ABSTRACT
Although dysbiosis in the gut microbiota is known to be involved in several inflammatory diseases, whether any specific bacterial taxa control host response to inflammatory stimuli is still elusive. Here, we hypothesized that dysbiotic indigenous taxa could be involved in modulating host response to inflammatory triggers. To test this hypothesis, we conducted experiments in germ-free (GF) mice and in mice colonized with dysbiotic taxa identified in conventional (CV) mice subjected to chemotherapy-induced mucositis. First, we report that the absence of microbiota decreased inflammation and damage in the small intestine after administration of the chemotherapeutic agent 5-fluorouracil (5-FU). Also, 5-FU induced a shift in CV microbiota resulting in higher amounts of Enterobacteriaceae, including E. coli, in feces and small intestine and tissue damage. Prevention of Enterobacteriaceae outgrowth by treating mice with ciprofloxacin resulted in diminished 5-FU-induced tissue damage, indicating that this bacterial group is necessary for 5-FU-induced inflammatory response. In addition, monocolonization of germ-free (GF) mice with E. coli led to reversal of the protective phenotype during 5-FU chemotherapy. E. coli monocolonization decreased the basal plasma corticosterone levels and blockade of glucocorticoid receptor in GF mice restored inflammation upon 5-FU treatment. In contrast, treatment of CV mice with ciprofloxacin, that presented reduction of Enterobacteriaceae and E. coli content, induced an increase in corticosterone levels. Altogether, these findings demonstrate that Enterobacteriaceae outgrowth during dysbiosis impacts inflammation and tissue injury in the small intestine. Importantly, indigenous Enterobacteriaceae modulates host production of the anti-inflammatory steroid corticosterone and, consequently, controls inflammatory responsiveness in mice.
KEYWORDS: Mucositis, gut microbiota, Escherichia coli, dysbiosis, inflammation, 5-FU, germ free, corticosterone
Introduction
Appropriate inflammatory responses are dependent on gut colonization1–3 and microbial composition.4 Several niches of the mammalian body are colonized, but the gastrointestinal tract (GIT) is the most widely colonized site.4 Approximately, 99% of the gastrointestinal microbiota of humans and mice are dominated by bacteria of the Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria phyla.5 Sustaining an indigenous microbiota is an integral part of maintaining host health and homeostasis.5–7 Changes in microbiota composition (dysbiosis) or function may cause immune system deregulation and lead to exacerbated inflammatory responses which can contribute to the development of various inflammatory bowel diseases (IBDs)8,9 and inflammation of the intestine after parasite infection.10,11 Although the microbiota lodge great variability between individuals at the species level, during IBD and parasite infection, Enterobacteriaceae overgrowth, including E. coli, is commonly found,10–14 suggesting that this group of microorganisms is involved in the inflammatory response characteristic of IBD. Chemotherapeutic agents also exert a detrimental effect on the intestinal microbial composition, leading to major shifts in numbers of several bacterial taxa in human and mice.15–18 Dysbiosis after chemotherapy commonly promotes a reduction of the diversity and richness of the bacterial community19 and usually coincides in time with the development of chemotherapy-induced mucositis19,20 what suggests that dysbiosis might promotes mucositis development during chemotherapy.
Our group has previously observed that mice without microbiota (GF mice) present greatly decreased local or systemic inflammatory responses after being stimulated with different inflammatory stimuli of sterile or infectious nature.1–3,21 Mice devoid of microbiota are able to perceive and respond to the inflammatory trigger, but in a skewed manner, by increasing the production of the anti-inflammatory mediator IL-10, in a lipoxin A4 (LXA4) and annexin 1 (ANXA-1)-dependent manner.1,2 Furthermore, absence of microbiota is associated with high levels of basal plasma corticosterone.22 Corticosterone is the major glucocorticoid present in the plasma of mice and has major anti-inflammatory actions23 due to its ability of inhibiting expression of multiple inflammatory genes and inducing expression of anti-inflammatory proteins, such as ANXA1 and IL-10.24,25 Therefore, in the absence of colonization by the microbiota, response to inflammatory triggers is shifted by up regulation of anti-inflammatory mediators.
Here, because dysbiosis associated with IBDs often fosters inflammation in the gut, we hypothesized that dysbiotic indigenous taxa could be involved in modulating host response to inflammatory triggers. To test this hypothesis, we conducted experiments in microbiota-deficient mice and in mice colonized with dysbiotic taxa identified in CV mice subjected to chemotherapy-induced mucositis. We showed that chemotherapeutic agents lead to dysbiosis in the gut microbiota, characterized by an increase of E. coli content. More importantly, E. coli monocolonization of GF mice was able to control the production of the anti-inflammatory steroid corticosterone and consequently to reverse the hyporesponsive phenotype of GF mice.
Results
Absence of microbiota protects mice from 5-FU-induced small intestine mucositis
Anticancer treatment often causes mucositis (a debilitating mucosal barrier injury and inflammation that affect the GIT), leukopenia and dysbiosis.15–19 Fluorouracil (5-FU) is a pyrimidine analogue that inhibits DNA and RNA synthesis, by inducing misincorporation in these macromolecules.26 By using a 5-FU-induced mucositis model in mice, we investigated whether the indigenous microbiota present in the GIT would be involved in 5-FU-induced inflammation and injury. To this end, we used conventional (CV), GF or CV mice treated from birth for 60 d with a cocktail of antibiotics (AB) to prevent gut colonization by the microbiota. CV mice treated with 5-FU (CV-5-FU) showed marked increase in clinical score when compared to the control group (Figure 1a). In small intestine of the CV-5-FU group, there was shortening of the intestinal length (Figure 1b), increase in eosinophil peroxidase (EPO) (Figure 1c) and myeloperoxidase (MPO) content (Figure 1d), indicating enhanced influx of eosinophils and neutrophils, respectively, into tissue. There was also heightened CXCL1 production in this group (Figure 1e). GF mice treated with 5-FU (GF-5-FU) presented no significant changes in all these parameters when compared to vehicle-treated GF group (GF-C) (Figure 1ae). This protection resulted in 100% survival of GF mice until 15 d after 5-FU treatment, while 100% of CV mice died until the 8th day after chemotherapy onset (data not shown). Results in AB-mice treated with 5-FU were similar to those observed in GF-5-FU mice, i.e., significant decrease of inflammation and tissue injury (Figure 1a–e).
Figure 2.
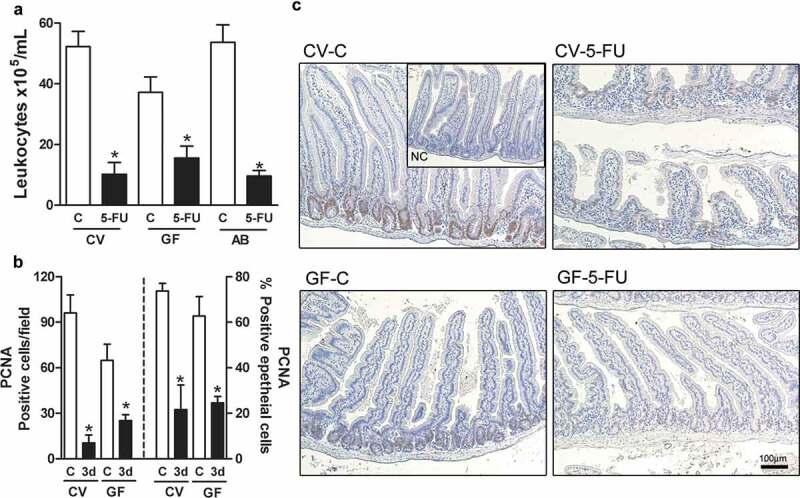
5-FU treatment induced decrease in leukocytes number in blood and in PCNA positive cell in small intestine of CV and GF groups. Leukocytes number in blood was reduced after 5-FU treatment of CV, GF, and AB groups when compared to respective control (a). The number of PCNA-positive epithelial cells was equally decreased in both CV and GF mice on 3rd day after 5 FU injection (b). These effects were not different between the several 5-FU-treated groups. These data were displayed in histological representation (10X) (c). NC: negative control staining. The data represent the mean ± SEM of 5–6 animals per group. *p < 0.05 vs respective control.
