ABSTRACT
Allergic asthma is a highly prevalent inflammatory disease of the lower airways, clinically characterized by airway hyperreactivity and deterioration of airway function. Immunomodulatory probiotic bacteria are increasingly being explored to prevent asthma development, alone or in combination with other treatments.
In this study, wild-type and recombinant probiotic Lactobacillus rhamnosus GR-1 were tested as preventive treatment of experimental allergic asthma in mice. Recombinant L. rhamnosus GR-1 was designed to produce the major birch pollen allergen Bet v 1, to promote allergen-specific immunomodulation. Administration of wild-type and recombinant L. rhamnosus GR-1 prevented the development of airway hyperreactivity. Recombinant L. rhamnosus GR-1 also prevented elevation of airway total cell counts, lymphocyte counts and lung IL-1β levels, while wild-type L. rhamnosus GR-1 inhibited airway eosinophilia. Of note, a shift in gut microbiome composition was observed after asthma development, which correlated with the severity of airway inflammation and airway hyperreactivity. In the groups that received L. rhamnosus GR-1, this asthma-associated shift in gut microbiome composition was not observed, indicating microbiome-modulating effects of this probiotic.
These data demonstrate that L. rhamnosus GR-1 can prevent airway function deterioration in allergic asthma. Bet v 1 expression by L. rhamnosus GR-1 further contributed to lower airway inflammation, although not solely through the expected reduction in T helper 2-associated responses, suggesting involvement of additional mechanisms. The beneficial effects of L. rhamnosus GR-1 correlate with increased gut microbiome resilience, which in turn is linked to protection of airway function, and thus further adds support to the existence of a gut-lung axis.
KEYWORDS: Probiotics, allergy, mouse model, gut microbiome, airway hyperreactivity, gut-lung axis, lactobacillus, microbiota, airway inflammation, birch pollen
Introduction
Allergic asthma presents a compelling challenge for public health due to its increasing prevalence in both developed and developing countries and the linked socio-economic costs.1,2 Airway hyperreactivity (AHR) and inflammation are the hallmark features of asthma.3 Recent data suggest that immune function development and susceptibility to disease are strongly influenced by the diverse community of resident microorganisms, or microbiota, in particular in the context of allergic disease.4 In fact, different members of the mammalian microbiota continuously undergo dynamic interactions with the immune, epithelial, and other host cells.5-7 Consequently, attempts have been made to positively steer the development of immune functions by administering specific microorganisms or probiotics, that can potentially prevent or treat allergic disease.8,9 Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”10 Various strains of the Lactobacillus rhamnosus phylogenetic group were shown to offer benefits in the context of allergic disease prevention and treatment, both in animal models and in human clinical trials.11-17 Up to now, allergy prevention with probiotic L. rhamnosus strains isolated from healthy humans has been primarily focused on the effects of L. rhamnosus GG (a gut isolate),18 while probiotic L. rhamnosus GR-1 (a urogenital isolate) was mostly used for urogenital health applications.19 Nonetheless, L. rhamnosus GR-1 was previously shown to have both local and systemic immunomodulatory effects.15,20
Due to their ability to influence the host immune system, recombinant L. rhamnosus strains are also increasingly explored as live delivery vehicles for prophylactic and therapeutic molecules to mucosal surfaces.20-22 Previously, pretreatment with Lactobacillus plantarum expressing allergenic proteins has led to improved allergen-specific outcomes in experimental mouse models of allergic disease induced by house dust mite and birch pollen. Prophylactic administration of wild-type Lactobacillus strains resulted in reduced signs of airway inflammation (e.g. eosinophilia and lung IL-5), while recombinant allergen expression additionally decreased allergen-specific IgE production associated with T helper 2 (Th2) responses, and increased levels of protective IgG2a and/or IgA antibodies.23,24 In addition to shifting the immune balance from Th2 activity associated with allergy development toward T helper 1 (Th1),23,24 probiotic effects in mouse models of allergic disease have also been linked to induction of regulatory responses, such as an increase in Forkhead box P3 (FoxP3) expression characteristic of regulatory T cells (Treg).25
Although the beneficial effects of L. rhamnosus GG co-administration with allergens have previously been investigated,12 to our knowledge, no studies evaluating recombinant L. rhamnosus GR-1 strains producing a clinically relevant allergen have been reported so far.
Therefore, in this study, live L. rhamnosus GR-1 was genetically modified to produce the major birch pollen allergen Bet v 1, to which 90–95% of birch pollen-allergic patients are sensitized.26 We analyzed the prophylactic effects of orally administered wild-type and recombinant L. rhamnosus GR-1 in a mouse model of birch pollen-induced allergic asthma, focusing on airway function and inflammation analysis, as well as linking allergic asthma severity with gut microbiome alterations.
Results
Recombinant L. rhamnosus GR-1 strain CMPG11250 produces the Bet v 1 pollen allergen
To assess the beneficial effects of expressing the major birch pollen allergen Bet v 1 in L. rhamnosus GR-1 that could contribute to allergen-specific immunomodulation, a recombinant L. rhamnosus GR-1 strain CMPG11250 was constructed and compared to wild-type L. rhamnosus GR-1. CMPG11250 carries the betv1 gene on the pCMPG11228 plasmid (Figure 1a) under the control of the nisin-inducible gene expression (NICE) system. Bet v 1 production in nisin-induced CMPG11250 was confirmed by Bet v 1-specific Western blotting (Figure 1b) and quantified at approximately 10.5 µg Bet v 1 per 1010 colony-forming units (CFU) of CMPG11250.
Figure 1.
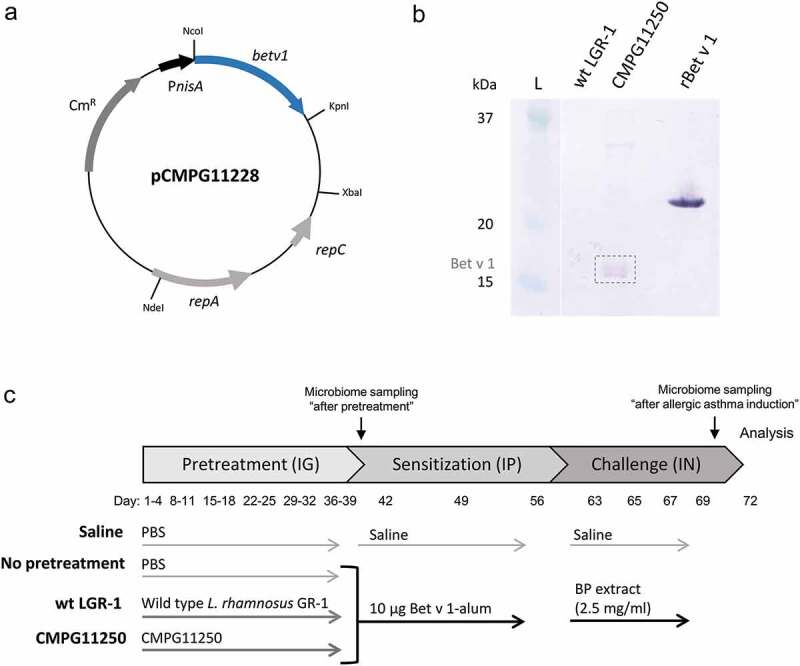
(ab) Production of Bet v 1 by the L. rhamnosus GR-1-derived strain CMPG11250 using the NICE expression system. (a) Map of the pCMPG11228 plasmid, a pMEC45 derivative carrying the betv1 gene under the control of the lactococcal inducible nisA promoter (PnisA). The lactococcal pSH71 replicon genes repA and repC and the chloramphenicol resistance cassette (CmR) are indicated. (b) Western blot analysis of Bet v 1 production (dotted rectangle) in the whole-cell protein fraction of nisin-induced CMPG11250. Whole cell protein fraction of wild-type L. rhamnosus GR-1 (wt LGR-1) is depicted for comparison. The L lane represents part of the Precision Plus Protein Kaleidoscope 10–250 kDa protein size ladder. Lanes between the L lane and the wt LGR-1 lane have been removed. rBet v 1 represents purified recombinant His-tagged Bet v 1 used as control. (c) Protocol for testing the effects of intragastric pretreatment with wild-type L. rhamnosus GR-1 (wt LGR-1), or recombinant CMPG11250, on allergic asthma. IG: intragastric instillations; IP: intraperitoneal injections; IN: intranasal instillations; Bet v 1-alum: rBet v 1 with aluminum hydroxide; BP: birch pollen; Microbiome sampling: time points of feces collection for microbiome analysis.
