Abstract
Even though effective drugs for treating hypertension are available, a great percentage of patients have inadequate control of their blood pressure. Unwanted side effects and inappropriate oral drug adherence are important factors that contribute to the global problem of uncontrolled hypertension. Vaccination could provide a revolutionary therapy with long-lasting effects, increasing patient compliance and therefore better control of high blood pressure. Nowadays, current immunization approaches against hypertension target renin, angiotensin I, angiotensin II, and angiotensin II type 1 receptor, key elements of the renin–angiotensin system. This article reviews the different vaccination attempts with proteins and peptides against the different molecules of the renin–angiotensin system in the last two decades, safety issues, and other novel prospects biomarkers in hypertension, and summarizes the potential of this immunomodulatory approach in clinical practice.
Keywords: Renin–angiotensin system, Vaccine, Hypertension, Immunomodulation, Therapy, Autoimmunity, Inflammation
Introduction
High blood pressure (BP) is the major risk factor for early death and disability worldwide [1]. Despite available, effective, and safe antihypertensive drugs, BP control remains unsatisfactory throughout the world with 50% or less of treated patients reaching recommended therapeutic goals [2]. The renin–angiotensin system (RAS) regulates BP and its activation plays a key role in common pathological disorders, including hypertension, heart failure, and renal failure.
Classical RAS pathway begins with release of renin from juxtaglomerular cells on the renal afferent arterioles as a response to renal hypoperfusion; renin then cleaves angiotensinogen (AGT) synthesized by the liver to produce angiotensin I (Ang I), an inactive peptide. In the next step of the system, Ang I is transformed to angiotensin II (Ang II) by endothelial angiotensin-converting enzyme (ACE), mostly in blood vessels of the lungs. Finally, Ang II promotes vasoconstriction as well as aldosterone release from the adrenal gland through the angiotensin II type 1 receptor (AT1R), effects that promote sodium retention and increased BP.
The juxtaglomerular apparatus initiates RAS by secreting renin in response to changes in BP and salt balance through two sensing systems: a renal baroreceptor and sodium chloride transport to the macula densa [3]. To prevent the harmful effects of excessive activation of RAS, drugs such as aliskiren interfere by blocking the active site of renin, inhibiting the formation of Ang I from AGT [4] or ACE inhibitors (ACEi) that impede the conversion of Ang I to Ang II.
Overactivation of RAS can lead to hypertension, which has been treated in the clinic with drugs that target the different molecules of the system, e.g., AT1R blockers (ARBs) prevent the interaction of Ang II with AT1R through competitive antagonism thus preventing Ang II harmful effects that act in a localized way like tissue remodeling, endothelial dysfunction, and fibrosis [5]. With the global increase in life expectancy, chronic diseases have become the greatest cause of morbidity and mortality worldwide. Lowering BP to therapeutic goals endorsed by most guidelines is the most successful way to prevent end organ damage and reduce the cardiovascular mortality. Despite the availability of effective antihypertensive drugs, less than 50% of patients with hypertension are controlled due to different aspects such as (1) improper or poor adherence to treatment, (2) physician inertia, and (3) the high costs in drug treatments, particularly in developing countries. Therefore, new approaches to solve poor patient compliance and reduce the global burden of hypertension are needed and continue to be explored to improve patient wellness.
Vaccines are one of the great achievements of modern medicine, primarily used to provide immunity against bacterial and viral pathogens; nevertheless, vaccination is now being rediscovered to treat chronic diseases like diabetes, cancer, and hypertension. A therapeutic vaccine against “hypertension” could have two major advantages over conventional therapy. First, therapeutic vaccination targeting RAS could overcome the need for daily medication and provide long-lasting effects in the range of months, improving patient compliance. In second place, patients would not suffer side effects of conventional therapy that impact on quality of life like diarrhea, dizziness, and fatigue with renin inhibitors; cough, angioedema, and anemia with ACEi; and renal insufficiency with ARBs [6, 7].
Unlike traditional vaccines directed against infectious pathogens, novel therapeutic vaccines target self-antigens that promote cardiovascular diseases. Current immunization approaches against hypertension target RAS molecules, where it is intended that the vaccine (antigen) must be identified by B cells and T cells to originate antibody production, expecting high affinity antibodies to neutralize the different peptides of RAS in plasma reducing its effects or in the case of the AT1R impede its binding to Ang II. However, under physiological circumstances, renin, Ang I, Ang II, and AT1R do not have T-cell epitopes due to immunological self-tolerance and antibodies against these molecules are not usually produced. To circumvent this immunological tolerance, currently therapeutic vaccines use carrier proteins that provide T-cell epitopes and adjuvants that enhance the immune response (Table 1).
Table 1.
Carrier proteins and adjuvants used in vaccines against RAS
| Carrier | |
| Keyhole limpet hemocyanin | A huge protein molecule obtained from the hemolymph of Megathura crenulate, a marine mollusk. KLH is a greatly immunogenic T cell–dependent antigen |
| Tetanus toxoid | Produced by formaldehyde detoxification of tetanus toxin made by Clostridium tetani. Tetanus toxoid conjugate vaccines have shown to be immunogenic and safe |
| Bovine serum albumin | A serum albumin protein (BSA) derived from cows that can react with crosslinkers for the coupling of peptides. Therefore, BSA has been used as an immunogenic carrier protein |
| Virus-like particle | Multiprotein structures that imitate the conformation and organization of genuine native viruses but lack the viral genome. They can be engineered to display multiple peptides in order to optimize the antigenicity and immunogenicity |
| Pneumococcal surface protein A | A highly immunogenic surface protein of Streptococcus pneumoniae efficient at eliciting T-cell immune responses and antibodies |
| Adjuvant | |
| Freund’s adjuvant | Freund’s complete adjuvant is composed of heat-killed and dried mycobacterial cells, mannide monooleate (a surfactant agent), and a light mineral oil. The mycobacteria in Complete Freund’s adjuvant gather macrophages and other cells to the injection site, which magnifies the immune response. Not approved for human use due to its toxicity |
| Aluminum hydroxide | The most frequently used chemical as adjuvant. The mechanism of how aluminum hydroxide–based adjuvants produce their beneficial effects is still not entirely understood |
| CoVaccine HT | An oil-in-water adjuvant composed of sucrose fatty acid sulfate ester (SFASE) immobilized on oil droplets of a submicron emulsion of squalane-in-water |
| Cyclic diguanylate monophosphate | A well-conserved second messenger found in multiple bacterial species and a potent activator of both humoral and Th1-like immune responses |
In this review, we will only focus on peptide vaccines against RAS from 2000 to date. A detailed historical perspective can be found in the following reviews [8–13].
Vaccines as preventive medicine and therapy
The low compliance by the patients to antihypertensive drug therapy, undesirable side effects, and economic burden is an area of opportunity where therapeutic vaccines could be the solution. Vaccines are one of the great achievements of modern medicine and are used to provide immunity against bacterial and viral pathogens. Recent immunological insights have opened the therapeutic window of vaccines, moreover the simplistic coverage to prevent infections. The first approach was set in the cancer scenario by recognition of tumor rejection antigens by determining tumor-associated antigens that trigger T-cell responses [14].
Successful immunotherapy for chronic illnesses, such as hypertension, demand (1) use of suitable target antigens, (2) effective interaction between the antigenic peptide and antigen-presenting cell to mount the desired immune response, and (c) concomitant blockade of negative regulatory mechanisms that hinder desired effects [14].