Figure 1.
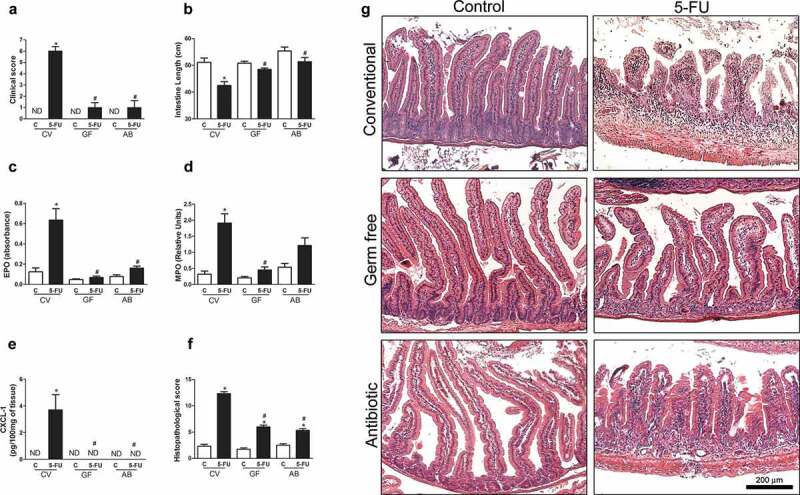
Absence of microbiota protected mice from 5-FU-induced small intestine injury and inflammation. Conventional (CV), germ free (GF) and newborn mice treated with a cocktail of antibiotics for 60 d (AB) received 1 injection of 5-FU or saline for 3 consecutive days and were euthanized 48 h after the last injection. 5-FU treatment in CV mice was associated with increased clinical score (a), intestinal shortening (b), EPO (c) and MPO (d) activities and CXCL-1 (e) production in small intestines when compared to CV-C mice. No changes were observed after 5-FU treatment of GF and AB mice when compared to respective control groups. Histopathological score (f) of the small intestine of the control or 5-FU-treated CV, GF, and AB mice were described in detail in supplementary materials and methods section. There was increase in histopathological score of CV mice after 5-FU treatment compared to the control group, but a little change was observed in GF and AB animals. These data were displayed in histological representation (10X) (g). ND = not detectable. The data represent the mean ± SEM of 5–6 animals per group. *p < 0.05 vs respective control, #p < 0.05 vs CV-5-FU.
Histological analysis revealed significant differences in the morphology of the small intestine of CV and GF control mice (Figure 1g), as previously reported.27,28 GF control mice showed higher and narrower villi and fewer cells in the lamina propria, as compared to CV-C mice. Administration of 5-FU to CV mice induced major changes in gut morphology, characterized by inflammatory influx, height reduction and rounding of villi, disappearance of Lieberkühn glands, hyperemia and edema (Figure 1g). However, in GF and AB mice there were no marked changes in tissue architecture after treatment with 5-FU (Figure 1g). The damage induced by 5-FU in all groups was quantified and represented on Figure 1f. Clearly, GF mice or CV mice treated with AB presented reduced tissue damage when compared to CV mice (Figure 1f).
5-FU-treatment results in inhibition of cellular division affecting mainly cells with high division rates, especially leukocytes and gut basal cells.26 We evaluated whether the protective phenotype of GF and AB was due to impaired antiproliferative effects of 5-FU. Leukocyte counts were reduced similarly in CV, GF and AB mice after 5-FU treatment (Figure 2a). PCNA staining in basal epithelial cells was equally decreased in both CV and GF mice on the 3rd day after 5-FU injection (Figure 2b,c).
In order to evaluate whether prior life-long contact or presence of microbiota would be required to exacerbate 5-FU-induced tissue damage, CV adult mice were treated for 7 (7d) or 30 (30 d) days with a cocktail of antibiotics before 5-FU treatment. We observed prevention of shortening in intestinal length in both groups treated with antibiotics when compared to control (V) mice (V = 41.5 ± 1.94 cm; 7d = 51.13 ± 0.85 cm; 30d = 50.83 ± 3.9 cm; n = 5). In addition, treatment with 5-FU was associated with low levels of EPO (V = 1.57 ± 0.30; 7d = 0.11 ± 0.04; 30d = 0.52 ± 0.12; n = 5) and MPO (V = 3.72 ± 0.49; 7d = 1.19 ± 0.28; 30d = 1.59 ± 0.38; n = 5) activities in both antibiotics treated groups given 5-FU. Furthermore, histopathological analyses showed greater preservation of intestinal structures in mice treated with antibiotics when compared control group (V = 9 ± 0.58 points; 7d = 4.3 ± 0.67 points; 30d = 3.67 ± 0.33 points; n = 5) These results suggest that acute absence of the microbiota is enough to impair the damage caused by 5-FU.
Next, we analyzed the phenotype of GF mice subjected to chemotherapy after replacement of intestinal microbiota by gavage with fecal homogenates obtained from CV mice (CV→GF-conventionalization). Thirty days later, microbiota replacement had promoted reversion of the protective phenotype during 5-FU-induced mucositis (Figure 3). CV→GF mice treated with 5-FU (CV→GF-5-FU) presented increase in clinical score when compared to GF-5-FU (Figure 3a). We have also observed intestine shortening after conventionalization when compared to GF mice that received the chemotherapy (Figure 3b). In addition, CV→GF-5-FU presented increase in EPO (Figure 3c) and MPO (Figure 3d) activities and CXCL1 (Figure 3e) concentration in small intestines when compared to control and GF-5-FU groups. Histopathological analyses showed greater damage of intestinal structure in CV→GF-5-FU mice when compared to GF-5-FU group (Figure 3f,g). Altogether, these data show that presence of indigenous microbiota is necessary for the development and severity of 5-FU-induced mucositis in mice.
Figure 3.
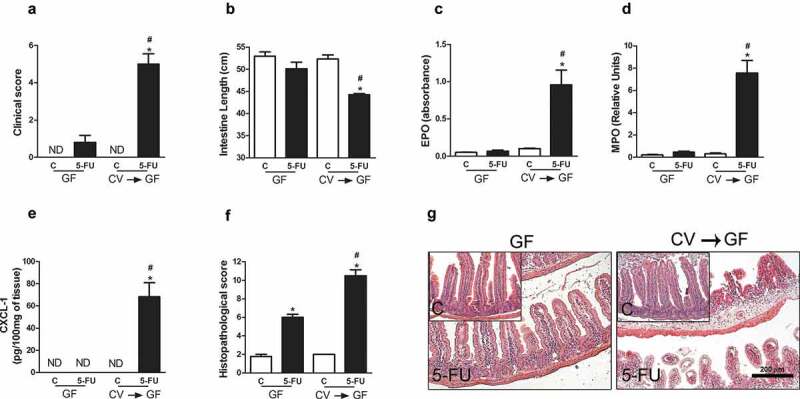
Microbiota reposition reversed the resistance of GF mice to 5-FU-induced small intestine injury and inflammation. GF mice were conventionalized with feces from CV mice (CV→GF) for 30 d and treated with 5-FU chemotherapy or saline. CV→GF mice treated with 5-FU showed increase in clinical score (a) shortening of the intestines (b), activity of EPO (c) and MPO (d) enzymes and in the concentration of CXCL1 (e). Histopathological score (f) of the gut of control or chemotherapy-treated CV→GF and GF mice. There was increased histopathological score in CV→GF mice after 5-FU treatment compared to control and GF-5-FU groups. These data were displayed in histological representation (10X) (g). The data represent the mean ± SEM of six animals per group. *p < 0.05 vs respective control, #p < 0.05 vs GF-5-FU.