L. rhamnosus GR-1 and CMPG11250 prevent airway function deterioration in mice
Subsequently, the effects of a preventive treatment with wild-type L. rhamnosus GR-1 and recombinant CMPG11250 were explored in a mouse model in which experimental allergic asthma was induced by Bet v 1 and birch pollen administration (Figure 1c). Wild-type L. rhamnosus GR-1 or CMPG11250 were administered 4 times a week for 6 weeks, followed by allergic sensitization with recombinant Bet v 1 with aluminum hydroxide (rBet v 1-alum) on days 42, 49 and 56, and allergic asthma induction by nasal application of birch pollen extract on days 63, 65, 67 and 69.
Both L. rhamnosus GR-1 and CMPG11250 preventive administration resulted in a significantly lower AHR (Figure 2a,b), which was reflected by a lower increase in airway resistance in response to increasing doses of methacholine. AHR is a hallmark manifestation of allergic asthma in human patients. Furthermore, CMPG11250 prevented the drop in forced expiratory volume at 0.2 s (FEV0.2%) expressed as a percentage of the baseline (Figure 2c). The provocative concentration of methacholine causing a 20% fall in FEV (PC20-FEV0.2%) was calculated to identify hyperreactive mice (Figure 2d). Most mice in the not pretreated asthmatic group were classified as hyperreactive, in contrast with only one mouse pretreated with wild-type L. rhamnosus GR-1, and none of the mice pretreated with CMPG11250 (significant when compared to the not pretreated asthmatic group).
Figure 2.
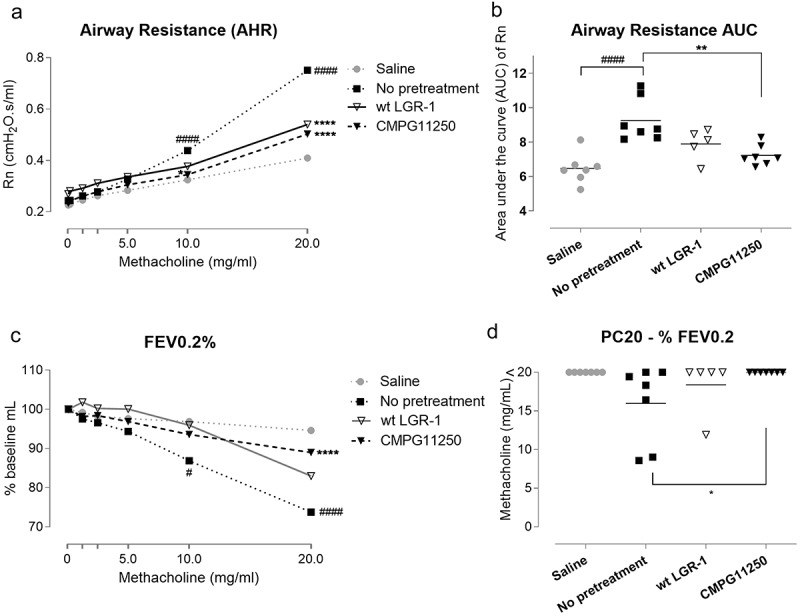
Airway function measurements following preventive treatment with wild-type L. rhamnosus GR-1 and CMPG11250, and allergic asthma induction. (a) Airway resistance (Rn) measurements in response to methacholine (0-20 mg/ml) as a reflection of airway hyperreactivity (AHR), and (b) Airway resistance (Rn) area under the curve (AUC) individual values and group means. (c) Forced expiratory volume as a percentage of baseline measurements at 0.2 s (FEV0.2%) in response to methacholine (0-20 mg/ml). (d) The concentration of provocative compound methacholine (PC20) causing a 20% drop in FEV0.2%. Mice are classified as hyperreactive if PC20 is lower than 20 mg/ml. Groups are labeled according to Figure 1c as follows: Saline: no asthma induction, not pretreated; No pretreatment: allergic asthma, not pretreated; wt LGR-1: allergic asthma, pretreated with wild-type L. rhamnosus GR-1; CMPG11250: allergic asthma, pretreated with recombinant L. rhamnosus GR-1 producing Bet v 1 (CMPG11250). Data are depicted as means. ##p < .01 and ####p < .0001 compared to the saline group; *p < .05, **p < .01 and ****p < .0001 compared to the no pretreatment group, n = 5–7 mice per group.
L. rhamnosus GR-1 and CMPG11250 attenuate airway inflammation, but not allergic sensitization
Infiltration of immune cells, such as eosinophils, into the airways, and the related pro-inflammatory cytokine production can further contribute to the decline in airway function through mediating tissue damage and remodeling.2,3 To assess the effects of wild-type L. rhamnosus GR-1 and CMPG11250 administration on airway inflammation, total and differential cell counts in the bronchoalveolar lavage fluid (BALF) (Figure 3ac and Figure S1A-B) were first performed. Significantly lower total cell (Figure 3a) and lymphocyte (Figure 3c) counts were observed for CMPG11250-pretreated mice relative to the not pretreated asthmatic control, while only a trend was observed toward lower eosinophilia (Figure 3b). These effects on total cell counts and lymphocytes were less pronounced after wild-type L. rhamnosus GR-1 pretreatment. However, prophylactic L. rhamnosus GR-1 administration led to significantly lower eosinophilia (Figure 3b), which is a typical feature of type 2 airway inflammation in half of asthmatic patients.2 No significant effect was found on macrophage and neutrophil counts (Figure S1A-B).
Figure 3.
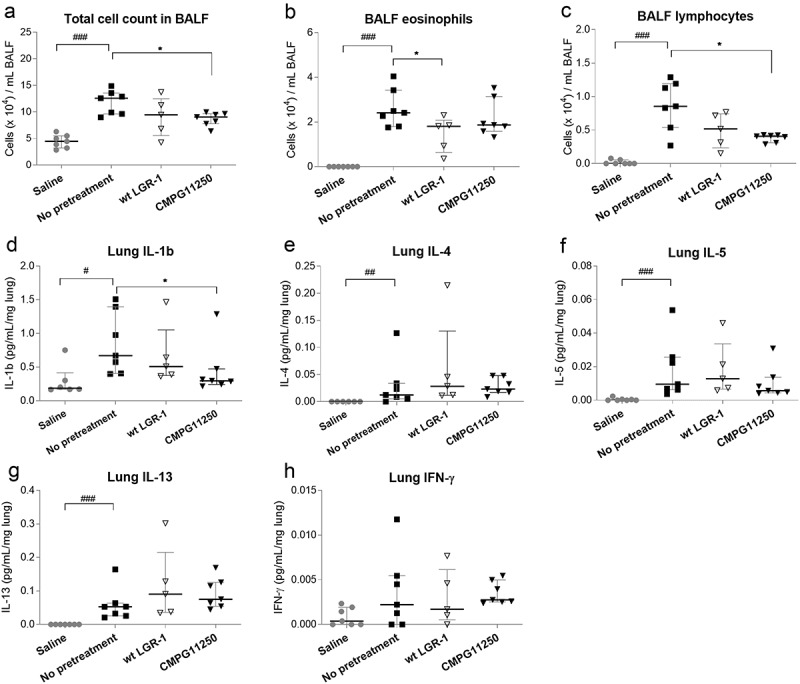
Total and differential cell counts in BALF (ac) and cytokine levels in lung homogenates (dh) following preventive treatment with wild-type L. rhamnosus GR-1 and recombinant CMPG11250 in a mouse model of allergic asthma. Total BALF cell count (a), eosinophil (b), and lymphocyte (c) counts per mL BALF are depicted, as well as levels of IL-1β (d), the Th2-associated cytokines IL-4 (e), IL-5 (f) and IL-13 (g), and the Th1-associated cytokine IFN-γ (h) in lung tissue. Groups are labeled according to Figure 1c as follows: Saline: no asthma induction, not pretreated; No pretreatment: allergic asthma, not pretreated; wt LGR-1: allergic asthma, pretreated with wild-type L. rhamnosus GR-1; CMPG11250: allergic asthma, pretreated with recombinant L. rhamnosus GR-1 producing Bet v 1 (CMPG11250). Data are depicted as median with interquartile range. #p < .05, ##p < .01 and ###p < .001 compared to the saline group; *p < .05 compared to the no pretreatment group; n = 5–7 mice per group.