In order to identify the appropriate target antigens, researchers chose those biomarkers that have been proven in in vivo models as in patients to be correlated to certain diseases. These antigens must be able to be presented in the human major histocompatibility complex (MHC) and counter-regulate the detrimental effects that end in developing or end-stage disease. It also depends on the chemical similarity the human antigen shares from mice, although actually this is the animal model used to identify and propose targeted antigens [14].
Vaccines, in order to be therapeutically useful, must be able to induce immune memory thus as T-cell specific for the epitope of the peptide of interest and B cells capable of secreting immunoglobulins also specific for the same epitope. The challenge in producing such is identifying the epitope of interest and the combination with the proper carrier and/or adjuvant [14].
Vaccine vectors become important because they influence the optimization of antigenic presentation. Vectors have been a developing stream to reach the desired target and avoid negative regulatory mechanism. These are, for example, protein adjuvants, loaded dendritic cells, nucleic acids, or recombinant viruses [14].
Targeted antigens: biological markers in hypertension
Different studies have shown that the immune system plays a role in the development of essential hypertension. It requires antigen presentation and co-stimulation, T cell–driven inflammation, and agonistic antibodies against AT1R and adrenergic receptors have supported the role of acquired immunity in hypertension [15]. Vascular dysfunction triggered by Ang II is related to NK cells and macrophages accumulation in the aortic wall [15].
In order to identify targeted antigens toward which the vaccines will work, we take into hand circulating molecules related to hypertension. Related to RAS, there are several components measured and correlated with cardiovascular pathologies that can be used (Table 2).
Table 2.
Overview of clinical and preclinical trials of vaccines targeting RAS
| Author (year) | Type of vaccine | Target | Phase of trial | Sample size | Outcome | Notes |
|---|---|---|---|---|---|---|
| Qiu et al. (2013) [16] | Human renin peptide (hR32), KLH and Freund’s adjuvant | Renin | Preclinical |
SD rats (n = 35) SHR (n = 48) WKY |
Decreased SBP of SHR up to –15 mmHg | No significant immune-mediated injury was observed |
| Gardiner et al. (2000) [17] |
“PMD-2850” Ang I peptide analogue, TT and aluminum hydroxide |
Ang I | Preclinical | SD rats (n = 82) | Suppressed responses to exogenous Ang I but had no effect to Ang II | Antibodies also cross-reacted with AGT |
| Downham et al. (2003) [18] | “PMD-3117” Ang I peptide analogue, KLH and aluminum hydroxide and PMD-2850 | Ang I | Preclinical | SD rats (n = 24) | Both vaccines produced comparable inhibition of the pressor effects of Ang I in rats | KLH is an acceptable substitute to TT as a carrier protein |
| Phase I clinical trial | Healthy, male, human volunteers (n = 50) | PMD-3117 induced production of anti-Ang I IgG, but no significantly effect on MAP | Sufficient anti-Ang I IgG molecules are needed to have an effect on blood pressure | |||
| Brown et al. (2004) [19] | PMD-3117 | Ang I | Phase II clinical trial | Patients with essential hypertension (n = 27) | Vaccination did not influence blood pressure | Well tolerated, the most common adverse events were transient local injection-site reactions |
| Turkie et al. (2016) [20] | PMD-3117 and CoVaccine HT | Ang I | Phase II clinical trial | Patients with moderate to mild hypertension (n = 20) | Study prematurely terminated as a consequence of adverse effects | The adverse effects were most likely caused by the adjuvant |
| Hong et al. (2009) [21] |
“AngI-R” Modified Ang I, BSA, aluminum hydroxide, and Freund’s adjuvant |
Ang I | Preclinical | SHR (n = 27) | Lowered the SBP by –15 mmHg | Two SHR in vaccinated group had vasculitis in the kidney |
| Ambül et al. (2007) [22] |
“CYT006-AngQb” Peptide derived from Ang II, VLP, and aluminum hydroxide |
Ang II | Preclinical | SHR (n = 42) |
SBP showed a reduction of up to –21 mmHg No change in blood pressure Ang II-specific antibodies were raised |
No manifestations of inflammation were identified in the kidney Well tolerated, no signs of inflammation or immune-complex formation |
| Phase I clinical trial | Healthy male subjects (n = 16) | |||||
| Tissot et al. (2008) [23] | CYT006-AngQb | Ang II | Phase II clinical trial | Patients with moderate to mild hypertension (n = 72) | Reduction in mean ambulatory daytime blood pressure by –9/–4 mmHg | Most side effects were influenza-like symptoms and transient, mild reactions at the injection site |
| Cytos Biotechnology (2009) [24] | CYT006-AngQb | Ang II | Phase II clinical trial | Patients with moderate to mild hypertension | Blood pressure reductions much lower at –2.3/–0.4 mmHg | Accelerated treatment induces higher antibody titers but lower antibody affinities |
| Ou et al. (2013) [25] |
“pHAV-4Ang IIs” Ang II, HAVLP, and Freund’s adjuvant |
Ang II | Preclinical | SHR (n = 20) | Reduction in SBP and DBP by –23/–12 mmHg | Reduction in Ang II levels up to 87 pg/ml |
| Nakagami et al. (2013) [26] |
“Ang II-KLH conjugate” Ang II peptide, KLH, and Freund’s adjuvant |
Ang II | Preclinical | C57/BL6J mice (n = 6–8 mice per group) | Attenuated Ang II–induced cardiac remodeling and hypertension | No pathological changes, nor macrophage or T-cell infiltrations were found in kidney or heart |
| SHRs (n = 10) | SBP was significantly decreased | |||||
| Watanabe et al. (2017) [27] | Ang II-KLH conjugate | Ang II | Preclinical | Rat experimental MI model in SD rats (n = 86) | Decreased cardiac remodeling that result in heart failure | No visible pathological changes were observed |
| Zhu et al. (2006) [28] |
“ATR12181” Extracellular portion of the rat AT1A, TT, and Freund’s adjuvant |
AT1R | Preclinical | SHR (n = 12) | A –17 mmHg reduction of SBP, reduced cardiac hypertrophy, and attenuation of kidney injuries | Signs of autoimmune diseases were not observed in heart and kidney |
| Li et al. (2014) [29] | ATR12181 | AT1R | Preclinical |
SHR (n = 35) Wistar rats (n = 28) |
Decreased SBP of immunized SHRs to a similar extent to losartan | Ameliorated the remodeling of small arteries to a similar degree to losartan |
| Azegami et al. (2012) [30] |
“AT1 receptor” Extracellular portion of the rat AT1A, KLH, and Freund’s adjuvant |
AT1R | Preclinical | SHRs (n = 90) | Significant decrease in SBP comparable with hydralazine and candesartan | Suppressed proteinuria, glomerular injury was significantly decreased |
| Chen et al. (2013) [31] |
“ATRQβ-001” Peptide (ATR-001) derived from human AT1R and VLP |
AT1R | Preclinical |
Ang II–induced hypertensive Balb/c mice (n = 40) SHR (n = 36) WKY |
Reduced blood pressure of Ang II–induced hypertensive mice up to –35 mmHg and that of SHRs up to –19 mmHg | No significant immune-mediated injury was detected |
| Zhou et al. (2016) [32] | ATRQβ-001 | AT1R | Preclinical |
ApoE−/− mice (n = 40) C57BL/6 mice (n = 10) |
Significantly reduced the lesion area and increased the stability of atherosclerotic plaque | No obvious immune-mediated injury was detected in the kidney or heart |
| Pan et al. (2018) [33] | ATRQβ-001 and aluminum hydroxide | AT1R | Preclinical | C57BL/6 mice (n = 70) | Prevented cardiac remodeling and improved postinfarct survival in a mouse model of AMI | Decrease in macrophage infiltration, fibrosis, apoptosis, and expression of pro-inflammatory cytokines |
| Hu et al. (2017) [34] | ATR-AP205-001 peptide from human AT1R displayed on VLP | AT1R | Preclinical | BALB/c mice (n = 76) | Induced humoral immunity through collaboration of follicular helper T cells, follicular dendritic cells, and B cells | No inflammatory injury was observed in the kidney and heart |
| Azegami et al. (2017) [35] |
“AT1R–PspA” AT1R peptide, PspA and cyclic di-GMP |
AT1R | Preclinical |
SHRs (n = 63) BALB/cAJcl mice (n = 20) |
Significantly decreased SBP by a maximum of –19 mmHg | Protected from lethal pneumococcal infection |
KLH keyhole limpet hemocyanin, SD rats Sprague–Dawley rats, SHR spontaneously hypertensive rats, WKY Wistar-Kyoto rats, SBP systolic blood pressure, TT tetanus toxoid, Ang I angiotensin I, Ang II angiotensin II, AGT angiotensinogen, MAP mean arterial pressure, VLP virus-like particle, DBP diastolic blood pressure, HAVLP hepatitis A virus–like particle, MI myocardial infarction, AT1R angiotensin II type 1 receptor, ApoE−/− apolipoprotein E-null mice, AMI acute myocardial infarction, Cyclic di-GMP cyclic diguanylate monophosphate
Renin
Produced and secreted by juxtaglomerular cells in the kidneys, renin is an aspartic protease that functions as a rate-limiting phase in RAS cascade. It has been proposed as a biomarker because it is found in plasma with a t1/2 of 90 min more or less. Its correlation with Ang II, which is the key player on vascular dysfunction, is quite accurate, although it is a trustable biomarker in patients prone to hypertension.