Enterobacteria exacerbates 5-FU-induced small intestine injury
Because Enterobacteriaceae overgrowth, including E. coli, is commonly found during inflammatory condition mainly in large intestine,11–14 we evaluated whether there would be any changes in this bacteria group in CV mice upon mucositis induction. Indeed, plating of stools on selective solid media showed increase in the content of Enterobacteriaceae group on the 5th day after 5-FU treatment (Figure 4a). We observed increased Escherichia coli relative content, an important member of enterobacteria group, in stools of all CV mice on the 5th day after 5-FU treatment when compared to day 0 (Figure 4b). Similar results were observed in the luminal contents of the small intestine (Figure 4d,e). No changes were observed in the relative content of the Firmicutes and Bacteroidetes phyla after 5-FU treatment (Figure 4c).
Figure 4.
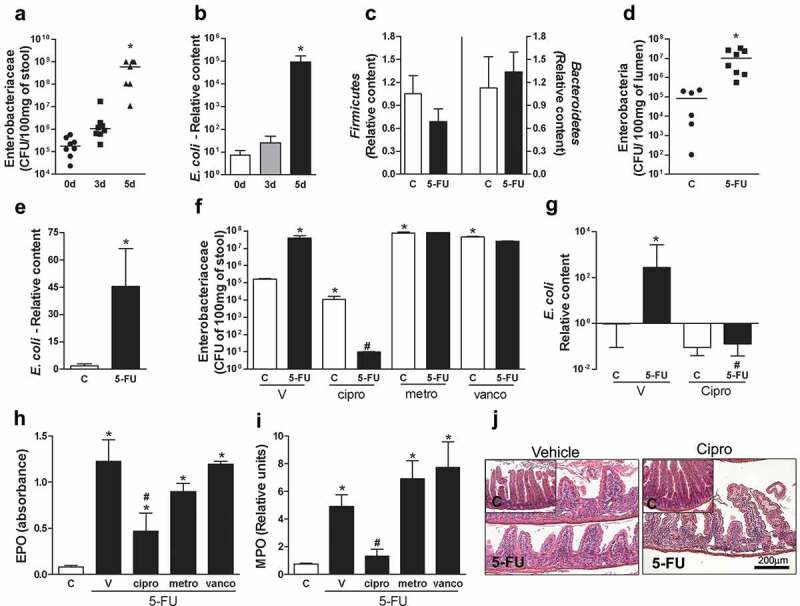
Enterobacteriaceae and E. coli amount were increased and contributed to exacerbated 5-FU-induced injury and inflammation. Treatment with chemotherapy resulted in significant increase in the number of Enterobacteriaceae CFU (a) when compared to the day zero. PCR analysis revealed a significant increase in E. coli relative content in feces of mice after 5-FU treatment (b). However, no change was observed in relative content of Bacteroidetes and Firmicutis (c) phyla. Enterobacteriaceae relative content (d) and E. coli relative content (e) were increased in lumen content after 5-FU treatment. Mice were treated with ciprofloxacin (cipro) to prevent Enterobacteriaceae increase during 5-FU treatment. Cipro treatment but not vancomycin (vanco) or metronidazole (metro) treatments promoted decrease of Enterobacteriaceae in feces of control and 5-FU mice (f). E. coli relative content was reduced by cipro treatment in control and 5-FU groups (g). Cipro treatment was able to prevent increased EPO (h) and MPO (i) activity in small intestines after 5-FU injection when compared to 5-FU-Vehicle (v) mice. Histological representation of cipro-5-FU displayed smaller changes in small intestine architecture when compared to the group treated with V-5-FU (10X) (j). The data represent the mean ± SEM of 4–6 animals per group in B, C, E-J. In A and D, the data represent the median. *p < 0.05 vs control, #p < 0.05 vs 5-FU-V.
To examine whether the Enterobacteriaceae group was associated to severity of 5-FU-induced mucositis, we treated CV mice with ciprofloxacin antibiotic (50 mg/kg twice a day (12/12 h), p.o.), the antibiotic of choice to treat patients infected with Enterobacteriaceae.27 Ciprofloxacin treatment caused a decrease of Enterobacteriaceae content in feces of the control and 5-FU groups (Figure 4f), but a greater reduction was observed in cipro-5-FU-treated mice. PCR methods confirmed that cipro was effective in preventing E. coli outgrowth in the cipro-5-FU group (Figure 4g). EPO (Figure 4h) and MPO (Figure 4i) activities were reduced in cipro-treated mice, when compared to CV mice given 5-FU. Histopathological analyses corroborated the previous data and showed decreased intestinal injury in cipro-treated mice exposed to 5-FU (Figure 4j).
To assess whether specific reduction of Enterobacteriaceae load or decrease of other bacteria taxa was sufficient to protect from 5-FU-induced small intestine damage, we included two other groups of animals treated with metronidazole (metro) or vancomycin (vanco). Metronidazole is used to treat infections with Bacteroidetes members and anaerobic bacteria such as Bacteroides fragilis28 and vancomycin acts on Gram-positive bacteria, including those belonging to the Firmicutes Phylum.29 Metro and vanco treatments promoted reduction of the relative presence of Bacteroidetes and Firmicutes to undetectable levels, respectively (data not shown). On the other hand, treatment with metro or vanco was accompanied by an increase in Enterobacteriaceae load (Figure 4f). There were no differences in EPO (Figure 4h) and MPO (Figure 4i) activities between vanco-5-FU, metro-5-FU and CV-5-FU groups. These data demonstrate that increase in Enterobacteriaceae and E. coli content contributed to the exacerbation of chemotherapy-induced intestinal injury and that preventing increase of these taxa, but not Firmicutes or Bacteroidetes, was associated with partial improvement of 5-FU-induced small intestine damage.
Next, we evaluated whether monocolonization with E. coli would be sufficient to cause tissue inflammation and damage upon administration of 5-FU. For this, GF mice were monocolonized with E. coli (ATCC 25922) or E. coli isolated from stool of CV mice with mucositis (ICVM) or B. fragilis (BF) (used as control). Although, administration of 5-FU to GF mice caused little intestinal damage, monocolonization of GF mice with E. coli ATCC or ICVM led to greatly elevated clinical score (Figure 5a), intestinal shortening (Figure 5b), MPO activity (Figure 5c) and histopathological injury (Figure 5d,e) after 5-FU treatment. Otherwise, there were no differences in response to chemotherapy treatment between B. fragilis-monocolonized mice and GF mice (Figure 5ae). Altogether, these findings support the conclusion that E. coli in the gut is necessary and sufficient for chemotherapy-induced mucositis.
Figure 5.