Pretreatment with CMPG11250 also significantly reduced pro-inflammatory IL-1β levels in lung tissues (Figure 3d) compared to the not pretreated group. Th2-associated cytokines IL-4, IL-5 and IL-13 implicated in allergic asthma development (Figure 3e-g), pro-inflammatory cytokines IL-17, IL-33 and GF-CSF (Figure S1D-F), and the regulatory cytokine IL-10 (Figure S1 C) were analyzed in lung tissue homogenates, but no statistically significant effects of pretreatment were observed. The increase in frequencies of FoxP3+ cells as a percentage of CD4+ CD45+ cells in MLNs, which would indicate Treg cells, was likewise not significantly altered after L. rhamnosus GR-1 or CMPG11250 preventive treatment followed by allergic asthma induction (Figure S1 G). A tendency toward increase was observed in Th1-associated cytokine IFN-γ in lung tissues of the CMPG11250 group (Figure 3h).
Effects of L. rhamnosus administration on allergen-specific antibody production were evaluated by measuring serum levels of Bet v 1-reactive IgG1 and total IgE and Bet v 1-reactive IgG2a. Production of Bet v 1-specific IgG1 and total IgE were observed in the not pretreated group that was submitted to the allergic asthma induction protocol (Figure 4a,d), while no significant Bet v 1-specific IgG2a production was detected in this group (Figure 4b). Interestingly, the presence of recombinant expression of Bet v 1 in CMPG11250 correlated with a significantly higher Bet v 1-specific IgG1 (associated with Th2 allergic responses in mice), compared to the not pretreated asthmatic group (Figure 4a). However, there was also a trend toward higher levels of the Bet v 1-specific IgG2a (Figure 4b), which can be an indicator of Th1-associated immune responses. As would be expected during Bet v 1-specific immunotherapy resulting in an immune shift away from the allergenic Th2 responses toward Th1, the ratios of Bet v 1-specific IgG1 to IgG2a (Figure 4c), and total IgE to Bet v 1-specific IgG2a (Figure 4e) showed a decreasing trend in the CMPG11250 group, and to a lesser extent in the L. rhamnosus GR-1 pretreatment group.
Figure 4.
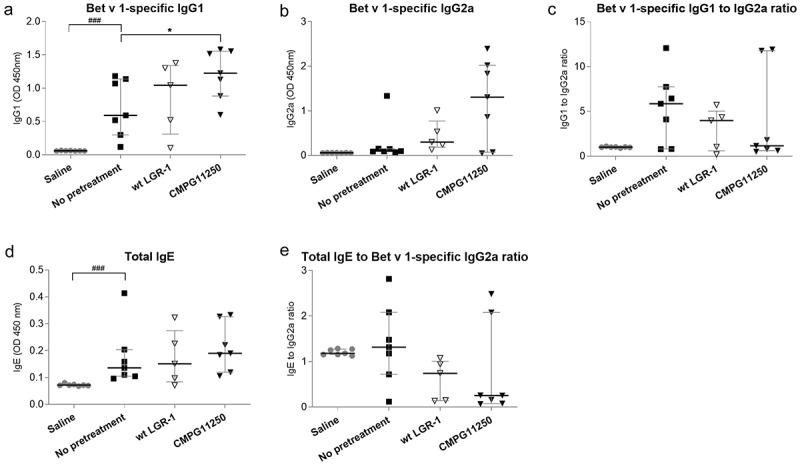
Serum antibody levels following preventive treatment with wild-type L. rhamnosus GR-1 and recombinant CMPG11250 in a mouse model of allergic asthma. Bet v 1-specific IgG1 (a), Bet v 1-specific IgG2a (b), ratios of Bet v 1-specific IgG1 to IgG2a (c), total IgE (d), and ratios of total IgE to Bet v 1-specific IgG2a (e) after L. rhamnosus preventive treatment and allergic asthma induction. Groups are labeled according to Figure 1c as follows: Saline: no asthma induction, not pretreated; No pretreatment: allergic asthma, not pretreated; wt LGR-1: allergic asthma, pretreated with wild-type L. rhamnosus GR-1; CMPG11250: allergic asthma, pretreated with recombinant L. rhamnosus GR-1 producing Bet v 1 (CMPG11250). Data are expressed as absorbance at 450 nm and depicted as median with interquartile range. ###p < .001 compared to the saline group; *p < .05 compared to the no pretreatment group, n = 5–7 mice per group.
Pretreatment with L. rhamnosus GR-1 and CMPG11250 is linked with increased gut microbiome resilience
To gain more insight into the link between allergic asthma induction and the murine gut microbiome, fecal samples were collected for microbiome analysis after pretreatment and after allergic asthma induction (Figure 1c). First, changes in alpha diversity (Inverse Simpson metric) reflecting the bacterial taxa richness at the level of individual mice were assessed by subtracting the alpha diversity before asthma induction from the diversity after asthma induction (Figure 5a). Significantly lower alpha diversity was observed in the not pretreated asthmatic group, but not in the groups pretreated with wild-type L. rhamnosus GR-1 or CMPG11250, when compared to the saline group. As the Inverse Simpson metric in the context of alpha diversity measurement does not take into account the similarities in taxon composition between samples, the beta diversity was calculated between all samples of the same group (Figure S2). For the non-pretreated asthmatic group, there was a clear shift in the microbiome composition after the allergy induction reflected by two different clusters before and after asthma induction (Figure S2, No pretreatment panel). This separate clustering was not present in the L. rhamnosus GR-1 or CMPG11250 treatment groups.
Figure 5.