Renin generation at tissues rely on renin taken from blood. Plasma circulating and tissue renin are greatly related, both under normal and pathological conditions. High plasma renin activity levels in hypertensive patients are correlated with a greater risk of stroke and myocardial infarction. Augmented renin levels may be the outcome of generalized vascular disease, which includes renovascular diseases [36]. The Intermountain Heart Collaborative Study followed 1165 patients with coronary artery disease for 3 years. The results showed that increased baseline plasma renin activity is linked with myocardial infarction and heart failure [36]. This also correlates with the fact that the offspring of patients with hypertension and premature cardiovascular events during their life had impaired renin suppression capacity when tested with a salt loading [37].
When measuring the effect of renin on the circulatory system, there are two options of great interest. The first one is the plasma renin activity, which reflects Ang I generated by the renin; thus, it is a readout of renin activity, dependent on AGT and renin concentration [37]. The second option is to measure directly the plasma renin concentration by direct immunoassay, specifically the enzymatically active renin [37]. Both options have been related to increased cardiovascular events in patients with hypertension. Nevertheless, there is still debate on whether it can predict the risk of developing hypertension or if it is a response to the altered circulatory system [37]. Because Ang II triggers renin release from the collecting duct, contrary to the juxtaglomerular apparatus, instead of measuring plasma levels, urinary levels of renin release could reflect the end feedback status of RAS system [36]. However, renin clearly predicts the risk of developing hypertension and its accompanying comorbidities. Therefore, its inhibition is ideal to prevent the detrimental effects of its overactivation.
The earliest human study of a renin vaccine was performed by administering hog renin to human subjects in 1951. In this pioneer study, patients with essential hypertension produced antibodies against renin but was unsuccessful reducing BP. This outcome was later elucidated by the fact that anti-hog renin antibodies were unable to cross-react to human renin [38]. In 1987, a vaccine composed of human renin and Freund’s adjuvant was made. This vaccine was tested on marmosets which developed high titers of antibodies and a significant reduction on systolic BP (SBP) from 125 ± 13 mmHg to 87 ± 8 mmHg. However, the animals also developed an autoimmune disease in the kidneys distinguished by immunoglobulin deposition and macrophage infiltration [39].
The same team conducted a similar investigation in spontaneously hypertensive rats (SHR) using a vaccine composed of mouse renin and Freund’s adjuvant. SHR produced anti-renin antibodies in response to the vaccine accompanied with a significant drop in BP. Unfortunately, these SHR also developed chronic autoimmune interstitial nephritis distinguished by the presence of immunoglobulins, mononuclear cell infiltration, and fibrosis all over the juxtaglomerular apparatus [40].
The results from these studies halted further search for a renin vaccine until 2013, when a vaccine composed of human renin peptide (hR32), keyhole limpet hemocyanin (KLH), and Freund’s adjuvant was developed. The vaccine was tested in Sprague–Dawley (SD) rats and SHR to evaluate the antihypertensive effect. SD rats subcutaneously immunized on days 0, 14, 28, 42, and 56 developed high antibody titers but had no effect on SBP. SHR were subcutaneously injected on weeks 6, 8, and 10. Antibody titers in SHR reached the peak on day 35, reduced plasma renin activity, and decreased SBP up to –15 mmHg. This reduction of SBP in SHR but not in SD rats may be attributed to the presence of normal plasma renin activity in SD rats. Wistar-Kyoto rats (WKY) were also subcutaneously immunized on days 0, 14, and 28 to assess the safety of the vaccine. No IgG or immune-complex deposition, cell proliferation in the mesangial region, infiltration from macrophages or B cells in the glomeruli was detected on the kidneys of SHR and WKY. The first vaccines against renin used the complete protein of 340 amino acids as the main antigen. hR32 peptide is 7 amino acids in length, shorter than the minimal T-cell epitope; therefore, a CD8+ cytotoxic T-cell response was highly unlikely on vaccinated SHR and WKY [16].
Higher circulating concentrations of pro-renin (precursor of renin), another component of RAS, could also have a prognostic and predictive value [36]. In patients with hypertension, renin levels could be normal or even low, but prorenin concentrations are highly raised and correspond with the degree of progression of the illness. Although levels do not correlate with cardiovascular events, kidney dysfunction and the high prevalence of patients with coexisting hypertension and diabetes make this marker an interesting target [36].
Angiotensin I
The next target for therapeutic vaccines in RAS cascade is the inactive decapeptide Ang I. In 2000, a vaccine against Ang I called “PMD-2850” composed of an Ang I peptide analogue conjugated with a tetanus toxoid (TT) carrier protein and adjuvanted with aluminum hydroxide was developed. SD rats immunized with PMD-2850 on days 0, 21, and 42 produced anti-angiotensin antibody titers. Interestingly, the antibodies also cross-reacted with AGT. The vaccine significantly lowered mean arterial pressure (MAP) following exogenous Ang I administration but without significant effect on MAP after exogenous Ang II administration [17].
