ABSTRACT
Background and aims
As the importance of gut–brain interactions increases, understanding how specific gut microbes interact with the enteric nervous system (ENS), which is the first point of neuronal exposure becomes critical. Our aim was to understand how the dominant human gut bacterium Bacteroides thetaiotaomicron (Bt) regulates anatomical and functional characteristics of the ENS.
Methods
Neuronal cell populations, as well as enteroendocrine cells, were assessed in proximal colonic sections using fluorescent immunohistochemistry in specific pathogen-free (SPF), germ-free (GF) and Bt conventionalized-germ-free mice (Bt-CONV). RNA expression of tight junction proteins and toll-like receptors (TLR) were measured using qPCR. Colonic motility was analyzed using in vitro colonic manometry.
Results
Decreased neuronal and vagal afferent innervation observed in GF mice was normalized by Bt-CONV with increased neuronal staining in mucosa and myenteric plexus. Bt-CONV also restored expression of nitric oxide synthase expressing inhibitory neurons and of choline acetyltransferase and substance P expressing excitatory motor neurons comparable to those of SPF mice. Neurite outgrowth and glial cells were upregulated by Bt-CONV. RNA expression of tight junction protein claudin 3 was downregulated while TLR2 was upregulated by Bt-CONV. The enteroendocrine cell subtypes L-cells and enterochromaffin cells were reduced in GF mice, with Bt-CONV restoring L-cell numbers. Motility as measured by colonic migrating motor complexes (CMMCs) increased in GF and Bt-CONV.
Conclusion
Bt, common gut bacteria, is critical in regulating enteric neuronal and enteroendocrine cell populations, and neurogenic colonic activity. This highlights the potential use of this resident gut bacteria for maintaining healthy gut function.
KEYWORDS: Bacteroides thetaiotaomicron, gut microbiome, enteric nervous system, neuronal plasticity, colonic motility
Introduction
The lower gastrointestinal tract (GIT) is heavily colonized with bacteria ranging in concentration from 1011–1014 per gram.1,2 The gut-brain axis (GBA), which encompasses the gut microbiota, is a bidirectional system with communication between the microbial quorum and the central nervous system (CNS), neuroendocrine and neuroimmune system, autonomic nervous system and enteric nervous system.3 Gut microbes and their metabolic products can influence the enteric nervous system (ENS) either via direct or indirect interactions.4 Studying germ-free (GF) mice has shown host–microbe interactions and the influence of the microbiome on neuron size, architecture and colon function.5,6 However, despite these advances, there is no suggestion of how specific bacteria induce these changes. It is likely that the first point of any cognate interaction is via pattern recognition receptors such as toll-like receptors (TLRs) expressed on the intestinal epithelium.7 TLRs recognize different types of highly conserved or ubiquitous microbial antigens, for example, TLR2 recognizes gram-negative bacteria by their membrane lipopolysaccharides (LPS).8 The Bacteroides genus makes up 50% of the typical Western gut community9 which contains as much as 300 mg of LPS.10 Bacteroides thetaiotaomicron (Bt) principally resides in and dominates the human GIT suggesting evolutionary adaptations for this specific environment.11 Of note, preclinical studies of colitis in mice have shown Bt reduces inflammation in Crohn’s disease by promoting mucosal barrier function and thus limiting pathogenic invasion.12-14 Furthermore, Bt displays efficacy in preclinical models of inflammatory bowel disease (IBD) by protecting against weight loss, histopathological changes and inflammatory markers, and may, therefore, be an alternative treatment for gut inflammation.15
The importance of the ENS in GIT function is demonstrated by the density and diversity of neuronal populations.16,17 Changes to enteric neuronal populations result in significant effects on global colonic function leading to defects in motility.18,19 Enteric neurons are classified using neurochemical markers that distinguish subtypes based on morphology, function, electrical properties, diameter and length of axonal projections.20,21 Primary functions such as motility, secretion, blood flow and intestinal permeability are coordinated by intrinsic primary afferent neurons (IPANs), motorneurons (muscle, secretomotor and secretomotor/vasodilator) and interneurons within the enteric neuronal ganglia.22 Extrinsic reflexes, in particular those via the vagus nerve, add a level of control and enable communication with the CNS. Effect of microbes on this system depends on access via epithelial cells, tight junction proteins and the mucus layer, which form an important physical barrier that protects the underlying cell layers including the ENS from microbial invasion but also to enable the ENS to respond to stimuli within the intestinal milieu.23
Bt is a bacterial species with relevance to mammalian GI function and is therefore ideally suited to investigate mechanisms of microbe-ENS interactions relevant to healthy gut function. We have examined the effects of Bt by comparing specific pathogen-free (SPF), germ-free (GF) and Bt mono-colonized GF mice (Bt-CONV) to identify firstly the gut phenotype of GF mice, then to determine if changes in neurochemistry and colonic function can be restored by Bt, and finally to further understand the pathway by which commensal microbes influence the ENS. Our findings suggest that the microbiota, exemplified by Bt, exerts significant influence on the ENS by altering the neurochemical phenotype of enteric neurons and subsequent aberrant colonic motility. Importantly, Bt alone is able to normalize both the anatomical deficits and physiological changes, suggesting this is a key microbe necessary for maintaining the healthy gut function, and potentially useful in future disease treatment.
Results
Germ-free mice have impaired innervation which is restored by Bt conventionalization
The pan-neuronal marker PGP9.5 was used to assess overall neuronal density and the selective afferent fiber marker calretinin to assess changes to nerve fiber number and density. In GF mice, the expression of PGP9.5 was significantly decreased in the mucosa and myenteric plexus (MYP) (Figure 1 a)
Figure 1.
