, Corl BA, Vogel H, et al. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR γ to suppress colitis. PLoS One. 2012;7:e31238. doi: 10.1371/journal.pone.0031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanter JE, Goodspeed L, Wang S, Kramer F, Wietecha T, Gomes-Kjerulf D, Subramanian S, O’Brien KD, Den Hartigh LJ. 10,12 conjugated linoleic acid-driven weight loss is protective against atherosclerosis in mice and is associated with alternative macrophage enrichment in perivascular adipose tissue. Nutrients. 2018;10(10). pII: E1416. doi: 10.3390/nu10101416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans NP, Misyak SA, Schmelz EM, Guri AJ, Hontecillas R, Bassaganya-Riera J. Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARgamma. J Nutr. 2010;140:515–521. doi: 10.3945/jn.109.115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinohara N, Tsuduki T, Ito J, Honma T, Kijima R, Sugawara S, Arai T, Yamasaki M, Ikezaki A, Yokoyama M, et al. Jacaric acid, a linolenic acid isomer with a conjugated triene system, has a strong antitumor effect in vitro and in vivo. Biochim Biophys Acta. 2012;1821:980–988. doi: 10.1016/j.bbalip.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara N, Ito J, Tsuduki T, Honma T, Kijima R, Sugawara S, Arai T, Yamasaki M, Ikezaki A, Yokoyama M, et al. jacaric acid, a linolenic acid isomer with a conjugated triene system, reduces stearoyl-CoA desaturase expression in liver of mice. J Oleo Sci. 2012;61:433–441. doi: 10.5650/jos.61.433. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal MP. Trans fatty acids – A risk factor for cardiovascular disease. Pak J Med Sci. 2014;30:194–197. doi: 10.12669/pjms.306.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogawa J, Kishino S, Ando A, Sugimoto S, Mihara K, Shimizu S. Production of conjugated fatty acids by lactic acid bacteria. J Biosci Bioeng. 2005;100:355–364. doi: 10.1263/jbb.100.355. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto J, Mizukure T, Park S-B, Kishino S, Kimura I, Hirano K, Bergamo P, Rossi M, Suzuki T, Arita M, et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J Biol Chem. 2015;290:2902–2918. doi: 10.1074/jbc.M114.610733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sulijaya B, Takahashi N, Yamada M, Yokoji M, Sato K, Aoki-Nonaka Y, Nakajima T, Kishino S, Ogawa J, Yamazaki K. The anti-inflammatory effect of 10-oxo-trans-11-octadecenoic acid (KetoC) on RAW 264.7 cells stimulated with Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. 2018;53:777–784. doi: 10.1111/jre.12564. [DOI] [PubMed] [Google Scholar]
- 54.Furumoto H, Nanthirudjanar T, Kume T, Izumi Y, Park S-B, Kitamura N, Kishino S, Ogawa J, Hirata T, Sugawara T. 10-Oxo-trans-11-octadecenoic acid generated from linoleic acid by a gut lactic acid bacterium Lactobacillus plantarum is cytoprotective against oxidative stress. Toxicol Appl Pharmacol. 2016;296:1–9. doi: 10.1016/j.taap.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Ohue-Kitano R, Yasuoka Y, Goto T, Kitamura N, Park S-B, Kishino S, Kimura I, Kasubuchi M, Takahashi H, Li Y, et al. α-Linolenic acid-derived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. FASEB J Off Publ Fed Am Soc Exp Biol. 2018;32:304–318. [DOI] [PubMed] [Google Scholar]