Since TT is a usual immunogen in man, it may exhibit limited effectiveness as a carrier protein in a vaccine due to epitopic suppression. This effect results in a suppressed immune response against the main antigen conjugated to TT by pre-existing immunity against this same carrier protein [41]. To further investigate this issue, PMD-2850 was compared with another 12 amino acid vaccine composed of an analogue of Ang I conjugated with KLH and aluminum hydroxide adjuvant named “PMD-3117.” SD rats were immunized with PMD-2850 and PMD-3117 on days 0, 21, and 42. Both vaccines had an equivalent effect on production of IgG titers against Ang I and attenuated MAP increase following exogenous Ang I administration. Therefore, KLH proved to be an adequate alternative for TT as a carrier protein. Both vaccines were also tested in a phase Ia clinical trial on healthy, male, human volunteers (age 18–45 years), but no anti-Ang I IgG was found after the first dose. A second dose of PMD-3117 in a phase Ib clinical trial on human volunteers originated production of anti-Ang I IgG, but no significant effect on MAP was observed following exogenous Ang I or Ang II administration. The decrease in MAP after exogenous Ang I administration seen on the SD rats and not in the human volunteers may be attributable to the lower anti-AI IgG titers (≤18,700) of humans compared with the anti-AI IgG titers (67,410 ± 7393) in rats [18]. PMD-3117 vaccine was tested in a phase II clinical trial on patients with essential hypertension (age 18–70 years). Patients were assigned to receive randomly three subcutaneous immunizations on days 1, 22, and 43 or four on days 1, 15, 29, and 43. Antibody induction against Ang I was detected in both regimes, after the second injection. No significant difference was observed between the two regimes, suggesting no benefit from using more than three injections to induce immunization. The median t1/2 for anti-Ang I antibodies was 85 days. Vaccination did not influence BP readings, but renin measurements had a significant elevation, probably an effect of the negative feedback of Ang II on renin secretion. Plasma aldosterone had no significant changes, but urine aldosterone was decreased by 6% on the groups that received PMD-3117. These results reflect some degree of blockade of RAS, but to have a significant impact on BP, a greater increase of anti-Ang I titers must be achieved. PMD-3117 was well tolerated; slight erythema and swelling surrounding the injection site was the most common adverse reaction [19]. A novel formulation of the PMD-3117 vaccine in combination with CoVaccine HT as an adjuvant was developed by BTG International Inc. and tested in a phase II trial on patients (age 35 to 70 years) with mild and moderate hypertension. Participants were randomly divided in two groups to receive either PMD-3117 with CoVaccine HT or CoVaccine HT adjuvant alone. Unfortunately, the study was prematurely terminated as a consequence of dose-limiting adverse effects. Of the serious adverse effects, cellulitis occurred in one patient of the control group; other common adverse effects were tachycardia, chills, headache, and pain in the injection site. The adverse effects were found on both the treatment and control groups, and therefore were most likely caused by the adjuvant [20].
A vaccine called “Ang I-R” was designed by modifying the structure of Ang I at its C terminus, conjugated with bovine serum albumin, aluminum hydroxide, and Freund’s adjuvant. SHR subcutaneous immunized at 0, 4, 8, and 12 weeks showed increased anti-Ang I and anti-Ang II antibody titers. High concentration and affinity of the antibodies led to a significant blockade of RAS, which resulted in a decrease in total Ang I and Ang II levels with a subsequent decrease in SBP by –15 mmHg. This work also showed that this antihypertensive agent did not cause damage to the body by histological studies, making the vaccine a safe agent for target organs [21].
Angiotensin II
ACE removes two amino acids at the C-terminal from Ang I to produce the octapeptide Ang II, the next target of therapeutic vaccines. A vaccine composed of a peptide derived from Ang II conjugated to virus-like particles (VLP) and aluminum hydroxide as adjuvant named CYT006-AngQb was developed. The application of VLP for vaccines is a relatively new technology; Gardisil and Cervavix are VLP-based vaccines currently used to prevent human papilloma virus infection. Since their approval by the FDA in 2006 and 2009, both have maintained good safety profiles [42]. This new vaccine technology conjugates antigens to the surface of highly repetitive structure of VLP and is successful in provoking strong B-cell responses against self-antigens. SHR immunized subcutaneously on days 0, 14, and 28 promoted a strong antibody response against Ang II with an average t1/2 of 13–19 days. SBP measured by the tail-cuff method showed a reduction of up to –21 mmHg, and measured by telemetry a reduction of –15 mmHg. This reduction in SBP was followed by a ninefold increase in the total Ang II concentration in vaccinated SHR. However, the quantity of antibodies was great enough to counter the increase in Ang II, and thus decrease BP. No evidence of inflammation was detected in the kidney, indicating no inflammatory immune complex deposition. In a phase I clinical trial, CYT006-AngQb was evaluated in healthy male subjects (age 22–52 years) as single-dose regimen to evaluate safety, tolerability, and immunogenicity. Although all volunteers receiving CYT006-AngQb showed high IgG titers against Ang II with an average t1/2 of 19 days, no significant variation occurred in BP in these normotensive volunteers. Vaccination with CYT006-AngQb was well tolerated, no findings of inflammation or immune complex formation was detected and local adverse events included erythema, edema, pain, and induration at the injection site [22]. In a phase II trial, patients (18–65 years) with mild to moderate hypertension received three subcutaneous injections of CYT006-AngQb at weeks 0, 4, and 12. All volunteers receiving CYT006-AngQb developed high IgG titers against Ang II with an average t1/2 of 17 weeks and an average affinity to Ang II of 1–5 nM. The vaccine caused a decrease in mean ambulatory BP of –9.0/–4.0 mmHg (SBP/DBP), this reduction was more pronounced during the early-morning BP surge at 08:00 a.m. with a reduction of –25/−13 mmHg. Typically, BP falls to a minimum between 01:00 and 02:00 a.m. and subsequently increases until early in the morning, an event termed early-morning BP surge when RAS is most active and nearly all cardiovascular events occur [43]. The BP reduction induced by the vaccine CYT006-AngQb caused a significant rise in mean renin from 5.1 to 6.3 pg/mL as an effect of blocking the negative feedback effect of Ang II on renin secretion. No variations in mean levels of the complement proteins C1, C3, and C3a, or a significant increase in activated T cells were found reflecting no immune complex deposition or uncontrolled T-cell activation. Most side effects were transitory and mild including local injection-site reactions, influenza-like illness, rigors, increased body temperature, and pyrexia [23]. In a second phase II study, an accelerated treatment regimen with CYT006-AngQb immunizations at weeks 0, 2, 4, 6, and 10 was evaluated. Although a fivefold higher antibody titer was seen in the second study compared with the first study, BP reductions in the second study were much lower at –2.3/–0.4 mmHg. This discrepancy was consequence of significantly lower antibody affinities and consequently the quantity of Ang II sequestered in the blood of vaccinated individuals was on average 33% lower. The authors concluded that an accelerated treatment regimen result in the induction of higher antibody titers but lower antibody affinities, thereby creating a lesser ability for sequestering Ang II in the blood and a smaller BP reduction [24].
A vaccine called “pHAV-4Ang IIs” which presents consecutive Ang II peptides repetitions as the functional epitope on the surface of the hepatitis A virus-like particle and Freund’s adjuvant was designed. SHR received an intramuscular immunization and a booster 3 weeks later of pHAV-4Ang IIs vaccine. The vaccine was able to induce Ang II–specific IgG antibodies in vaccinated SHR, suggesting that pHAV-4AngIIs was able to break immunological tolerance. These antibodies could effectively neutralize serum Ang II, as seen by a significant reduction in Ang II levels up to 87 pg/mL and a significant decrease in SBP and DBP up to –23/–12 mmHg [25].