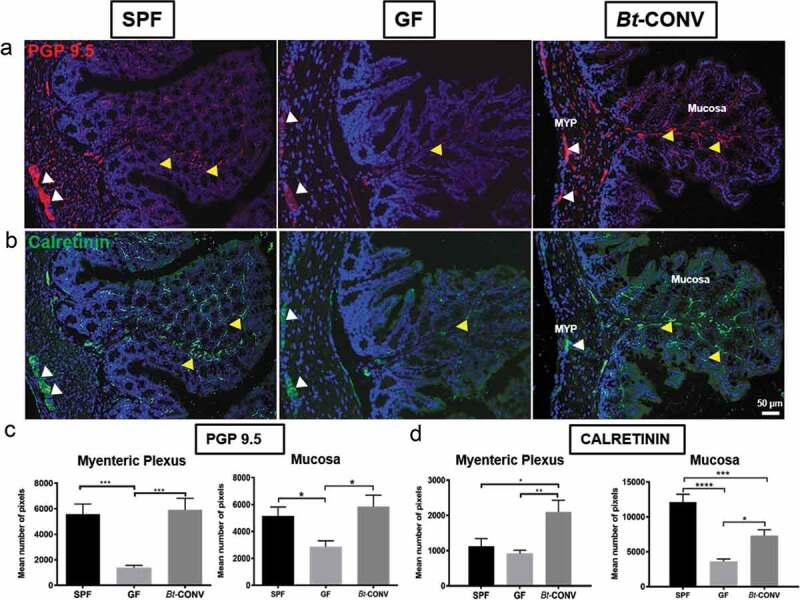
Neuronal innervation is promoted by Bt-CONV.
Expression of pan-neuronal marker PGP9.5 (red) is decreased in MYP and mucosa in GF mice compared to SPF, while, mono-colonization with Bt significantly increases PGP9.5 to SPF levels (a). Vagal afferent marker calretinin (green) shows reduced staining in GF mice compared to SPF only in the mucosa and Bt increases calretinin expression in both MYP and mucosa to similar levels of SPF (b). Quantification of PGP9.5/calretinin immunoreactivity (IR) in colonic mucosa showed a significant increase in the number of positive pixels in Bt-CONV compared to GF (c and d). N = 3–5. Yellow arrows indicate positive MYP-IR, white arrows indicate positive mucosal-IR. Error bars show SEM, and significance is p < .05 as denoted by *. Scale bar = 50 μm.
Bt conventionalization induces differential regulation of neuronal subtypes
To understand the specific role of Bt influencing neuronal subtypes, neurons were labeled according to their neurochemical profile. We and others have shown a distinct and diverse neurochemical profile of intrinsic and extrinsic neurons innervating the GIT24,25 that regulates neurogenic colonic processes.26,27 Expression of nitric oxide synthase (NOS), an enzyme that catalyzes the production of nitric oxide (NO), was used to examine the production of this major inhibitory neurotransmitter, in sections of colon from each animal group. NOS expression in the MYP significantly decreased in GF mice (Figure 2a, b) while Bt conventionalization restored expression to that seen in SPF mice (Figure 2b). By contrast, expression of the excitatory neurotransmitter acetylcholine (ACh) detected via its catalyzing enzyme choline acetyltransferase (ChAT), was significantly increased within the myenteric plexus of GF mice (Figure 2c) but was normalized after Bt conventionalization (Figure 2d). Expression of substance P (SP), another excitatory neurotransmitter co-released with ACh, was unchanged in GF mice in both the MYP and mucosa (Figure 2e, f). Following Bt-CONV, SP expression significantly increased in both mucosa and MYP (Figure 2g). Expression of sensory neurons identified by calcitonin gene-related peptide (CGRP) was unchanged in the MYP of both GF and Bt-CONV compared to SPF. However, Bt-CONV significantly decreased mucosally expressed CGRP compared to GF mice (Figure 2d, h). These data reveal that Bt can independently regulate the expression of specific neurotransmitters in the adult colon and normalize neuronal subpopulations after colonizing the GIT.
Figure 2.
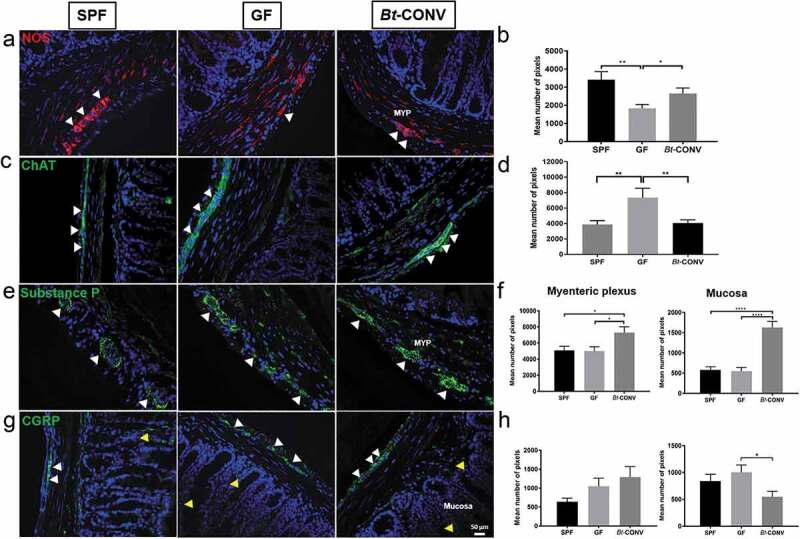
Bt normalizes expression of neuronal subtype post-GF perturbations.
Expression of the inhibitory neurotransmitter NOS (red) is significantly decreased in MYP in GF conditions when compared to SPF, while Bt significantly induces proliferation of NOS-IR compared to GF (a and b). Excitatory cholinergic marker ChAT is increased in MYP GF conditions compared to SPF, while Bt reduces the innervation to SPF levels (c and d). Expression of substance P is unchanged in both MYP and mucosa in GF mice compared to SPF; however, Bt significantly increases substance P-IR in both colonic regions compared to GF (e and f). There are no changes in expression pattern for CGRP in the mucosa and MYP except for a significant reduction in the MYP by Bt compared to SPF (g and h). N = 3–5. Yellow arrows indicate positive mucosal-IR, white arrows indicate positive MYP-IR. Error bars show SEM, and significance is p < .05 as denoted by *. Scale bar = 50 μm.