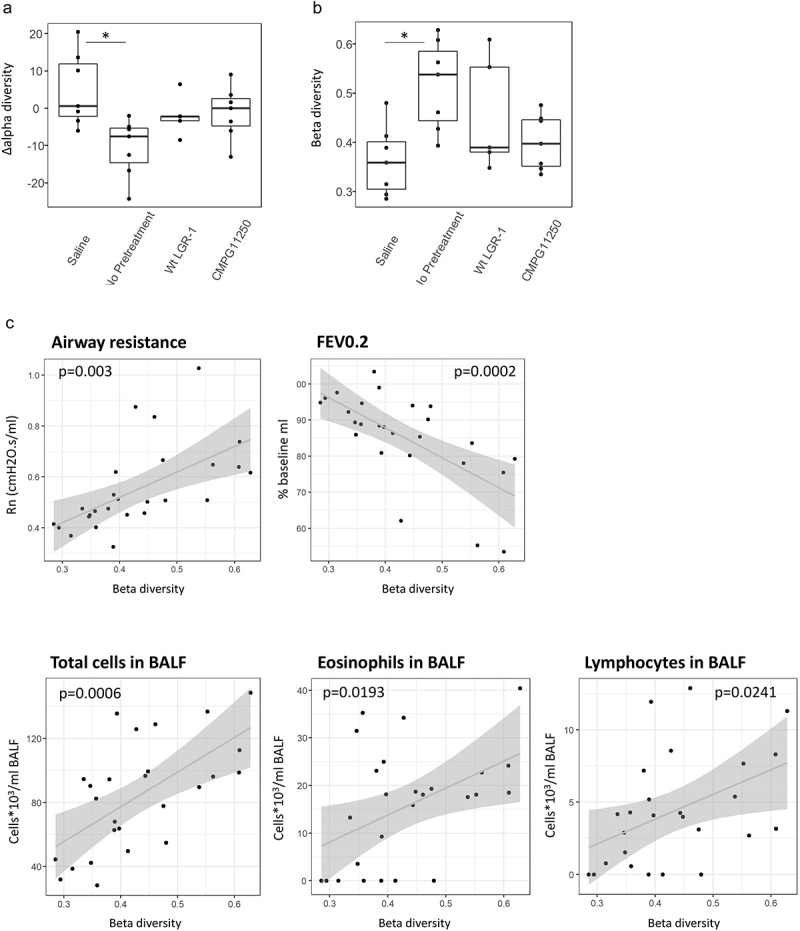
(a) Change in alpha diversity (Inverse Simpson) and (b) community shift reflected as Beta diversity (Bray-Curtis) between microbiome composition after pretreatment and after allergic asthma induction. The position of dot for each mouse reflects a temporal shift in microbiome composition (e.g. the gradient between 0 and 1 from very similar to very dissimilar in Panel b). Crossbar depicts median per group; *p < .05 compared to the saline group; n = 5–7 mice per group; (c) Correlation between the calculated beta diversity reflecting a shift in microbiome composition before and after allergic asthma induction, and allergic asthma parameters. Beta diversity (Bray-Curtis) values between samples of individual mice collected after pretreatment and after allergic asthma induction are depicted as dots per mouse. Airway resistance: measure of AHR in response to methacholine; FEV0.2: FEV0.2% of baseline; Total cells BALF: lung total cell counts per ml BALF; Lymphocytes BALF: lung lymphocyte counts per ml BALF; Eosinophils BALF: lung eosinophil counts per ml BALF. Groups are labeled according to figure 1c as follows: Saline: no asthma induction, not pretreated; No pretreatment: allergic asthma, not pretreated; wt LGR-1: allergic asthma, pretreated with wild-type L. rhamnosus GR-1; CMPG11250: allergic asthma, pretreated with recombinant L. rhamnosus GR-1 producing Bet v 1 (CMPG11250).
To gain insight into this change at the level of individual mice, beta diversity (Bray-Curtis) between individual mouse fecal samples before and after asthma induction was calculated. A significantly higher beta diversity, reflecting a significant change in microbiome composition before and after asthma induction, was observed in the not pretreated group compared to the saline group (Figure 5b, gradient from 0 to 1 reflecting, respectively, very similar to very dissimilar communities). This was not the case for the wild-type L. rhamnosus GR-1 and CMPG11250 groups. These treated groups thus appeared to show a higher gut microbiome resilience after asthma induction reflected by a smaller shift in microbiome composition.
To explain the microbiome composition shift for each mouse group, we focused on specific taxa that showed a significant fold change between the two time points (Table S2). A significant increase (log fold 2.05–3.06) in abundance of Lachnospiraceae (genera Roseburia and Acetatifactor) and Ruminococcaceae (genus Ruminiclostridium_5) was detected at the time point after asthma induction compared to the time point after pretreatment in the not pretreated asthmatic mice. Interestingly, a notable decrease (log fold −4.84) in Bacteroidales_S24-7 was observed in the CMPG11250 group between the two time points. For the saline and wild-type L. rhamnosus GR-1 groups, no taxa were significantly different in abundance after pretreatment and after asthma induction.
We also assessed the effect of L. rhamnosus GR-1 and CMPG11250 administration specifically on the gut Lactobacillus communities. After pretreatment, there was a clear difference between control groups (0%) and Lactobacillus-treated groups (9.7%) in the share of the Lactobacillus ASV classified as belonging to the Lactobacillus casei group (species rhamnosus/casei/reuteri) within the Lactobacillus community. The overall relative abundance of lactobacilli was significantly higher (p = .02) at the time point after pretreatment in the groups that received L. rhamnosus GR-1 and CMPG11250 (0.0044%) compared to the groups that were not pretreated with lactobacilli (0%).
The relative abundances of the 15 most abundant microbial amplicon sequence variants (ASVs) were also visualized for each mouse at both time points (Figure S3). As notable heterogeneity was observed between the mice (Figure S3), the influence of different factors on the variability of the microbiome data was subsequently determined. Hereto, we performed a permutational multivariate analysis of variance on a Bray-Curtis distance matrix in which the microbiome composition differences between all mice at both time points were summarized (Table S3). Individual mice were the most significant source of microbiome variability, as the influence of individual mice could explain 37% of the observed variability in the microbiome data. The second important factor was the type of pretreatment, which was responsible for 11% of the observed microbiome variability. Finally, the time point of feces collection (after pretreatment or after asthma induction) could explain 3% of the microbiome variability.
Gut microbiome composition resilience inversely correlates with allergic asthma severity
We then correlated the changes in microbiome composition with the various allergic asthma parameters reflecting airway inflammation and airway function. Hereto, a Pearson correlation analysis was performed of the calculated beta diversity reflecting the shift in microbiome composition from the time point after pretreatment to that after allergic asthma induction per mouse, with each of the measured allergic asthma parameters (Figure 5c). Significant positive correlations were found between higher beta diversity, reflecting a shift in microbiome composition, and AHR, drop in FEV0.2%, BALF total cell counts, eosinophil counts, and lymphocyte counts (Figure 5c).
Discussion
In this study, the effects of intragastric pretreatment with wild-type probiotic L. rhamnosus GR-1 and the corresponding recombinant CMPG11250 strain producing Bet v 1 were explored in a mouse model of allergic asthma. We showed that both the recombinant CMPG11250 strain and wild-type L. rhamnosus GR-1 prevented the development of AHR in our mouse model. The significantly lower AHR reflected both in airway resistance (Rn) and FEV, as a result of oral probiotic administration is a clinically relevant finding, as this parameter has a direct impact on the asthmatic patient’s quality of life.3 Furthermore, our results support FEV measurement next to the classical assessment of airway resistance typically used in animal models for asthma research,13,25,27 which could improve the translational value of pre-clinical animal models.28,29
Besides the changes in hyperreactivity, pretreatment with CMPG11250 in our mouse model resulted in lower airway inflammation as evidenced by significantly lower BALF total cell and lymphocyte counts, and lung IL-1β levels. These mechanisms are summarized in Figure 6. It has previously been described that oral administration of certain strains belonging to the Lactobacillus genus complex, alone or in combination with allergens for allergen-specific immunotherapy, can prevent allergic asthma development in mouse models through modulation of Th2 immune responses.13,25,27 In our model, only wild-type L. rhamnosus GR-1 had a significant prophylactic effect on airway eosinophilia typical for type 2 asthmatic airway inflammation,2 while the effect on Th2 inflammatory markers as a result of CMPG11250 pretreatment was less pronounced. This lack of effect on Th2 markers can potentially be explained by the particular combination of the Bet v 1 antigen with the L. rhamnosus GR-1, as well as the dose and method of probiotic administration. It should also be noted that the birch pollen extract used to induce allergic asthma in our mouse model likely contained other (minor) allergens in addition to Bet v 1, such as Bet v 2, that could play an additional role in allergy induction.26 The nature of the antigen,23 the probiotic strain used,11,24,30 and the probiotic dose and administration route have all been shown to affect the probiotic treatment outcomes in mouse models of allergic disease.30 Although there was a discordancy between effects on hyperreactivity and those on Th2 inflammation for CMPG11250, previous studies suggest that AHR and allergic airway inflammation might be two separate characteristics of asthma that do not necessarily follow the same trend.31 For example, while the exact role of IL-1β in the development of allergic asthma is unclear, it might contribute to airway inflammation by exacerbating the innate immune response.32 IL-1β was previously shown to induce AHR through direct alteration of mitogen-activated protein kinase (MAPK) pathways in airway cells.33 Probiotic lactobacilli are also capable of influencing innate immune responses,5-7 and wild-type L. rhamnosus GR-1 was recently shown to locally inhibit pathogen-induced IL-1β production in bovine mammary epithelial cells,34 although this has not yet been described for allergic airway disease.