A vaccine composed of an Ang II peptide conjugated with KLH and Freund’s adjuvant was developed. C57/BL6J mice received three subcutaneous injections at 2-week intervals which successfully promoted the production of anti-Ang II antibodies. These antibodies were able to block Ang II signaling in human aortic smooth muscle cells as seen by a significant decrease in Ang II–induced ERK phosphorylation and c-fos promoter activity. This translated in an Ang II–infused model as a significant decline in SBP and significant reductions in cardiac hypertrophy and fibrosis on immunized mice. T-cell proliferation and ELISPOT assays were used to examine whether Ang II had the capacity to activate a T cell–mediated immune response. Splenocytes from vaccinated mice responded to KLH and Ang II-KLH, but not to Ang II, which demonstrated that T-cell activation was not induced by Ang II since Ang II lacks the foreign T-cell epitopes provided by the carrier protein. Furthermore, no pathological changes, T-cell or macrophage infiltrations were noticed by histochemical analysis in kidney or heart. The vaccine was also applied to SHR three times on days 0, 14, and 21 as a therapeutic model. The titer of anti-Ang II antibody significantly increased while SBP was significantly decreased on immunized SHR [26].
The detrimental effects of Ang II via AT1R induce not only high BP but also inflammatory, hypertrophic, and fibrotic reactions. Ang II performs a pivotal role in the pathogenesis of cardiac remodeling after myocardial infarction, which causes heart failure. Further investigation if the Ang II-KLH conjugate vaccine could prevent cardiac remodeling in an experimental model of myocardial infarction was performed. SD rats were subcutaneously injected three times on days 0, 14, and 21. The vaccine was able to elicit anti-Ang II antibodies with a t1/2 of 42 days. These antibodies were capable to block the Ang II–induced cardiac remodeling-associated reaction in rat neonatal cardiac fibroblasts as seen by inhibition of Ang II–induced expression of NOX4, phosphorylation of c-Jun and NF-κB p65, AT1R mRNA expression, MMP-2 activity, and collagen production. After vaccination, myocardial infarction was induced on rats on day 28 by ligating the left anterior descending coronary artery to analyze the protective effect of the vaccine on a heart failure model. On day 56, an echocardiogram showed an improved regional wall motion of the non-infarct area and a significantly improved left ventricular ejection fraction on vaccinated rats although the antero-lateral wall motion remained impaired. Histopathologically, hypertrophy of the cardiomyocytes in the non-infarct area, the number of infiltrating macrophages, and the myocardial infarction–induced increase of cardiac collagen level were significantly suppressed. The present study concluded that the Ang II-KLH vaccine diminished the cardiac remodeling that results in heart failure, improving cardiac function and suppressing adverse pathological changes in a rat model of myocardial infarction. In regard to the safety of the Ang II-KLH vaccine, no visible pathological changes were observed in various organs (heart, lung, liver, spleen, and kidney) and did not affect the levels of serum cystatin C, plasma brain natriuretic peptide-45, and serum albumin which reflect renal, cardiac, and hepatic function, respectively [27].
Angiotensin II type 1 receptor (AT1R)
Most of the known effects of Ang II are mediated through AT1R, a member of the seven-transmembrane G protein-coupled receptor family. AT1R is mainly expressed in various tissues of the cardiovascular system including kidney, heart, endothelium, and vascular smooth muscle. AT1R promotes various intracellular signaling pathways resulting in hypertension, endothelial dysfunction, vascular remodeling, and end organ damage [44, 45]. It is the last target of the therapeutic vaccines designed so far.
A vaccine called “ATR12181” composed of a seven amino acid sequence from the second extracellular loop of the rat AT1A receptor conjugated to TT as a carrier protein and Freund’s adjuvant was developed. SHR were subcutaneously immunized at weeks 0, 4, 8, 12, 16, 24, 32, 40, and 52. The vaccine was able to induce antibody production against the peptide ATR12181. A reduction of −17 mmHg in SBP was detected in vaccinated SHR. These authors hypothesize that the antibodies produced by the vaccine, which had no agonistic effect, hindered Ang II binding to the receptor. Necropsy of immunized SHR revealed a significant attenuation of left ventricular hypertrophy and fibrosis in the heart, and in the kidney a significant attenuation of glomerular damage and interstitial fibrosis. The mRNA expression of c-jun and c-fos, which promote cardiac remodeling induced by Ang II through AT1R, were decreased significantly in heart and kidneys. ATR12181 vaccine was found safe as in the biopsied sections of heart and kidney, there were no signs of autoimmune diseases [28]. A 16-month long-term investigation on the efficacy and safety of the vaccine in SHR yielded similar results, the ATR12181 vaccine could effectively reduce SBP up to –17 mmHg and ameliorated the remodeling of target organs. Morphological examinations of the heart, kidneys, lungs, brain, and liver did not find any signs of autoimmune damage [46]. To study the effect on BP and small artery remodeling of the ATR12181 vaccine, the ATR12181 vaccine was compared with losartan. To this end, SHR were immunized repeatedly by subcutaneous injection at weeks 0, 2, 4, 8, 12, 16, and 20. The antibodies against the AT1R appeared 1 month after immunization and decreased SBP of immunized SHR to a similar extent to losartan. The antibodies could also inhibit the increased proliferation of vascular smooth muscle cells (VSMCs) induced by Ang II signaling; this translated as a thinner media and a larger lumen of the mesenteric artery on immunized SHR similar to losartan treatment. From these results, the present study concluded that immunization with ATR12181 was able to lower BP and ameliorate the remodeling of small arteries to a similar degree to losartan in a rat model of hypertension [29].
RAS is well known to have a major role in the pathogenesis of hypertensive renal injury. Hypertension-induced renal injury in patients with essential hypertension may lead to nephrosclerosis if left untreated. A vaccine using the same seven amino acid sequence (amino acids 181–187) from the rat AT1a receptor as the ATR12181 vaccine coupled to KLH as a carrier protein and Freund’s adjuvant was designed. The AT1 vaccine was compared with candesartan and hydralazine in a NG-nitro-l-arginine methyl ester (L-NAME) model of hypertensive nephrosclerosis. SHR received three injections of the AT1 vaccine at age 4, 6, and 8 weeks which provoked a significant elevation in AT1 antibody titers and a significant reduction in SBP similar to hydralazine and candesartan. The antibodies from vaccinated SHR were capable to block Ang II signaling in rat aortic VSMCs as seen by a suppressed Ang II–induced ERK phosphorylation. L-NAME was administered to SHR from 18 to 21 weeks of age to induce proteinuria and renal injury. This increase in proteinuria was fully suppressed in SHR vaccinated with the AT1 vaccine and treated with candesartan. Glomerulosclerosis and vascular damage were significantly suppressed on immunized SHR on histopathological examination of the kidneys. This work concluded that AT1 vaccination is as effective as treatment with ARBs for attenuation of hypertension and prevention of L-NAME-induced nephropathy in SHR [30].