Bt conventionalization increases neuronal budding and glial cell expression
Our observation that Bt-CONV increases expression of specific nerve fibers suggested that this increase may be due to neuronal outgrowth. This possibility was tested by labeling with GAP43, a marker for neuronal budding in the colon.28 Furthermore, we used S100β, a marker of glial cells and glial processes since it is well known that glial cells produce neurotrophic factors including neural growth factors that are known to contribute to neurite outgrowth measured by GAP43.28,29 GAP43 expression was significantly increased in mucosal nerve fibers of Bt-CONV mice compared to GF mice (Figure 3a, b) while cell bodies within the myenteric plexus showed a significant increase in GAP43-IR in Bt-CONV compared to SPF mice (Figure 3c, d). Mucosal glial cells stained with S100β were significantly lower in GF mice and Bt-CONV restored expression to levels seen in SPF mice (Figure 3e, f). Interestingly, the punctate expression of S100β in the myenteric glial cells significantly increased following Bt-CONV compared to SPF and GF mice (Figure 3g, h).
Figure 3.
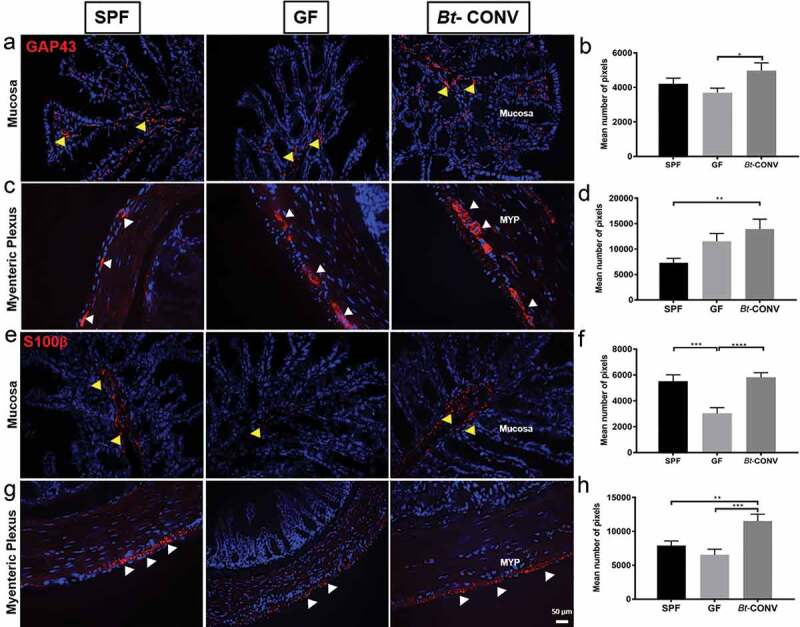
Bt-CONV increases neuronal budding and glial cells.
Expression of budding marker GAP43 (red) is significantly increased after Bt conventionalization compared to GF (mucosa-a and b) and SPF (MYP-c and d). Glial cell marker S100β (red) is significantly decreased in GF conditions in the mucosa compared to SPF (e and f) and Bt restores GAP43-IR to SPF levels. In MYP, S100β is unchanged in GF conditions compared to SPF; however, Bt-CONV showed a significant increase in the number of IR pixels compared to GF conditions (g and h). N = 3–5. Yellow arrows indicate positive mucosal-IR, white arrows indicate positive MYP-IR. Error bars show SEM, and significance is p < .05 as denoted by *. Scale bar = 50 μm.
Bt conventionalization specifically downregulates the tight junction protein claudin 3 while upregulating TLR2 expression
We next asked if the effect of Bt was mediated by the paracellular movement of bacterial components directly interacting with nerves by assessing the expression of tight junction proteins important for maintaining epithelial barrier integrity. qRT-PCR data revealed GF mice had similar levels of expression of claudin 3 (Figure 4a), claudin 7 (Figure 4b), occludin 1 (Figure 4c), tight junction protein (TJP, Figure 4d) and junction adhesion molecule (JAM) Figure 4e). After Bt-CONV claudin 3 expression significantly decreased (Figure 4a), TLRs can directly interact with microbial components to facilitate communication between microbes and host cells. Therefore, we assessed TLR expression to understand if Bt can alter signaling via this mechanism. Of the TLRs studied, GF mice had significantly lower mRNA expression of TLR2 which was recovered after Bt-CONV (Figure 4g). Expression of TLR1 (Figure 4f), TLR4 (Figure 4h), TLR5 (Figure 4i) and TLR9 (Figure 4j) was comparable between SPF, GF and Bt-CONV. These data show Bt is likely to communicate via TLR2 expression.
Figure 4.
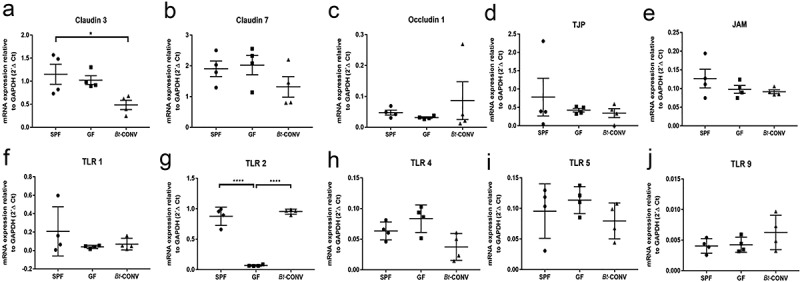
Bt-CONV specifically downregulates claudin 3 and upregulates TLR2 mRNA expression.
mRNA expression levels in claudin 3-a, claudin 7-b and occludin 1-c, tight junction protein (TJP-d), junctional adhesion molecule (JAM-e) in SPF, GF and Bt-CONV. Claudin 3 mRNA is significantly decreased in Bt-CONV group only (a). mRNA expression levels in toll-like receptor 1 (TLR1-f), toll-like receptor 2 (TLR2-g), toll-like receptor 4 (TLR1-h), toll-like receptor 5 (TLR5-i) and toll-like receptor 9 (TLR9-j) in SPF, GF and Bt-CONV. TLR2 mRNA is significantly decreased in GF compared to SPF but when conventionalized by Bt the expression is significantly increased compared to GF conditions (b). The expression is described relative to GAPDH and represents N = 4. Error bars show SEM, and significance is p < .05 as denoted by *. The expression is described relative to GAPDH and represents N = 4. Error bars show SEM, and significance is p < .05 as denoted by *.