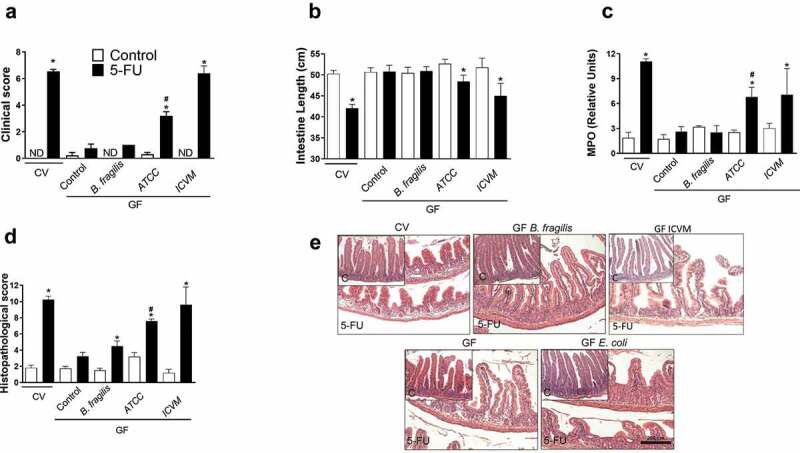
Moncolonization of GF mice with E. coli, but not B. fragilis, was enough to reverse the hyporresponsiveness of GF mice to 5-FU-induced small intestine injury and inflammation. GF mice monocolonized with both E. coli ATCC 25922 or ICVM for 7 d presented increased clinical score of the disease after 5-FU treatment when compared to its respective the control group and GF-5-FU (a). GF monocolonized with both E. coli ATCC 25922 or ICVM showed shortening of the intestinal length (b) and increased MPO activity (c) after chemotherapy treatment compared to respective the control and GF-5-FU groups. There was increased histopathological score in GF mice monocolonized with both E. coli ATCC 25922 or ICVM and submitted to 5-FU treatment compared to control and GF-5-FU groups (d). These data were displayed in histological representation (10X) (e). GF monocolonized with B. fragilis have presented similar results to GF mice (a-e). The data represent the mean ± SEM of 5–6 animals per group. *p < 0.05 vs respective control, #p < 0.05 vs GF-5-FU.
Next, we evaluated whether LPS from E. coli is sufficient to cause tissue inflammation and damage upon administration of 5-FU. To address this question, we conducted experiments involving administration of LPS (30ug/mL) from E. coli O111:B4 to GF mice in the drinking water and subsequent mucositis induction by 5-FU injection. This LPS administration protocol was not able to revert the GF phenotype upon mucositis induction as shown by clinical score (Fig S1A), intestine length (Fig S1B) and MPO activity in tissue (Fig S1 C). It suggests that other structural compounds or even alive E. coli may be required for reversion of GF phenotype and corticosterone production.
Enterobacteriaceae controls host response to chemotherapy-induced mucositis or other inflammatory stimuli by decreasing basal plasma corticosterone levels
The indigenous microbiota interferes with several physiologic functions of their host, including the production of glucocorticoids22,30 and the control of basal plasma corticosterone levels (Figure 6a). Anti-inflammatory effects of glucocorticoids are attributed to several mechanisms including increasing the synthesis of Annexin-1 (anxa-1) and Il-10.23–25 Indeed, we have observed that expression of Anxa-1 (Figure 6b) and Il-10 (Figure 6c) were increased in the gut of GF mice after chemotherapy at earlier time points. Therefore, GF mice respond to chemotherapy-treatment by early upregulating anti-inflammatory mediators known to be induced by corticosterone.
Figure 6.
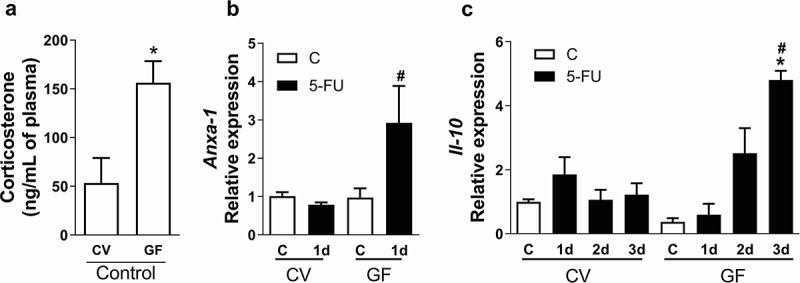
Corticosterone levels, Anxa-1 and Il-10 relative expression were increased early in gut of GF mice after chemotherapy injection. Corticosterone level was increased in plasma of GF mice when compared to CV mice (a). Anxa-1 expression was increased 6 h after first injection of 5-FU in GF mice when compared to CV-5-FU and GF-C (b). Il-10 expression was increased 3 d after 5-FU treatment only in GF mice when compared to CV-5-FU and GF-C (v) (c). The data represent the mean ± SEM of 4–5 animals per group. *p < 0.05 vs control. # in B p < 0.05 vs GF-5-FU. # in C p < 0.05 vs V-5-FU.
To study whether the presence of glucocorticoids could protect the CV mice to 5-FU-induced injury, we treated mice with dexamethasone, a synthetic glucocorticoid analogue. Prevention of intestinal shortening (Figure 7a), decrease in EPO (Figure 7b) and MPO activities (Figure 7c), and CXCL1 levels (Figure 7d) were evident in CV mice treated with 5-FU and dexamethasone when compared to vehicle-treated CV-5-FU mice. Next, we sought to address whether the action of glucocorticoids could account for the resistance of GF mice to 5-FU-induced injury. For this, we treated GF mice with a glucocorticoid receptor antagonist (RU486).31 Intestinal shortening (Figure 8a), increase in MPO activity (Figure 8b), IL-1β, and CCL24 levels (Figure 8c) were evident in GF mice treated with 5-FU and RU486 when compared to vehicle-treated GF-5-FU mice. Therefore, endogenous release of corticoids account for the resistance of GF mice to 5-FU-induced mucositis.
Figure 7.
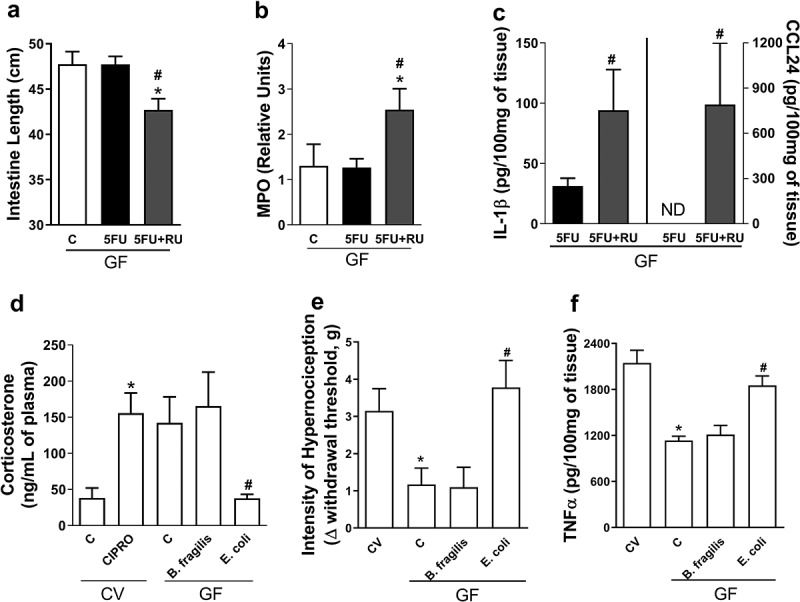
Dexamethasone treatment protects conventional mice from small intestine 5-FU-induced damage. Concomitant treatment with dexamethasone (2 mg/Kg, i.p.) and 5-FU was associated with prevention of the small intestine shortening when compared to the group treated only with 5-FU (a). There were also prevention of heightened EPO (b) and MPO (c) activities and CXCL1 chemokine production (d) in dexa-5-FU group. The data represent the mean ± SEM of 5 animals per group. *p < 0.05 vs control. #p < 0.05 vs 5-FU-V.