The same team that developed the ATR12181 vaccine designed another novel vaccine called “ATRQβ-001,” composed of a human AT1R-derived peptide, conjugated with Qβ bacteriophage VLP to improve its immunogenicity. The vaccine was evaluated in two animal models of hypertension; Ang II–induced hypertensive Balb/c mice were immunized subcutaneously on days 0 and 14, and SHR on days 0, 14, and 28. Both animal models developed ATR-001–specific antibodies with an average t1/2 of 14.4 days. The ATRQβ-001 vaccine significantly reduced the SBP of Ang II–induced hypertensive mice up to –35 mmHg and that of SHR up to –19 mmHg; this BP decrease was similar to valsartan treatment. The vaccine significantly attenuated left ventricular hypertrophy and fibrosis induced by Ang II perfusion in the heart to the same extent as valsartan treatment. Both plasma renin activity and plasma Ang II concentration were significantly increased with valsartan therapy, whereas no significant increase was observed with the ATRQβ-001 vaccine. The anti–ATR-001 antibody specifically bound to AT1R in rat small arterial smooth muscle cells and human embryonic kidney 293 cells that stably expressed the human AT1R. The ATR-001–specific antibody also effectively inhibited Ca2+-dependent signaling events, including protein kinase C-α translocation, ERK phosphorylation, and increase of intracellular Ca2+, induced by Ang II. Interestingly, no competitive binding with AT1R was detected between anti–ATR-001 and Ang II, unlike losartan which competed with Ang II to bind to AT1R. Ang II binding with AT1R induces a specific conformational change, followed by AT1R activation and signal transduction. These authors hypothesized that the anti–ATR-001 antibody may produce a conformational rigidification of the AT1R, which in the end blocked the activation effect of Ang II, despite the fact that it could not prevent the binding of Ang II with AT1R. WKY and normal Balb/c mice were also immunized to determine the safety of the ATRQβ-001 vaccine. No obvious tissue damage or inflammatory cell infiltration were seen in the heart, lung, liver, spleen, or kidney [31]. Lipid accumulation in the blood vessels increases the expression of RAS components; simultaneously, activation of RAS provokes accumulation of low-density lipoproteins, especially the oxidatively modified form, in the blood vessels [47]. This interaction between dyslipidemia and RAS gave rise to the question if the ATRQβ-001 vaccine, which blocked the effect of Ang II via AT1R, could prevent atherosclerosis in apolipoprotein E-null (ApoE−/−) mice. The ApoE−/− mice were immunized subcutaneously on days 0, 14, 28, 56, 94, 122, and 150 and fed a western-type diet from day 20. The ATRQβ-001 vaccine had no effect on the lipid profile, but plaque formation in the aorta and aortic sinus was significantly decreased by 28% compared with 29% with valsartan treatment. Expressions of VCAM-1 and MCP-1 involved with migration and adhesion of leukocytes to the endothelium were dramatically attenuated in the aorta of the vaccinated mice. This contributed to a suppressed accumulation of macrophages in the lesion area, ultimately attenuating the inflammatory infiltration in plaque area and promoting the stability of atherosclerotic plaque as reflected by an increase in smooth muscle cells and collagen. Anti-ATR antibodies significantly decreased apoptosis in the atherosclerotic lesions of ApoE−/− mice and of human coronary artery endothelial cells induced by Ang II. Endocytosis of oxidized low-density lipoprotein performs an important role in the development of atherosclerosis and Ang II is recognized to upregulate the level of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) [48]. Expressions of LOX-1 and AT1R were significantly decreased in the aorta of immunized ApoE−/− mice and pretreatment with anti-ATR antibodies markedly reduced the expression of LOX-1 in human coronary artery endothelial cells induced by Ang II. These results suggest that the ATRQb-001 vaccine significantly attenuated the development of atherosclerosis in ApoE−/− mice. No obvious immune-mediated injury was detected under light microscopy in the heart or kidney [32].
After acute myocardial infarction, the activation of RAS performs a key role in ventricular remodeling through its pro-inflammatory and pro-fibrotic effects. Investigation whether the ATRQβ-001 vaccine, which could block Ang II signaling via the AT1R, would improve myocardial function and block cardiac remodeling after acute myocardial infarction was conducted. C57BL/6 mice were vaccinated subcutaneously on days 0, 14, 28, 56, and 84 with the ATRQβ-001 vaccine and aluminum hydroxide as adjuvant. Myocardial infarction was induced by ligating the left anterior descending coronary artery on day 19. The ATRQβ-001 vaccine was able to elicit anti–ATR-001 antibodies and significantly reduced post-myocardial infarction death with increased survival rates of up to 80%, greater than the 70% with valsartan treatment. Even though the infarct size reduction was not significant with either ATRQβ-001 vaccine or valsartan, echocardiography revealed a remarkably improved left ventricular ejection fraction with both treatment options. The ATRQβ-001 vaccine could modulate the immune response as seen by a decrease in macrophage infiltration, fibrosis, apoptosis, and expression of pro-inflammatory cytokines, and increasing anti-inflammatory cytokines post-myocardial infarction. These data suggest that the ATRQβ-001 vaccine enhanced post-myocardial infarction survival and prevented cardiac remodeling in a mice model of myocardial infarction [33]. The ATRQβ-001 vaccine may be a revolutionary approach for treating not only hypertension but also atherosclerosis and ventricular remodeling after myocardial infarction.
The same group that developed the ATR12181 and ATRQβ-001 vaccines designed another vaccine against AT1R, called “ATR-AP205-001,” composed of a seven amino acid peptide from human AT1R displayed on VLP. Ang II–induced hypertensive Balb/c mice were immunized subcutaneously on days 0 and 14 to investigate immunological profile and safety of the VLP-based vaccine. The vaccine was able to induce anti-ATR001 antibody titers, reduce SBP, significantly attenuate myocardium fibrosis, and prevent excess heart hypertrophy. To track the distribution of the vaccine in the lymph nodes, BALB/c mice were subcutaneously injected with FITC-labeled ATR-AP205-001. The ATR-AP205-001 vaccine arrived at the subcapsular area, and was later transported mainly by B cells to the follicle dendritic cells area and enhanced formation of germinal centers. T-cell differentiation was further assessed, and ATR-AP205-001 strongly increased Tfh cell differentiation in the lymph nodes; this subset focuses on promoting germinal center formation and high affinity B-cells screening, without biased Th17/Th2/Th1 cell and Treg cell differentiation. ATR-AP205-001 was able to interact with B cells which presented the antigens to follicle dendritic cells, and the end result was a strong germinal center reaction which produced specific antibodies and long-lasting B-cell memory. For long-term safety observation, normal BALB/c mice were vaccinated five times with a dosing interval of 14 days; no inflammatory injury was seen in the heart and kidney [34].
In order to avoid adverse effects, like pain, induration, and edema at the injection site, of intramuscular and subcutaneous vaccines, an intranasal vaccine against AT1R called “AT1R–PspA” was developed. The main antigen of the vaccine was a peptide (amino acids 181–187) of rat AT1R conjugated to pneumococcal surface protein A (PspA), a Streptococcus pneumoniae surface protein, as a carrier protein in order to target both control of hypertension and prevention of pneumonia. A nanometer-sized hydrogel (nanogel) consisting of a cationic cholesteryl-group-bearing pullulan was used to transport the AT1R–PspA antigen to dendritic cells in the nasal epithelium and cyclic diguanylate monophosphate as adjuvant to improve the immune response. SHR were intranasally immunized on five occasions at 1-week intervals. The vaccine successfully elevated serum IgG antibody titers directed toward both AT1R and PspA. The AT1R–PspA vaccine diminished the SBP of immunized SHR by a maximum of –19 mmHg, and this effect persisted for 10 weeks after the final immunization. The IgG antibodies against AT1R could also inhibit Ang II signaling via AT1R in rat aortic VSMCs as seen by a suppressed Ang II–induced phosphorylation of ERK. Serum IgG antibodies directed toward PspA were able to protect passively immunized BALB/cAJcl mice from lethal pneumococcal infection as seen by an increased survival rate of vaccinated mice of 80% compared with 0% of the control mice. These results suggest that the AT1R–PspA vaccine reduced the development of hypertension in SHR and protected mice from lethal pneumococcal infection [35].