Bt conventionalization regulates L-cells of the enteroendocrine cell lineage
Enteroendocrine cells (EEC) are specialized epithelial cells that store and release hormones and neurotransmitters capable of communicating with enteric neurons. In GF mice, GLP-1 immunoreactive (IR) L-cells doubled in number compared to SPF mice, and Bt normalized cell numbers within 3 d of colonization (Figure 5a, b). GF conditions also increased the expression of 5-HT containing enterochromaffin (EC) cells when compared to SPF mice (Figure 5c, d). However, this was not normalized by Bt. We also visualized ChAT and acetylcholine containing tuft cells.30 The total number of ChAT expressing cells was comparable in GF and Bt-CONV mice (Figure 5e, f). The increased EC cell population is likely due to reduced release of mediators and not increased proliferation as we observed reduced expression of LGR5 (marker of intestinal stem cells31) and ATOH-1, transcription factor required for EEC differentiation (Supplementary Figure 1a, b).32 To confirm the change in GLP-1 and 5-HT cells, we normalized the data against a total number of epithelial cells per field of view. The results were parallel in each form of analysis (Supplementary Figure 2a, b). Thus, the observed changes in epithelial cells by Bt are a true reflection rather than a change in overall epithelial cell proliferation. Additionally, there were no changes in villi length across all mouse groups (data not shown).
Figure 5.
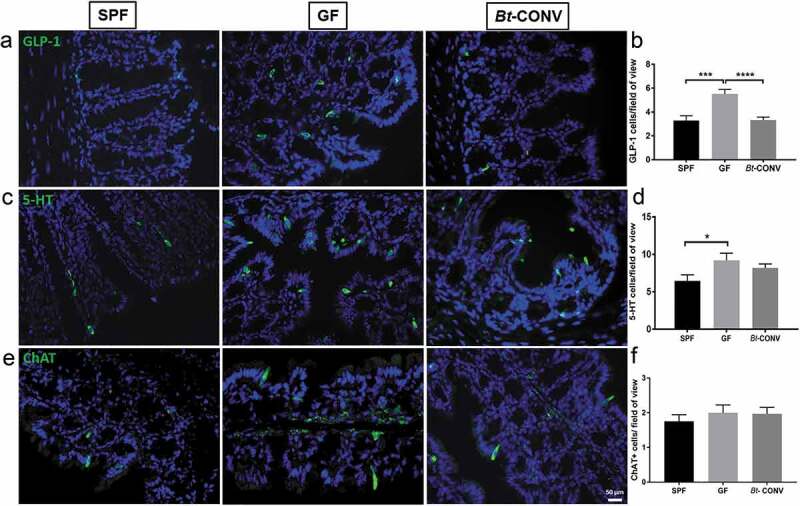
Bt-CONV specifically normalizes L-cells only.
GLP-1 found in L cells is increased in GF conditions; however, this is restored by Bt-CONV back to SPF levels (a and b). 5-HT containing EC cells is similarly increased in GF mice, however, unaltered in Bt-CONV (c and d). ChAT containing tuft cells is unchanged across all groups (e and f). N = 3–5. Error bars show SEM, and significance is p < .05 as denoted by *. Scale bar = 50 μm.
Coordinated colonic contractions are regulated by Bt
The differential regulation of enteric neurons by Bt suggested a possible role in neurogenic control of motility. To investigate this, we used colonic manometry to measure the complex motor control of the colon.33 Raw traces demonstrate colonic contractions from proximal to distal colon in SPF (Figure 6a), GF (Figure 6b) and Bt-CONV mice (Figure 6c). The rate of contraction, denoted by the number of peaks/min, was significantly increased in the proximal colon of GF and Bt-CONV mice compared to SPF mice, while all other regions were similar in all groups of animals (Figure 6e). The strength of contractions as measured amplitude of pressure waves was unchanged across all groups of mice (Figure 6f). CMMC analysis gives an understanding of the regulatory processes involved in colonic motility, including the direction of contraction and propagation velocity. A CMMC was taken as contractions that propagated either anterograde (orally), retrograde (aborally), or remained static (simultaneous) across at least three channels covering approximately half the length of the colon.34 The number of CMMCs was significantly increased in both GF and Bt-CONV mice compared to those seen in SPF mice (Figure 6d). Propagation velocity of CMMC, which measures the speed at which the CMMCs migrated, showed no change across all groups of animals (Supplementary Figure 2a). Finally, there were no discernable differences between groups in the change in direction of contraction, which would indicate the disorganization of neurogenic colonic control (Supplementary Figure 2b). Collectively, our functional analysis shows that Bt alters region-specific contractions in the proximal colon and is able to increase colonic motor complexes suggesting an important role in colonic motility.
Figure 6.
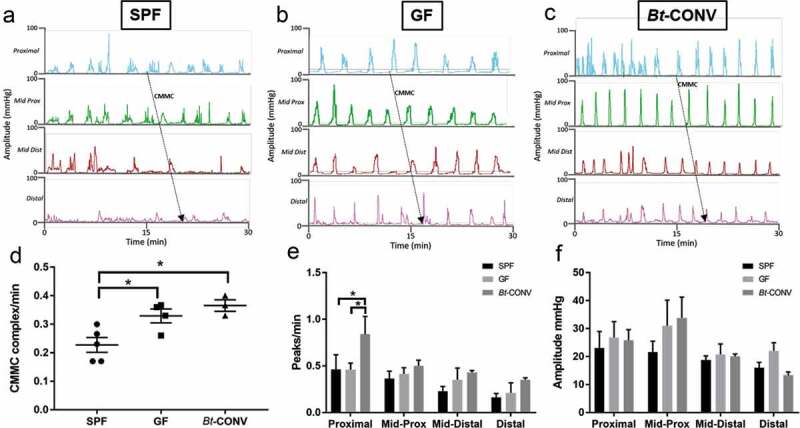
Coordinated colonic contractions are regulated by Bt.