Figure 8.
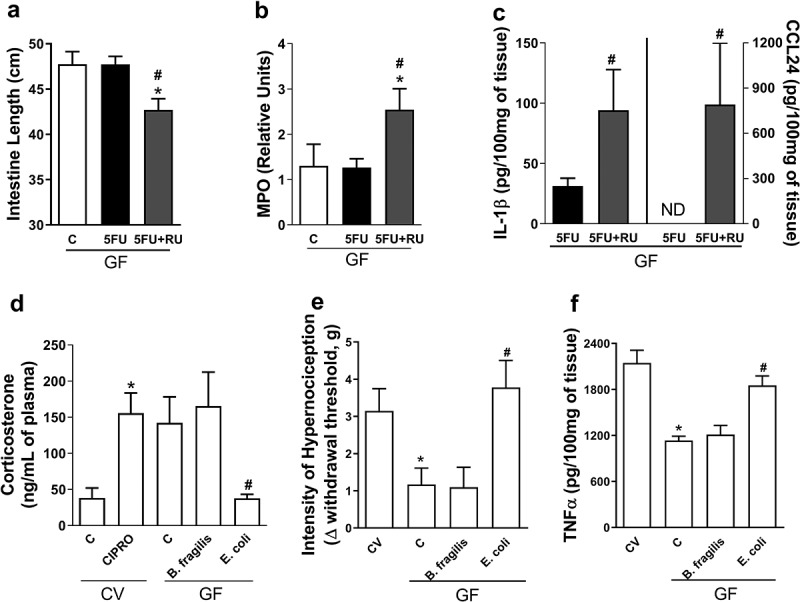
Plasma corticosterone basal levels modulate 5-FU-induced injury and inflammation and Enterobacteriaceae and E. coli were able to reduce basal plasma levels of this hormone. GF mice treated with glucocorticoid receptor antagonist (RU486) and 5-FU presented small intestines shortening (a) and increased MPO activity (b), IL-1β and CCL24 (c) levels in small intestines when compared to vehicle-treated GF-5-FU group. GF mice monolonizated with E. coli for 7 d, but not with B. fragilis, showed reduction of basal levels of corticosterone, when compared to GF mice (d) while cipro treatment in CV mice promoted increase in corticosterone concentration. Carrageneen injection on GF mice induced a diminished hypernociception in relation to CV mice (e). GF mice monocolonized with B. fragilis for 7 d and injected with carrageenan showed a similar result. However, carrageenan injection in GF mice monocolonized with E. coli caused higher hyperalgesia compared to GF group. After injection of carrageenan, GF and B. fragilis group had a lower increase in TNF-α concentration in paw when compared to CV animals (f). On the other hand, GF mice monocolonized with E. coli presented increased concentration of TNF-α to levels similar to CV animals. The data represent the mean ± SEM of 5–6 animals per group. *p < 0.05 vs CV-C, #p < 0.05 vs GF-5-FU. In D-GF*p < 0.05 vs CV-C, #p < 0.05 vs GF-C.
Since Enterobacteriaceae and E. coli exacerbated chemotherapy-induced tissue injury in CV mice and reversed the protective phenotype of monocolonized GF mice, our next step was to evaluate whether this bacteria family was able to modulate corticosterone concentration in both conditions. As shown in Figure 8d, E. coli-monocolonized mice showed reduced basal levels of corticosterone in plasma when compared to GF and B. fragilis-monocolonized groups. On the other hand, treatment of CV mice with ciprofloxacin led to increased corticosterone levels as compared to untreated CV mice.
Finally, we assessed whether the reduced corticosterone levels found in mice monocolonized with E. coli would impact in inflammatory responses in sites other than the intestine. For this, we subjected E. coli- or B. fragilis-monocolonized mice to an inflammatory hyperalgesia model induced by the intraplantar injection of carrageenan. As demonstrated previously,21 we observed that hypernociceptive responses are lower in GF animals than in CV mice (Figure 8e). In addition, GF animals had lower increase in the concentration of TNF-α after injection of carrageenan (figure 8f). Interestingly, GF mice monocolonized with E. coli showed increased hypernociceptive responses and enhanced TNF-α production in response to carrageenan injection as compared to the GF group. However, monocolonization with B. fragilis had no effect on the phenotype of GF mice, that persisted with low intensity of the hypernociceptive response and low concentration of TNF-α (Figure 5f,g). These results indicate that monocolonization with E. coli, but not B. fragilis, is able to change systemic corticosterone production and the hyporesponsive phenotype of GF mice both locally in the gut and systemically.
Discussion
The present study was conducted to investigate the relevance of the intestinal microbiota for the inflammatory response and small intestine injury induced by chemotherapy and to determine the mechanisms involved in the control of host inflammatory responsiveness by specific bacterial taxa colonizing the mammalian gut. We found that (i) the presence of the microbiota is involved in the development and severity of 5-FU chemotherapy-induced mucositis; (ii) 5-FU-treated CV mice have an increase in E. coli and Enterobacteriaceae content; (iii) Enterobacteriaceae, including E. coli, contributes to the exacerbation of gut damage and inflammation during 5-FU-induced mucositis; (iv) Enterobacteriaceae content in the intestinal microbiota shifts plasma levels of corticosterone and modulates the response to inflammatory stimuli.
First, we used GF mice and antibiotic-treated animals for either depletion or reduction of microbiota load to study whether microbiota presence was required for exacerbated inflammation and tissue damage in 5-FU-induced mucositis. Interestingly, we showed that all the groups without microbiota, even the one that received a short-term treatment schedule, were resistant to 5-FU-induced gut injury and presented decreased neutrophil and eosinophil accumulation, and reduced levels of CXCL1 in the intestine when compared to CV mice that received the same chemotherapy treatment. Some researchers have already shown that GF mice are markedly resistant to mucositis induced by chemotherapy or radiation when compared to CV mice.30,32,33 We confirmed that the microbiota is important to 5-FU-induced gut damage through conventionalization of GF mice after gavage with feces from CV mice. In this group, it was possible to observe restored neutrophil and eosinophil influx into intestinal tissue after 5-FU treatment. Similar results were observed after irinotecan or radiation treatment of conventionalized GF mice.30,33 Altogether, we demonstrated that intestinal microbiota presence plays a key role in increasing 5-FU-induced inflammation and damage in the small intestine.
Van Vliet et al. (2010) have hypothesized that the microbiota has a pivotal role in mucositis, once chemotherapeutics can deregulate intestinal microbiota homeostasis.17 Furthermore, other factors can also deregulate the intestinal microbiota, including inflammation34 and antimicrobial peptides.11 Shifts in microbiota composition differ along the GI tract and according to chemotherapy regime.18,35,36 Usually, after chemotherapy in patients and in rodent models, there is outgrowth of opportunistic bacteria with a concomitant reduction in the content of bacterial taxa responsible for the maintenance of the intestinal ecosystem.18,37 Overall, some common alterations include increase in the total number of facultative anaerobes in the colon, such as Enterobacteriaceae, and decrease in Firmicutes members.18,36,37 Although, there are differences in the shifts in bacterial groups in previous reports, the anticancer treatments have in common a reduction of the diversity and richness of bacterial community.19 In our model, we observed an expressive increase in Enterobacteriaceae and E. coli amount in feces and lumen content of mice after 5-FU-treatment, but no significant changes in the content of Bacteroidetes and Firmicutes phyla during disease. This microbiota deregulation can be induced by chemotherapeutics, but other factors can also deregulate the intestinal microbiota, including inflammation.34 Winter and colleagues34 have shown that metabolites from inflammation in the colon can selectively enhances the growth of commensal Enterobacteriaceae promoting dysbiosis. The same process may promote Enterobacteriaceae and E. coli growth in small intestine. Although our findings support the conclusion that chemotherapy induces dysbiosis in gut microbiota, there is still a long way before understanding the shifts in gut microbiota and its causes during mucositis.