Figure 6.
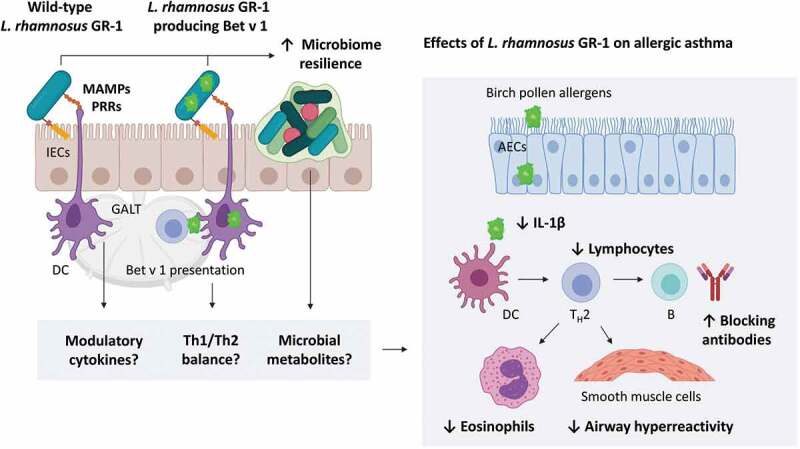
Proposed mechanisms of probiotic action of wild-type and recombinant L. rhamnosus GR-1 in the mouse model of birch pollen-induced allergic asthma. Beneficial microbes can interact with intestinal epithelial cells (IECs) and dendritic cells (DC) through microbe-associated molecular patterns (MAMPs) recognized by pattern recognition receptors (PRRs).5–7 In the gut, these and other effects mediated by beneficial bacteria translate to immune signals processed in the gut-associated lymphoid tissue (GALT). Immune cells and signals (e.g. modulatory cytokines) and microbial metabolites can circulate through the blood and lymphatic system, resulting in distant beneficial effects in the asthmatic airways. ↑ indicates enhancement while ↓ indicates inhibition by pretreatment with wild-type or recombinant L. rhamnosus GR-1. AECs: airway epithelial cells; TH2: T helper 2 cell; B: B cell; IL-1β: Interleukin 1 beta.
Preventive administration of the recombinant L. rhamnosus GR-1 strain CMPG1250 expressing Bet v 1 also resulted in a stimulation of Bet v 1-specific immune responses (Figure 6), as demonstrated by an increase in Bet v 1-reactive systemic IgG levels. This is not unexpected, as it has previously been shown that recombinant Lactobacillus strains producing an antigen, such as Helicobacter pylori urease B subunit, can increase systemic IgG levels as a result of oral vaccination.35 In our mouse model, the higher Bet v 1-specific antibody response was in favor of the Th1 immunity in the majority of the CMPG11250-pretreated mice, reflected by a higher ratio of Bet v 1-specific IgG2a to IgG1, and to total IgE. Together with the trend toward increased levels of lung IFN-γ, this might reflect a subtle shift in Th1/Th2 balance toward Th1 as a result of CMPG11250 administration. Importantly, the immunostimulatory effect of CMPG11250 did not result in increased allergic airway inflammation. These results further support previous studies in which the lowering of allergen-specific IgG1 or IgE antibody levels was not necessarily a prerequisite for successful preventive therapy of allergic disease with wild-type of recombinant lactic acid bacteria.25,30,36 Taken together, orally administered L. rhamnosus GR-1 could serve as an effective oral probiotic for the prevention of allergic airway disease, but its use as a delivery vector for allergens in the context of allergen-specific immunotherapy requires further investigation.
In addition to the immunological effects of probiotic administration, we observed a strong connection between the changes in the fecal microbiome and allergic asthma severity in our mouse model. Microbiome changes have previously been described in a range of respiratory diseases, and a powerful gut-lung microbiome axis has recently been defined.37 Indeed, we could demonstrate that allergic asthma induction on its own was linked to a shift in the gut microbiome composition and a significant decrease in gut microbiome diversity. Likewise, lower gut microbiome diversity has previously been linked to the development of allergic asthma in children.38 The most significant changes in abundance of specific taxa after allergic asthma induction in our study were due to the members of the Lachnospiraceae and Ruminococcaceae families, which is in line with recent observations in a mouse model of ovalbumin-induced allergic airway disease.39 The higher abundancy of Lachnospiraceae spp. was also hypothesized to be involved in an increased atopic dermatitis induction in mice.40 Importantly, the latter study demonstrated a causative link between microbiome composition and the levels of IL-1β and other inflammatory biomarkers in experimental allergic disease. Similarly to our results, this was not always linked to an effect on serum IgE levels.
Furthermore, in our study, pretreatment with wild-type L. rhamnosus GR-1 and CMPG11250 that improved AHR was associated with protection from or resilience to the observed allergic asthma-linked shift in microbiome composition, and a temporary increase in relative lactobacilli abundance. Recently, comparable results were obtained in a mouse model of colitis, where Lactobacillus fermentum strains KBL374 and KBL375 restored gut microbiota diversity diminished by colitis induction, and contributed to an increase in the Lactobacillus population.41 In addition, we found a significant positive correlation between the magnitude of the gut microbiome composition shift and the development of hallmark allergic asthma manifestations, such as AHR and elevated airway inflammatory cell counts. We cannot be certain whether these correlations are a result of local and distant microbiota influences on the immune system, or the impact of the immune system and airway disease-associated inflammation on the gut microbiome. However, the former link is plausible, as perturbations in the gut microbiome have previously been linked to allergic disease in clinical studies.14,42,43 Beneficial microbes can directly influence immune and epithelial cells through microbe-associated molecular patterns that interact with host pattern recognition receptors (Figure 6).5-7 Furthermore, it has been hypothesized that gut microbial metabolites can mediate the probiotic effects on birch pollen allergy at the airway level.44 Future experiments (e.g. in germ-free mouse models) are required to elucidate the role of various beneficial microbial compounds in the complex gut-lung cross-talk. In addition, numerous studies demonstrate that shifts in airway microbiome composition correlate with allergic and other inflammatory airway diseases.45,46 Mapping both the airway and the gut microbiome will contribute to a comprehensive picture of microbial influence on local and systemic immunity in future work. Further research is required to gain insight into the exact mechanisms of how improved host-microbiome composition stability mediated through the administration of probiotic lactobacilli could be linked to clinical benefits in the host.
Conclusion
Intragastric pretreatment with L. rhamnosus GR-1 significantly prevented AHR development in the context of allergic asthma. Recombinant expression of Bet v 1 by CMPG11250 showed a beneficial effect on several immunological airway parameters but was not found to be crucial, since also wild-type L. rhamnosus GR-1 showed benefits against allergic asthma. This outcome of pretreatment with probiotics could be considered important in a clinical setting, as prevention of AHR development and the concomitant symptoms is the primary goal of allergic asthma prophylaxis. Furthermore, both wild-type and recombinant L. rhamnosus GR-1 strains were also able to prevent the microbiome disturbance observed in the asthmatic animals, suggesting a link between airway function stability and gut microbiome composition resilience, and thus supporting the existence of the gut-lung axis.