Raw traces showing manometric pressure recordings in the whole colon in SPF (a), GF (b) and Bt-CONV (c). The number of colonic migrating motor complexes (CMMCs)/min is significantly increased in GF conditions compared to SPF and further upregulated in Bt-CONV compared to GF conditions (d). The frequency of contractions (peaks/min) is only increased in Bt-CONV compared to SPF and GF only in the proximal colon. The amplitude of contractions across all colonic regions and mouse groups is unchanged. N = 5. Error bars show SEM, and significance is p < .05 as denoted by *.
Discussion
An understanding of the mechanisms by which the colonic microbial environment influences GI physiology is needed before we can advance the treatments of many conditions. Here we show that the microbial quorum is critical for normal colonic ENS innervation as germ-free mice have significantly reduced neuronal populations in both mucosal and muscle layers. Importantly, we show that the prominent gut bacterium Bt is critical for colonic ENS innervation as recolonization normalizes neuronal populations. We also show that the microbial environment influences colonic motility and Bt increases propulsive contraction frequency.
Using the pan-neuronal marker PGP9.5, we observed that overall neuronal innervation was reduced in both the colonic mucosa and muscle layers (including the myenteric plexus) in GF compared to SPF mice. Interestingly, expression of the calcium-binding protein, calretinin, was reduced only in myenteric neurons and not in mucosal fibers of GF mice. The colonic myenteric plexus tightly regulates functions such as motility that responds to the luminal environment, via neuronally mediated pathways. Calretinin is an important neurochemical marker for two reasons; firstly, it is a protein that is primarily expressed in mucosal vagal afferent fibers35 and secondly, calretinin-positive myenteric neurons expressed in the colon36 induce neurogenic contractions.37Therefore, changes to myenteric calretinin-positive neurons are important predictors of changes to reflexes controlling motility. Thus, it was important to know how specific types of motor neurons, i.e. excitatory and inhibitory, were altered by the microbial environment and by Bt. Nitric oxide is the primary inhibitory neurotransmitter found in motor neurons that inhibits smooth muscle contractility, while acetylcholine is the primary excitatory neurotransmitter that promotes peristalsis.38 Our data show that Bt alone is sufficient for normal regulation and expression of these neurotransmitters as prior to Bt conventionalization GF mice had two-fold higher expression levels of the enzyme ChAT which synthesizes acetylcholine. Conversely, expression of NOS, that catalyzes the production of nitric oxide from L-arginine, was significantly reduced in myenteric plexus of GF mice compared to SPF mice. The effect of Bt in inducing such changes to the neurochemical profile of myenteric neurons was confirmed at a functional level by colonic manometry experiments. GF mice had an increased number of CMMCs compared to the SPF group. This agrees with the substantial increase of ChAT and therefore acetylcholine in GF mice as acetylcholine initiates neuronal activity to promote smooth muscle contraction leading to CMMC generation.38 Decreased nitrergic neurons, as evidenced by reduced NOS expression in GF mice, are likely to also contribute to the increased migration observed in GF mice as nitrergic neurons inhibit both smooth muscle contractility and the activity of ChAT-positive cholinergic neurons.38 Therefore, the increase in colonic motility in GF mice is likely via increased activity of motor neurons combined with reduced inhibitory activity.
Our data shows the specific role of a single bacterium-Bt and its role in influencing neuronal subpopulations and colonic motor function. Indeed, these data begin to delineate the impact of specific bacteria and adds to existing data demonstrating the influence of whole bacterial quorum on enteric neuronal maturation.39 Neurochemical data show that Bt regulates populations of excitatory and inhibitory motor neurons via ChAT and NOS, respectively. However, expression of SP, an excitatory neurotransmitter that is co-released with acetylcholine40 is unaltered in GF mice but is specifically up-regulated by Bt in both mucosa and myenteric plexus. SP increases propulsive motility by activating NK1 receptors on smooth muscle cells41,42 and promotes the initial peristaltic contraction to increase peristaltic frequency via NK2 and NK3 receptors on circular muscle and enteric nerves, respectively.43 Our ex vivo colonic manometry experiments in Bt conventionalized mice showed increased CMMCs and higher amplitude contractions in the proximal colon. These data concur with increased expression of SP observed in the Bt-CONV group as this neurotransmitter is likely to be activating neuromuscular and enteric NK1-3 receptors to promote colonic motility. Interestingly, a previous study using mice mono-colonized with Lactobacillus rhamnosus showed this specific bacterium influences ENS signaling and function via increased ileal contractions, transit time and stool frequency.44 This is in agreement with our study whereby Bt exerts a similar effect on the colonic motility as evidenced by increased CMMC. Collectively, the emerging data suggest specific bacteria may exert different effects on ENS activity.
To understand the mechanisms by which the intestinal microbiota in general, and Bt specifically, influences the makeup of the neuronal populations of the colon, we asked if changes occur to enteric glial cells (EGCs) and neuronal budding. EGCs specifically express the calcium-binding protein S100β and it is therefore used as a marker for these cells.45 We found that both mucosal EGCs (mEGCs) and myenteric EGC labeling via S100β are reduced in the colon of GF mice compared to SPF mice. These data agree with Kabouridis et al. who reported reduced S100β staining within villus structures of GF mice, although this was restricted to the ileum.46 Colonization with Bt was able to restore both mEGC and ganglionic EGCs of the myenteric plexus demonstrating that this bacterium is a critical regulator of EGC proliferation. Furthermore, the neuronal budding marker, GAP43 was unaffected in GF mice but upregulated by Bt. Only recently has the concept of neurogenesis in adult ENS been confirmed whereby myenteric neurons undergo apoptosis and regeneration by myenteric stem cells expressing Nestin+.47 Collectively, our data support this observation and suggest Bt has a significant role in maintaining neuronal numbers, the first time an effect of a single bacteria of colonic neuronal populations has been demonstrated.