Causal relationship between intestinal bacteria dysbiosis and mucositis is still a matter of debate. Findings of other groups have shown that there are marked changes in microbiota composition (dysbiosis) coincidentally in time of the development of chemotherapy-induced mucositis injury in humans and in animal models.32–37 We observed, for the first time, to our knowledge, direct relationship between dysbiotic Enterobacteriaceae and 5-FU-induced mucositis injury. We showed that treatment of CV mice with ciprofloxacin prevented Enterobacteriaceae increase after 5-FU treatment and resulted in markedly diminished intestine injury or inflammation. Interestingly, intestines of GF mice monocolonized with E. coli presented clear signs of tissue damage and inflammation. Moreover, the pivotal role of E. coli expansion in the severity of mucositis is clearly demonstrated by reversion of GF hyporesponsiveness after monocolonization with E. coli isolated from CV mice with mucositis. Uncontrolled expansion of the Enterobacteriaceae family was also required for the Toxoplasma gondii-induced small intestinal pathology.11 Altogether, these data suggest that the shift in microbiota induced by chemotherapy, including the increase in Enterobacteriaceae content, may amplify the inflammatory mechanisms responsible for tissue injury during mucositis, such as demonstrated for others bowel diseases11-14 or bolster the effects of 5-FU through metabolic drug interconversion.38
Over the past years, our group has demonstrated that the microbiota is also essential for host ability to mount canonical acute inflammatory responses.1–3,21 The overall picture that arose from these past studies is that the microbiota fine-tunes host inflammatory responsiveness: in the absence of colonization, mice respond to inflammatory triggers by up-regulating production of anti-inflammatory molecules such as ANXA-1, LXA4, and IL-10 and intestinal colonization by the microbiota blocks this skewed response, favoring pro-inflammatory mediator production and leukocyte mobilization to the gut. It is known that the microbiota has the ability to control the secretion of glucocorticoids by epithelial cells in the gut22 and interferes in adrenal-released corticosterone levels upon stress.39 We found that the high plasma corticosterone levels observed in GF mice is important for attenuating intestinal damage in mucositis. Hence, the response of GF mice to chemotherapy-induced mucositis also follows this previously described pattern. GF treated with glucocorticoid receptor antagonist (RU486) presented partial reversal of the protective phenotype of these animals after 5-FU injection lesion. Indeed, we and others have shown that administration of a synthetic glucocorticoid, dexamethasone, to CV animals resulted in decreased tissue injury and inflammation induced by 5-FU.40
The presence and action of glucocorticoids may account for many of the results presented by the GF-5-FU group, because glucocorticoids may reduce leukocyte influx to tissues and inhibit the synthesis of several proinflammatory cytokines, in addition to increasing the synthesis of anti-inflammatory molecules such as ANXA-1 and IL-10.23–25 Thus, it is possible to suggest that the higher plasma basal corticosterone concentration in GF animals prevented the development of mucositis lesions by promoting early expression of anti-inflammatory molecules and inhibiting the synthesis of pro-inflammatory mediators. During chemotherapy, there is an early increase in ANXA-1 and IL-10 up-regulation (1st and 3rd days, respectively) in tissues of GF mice. These data suggest that in the absence of microbiota, the inflammatory response to mucositis induction is altered due to increased ANXA-1 and IL-10 upon chemotherapy. Other researchers have previously shown that increased IL-10 production is associated to decreased chemotherapy-induced injury in CV mice,41,42 but the role of IL-10 on chemotherapy-induced mucositis is still unknown. Nevertheless, our findings suggest that the IL-10-skewed response of GF mice to 5-FU injection would be able to prevent the production of pro-inflammatory mediators and intestinal damage.
Interestingly, GF mice monocolonized with E. coli or B. fragilis showed divergence in the control of plasma corticosterone concentration and in the response to 5-FU chemotherapy. While E. coli monocolonization led to reduced levels of this hormone, B. fragilis monoassociation did not change plasma corticosterone concentration. In accordance, E. coli-associated mice responded to 5-FU treatment and carrageenan injection by upregulating pro-inflammatory mediators and inducing leukocyte recruitment to tissue, while B. fragilis-colonized mice responded in the same way as GF hosts. Furthermore, CV mice treated with ciprofloxacin that presented reduction of Enterobacteriaceae and E. coli content and increased plasma corticosterone concentrations were protected from 5-FU induced intestinal inflammation. These findings suggest that Enterobacteriaceae may favor inflammation by reducing basal plasma corticosterone levels. Our group has previously observed that GF mice previously injected with LPS from E. coli presented increase in local or systemic inflammatory responses after being exposed to stimuli of sterile or infectious nature.3 However, we observed that LPS (30ug/mL) from E. coli, when given in drinking water following the protocol previously shown to enhance colitis disease43 do not revert the phenotype of GF mice submitted to 5-FU-induced mucositis. It suggests that TLR4 activation may not be sufficient or that other structural components or even alive E. coli may be required for reversion of GF phenotype and corticosterone production.
Altogether, our results allow us to conclude that microbiota colonization shifts the way the host perceives and respond to inflammatory triggers by modulating the basal corticosterone concentration in circulation. More specifically, Enterobacteriaceae and E. coli are able to reduce basal corticosterone levels and to exacerbate host response to several inflammatory stimuli, including chemotherapy-induced mucositis. In general, this work shows that the maintenance of the indigenous gut microbiota is important to contain or even prevent the side effect of chemotherapy on intestinal mucosa.
Materials and methods
Animals
Germ free Swiss/NIH mice were derived from a GF nucleus from Taconic Farms and maintained in flexible plastic isolators (Standard Safety Equipment). Conventional Swiss mice are derived from GF matrices, and considered conventional only after two generations in the conventional facility. All animals were 6- to 8-week-old males and females. GF condition was monitored by collection of feces, which were homogenized in PBS, serially diluted, and then plated on brain heart infusion (BHI) or thioglycolate broth for 24 h at 37°C in aerobic or anaerobic conditions to determine the absence of intestinal microbes. Animals were age matched and maintained according to the ethical guidelines of our institution, and the experimental protocol was approved by the Ethics Committee in Animal Experimentation of the Federal University of Minas Gerais.
Depletion of gut microbiota
Mice were treated with antibiotics as previously described.44 Briefly, conventional Swiss newborn mice were continuously provided with ampicillin (2 g/L), vancomycin (0.5 g/L), neomycin (2 g/L), metronidazole (1 g/L), and ciprofloxacin (0.2 g/L) in drinking water ad libitum for six weeks prior to experimentation. Fresh antibiotics were supplied twice a week. Adult mice were submitted to similar protocols for 7 or 30 d before experimentation. During 5-FU treatment, the cocktail of antibiotics was kept in the drinking water.