Other novel potential biomarkers in hypertension
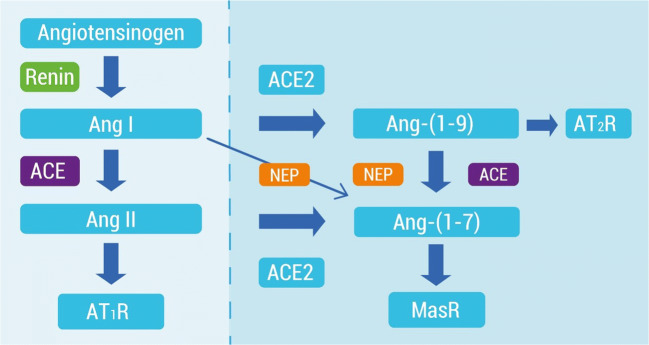
In the classical RAS pathway, the enzymes, peptides, and receptors, ACE, Ang I, Ang II, and AT1R, respectively, have been recognized and used as biomarkers for therapies of cardiovascular diseases such as hypertension. However, the physiological implications of this system have been studied over time since other non-classical components have been included in this system such as angiotensin-(1–7) (Ang-(1–7)) and angiotensin-(1–9) (Ang-(1-9)) peptides, as well as new enzymes, the homologous angiotensin I-converting enzyme (ACE2) and the Mas receptor (MasR).
ACE2 differs from ACE both in its substrate specificity and in its function since ACE2 hydrolyzes Ang II and Ang I to produce Ang-(1–7) and Ang-(1–9), respectively (Fig. 1). Furthermore, Ang-(1–9) by ACE action becomes Ang-(1–7). Both Ang-(1–7) and Ang-(1–9) exert their effects by binding to the MasR and AT2 receptor (AT2R), respectively. The stimulation of both receptors has shown beneficial effects in the pathophysiology of cardiovascular diseases, such as fibrosis, vasodilatory, diuretic, and anti-proliferative effects, hypertrophy, hypertension, and counter-regulating the effect of Ang II. For this reason, in the use of vaccines against the classic ACE/AngII/AT1R system, it would be interesting to specify what are the roles performed by the components of the counter-regulatory axis. The effects of the non-classical axis could be accentuated by activating an immune response to attenuate the actions of the effectors of the classical RAS. The findings of potential biomarkers of hypertension from the non-classical axis of RAS are described below.
Fig. 1.
Schematic representation of classical RAS (left) and the counter-regulating RAS axis (right). Ang I angiotensin I, Ang II angiotensin II, AT1R angiotensin II type 1 receptor, ACE2 angiotensin-converting enzyme 2, Ang-(1–7) angiotensin-(1–7), Ang-(1–9) angiotensin-(1–9), NEP neutral endopeptidase, MasR Mas receptor
Soluble angiotensin-converting enzyme 2 (sACE2)
ACE2, the homologue of ACE, works by stimulating the conversion of Ang II to Ang-(1–7), a peptide that binds to the MasR [49]. ACE2 is an integral membrane carboxypeptidase that has a net effect similar to the action of AT1R blockers [49]. Furthermore, ACE2 can be cleaved to sACE2 (the soluble form) that can be easily measured in urine and plasma. In this form, the plasma membrane-bound sACE2 may provide vasoprotective and anti-proliferative actions to counteract RAS [50]. Increased sACE2 activity correlates with heart failure and hypertension [51]. sACE2 seems to have a protective role on the regulation of RAS. Elevated levels of Ang-(1–7) have been found in patients with imminent progression to heart failure, suggesting its protective mechanism to control chronic activation of RAS [49]. Interestingly, sACE2 levels also correlate with disease severity. On the other hand, sACE2 concentration increases, while pulmonary ACE concentration decreases in HF with reduced ejection fraction patients, suggesting an opposite change in forms of these enzymes, depending on its tissue localization [50]. This interesting marker can be easily measured in serum and elevated concentration of sACE2 could be assessed in patients with great cardiovascular risk before developing heart failure [52].
Angiotensin-(1–7)
The use of ACEi as first-line therapies that suppress the canonical RAS pathway led to the discovery of metabolites of AngI comprising 5, 7, 8, or 9 amino acid residues. Some of them have shown to have antagonistic actions to Ang II, the main end-effector of the classical RAS [53]. In 1988, the formation of Ang-(1–7) from Ang I was described by an ACE-independent pathway. This heptapeptide was recovered in lyophilized canine brains. Later on, it was found that Ang-(1–7) is primarily formed as a metabolite from Ang II by ACE2. However, ACE2 can also shift Ang I to Ang-(1–9), which is then cleaved by neutral endopeptidase (NEP) or again by ACE2 to generate Ang-(1–7) (Fig. 1).
Ang-(1–7) plays a key role in the non-canonical RAS, producing a vast array of physiological actions, many of them contrary to those attributed to Ang II [54, 55]. Ang-(1–7) has emerged as a key cardioprotective peptide against heart failure with preserved and reduced ejection fraction [53]. Ang-(1–7) has anti-inflammatory and anti-remodeling effect on cardiomyocytes, and cardiac fibroblasts which could slow down the mechanisms of cellular injury involved in the development of heart failure [54, 55]. Using Ang-(1–7) as a vaccine antigen may be detrimental to its positive effects relative to those of Ang II. The ACE/Ang II/AT1R pathway leads to pro-oxidative and pro-inflammatory effects, being counteracted by ACE2/Ang-(1–7)/MasR pathway. Cerebroventricular administration of a purified Ang-(1–7) antibody in female transgenic hypertensive rats caused specific elevations in BP associated with tachycardia [56]. However, administration of the Ang II antibody caused hypotension and bradycardia, a hemodynamic response opposite to that obtained with the Ang-(1–7) antibody. Therefore, activation of the immune system against Ang-(1–7) is not beneficial after these results.
The therapeutic hypertensive vaccine against AT1R, ATRQβ-001, was studied. They showed that the ATRQβ-001 vaccine suppressed renal activation of Ang II/AT1R together with an upregulation of ACE2/Ang-(1–7)/MasR expression, similar to the treatment with olmesartan (AT1R antagonist) in diabetic SD rats [57].
Angiotensin-(1–9)
Studies of the non-classical RAS axis were initially focused on the protective and opposite implications of Ang-(1–7) with respect to Ang II. Ang-(1–9) was believed to be only a precursor for the production of Ang-(1–7). However, this bioactive peptide has shown to have beneficial and protective effects similar to Ang-(1–7). Chronic enalapril treatment decreased Ang II levels with a concomitant raise in Ang-(1–9) amounts [58]. Before 2006, the biological activity of Ang-(1–9) was unknown, but the accumulated experimental evidence to date show that Ang-(1–9) protects the blood vessels and heart from detrimental cardiovascular remodeling in patients with heart failure and/or hypertension. These consequences are prevented by the AT2 receptor antagonist PD23319 but not with and Ang-(1–7) receptor (MasR) blocker. Similarly to Ang-(1–7), a reduction of Ang-(1–9) levels could be harmful if this peptide is employed as an antigen in vaccine development because Ang-(1–9) can prevent and/or reduce hypertension and organ injury induced by Ang II.
Safety issues
Hypersensitivity reactions are inflammatory and immune reactions that are detrimental to the host. The classic classification for hypersensitivity reactions divides them into four types: IgE-mediated hypersensitivity or type I, antibody-mediated cytotoxic hypersensitivity or type II, immune complex–mediated hypersensitivity or type II, and cell-mediated hypersensitivity or type IV [59].