As it was clear that Bt had an effect on the ENS at both structural and functional levels, we asked if this was a direct effect on neurons or indirect effect via an intermediary signaling pathway. Although other studies in GF mice show both a positive and negative effect of the microbiota on tight junction expression in the colon and brain, respectively,48,49 our study assesses a broader array of constituents of tight junction complexes to give additional insights into the overall effect on barrier function homeostasis. We observed strikingly that amongst TLRs, TLR2 expression was downregulated in GF mice, with Bt conventionalization restoring expression levels. We observed a change in claudin 3 expression exclusively without any changes in other epithelial tight junction proteins. The data suggest Bacteroides may be interacting with TLR-2 to induce downstream effects on claudin 3, similar to other gram-negative bacteria8. For example, C.Rodentium (enteric bacterial pathogen) is a gram negative bacteria that specifically activates TLR-2. This study demonstrated that TLR2–/– mice suffered impaired epithelial barrier function mediated via claudin‐3 in a model of colitis. The epithelium was unable to respond appropriately due to barrier dysfunction mediated by the delocalisation of a single transmembrane protein- claudin 38.Similarly, Bacteroides, a stable gut resident specifically alters expression of claudin 3.
We also observed that amongst enteroendocrine cells studied, GLP-1 containing L-cells were influenced by Bt. Previous studies have shown a number of TLRs, including TLR2 are expressed in the mouse colon,50 and by human L-cells.51,52 Therefore, normalization of neuronal populations by Bt may be mediated by induction of TLR2 recognition of outer membrane lipoligosaccharides8,10 leading to activation of L-cells containing GLP-1 and PYY. Binding of PYY to Y2 receptors on enteric neurons, as has been shown in the secretomotor neuron subtype,53 and binding of GLP-1 to GLP1 R on calretinin-positive fibers (data not shown) may influence neurotransmitter expression and release, as well as modulate neurogenesis pathways. Additionally, treating mice with an LPS antagonist will confirm whether Bt is indeed acting via TLR-2-mediated pathway.
This initial report describing the effects of Bt on ENS structure and function has limitations that arise from access, cost and feasibility of studying germ-free and mono-colonized mice. For example, while we have identified changes to neurochemical profiles, alterations to extrinsic vs. intrinsic populations induced by Bt will further add to understanding how a single bacteria can influence the neuroanatomy. Furthermore, whole-mount studies will assist in understanding if Bt regulates neuronal architecture of ganglia, fibers or both, as well as glial processes and cells. Finally, colonic motility is neuronally regulated by both motor and secretory neurons. Limited access to germ-free mice allowed us to perform manometry studies only; however, secretory experiments such as measuring changes to short-circuit current in Ussing chamber experiments would be useful.
Collectively, our study reveals a critical role for Bt in regulating enteric neuronal cell populations and neurogenic gastrointestinal activity. These findings demonstrate that a specific, dominant gut microbe has a significant impact on healthy gut motility by maintaining ENS and enteroendocrine cell populations. As such, it is now important to understand if Bt (including dysbiosis leading to altered Bt populations) may impact gastrointestinal symptoms leading to abnormal motility by inducing changes in neuronal plasticity.
Materials and methods
Mouse tissue
All animal experiments were conducted in strict accordance with the Home Office Animals (Scientific Procedures) Act 1986. C57BL/6 mice, aged 10–12 weeks, were housed and maintained in SPF conditions at the University of East Anglia, Norwich. C57BL/6 GF mice were maintained in sterile isolators in the UEA animal facility and GF status was continuously monitored by microscopy, anaerobic and aerobic culturing, and PCR for bacterial contamination. GF mice were conventionalized, by administrating 0.1 ml of a Bacteroides thetaiotaomicron (VPI-5482) (Bt-CONV) suspension containing 1 × 108 colony-forming units (CFU) in sterile phosphate-buffered saline (PBS) by oral gavage. Fresh feces were collected into sterile containers, weighed and homogenized in PBS. Serial dilutions of the supernatants were plated onto Brain Heart Infusion (BHI) agar supplemented with 0.001% hemin and incubated anaerobically at 37°C with CFU counted 48 h later. Tissue collected for immunohistochemical studies was flushed with PBS and cut into segments as proximal, mid and distal colon prior to fixation in 4% paraformaldehyde (PFA). Thus, we could not measure the length of the whole, intact colon. However, it has been demonstrated colonic length is unchanged between germ-free and mice colonized with human gut microbes.54
Immunohistochemistry
Immunolabelling of protein gene product 9.5 (PGP 9.5, Agilent Dako, Z5116, 1 in 500), calretinin (Swant, CG1, 1:500), nitric oxide synthase (NOS, Genetex, GTX89962, 1:400), choline acetyltransferase (ChAT, Merck Millipore, AB144P, 1 in 400), substance P (SP, Novus Biologicals, NC1/34, 1:500), calcitonin gene-related peptide (CGRP, Abcam, ab36001, 1 in 400), GAP-43 (Abcam, ab16053, 1:500) and S100β (Abcam, ab41548, 1:500) was assessed in the proximal colon of each mouse group. In brief, tissue was fixed in 4% paraformaldehyde, sections were prepared, then blocked in universal blocking agent and incubated overnight with a specific neuronal marker. Tissues were then washed and incubated with species-specific AlexaFluor conjugated secondary antibodies. Images were obtained using Metamorph software on an Olympus MM Leica with 10x and 40x objectives.
From each mouse group, 20 sections were analyzed in total. From each section, five images at 40x were captured; therefore, the total number of images captured covered the entire section. Positive immunolabeling was measured by pixel analysis using Image J with a plugin called JACoP. Regions of interest (ROI) from images were obtained for mucosa and myenteric plexus using the ‘Freehand’ selection tool on Image J to separate these regions from surrounding tissue structures such as sub-mucosal plexus, musculature and connective tissue. To determine whether a nerve/neuronal ganglia were positively stained, an approximate threshold of 20% above background was set as the baseline. The background was defined as 0% (which was black), and the brightest area of the image was 100%. No primary antibody controls were performed alongside each experiment to confirm the specificity of the primary antibody, which resulted in no fluorescence above background.
Villi length (distance from the base of the crypt to the top of the villi) was measured using the ruler tool on Image J. We measured the distance from the base of the crypt to the top of the villi.
Immunopositive cells for GLP-1, 5-HT and ChAT containing tuft cells were manually counted in each section and averaged over five fields of view. We also counted GLP-1 and 5-HT positive cells relative to the total number of epithelial cells in each image.