Experimental intestinal mucositis
For induction of mucositis, we used 5-fluorouracil (5-FU), a chemotherapy utilized for the treatment of solid cancers.26 Saline or 5-FU (450 mg/kg) was given intraperitoneally (i.p.) once a day for three consecutive days. Five days after beginning the 5-FU treatment, mice were anesthetized and euthanized by cervical dislocation and blood samples and intestines were collected for analysis.
Determination of clinical score
Scoring for stool consistency and occult blood were done as previously described.45 In brief, stool scores were determined as follows: 0, well-formed pellets and negative occult blood test; 1, semi-formed stools and negative occult blood test; 2, normal stool (consistent) and traces of blood in the occult blood test; 3, semi-formed stool and positive occult blood test; 4, liquid stools and positive occult blood test; 5, semi-formed stool, negative occult blood test and signs of morbidity; 6, semi-formed stool, positive occult blood test and signs of morbidity; 7, liquid stools, positive occult blood test and signs of morbidity. Signs of morbidity included the creepy, hunched posture and reduced mobility.
Determination of the EPO and MPO activities
The extent of tissue eosinophil infiltration was assessed by measuring EPO as previously described.45 Briefly, intestines were weighted and homogenized with PBS and centrifuged at 7,826 x g for 10 minutes. The supernatant was discarded, and the erythrocytes were lysed. The pellet was suspended in 1.9 mL of 0.5% hexadecyltrimethyl ammonium bromide in PBS, frozen three times in liquid nitrogen, and centrifuged at 4°C at 7,826 x g for 10 minutes. The supernatant was used in the enzymatic assay by the addition of an equal amount of substrate (1.5 mmol/L o-phenylenediamine and 6.6 mmol/L H2O2 in 0.075 mmol/L Tris-HCl (pH 8)). The reaction was stopped with 50 µL of 1 M H2SO4, and the absorbance was read at 492 nm.
The extent of neutrophil accumulation in the intestine was measured by assaying MPO activity, as described previously.2 Briefly, a portion of intestine of animals were removed and intestine were weighted and homogenized with PBS and centrifuged at 7,826 x g for 10 minutes. The supernatant was discarded, and the erythrocytes were lysed. The pellet was suspended in 1.9 mL of 0.5% hexadecyltrimethyl ammonium bromide in 0.05 M Na3PO4 buffer (pH = 5.4), frozen three times in liquid nitrogen, and centrifuged at 4°C at 7,826 x g for 10 min. The supernatant was used in the enzymatic assay by the addition of an equal amount of substrate (0.4 M of tetramethylbenzidine in DMSO and 0.002% H2O2). The reaction was stopped with 50 µL of 1 M H2SO4, and the absorbance was read at 450 nm. Results were expressed as the relative unit that denotes activity of MPO related to casein-elicited murine peritoneal neutrophils processed in the same way.
Measurement of cytokine concentrations in intestine
The concentration of CXCL1, CCL24, IL-10, TNF-α, and IL-1β were measured in intestine of mice using commercially available antibodies and according to the procedures supplied by the manufacturer (R&D Systems).
Histopathological analysis
Intestine samples were immediately fixed in 10% buffered formalin. Tissue sections were stained with H&E. The histologic score was obtained based on the intensity of mononuclear and polymorphonuclear infiltrates in the lamina propria, changes in the architecture of the mucosa, decreased villus height, hyperemia and edema as previously described.45 For each parameter, the changes were graded according to the following scale: 0, absent; 1, mild; 2, moderate; and 3, intense. The inflammation score was represented by numbers from 0 (normal) to 14 (highly altered). The histological analysis was performed by a single examiner masked to the experimental group’s status.
Immunohistochemistry for proliferating cell nuclear antigen (PCNA)
Tissue sections from small intestine were incubated with primary antibody to PCNA (1:750; rabbit mAb; Cell Signaling). The secondary antibody (Cell Signaling) peroxidase conjugated was used consecutively. The reaction development was performed by incubation in diaminobenzidine solution with hydrogen peroxide. Subsequently, these sections were counterstained with hematoxylin and used for analysis.
Conventionalization of GF mice
GF mice were conventionalized as previously described.2 Briefly, fecal samples removed from CV mice were homogenized in saline (10%) and administered by oral gavage to GF mice. Thirty days later, 5-FU-treatment was given to these animals, as described above.
Bacterial culturing
For analysis of Enterobactericaeae, stools and lumen content of small intestine were separated and homogenized in sterile PBS. The homogenates were serially diluted and plated on the MacConkey solid medium. Plates were incubated aerobically or anaerobically at 37°C for 24 h.
Isolation of bacterial genomic DNA and microbiota analysis by quantitative PCR
Stool and lumen content of small intestines of mice were collected on first, third and 5th day along 5-FU treatment. Genomic DNA was extracted from feces pellets with the Qiagen Stool Kit. Quantitative PCR was performed to quantify the abundance of 16 S rRNA sequences of bacteria.
Reverse transcription and real-time PCR for gene expression assay
Total RNA from gut was prepared using Trizol (Thermofisher Scientific). Il-10, Anxa-1 and Rpl-4 (housekeep gene) cDNA were amplified using specific primers (Invitrogen) and SYBER green reagent (Applied Biosystems) in a 7500 Fast Real-Time PCR System (Applied Biosystems).
E. coli and Bacteroides fragilis monocolonized GF mice
GF mice were monocolonized with E. coli (ATCC: 25922) or E. coli isolated from stools of CV mice with mucositis (ICVM) or Bacteroides fragilis (ATCC: 25285) by oral gavage with 108 UFC/mL of each bacteria. Seven days later, 5-FU-treatment was conducted in these animals, as described above.
Treatment with LPS
LPS (30µg/mL) from E. coli (O111:B4) was given in drinking water to GF mice and 1 day later mice were submitted to 5-FU treatment.43 LPS treatment was kept in the drinking water throughout the entire experiment.
Treatment with dexamethasone or RU486
Mice were treated intraperitonially (i.p.) with 2 mg/Kg of dexamethasone or subcutaneously (s.c.) with RU 486 concomitant with 5-FU injection. Control animals were injected with saline or oil, respectively.
Plasma corticosterone analysis
Plasma corticosterone levels were measured from ad libitum fed mice. The blood was collected from cava vein among 7- and 8-h a.m. in heparinized tube. Corticosterone was measured by ELISA kit from Cayman.
Statistical analysis
All results are reported as mean ± SEM. Statistical analysis was performed using analysis of variance, followed by Newman–Keuls test. Unpaired t test was used to determine differences between two groups. p < 0.05 was considered statistically significant. Statistical analyses were performed using Prisim4 (GraphPad) software.