Materials and methods
Bacterial strains, plasmids, and growth conditions
Bacterial strains, plasmids, and primers used in this study are listed in Table S1. Wild-type and recombinant L. rhamnosus strains were cultured in de Man-Rogosa-Sharpe (MRS) medium (Difco) at 37°C. E. coli strains were grown at 37°C in Luria-Bertani broth with aeration. When appropriate, erythromycin, chloramphenicol, and kanamycin were supplied at, respectively, 5, 10, and 50 µg/ml. Production of Bet v 1 by recombinant L. rhamnosus strains was induced with commercial nisin from Lactococcus lactis (Sigma-Aldrich, N5764) as previously described.47 Construction of plasmids for Bet v 1 production (Table S1) and their transformation into E. coli and L. rhamnosus strains were performed using standard molecular techniques and previously described protocols.47-49 The betv1 gene was codon-optimized for L. rhamnosus, synthesized by GenScript (US), and cloned downstream of the T7 promoter in the pET-28-a(+) plasmid (Novagen), yielding pCMPG11221. The pCMPG11221 plasmid carrying betv1 was transformed into E. coli BL21 (DE3), resulting in the CMPG11221 strain for rBet v 1 production. The pCMPG11228 plasmid was constructed by cloning the betv1 gene downstream of the nisA promoter instead of the gfp gene in the pMEC45 plasmid (Figure 1a) using primers listed in Table S1. pCMPG11228 was electroporated into the CMPG11259 strain of L. rhamnosus GR-1,47 resulting in CMPG11250.
Large-scale protein production and purification of recombinant Bet v 1 in CMPG11221
rBet v 1 used for allergic sensitization in mice was produced in the E. coli BL21 (DE3)-derived CMPG11221 after induction with β-D-1-thiogalactopyranoside (IPTG), as previously described.49 Briefly, soluble total cell protein fraction was obtained from the cell pellet of CMPG11221 by centrifugation (4000 g, 20 min, 4°C), resuspension in non-denaturing lysis buffer and sonication.49 rBet v 1 was purified by affinity chromatography (ÄKTA Purifier System, GE Healthcare Life Sciences) using a HisTrapTM HP column (GE Healthcare) followed by size-exclusion chromatography on a HighloadTM 16/60 column packed with a SuperdexTM prep grade matrix (GE Healthcare). Purity of eluted fractions containing rBet v 1 was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining, as previously described.49
Bet v 1-specific western blotting
Protein electroblotting was performed on a polyvinylidene difluoride (PVDV) membrane (Novex) blocked in a 10% skimmed milk solution. Primary anti-Bet v 1 IgG murine antibody (MA-4B10; Indoor Biotechnologies) at 1/10000 dilution and secondary anti-mouse IgG alkaline phosphatase goat antibody (A3562; Sigma Life Science) at 1/6666 dilution were used. The membrane was scanned, and ImageJ software was used for Bet v 1 production quantification by CMPG11250 based on band intensity compared to the rBet v 1 control bands.
Experimental mouse model of intragastric preventive treatment with L. rhamnosus
Male BALB/c mice (BALB/cOlaHsd, 4–5 weeks old) were obtained from Envigo (The Netherlands). Animals were housed in a Specific Pathogen Free (SPF) facility in filter top cages and were provided with standard pelleted laboratory chow (Trouw Nutrition, Ghent, Belgium) and water ad libitum. Mouse weight was recorded daily. Approval was obtained from the Ethical Committee for Animal Experimentation (KU Leuven, Belgium) for all experimental procedures (P063-2014).
To prepare bacterial solutions for intragastric administration, Bet v 1 production in CMPG11250 was first induced with nisin as previously described.47 After 24 h, L. rhamnosus cultures were centrifuged (5 min, 3000 g, 4°C) and washed twice in sterile PBS. Approximately 1010 CFU of L. rhamnosus in 200 µl PBS per dose were administered to mice through intragastric intubation.
The experimental protocol is depicted in Figure 1c. Mice were preventively treated by intragastric instillations of CMPG11250 or L. rhamnosus GR-1 at 1010 CFU four times a week over the course of 6 weeks. The saline and no pretreatment groups received PBS. Subsequently, allergic sensitization was performed by three intraperitoneal injections of 10 μg rBet v 1 with 2 mg alum (Imject Alum; Thermo Fisher Scientific) in 100 μl saline (0.9% NaCl, B. Braun) on days 42, 49, and 56. Allergen challenges were performed after anesthesia with isoflurane (Schering-Plow Animal Health) by intranasal instillation of 40 μl of BP extract containing 2.5 mg total protein/ml (Greer®) on days 63, 65, 67 and 69. The saline group received mock sensitizations and challenges with saline. Mice were sacrificed on day 72. Airway hyperreactivity (AHR) measurements were performed. Blood for total IgE, Bet v 1-specific IgG1 and IgG2a determination, bronchoalveolar lavage fluid (BALF), and lung samples for cytokine analysis were collected and subsequently analyzed as previously described.11 In addition, mesenteric lymph nodes (MLNs) were isolated for Treg analysis.
Airway hyperreactivity (AHR) measurements
AHR measurements were performed using the Flexivent (Flexivent 7, SCIREQ, Montreal, Canada) as previously described.28,29 Mice were anesthetized by an intraperitoneal injection of pentobarbital (Nembutal®, Ceva) and artificially ventilated at a frequency of 150 breaths/min. The forced oscillation perturbation QP3 was used to measure airway resistance (Rn) and the negative pressure forced expiration (NPFE) was used to measure the forced expiratory volume at 0.2 sec (FEV0.2) in response to increasing doses of methacholine (Sigma-Aldrich®) solution (0, 1.25, 2.5, 5, 10, 20 mg/mL). The FEV0.2 data are presented as a percent of their own baseline for each mouse (FEV0.2%). Based on the FEV0.2% data, the concentration of the provocative agent methacholine causing a 20% fall in FEV (PC20-FEV0.2%) was calculated, allowing to classify the mice as hyperreactive.
Treg quantification in mesenteric lymph nodes (MLNs)
MLNs were aseptically isolated and single-cell suspensions were prepared in RPMI medium (BioWhittaker® Lonza) with 10% fetal calf serum (FCS) using a 40 µm cell strainer (BD Bioscience). 106 white blood cells were surface stained using anti-CD4+ (PerCP-Cy5.5) and anti-CD45+ (AF700), and intracellularly stained with anti-FoxP3+ (PE) using the fixation/permeabilization kit according to the manufacturer’s instructions (BD Biosciences). Percentage of anti-FoxP3-labeled cells within the CD+CD45+ cell population was determined by flow cytometry (FACSArray, BD Biosciences) and subsequent processing with FlowJo software (TreeStar, Inc.).
Fecal microbiome analysis
Fecal pellets were collected on day 40 after the final L. rhamnosus pretreatment administration before allergic asthma induction, and on day 70 after allergic asthma induction (Figure 1c). The QIAamp PowerFecal DNA Isolation Kit (QIAGEN) was used for microbial DNA extraction from the pellets. PCR amplification of the V4 region of the 16 S rRNA gene was then performed in duplicate for each sample using barcoded primers and the Phusion High-Fidelity DNA polymerase (Thermo Scientific), as previously described.50 The 16 S rRNA gene amplicons were subsequently purified with the SequalPrep Normalization plate kit (Invitrogen) and pooled into one library. Agarose gel extraction of the pooled library was performed with NucleoSpin® Gel and PCR Clean-up (Macherey Nagel) and the extracted library was diluted to 2 nM. Sequencing of the pooled amplicons was performed at the Center for Medical Genetics (Edegem, Belgium) with Illumina MiSeq using the MiSeq Reagent Kit v2 (Illumina). Data processing was performed using the DADA2 package (version 1.4) in the R environment.51,52 Further downstream analysis was performed using phyloseq (version 1.22.3) and in-house developed package tidyamplucons (https://github.com/SWittouck/tidyamplicons) and included: alpha diversity calculation based on the Inverse Simpson metric, beta diversity calculations based on Bray-Curtis distances and plotting taxonomic profiles of 15 most abundant ASVs. Permutational multivariate analysis of variance (PERMANOVA) was performed using adonis function embedded in the vegan package (version 2.5–2) on the Bray-Curtis distance matrix.53 Pearson correlation was used to correlate the gut microbiome shift to allergic asthma parameters. To find taxa that had a significant fold change between different time points for each group the Metagenomeseq package was implemented in the R environment. In short, samples were first divided based on the pretreatment received. Afterward, taxa were filtered so that all taxa were present no less than in two samples. Then, the normalization factor was calculated based on the data itself using the cumNorm function built in the package. Finally, a model was fit that determined which taxa were significantly different between timepoints after pretreatment and after asthma induction. To account for the false discovery rate (false positives), the Hochberg method is applied to adjust for multiple testing.