Quantitative polymerase chain reaction (qPCR)
RNA was extracted from each mouse tissues using an RNeasy Mini kit (Qiagen, Hilden, Germany). RNA quantity and quality were assessed using a NanoDrop, and cDNA was obtained using a High-Capacity cDNA Reverse Transcription Kit with an RNase inhibitor (Thermo Fisher Scientific). Quantitative real-time reverse transcription PCR (qRT-PCR), performed using SYBR® Green-based expression analysis, was used to assess the relative expression of tight junction consTotal target gene expression wastituents. Qiagen QuantiTect primer assays were used to assess the expression of tight junction protein (TJP, QT00493899), junctional adhesion molecule (JAM, QT00159481), claudin 3 (QT02419851), 7 (QT02420187) and occludin 1 (QT00111055). Expression of toll-like receptors was also assessed using the QuantiTect primer assays; TLR1 (QT00157430), 2 (QT00129752), 4 (QT00259042), 5 (QT00262549) and 9 (QT01043049) (Qiagen). Total target gene expression was determined relative to GAPDH (QT00199388) expression. Reactions were run on an Applied Biosystems 7500 Real-Time PCR system.
Motility studies
Colonic transit was determined using multi-lumen perfusion manometry. In brief, mouse colons were dissected and flushed with 4°C Krebs solution (NaCI, 120; KCl, 5.0; CaC12, 2.5; MgCl2, 1; NaH2PO4, 1; NaHCO3, 25; and glucose, 11 mM). Once clean the whole colon was explanted to an organ bath and the solution was gassed continuously with 95% O2 and 5% CO2 and maintained at 37°C. A multi-lumen manometric catheter was threaded from the oral end into the lumen of the colon for manometric recordings. Pressure waves were recorded at 4 side holes, 1 cm apart using a specialized medical measurement system (MMS-Sandhill scientific). Each channel was perfused with degassed saline at 0.10 mL channel.−1 Colonic tissue was allowed to equilibrate for 15 min before spontaneous recordings were measured for 30 min (N = 5 in each mouse group). Spontaneous pressure waves were recorded over a set period of time in each mouse group (30 mins). The total number of contractions and amplitude of contractions were calculated. CMMCs were calculated manually where the frequency, direction of propagation, i.e. retrograde, anterograde or synchronous was noted, as well as the propagation velocity calculated by dividing the distance between recording sites by the time measured between the onset of contractions (within 5%) as used previously by Fida et al.55 Recordings were measured using the Medical Measurement System (MMS) database analysis software (Ardmore Healthcare Ltd, UK).
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). For immunohistochemistry, statistical analysis was performed using a two-way ANOVA and a Mann–Whitney post hoc test with p < .05 defined as significant. Statistical analysis using a one-way ANOVA was performed for qRT-PCR expression studies. Motility studies were statistically analyzed with a two-way ANOVA using Sidak multiple comparison test to compare the two groups; p < .05 was taken to be significant. p < .05 = *, p < .01 = **, p < .001 = ***, p < .0001 = ****All statistical analysis was performed using GraphPad Prism (V.7.02, GraphPad Software, Inc).
Supplementary Material
Funding Statement
The author(s) gratefully acknowledge the support of the Biotechnology and Biological Sciences Research Council (BBSRC); this research was funded by the BBSRC Institute Strategic Programme Gut Microbes and Health BB/R012490/1 and its constituent project (BBS/E/F/000PR10355).
Author contribution
All authors had access to the study data and reviewed and approved the final manuscript. Study concept and design – MP, RA & LAB; Acquisition of data – MP, RA, NP, LB; Analysis and interpretation of data – MP, RA, NP, SC and LAB; Drafting of the manuscript – MP & RA; Critical revision of the manuscript for important intellectual content – all authors; Statistical analysis – RA & MP; Obtained funding – LAB & SC; Administrative, technical or material support – RS, AP, AG, & AB; Study supervision – MP, SC & LAB.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Derrien M. van Hylckama Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyland NP, Cryan JF. Microbe-host interactions: influence of the gut microbiota on the enteric nervous system. Dev Biol. 2016;417:182–187. doi: 10.1016/j.ydbio.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Husebye E, Hellstrom PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G368–80. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 6.Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 7.Koppel N, Balskus EP. Exploring and understanding the biochemical diversity of the human microbiota. Cell Chem Biol. 2016;23:18–30. doi: 10.1016/j.chembiol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, Vallance BA. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:388–403. doi: 10.1111/j.1462-5822.2007.01052.x. [DOI] [PubMed] [Google Scholar]
- 9.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson AN, Choudhury BP, Fischbach MA. The biosynthesis of lipooligosaccharide from bacteroides thetaiotaomicron. MBio. 2018;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 13.Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, Langella P, Thomas M. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 15.Delday M, Mulder I, Logan ET, Grant G. Bacteroides thetaiotaomicron ameliorates colon inflammation in preclinical models of Crohn’s disease. Inflamm Bowel Dis. 2019;25:85–96. doi: 10.1093/ibd/izy281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 17.Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130:2187–2198. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- 18.Taketomi T, Yoshiga D, Taniguchi K, Kobayashi T, Nonami A, Kato R, Sasaki M, Sasaki A, Ishibashi H, Moriyama M, Nakamura K, Nishimura J, Yoshimura A.Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci. 2005;8:855–857. doi: 10.1038/nn1485. [DOI] [PubMed] [Google Scholar]
- 19.Roberts RR, Bornstein JC, Bergner AJ, Young HM. Disturbances of colonic motility in mouse models of Hirschsprung’s disease. Am J Physiol Gastrointest Liver Physiol. 2008;294:G996–G1008. doi: 10.1152/ajpgi.00558.2007. [DOI] [PubMed] [Google Scholar]
- 20.Hansen MB. The enteric nervous system I: organisation and classification. Pharmacol Toxicol. 2003;92:105–113. doi: 10.1034/j.1600-0773.2003.t01-1-920301.x. [DOI] [PubMed] [Google Scholar]
- 21.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 22.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. [DOI] [PubMed] [Google Scholar]