Supplementary Material
Funding Statement
This work was supported by the Fundação do Amparo a Pesquisa do Estado de Minas Gerais, the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and the Instituto Nacional de Ciência e Tecnologia em Dengue e Interação Microrganismo Hospedeiro. C.T.F. was funded by the Jovens Talentos Scholarship (Bolsa Jovens Talentos) program of the Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Supplementary mateial
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM, et al. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 2007;179(12):8533–8543. doi: 10.4049/jimmunol.179.12.8533. [DOI] [PubMed] [Google Scholar]
- 2.Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM.. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173(6):4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- 3.Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM, Souza DG. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germ free mice. J Immunol. 2012a;188(3):1411–1420. doi: 10.1126/science.aad9378. [DOI] [PubMed] [Google Scholar]
- 4.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science. 2016;352(6285):535–538. doi: 10.1126/science.aad9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic disease and hematopoiesis. Nat Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 8.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W, Chen F, Liu Z, Cong Y. Microbiota-specific Th17 cells: yin and yang in regulation of inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(6):1473–1482. doi: 10.1097/MIB.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molloy MJ, Grainger JR, Bouladoux N, Hand TW, Koo LY, Naik S, Quinones M, Dzutsev AK, Gao JL, Trinchieri G, et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe. 2013;14(3):318–328. doi: 10.1016/j.chom.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raetz M, Hwang SH, Wilhelm CL, Kirkland D, Benson A, Sturge CR, Mirpuri J, Vaishnava S, Hou B, Defranco AL, et al. Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-γ-dependent elimination of Paneth cells. Nat Immunol. 2013;14(2):136–142. doi: 10.1038/ni.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. Isme J. 2007;1(5):403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 13.Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127(2):412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 14.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2(2):119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Stringer AM, Gibson RJ, Bowen JM, Keefe DM. Chemotherapy-induced mucositis: the role of gastrointestinal microflora and mucins in the luminal environment. J Support Oncol. 2007;5(6):259–267. PMID: 17624050. [PubMed] [Google Scholar]
- 16.Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E, Yokoi T, Kamataki T. Involvement of beta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res. 1996;56(16):3752–3757. PMID: 8706020. [PubMed] [Google Scholar]
- 17.van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6(5):e1000879. doi: 10.1371/journal.ppat.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Vliet MJ, Tissing WJ, Dun CA, Meessen NE, Kamps WA, de Bont ES, Harmsen HJ. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis. 2009;49(2):262–270. doi: 10.1086/599346. [DOI] [PubMed] [Google Scholar]
- 19.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetière MF.. Systematic review: the role of the gut microbiota in chemotherapy- or radiation- induced gastrointetinal mucositis – corrent evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40(5):409–421. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 20.Fijlstra M, Ferdous M, Koning AM, Rings EH, Harmsen HJ, Tissing WJ. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support Care Cancer. 2015;23(6):1513–1522. doi: 10.1007/s00520-014-2487-6. [DOI] [PubMed] [Google Scholar]
- 21.Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM, Ferreira SH, Cunha FQ, Silva TA, Nicoli JR, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci. 2008;05(6):2193–2197. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153(4):812–827. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9(1):62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 24.Barnes PJ. How corticosteroids control inflammation: quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148(3):245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol. 2007;275(1–2):79–97. doi: 10.1016/j.mce.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 27.Mortensen NP, Boisen N, Carey S, Kennel SJ, Fowlkes JD, Doktycz MJ, Nataro JP, Allison DP. Enteroaggregative Escherichia coli: surface protein dispersin increases bacterial uptake of ciprofloxacin. Int J Antimicrob Agents. 2013;42(5):462–465. doi: 10.1016/j.ijantimicag.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Löfmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50(1):16–23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 29.Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomicin: review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51(1):11–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci. 2005;102(37):13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang B, Trump RP, Shen Y, McNulty JA, Clifton LG, Stimpson SA, Lin P, Pahel GL. RU486 did not exacerbate cytokine release in mice challenged with LPS nor in db/db mice. BMC Pharmacol. 2008;12(8):7. doi: 10.1186/1471-2210-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandi G, Dabard J, Raibaud P, Di Battista M, Bridonneau C, Pisi AM, Morselli Labate AM, Pantaleo MA, De Vivo A, Biasco G. Intestinal microflora and digestive toxicity of irinotecan in mice. Clin Cancer Res. 2006;12(4):1299–1307. doi: 10.1158/1078-0432.CCR-05-0750. [DOI] [PubMed] [Google Scholar]
- 33.Pedroso SH, Vieira AT, Bastos RW, Oliveira JS, Cartelle CT, Arantes RM, Soares PM, Generoso SV, Cardoso VN, Teixeira MM, et al. Evaluation of mucositis induced by irinotecan after microbial colonization in germ-free mice. Microbiol. 2015;161(10):1950–1960. doi: 10.1099/mic.0.000149. [DOI] [PubMed] [Google Scholar]
- 34.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013b;339(6120):708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stringer AM, Gibson RJ, Bowen JM, Keefe DM. Chemotherapy-induced modifications to gastrointestinal microflora: evidence and implications of change. Curr Drug Metab. 2009;10(1):79–83. PMID: 19149515. doi: 10.2174/138920009787048419. [DOI] [PubMed] [Google Scholar]
- 36.Von Bultzingslowen I, Adlerberth I, Wold AE, Dahlén G, Jontell M. Oral and intestinal microflora in 5-fluorouracil treated rats, translocation to cervical and mesenteric lymph nodes and effects of probiotic bacteria. Oral Microbiol Immunol. 2003;18(5):278–284. PMID: 12930518. doi: 10.1034/j.1399-302X.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 37.Stringer AM Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Hamilton J, Keefe DM. Gastrointestinal microflora and mucins may play a critical role in the development of . 5-Fluorouracil-induced gastrointestinal mucositis. Exp Biol Med. 2009b;234(4):430–441. doi: 10.3181/0810-RM-301. [DOI] [PubMed] [Google Scholar]
- 38.Scott TA, Quintaneiro LM, Norvaisas P, Lui PP, Wilson MP, Leung KY, Herrera-Dominguez L, Sudiwala S, Pessia A, Clayton PT, et al. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell. 2017. 20;169(3):442–456.e18. doi: 10.1016/j.cell.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;1(558):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lara RN, da Guerra EN, de Melo NS. Macroscopic and microscopic effects of GaAIAs diode laser and dexamethasone therapies on oral mucositis induced by fluorouracil in rats. Oral Health Prev Dent. 2007;5(1):63–71. PMID: 17366763. [PubMed] [Google Scholar]
- 41.Justino PF, Melo LF, Nogueira AF, Morais CM, Mendes WO, Franco AX, Souza EP, Ribeiro RA, Souza MH, Soares PM. Regulatory role of Lactobacillus acidophilus on inflammation and gastric dysmotility in intestinal mucositis induced by 5-fluorouracil in mice. Cancer Chemother Pharmacol. 2015;75(3):559–567. doi: 10.1007/s00280-014-2663-x. [DOI] [PubMed] [Google Scholar]
- 42.de Araújo AA, Varela H, de Medeiros CA, de Castro Brito GA, de Lima KC, de Moura LM, RF. De Araújo Júnior. Azilsartan reduced TNF-α and IL-1β levels, increased IL-10 levels and upregulated VEGF, FGF, KGF, and TGF-α in an oral mucositis model. PLoS One. 2015;10(2):e0116799. doi: 10.1371/journal.pone.0116799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gronbach K, Flade I, Holst O, Lindner B, Ruscheweyh HJ, Wittmann A, Menz S, Schwiertz A, Adam P, Stecher B, et al. Endotoxicity of lipopolysaccharide as a determinant of T-Cell−mediated colitis induction in mice. Gastroenterology. 2014;146(3):765–775. doi: 10.1053/j.gastro.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 44.Marques PE, Oliveira AG, Pereira RV, David BA, Gomides LF, Saraiva AM, Pires DA, Novaes JT, Patricio DO, Cisalpino D, et al. Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice. Hepatology. 2015;61(1):348–360. doi: 10.1002/hep.27216. [DOI] [PubMed] [Google Scholar]
- 45.Arifa RD, Madeira MF, De Paula TP, Lima RL, Tavares LD, Menezes-Garcia Z, Fagundes CT, Rachid MA, Ryffel B, Zamboni DS, et al. Inflammasome activation is reactive oxygen species dependent and mediates irinotecan-induced mucositis through IL-1β and IL-18 in mice. Am J Pathol. 2014;184(7):2023–2034. doi: 10.1016/j.ajpath.2014.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