Statistical analysis
Statistical data analysis was performed using GraphPad Prism v.7 (La Jolla, CA, USA). Mann–Whitney U test was used for pairwise comparisons between two groups and Kruskal-Wallis test with post-hoc analysis was implemented to compare between multiple groups. Fisher’s exact test was used for PCD20-FEV% data. Two-way ANOVA with a Bonferroni post-hoc test was implemented for AHR data analysis. Statistical data analysis on the microbiome data was performed in the R environment (version 3.4.0) using the nonparametric Wilcoxon test for alpha and beta diversity metrics, and additional methods as described in the section “Fecal microbiome analysis.” Differences between groups were considered significant when p < .05.
Figure 6 is created with BioRender.com.
Supplementary Material
Acknowledgements
We acknowledge the valuable technical help of Tine Verhoeven, Jolien Mennens, Rob Dockx, Jonathan Cremer, Eline Oerlemans, Stijn Wittouck, Ahmad Kasran, and Anne-Charlotte Jonckheere during the course of this study.
Funding Statement
I.S. was supported by IWT-SB-Vlaanderen for her PhD scholarship, and by IOF POC (University of Antwerp) and IWT SBO financing during her postdoctoral work at the University of Antwerp. S.S. was the recipient of a KU Leuven Research Council grant (PDMK/14/189). S.L. was supported by the Fund for Scientific Research (FWO) Vlaanderen postdoctoral grant, the research grant KaN 28960, and the IWT-SBO ProCure Grant (IWT150052). M.P. was the recipient of an FWO Vlaanderen postdoctoral grant. J.C. was supported by a grant from FWO Vlaanderen. WVB is supported by a Dehousse scholarship from the University of Antwerp.
Abbreviations
- AHR
Airway hyperreactivity
- ASV
Amplicon sequence variant
- BALF
Bronchoalveolar lavage fluid
- BP
Birch pollen
- FEV0.2%
Forced expiratory volume at 0.2 s as percentage of baseline
- Ig
Immunoglobulin
- L. rhamnosus
Lactobacillus rhamnosus
- LGR-1
Lactobacillus rhamnosus GR-1
- MAMP
Microbe-associated molecular pattern
- MLN
Mesenteric lymph node
- PC20-FEV%
Provocative concentration of methacholine causing a 20% fall in FEV
- PCoA
Principal coordinate analysis
- Rn
Airway resistance
- Th
T helper
- Treg
Regulatory T cell
Authors contributions
I.S., J.C., Jozef V, S.L., M.P., and S.S. were involved in the conception of the study. I.S., J.C., M.P., S.L., S.S., and W.V.B. contributed to the design of the experimental work and/or the writing and the critical revision of the manuscript. I.S., W.V.B., F.D., and Jeroen V. performed the experimental work and processed the data. I.S., J.C., S.S., M.P., S.L., W.V.B., and Jeroen V. interpreted the data. All authors reviewed and corrected the manuscript.
Disclosure of potential conflicts of interest
M.P. consults for academic and industrial representatives in the field of microbiome and probiotics. She holds no share in any probiotic companies nor receives any profit-sharing linked to the field. Her clients had no role in drafting this manuscript or the decision to submit the work for publication. Other authors declare no conflict of interest.
Data availability
Sequencing data are available at the European Nucleotide Archive with the accession number PRJEB33230 (https://www.ebi.ac.uk/ena/data/view/PRJEB33230).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Meadows A, Kaambwa B, Novielli N, Huissoon A, Fry-Smith A, Meads C, Barton P, Dretzke J.. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol Assess. 2013;17(27):vi, xi–xiv, 1–322. doi: 10.3310/hta17270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018. 24;391(10122):783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 3.Chapman DG, Irvin CG. Mechanisms of airway hyper-responsiveness in asthma: the past, present and yet to come. Clin Exp Allergy. 2015;45(4):706–719. doi: 10.1111/cea.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. 2011;10:66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 6.Lebeer S, Bron PA, Marco ML, Van Pijkeren JP, O’Connell Motherway M, Hill C, Pot B, Roos S, Klaenhammer T. Identification of probiotic effector molecules: present state and future perspectives. Curr Opin Biotechnol. 2018;49:217–223. doi: 10.1016/j.copbio.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 8.Cuello-Garcia CA, Brozek JL, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Terracciano L, Gandhi S, Agarwal A, Zhang Y, Schünemann HJ. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952–961. doi: 10.1016/j.jaci.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg A, West CE, Prescott SL, Jenmalm MC. Pre- and probiotics for allergy prevention: time to revisit recommendations? Clin Exp Allergy. 2016;46:1506–1521. doi: 10.1111/cea.12838. [DOI] [PubMed] [Google Scholar]
- 10.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 11.Spacova I, Petrova MI, Fremau A, Pollaris L, Vanoirbeek J, Ceuppens JL, Seys S, Lebeer S. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy. 2019;74(1):100–110. doi: 10.1111/all.13502. [DOI] [PubMed] [Google Scholar]
- 12.Thang CL, Baurhoo B, Boye JI, Simpson BK, Zhao X. Effects of Lactobacillus rhamnosus GG supplementation on cow’s milk allergy in a mouse model. Allergy Asthma Clin Immunol. 2011;7(1):20. doi: 10.1186/1710-1492-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CT, Chen PJ, Lee YT, Ko JL, Lue KH. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. J Microbiol Immunol Infect. 2016;49(5):625–635. doi: 10.1016/j.jmii.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Kalliomäki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019–1021. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 15.Koyama T, Kirjavainen PV, Fisher C, Anukam K, Summers K, Hekmat S, Reid G. Development and pilot evaluation of a novel probiotic mixture for the management of seasonal allergic rhinitis. Can J Microbiol. 2010;56:730–738. doi: 10.1139/W10-061. [DOI] [PubMed] [Google Scholar]
- 16.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Simpson MR, Dotterud CK, Storro O, Johnsen R, Oien T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015;15:13. doi: 10.1186/s12895-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segers ME, Lebeer S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb Cell Fact. 2014;13 Suppl 1(Suppl1):S7. doi: 10.1186/1475-2859-13-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrova MI, Macklaim JM, Wuyts S, Verhoeven T, Vanderleyden J, Gloor GB, Lebeer S, Reid G. Comparative genomic and phenotypic analysis of the vaginal probiotic Lactobacillus rhamnosus GR-1. Front Microbiol. 2018;9:1278. doi: 10.3389/fmicb.2018.01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrova MI, van den Broek MFL, Spacova I, Verhoeven TLA, Balzarini J, Vanderleyden J, Schols D, Lebeer S. Engineering Lactobacillus rhamnosus GG and GR-1 to express HIV-inhibiting griffithsin. Int J Antimicrob Agents. 2018;52(5):599–607. doi: 10.1016/j.ijantimicag.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Álvarez B, Krogh-Andersen K, Tellgren-Roth C, Martínez N, Günaydın G, Lin Y, Martín MC, Álvarez MA, Hammarström L, Marcotte H, et al. An exopolysaccharide-deficient mutant of Lactobacillus rhamnosus GG efficiently displays a protective llama antibody fragment against rotavirus on its surface. Appl Environ Microbiol. 2015;81(17):5784–5793. doi: 10.1128/AEM.00945-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandasamy M, Selvakumari Jayasurya A, Moochhala S, Bay H-B, Lee K-Y MR. Lactobacillus rhamnosus GG secreting an antigen and Interleukin-2 translocates across the gastrointestinal tract and induces an antigen specific immune response. Microbiol Immunol. 2011;55:704–714. doi: 10.1111/j.1348-0421.2011.00370.x. [DOI] [PubMed] [Google Scholar]