- 23.Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, Guarner F. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4:17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin K, Heemskerk L, Herman R, Ainslie M, Rikers RM, Schmidt HG. Initial diagnostic hypotheses bias analytic information processing in non-visual domains. Med Educ. 2008;42:496–502. doi: 10.1111/j.1365-2923.2007.02994.x. [DOI] [PubMed] [Google Scholar]
- 25.Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- 26.Brierley SM, Jones RC 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 28.Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L, Bellacosa L, De Giorgio R, Le Berre-Scoul C, Aubert P, Neunlist M, De Ponti F, Stanghellini V, Barbara G. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–11 e4. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Hansebout CR, Su C, Reddy K, Zhang D, Jiang C, Rathbone MP, Jiang S.Enteric glia mediate neuronal outgrowth through release of neurotrophic factors. Neural Regen Res. 2012;7:2165–2175. doi: 10.3969/j..1673-5374.2012.028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schutz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, Chubanov V, Gudermann T, Diener M, Kummer W, Krasteva-Christ G, Weihe E.Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015;6:87. doi: 10.3389/fphys.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P.Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aktar R, Peiris M, Fikree A, Cibert-Goton V, Walmsley M, Tough IR, Watanabe, P. Araujo EJA, Mohammed SD, Delalande JM, Bulmer DC, Scott SM, Cox HM, Voermans NC, Aziz Q, Blackshaw LA.The extracellular matrix glycoprotein tenascin-X regulates peripheral sensory and motor neurones. J Physiol. 2018;596:4237–4251. doi: 10.1113/JP276300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarna SK. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology. 1985;89:894–913. doi: 10.1016/0016-5085(85)90589-X. [DOI] [PubMed] [Google Scholar]
- 35.Dutsch M, Eichhorn U, Worl J, Wank M, Berthoud HR, Neuhuber WL. Vagal and spinal afferent innervation of the rat esophagus: a combined retrograde tracing and immunocytochemical study with special emphasis on calcium-binding proteins. J Comp Neurol. 1998;398:289–307. doi:. [DOI] [PubMed] [Google Scholar]
- 36.Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- 37.Hibberd TJ, Feng J, Luo J, Yang P, Samineni VK, Gereau R, Kelley N, Hu H, Spencer NJ.Optogenetic induction of colonic motility in mice. Gastroenterology. 2018;155:514–28 e6. doi: 10.1053/j.gastro.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gould TW, Swope WA, Heredia DJ, Corrigan RD, Smith TK. Activity within specific enteric neurochemical subtypes is correlated with distinct patterns of gastrointestinal motility in the murine colon. Am J Physiol Gastrointest Liver Physiol. 2019;317:G210–G21. doi: 10.1152/ajpgi.00252.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Vadder F, Grasset E, Manneras Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Backhed F.Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018;115:6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maggi CA, Patacchini R, Meini S, Quartara L, Sisto A, Potier E, Giuliani S, Giachetti A.Comparison of tachykinin NK1 and NK2 receptors in the circular muscle of the guinea-pig ileum and proximal colon. Br J Pharmacol. 1994;112:150–160. doi: 10.1111/j.1476-5381.1994.tb13045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zagorodnyuk V, Santicioli P, Maggi CA. Tachykinin NK1 but not NK2 receptors mediate non-cholinergic excitatory junction potentials in the circular muscle of guinea-pig colon. Br J Pharmacol. 1993;110:795–803. doi: 10.1111/j.1476-5381.1993.tb13882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maggi CA, Holzer P, Giuliani S. Effect of omega-conotoxin on cholinergic and tachykininergic excitatory neurotransmission to the circular muscle of the guinea-pig colon. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:529–536. doi: 10.1007/BF00173023. [DOI] [PubMed] [Google Scholar]
- 43.Holzer P, Schluet W, Maggi CA. Substance P stimulates and inhibits intestinal peristalsis via distinct receptors. J Pharmacol Exp Ther. 1995;274:322–328. [PubMed] [Google Scholar]
- 44.Chandrasekharan B, Saeedi BJ, Alam A, Houser M, Srinivasan S, Tansey M, Jones R, Nusrat A, Neish AS. Interactions between commensal bacteria and enteric neurons, via fpr1 induction of ros, increase gastrointestinal motility in mice. Gastroenterology. 2019;157:179–92 e2. doi: 10.1053/j.gastro.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruhl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17:777–790. doi: 10.1111/j.1365-2982.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 46.Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, Liu L, Li Q, Saha M, Li C, Enikolopov G, Becker L, Rakhilin N, Anderson M, Shen X, Dong X, Butte MJ, Song H, Southard-Smith EM, Kapur RP, Bogunovic M, Pasricha PJ. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A. 2017;114:E3709–E18. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braniste V, Al-Asmakh M, Kowal C, Anuar F, A A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyas B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S.The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes CL, Dong J, Galipeau HJ, Jury J, McCarville J, Huang X, Wang XY, Naidoo A, Anbazhagan AN, Libertucci J, Sheridan C, Dudeja PK, Bowdish DME, Surette MG, Verdu EF. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci Rep. 2018;8:14184. doi: 10.1038/s41598-018-32366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Price AE, Shamardani K, Lugo KA, Deguine J, Roberts AW, Lee BL, Barton GM.A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity. 2018;49:560–75 e6. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogunovic M, Dave SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Mayer LF, Plevy SE.Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1770–83. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larraufie P, Dore J, Lapaque N, Blottiere HM. TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways. Cell Microbiol. 2017;19:2. [DOI] [PubMed] [Google Scholar]
- 53.Cox HM. Endogenous PYY and NPY mediate tonic Y1- and Y2-mediated absorption in human and mouse colon. Nutrition. 2008;24:900–906. doi: 10.1016/j.nut.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slezak K, Krupova Z, Rabot S, Loh G, Levenez F, Descamps A, Lepage P, Dore J, Bellier S, Blaut M. Association of germ-free mice with a simplified human intestinal microbiota results in a shortened intestine. Gut Microbes. 2014;5:176–182. doi: 10.4161/gmic.28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fida R, Bywater RA, Lyster DJ, Taylor GS. Chronotropic action of 5-hydroxytryptamine (5-HT) on colonic migrating motor complexes (CMMCs) in the isolated mouse colon. J Auton Nerv Syst. 2000;80:52–63. doi: 10.1016/S0165-1838(00)00074-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