- 23.Rigaux P, Daniel C, Hisbergues M, Muraille E, Hols P, Pot B, Pestel J, Jacquet A. Immunomodulatory properties of Lactobacillus plantarum and its use as a recombinant vaccine against mite allergy. Allergy. 2009;64:406–414. doi: 10.1111/j.1398-9995.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 24.Daniel C, Repa A, Wild C, Pollak A, Pot B, Breiteneder H, Wiedermann U, Mercenier A. Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy. 2006;61:812–819. doi: 10.1111/j.1398-9995.2006.01071.x. [DOI] [PubMed] [Google Scholar]
- 25.Feleszko W, Jaworska J, Rha R-D, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 26.Wensing M, Akkerdaas JH, van Leeuwen WA, Stapel SO, Bruijnzeel-Koomen CA, Aalberse RC, Bast BJ, Knulst AC, van Ree R. IgE to Bet v 1 and profilin: cross-reactivity patterns and clinical relevance. J Allergy Clin Immunol. 2002;110(3):435–442. doi: 10.1067/mai.2002.126380. [DOI] [PubMed] [Google Scholar]
- 27.Charng YC, Lin CC, Hsu CH. Inhibition of allergen-induced airway inflammation and hyperreactivity by recombinant lactic-acid bacteria. Vaccine. 2006;24:5931–5936. doi: 10.1016/j.vaccine.2005.07.107. [DOI] [PubMed] [Google Scholar]
- 28.Devos FC, Maaske A, Robichaud A, Pollaris L, Seys S, Lopez CA, Verbeken E, Tenbusch M, Lories R, Nemery B, et al. Forced expiration measurements in mouse models of obstructive and restrictive lung diseases. Respir Res. 2017;18(1):123. doi: 10.1186/s12931-017-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanoirbeek JAJ, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 30.Pellaton C, Nutten S, Thierry A-C, Boudousquié C, Barbier N, Blanchard C, Corthésy B, Mercenier A, Spertini F. Intragastric and intranasal administration of Lactobacillus paracasei NCC2461 modulates allergic airway inflammation in mice. Int J Inflam. 2012;686739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leuppi JD, Salome CM, Jenkins CR, Koskela H, Brannan JD, Anderson SD, Andersson M, Chan HK, Woolcock AJ. Markers of airway inflammation and airway hyperresponsiveness in patients with well-controlled asthma. Eur Respir J. 2001;18(3):444–450. doi: 10.1183/09031936.01.00058601. [DOI] [PubMed] [Google Scholar]
- 32.Allen IC, Jania CM, Wilson JE, Tekeppe EM, Hua X, Brickey WJ, Kwan M, Koller BH, Tilley SL, Ting JP. Analysis of NLRP3 in the development of allergic airway disease in mice. J Immunol. 2012;188(6):2884–2893. doi: 10.4049/jimmunol.1102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Cardell L-O AM. IL-1β induces murine airway 5-HT2A receptor hyperresponsiveness via a non-transcriptional MAPK-dependent mechanism. Respir Res. 2007;8(1):29. doi: 10.1186/1465-9921-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Q, Zhu Y-H, Xu J, Liu X, Duan C, Wang M-J, Wang J-F. Lactobacillus rhamnosus GR-1 ameliorates Escherichia coli-induced activation of NLRP3 and NLRC4 inflammasomes with differential requirement for ASC. Front Microbiol. 2018;9:1661. doi: 10.3389/fmicb.2018.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corthésy B, Boris S, Isler P, Grangette C, Mercenier A. Oral immunization of mice with lactic acid bacteria producing Helicobacter pylori urease B subunit partially protects against challenge with Helicobacter felis. J Infect Dis. 2005;192:1441–1449. doi: 10.1086/444425. [DOI] [PubMed] [Google Scholar]
- 36.Kruisselbrink A, Heijne Den Bak-Glashouwer M-J, Havenith CEG, Thole JER, Janssen R. Recombinant Lactobacillus plantarum inhibits house dust mite-specific T-cell responses. Clin Exp Immunol. 2001;126:2–8. doi: 10.1046/j.1365-2249.2001.01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 38.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 39.Barfod KK, Roggenbuck M, Al-Shuweli S, Fakih D, Sørensen SJ, Sørensen GL. Alterations of the murine gut microbiome in allergic airway disease are independent of surfactant protein D. Heliyon. 2017;3(3):e00262. doi: 10.1016/j.heliyon.2017.e00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zachariassen LF, Krych L, Engkilde K, Nielsen DS, Kot W, Hansen CHF, Hansen AK. Sensitivity to oxazolone induced dermatitis is transferable with gut microbiota in mice. Sci Rep. 2017;7:44385. doi: 10.1038/srep44385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang YJ, Kim W-K, Han DH, Lee L, Ko G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 2019;10:696–711. doi: 10.1080/19490976.2019.1589281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forno E, Onderdonk A, McCracken J, Litonjua A, Laskey D, Delaney M, Dubois AM, Gold DR, Ryan LM, Weiss ST, et al. Diversity of the gut microbiota and eczema in early life. Clin Mol Allergy. 2008;6:11. doi: 10.1186/1476-7961-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth cohort study. Gut. 2007;56(5):661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouwehand AC, Nermes M, Collado MC, Rautonen N, Salminen S, Isolauri E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J Gastroenterol. 2009;15(26):3261–3268. doi: 10.3748/wjg.15.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pulvirenti G, Parisi GF, Giallongo A, Papale M, Manti S, Savasta S, Licari A, Marseglia GL, Leonardi S. Lower airway microbiota. Front Pediatr. 2019;7:393. doi: 10.3389/fped.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Botterel F, Angebault C, Cabaret O, Stressmann FA, Costa J-M, Wallet F, Wallaert B, Bruce K, Delhaes L. Fungal and bacterial diversity of airway microbiota in adults with cystic fibrosis: concordance between conventional methods and ultra-deep sequencing, and their practical use in the clinical laboratory. Mycopathologia. 2018;183(1):171–183. doi: 10.1007/s11046-017-0185-x. [DOI] [PubMed] [Google Scholar]
- 47.Spacova I, Lievens E, Verhoeven T, Steenackers H, Vanderleyden J, Lebeer S, Petrova M. Expression of fluorescent proteins in Lactobacillus rhamnosus to study host-microbe and microbe-microbe interactions. Microb Biotechnol. 2018;11(2):317–331. doi: 10.1111/1751-7915.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor (N.Y): Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 49.Petrova MI, Imholz NCE, Verhoeven TL, Balzarini J, Van Damme EJM, Schols D, Vanderleyden J, Lebeer S. Lectin-like molecules of Lactobacillus rhamnosus GG inhibit pathogenic Escherichia coli and Salmonella biofilm formation. PLoS One. 2016;11(8):e0161337. doi: 10.1371/journal.pone.0161337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wuyts S, Van Beeck W, Oerlemans EFM, Wittouck S, Claes IJJ, De Boeck I, Weckx S, Lievens B, De Vuyst L, Lebeer S. Carrot juice fermentations as man-made microbial ecosystems dominated by lactic acid bacteria. Appl Environ Microbiol. 2018;84(12):e00134–18. doi: 10.1128/AEM.00134-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, et al.. vegan: community ecology package. R package version 2.5-2; 2018. Retrieved on June 17, 2018 from https://CRAN.R-project.org/package=vegan
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data are available at the European Nucleotide Archive with the accession number PRJEB33230 (https://www.ebi.ac.uk/ena/data/view/PRJEB33230).