IgE-mediated or type I hypersensitivity is triggered by interaction of an antigen with preformed IgE bound to the surface of basophils and mast cells; this causes degranulation of these cells, accompanied by the liberation of vasoactive substances. Clinical manifestations of IgE-mediated reactions are predominantly systemic and range from mild cutaneous symptoms (angioedema, urticaria, flushing) to anaphylaxis [60]. Vaccines have the potential to originate allergic reactions and nearly all components of the vaccine may be considered as possible triggers of an allergic reaction. Fortunately, estimates of immediate hypersensitivity reactions to established vaccines range from 1 per 500,000–1,000,000 doses for most vaccines [61]. PMD-3117 and CYT006-AngQb vaccines were well tolerated on clinical trials, and no IgE-mediated clinical manifestations were observed on immunized patients. The most common adverse events were transient local injection-site reactions common on injectable vaccines. However, the scope of these studies had a limited sample size and further investigation is needed to determine the incidence of allergic reactions in therapeutic vaccines.
Antibody-mediated or type II cytotoxic hypersensitivity takes place when IgM or IgG targeting a cell surface antigen mediates cell destruction via antibody-dependent cellular cytotoxicity or complement activation. The main immunoglobulin class produced after vaccination is IgG, so type II hypersensitivity remains a risk especially in AT1R vaccines where the main antigen is a receptor in the cell surface. No change in levels of complement system proteins were detected after immunization in clinical trials with CYT006-AngQb reflecting no complement activation. Visible pathological changes were not found in various organs on histopathological examination on animal models. Regardless, the possibility for antibody-dependent cell-mediated cytotoxicity produced by therapeutic vaccines will require to be further investigated.
Immune complex mediated or type III hypersensitivity is characterized by deposition of antigen–antibody complexes which causes complement activation and an inflammatory response. Early studies performed with renin vaccines in marmosets and SHR developed autoimmune disease in the kidneys, raising concerns of the potential damage of vaccination against a self-antigen. Antibodies aimed at larger polypeptide antigens are more probable to originate immune complexes. It is highly likely that the anti-renin antibodies induced by the vaccines in these studies formed immune complexes with renin, a 340 amino acid protein. Unlike renin, Ang I and Ang II are small peptide molecules, composed respectively of only 10 and 8 amino acids. Recent development of vaccines against RAS has focused on short peptides, 8 amino acids in length on average, a length which practically prevents the binding of two antibodies, simultaneously, to one peptide and consequently immune complex formation. Immune-complex deposition is commonly seen in the kidney, predominantly in the glomerular membrane. Kidney injury caused by immune complex was not identified in almost all vaccines against RAS. AngI-R was the exception where two of nine SHR vaccinated developed vasculitis in the kidney. No change in levels of complement system proteins were detected after immunization in clinical trials with CYT006-AngQb reflecting no complement activation.
In cell-mediated or type IV hypersensitivity, antigen exposure activates T cells, which subsequently mediate tissue damage. A crucial characteristic in the design of therapeutic vaccines against self-antigens is to be specific to B cells in order to avoid unintended autoreactive T cells. T cells respond to peptide epitopes presented through the MHC class I (CD8+ T cells) or class II (CD4+ T cells). The minimal length of a peptide that MHC I can recognize is of 8 amino acids and 10–12 amino acids for MHC II. Usage of peptides less than 8 amino acids as the main antigen of therapeutic vaccines should prevent T-cell responses, another reason to use short peptides as the main antigen. No T-cell infiltrations were found in the target organs of animals immunized with therapeutic vaccines against RAS. T-cell differentiation was evaluated in Balb/c mice vaccinated with ATR-AP205-001; no significant difference in CD4+/CD8+ T-cell ratio and no evident differentiation in Th17/Th2/Th1 biased were detected. Similarly, baseline levels of immune-cell subpopulations, including activated T cells, had no change after immunization with CYT006-AngQb in a clinical trial.
Another concern in vaccines against RAS is a self-perpetuating immune response. All current studies have shown that antibodies induced by vaccination gradually decline after reaching peak levels. This is explained by the fact that immunoglobulin response is not increased by endogenous renin, Ang I, Ang II, or AT1R since they lack the foreign T-cell epitopes provided by the carrier protein in the vaccine. Autoantibodies are the etiology of an array of different autoimmune diseases; however, they are also present in the serum of healthy persons without autoimmune disorders [62]. These autoantibodies are produced by self-reactive B cells that were not eliminated by negative selection [63].
Autoantibodies against G protein-coupled receptors may play an important role in the homeostasis of the immune system. Our understanding of autoreactive antibodies is changing; they may regulate clearance of apoptotic cells by binding to cellular antigens. Another example are autoantibodies against human endothelin receptor type A which have chemotactic activity and trigger IL-8 production, regulating neutrophil migration [62]. Recent studies appear to indicate that autoantibodies are not the only factor that triggers autoimmunity [64].
Dysregulation of the delicate balance of autoantibodies function and production may lead to autoimmune diseases. This homeostasis is more intricate than the simple quantity of autoantibodies since both decreased and increased concentrations of autoantibodies have been related with different autoimmune diseases. The different pathways from which autoantibodies may regulate the immune system and the different variables that could disrupt the equilibrium still need to be elucidated in healthy patients and patients with autoimmune diseases.
In the case of vaccines against AT1R exists the potential to induce agonistic antibodies leading to overstimulation of RAS instead of its blockade. Autoantibodies that activate AT1R are present in patients with preeclampsia [65]. None of the animal models immunized with a vaccine against AT1R developed hypertension. On the contrary, RAS was inhibited with a following reduction in BP. Nevertheless, autoantibodies against G protein-coupled receptors have been found on patients with rheumatic diseases [66]. Autoantibodies against a G protein-coupled receptor, like AT1R, were increased in a mouse model of systemic sclerosis following immunization of mice with human AT1R which induced pathological characteristics of systemic sclerosis [62].
Another example of dysregulation of autoantibodies homeostasis was found on patients with connective tissue diseases with autoantibodies against ACE2. ACE2 antagonizes the effect of ACE by degrading Ang II to Ang-(1–7) which is a vasodilator and has vasoprotective effects. Patients who had increased titers of autoantibodies targeting ACE2 had a tendency to present constrictive vasculopathies like persistent digital ischemia and pulmonary artery hypertension [67].
Finally, in case of an emergency with acute blood loss, the antibody longer t1/2 induced by vaccination could be a problem since RAS contributes to stability of BP during volume depletion. Oral drugs for hypertension like the renin inhibitor aliskiren or the ARB losartan with an average t1/2 of 24 h and 9 h, respectively, may be short compared with the antibody half-lives in the range of months. However, this difference is irrelevant on the setting of acute volume depletion where treatment needs to be established within hours. RAS blockade with oral drugs has not been related with severe adverse effects in the course of an acute hemorrhage and is highly probable that antibodies may have a similar safety profile in this scenario.
Conclusions
Hypertension can be managed appropriately with existing drugs. However, poor compliance is the principal reason for deficient BP control. The growing health and economic burden is an evident indication that additional therapeutic alternatives are needed. Therapeutic vaccination targeting components of RAS could overcome the need for daily medication and provide long-lasting effects that improve patient compliance. Current immunization approaches against hypertension are directed against renin, Ang I, Ang II, and AT1R (Fig. 2, Table 2).
Fig. 2.
Schematic representation of the classical RAS and counter-regulatory RAS with the vaccines blocking specific targets. Dashed lines indicate inhibitory actions
The success of therapeutic vaccines against RAS must overcome two major walls, safety and efficacy. Therapeutic vaccines have a small margin for error since effective and safe pharmaceuticals for management of hypertension are currently available. The multiple vaccines developed in the past two decades have proven to be secure so far in preclinical and clinical studies. The most frequent unfavorable events in clinical trials were transient local injection-site reactions. However, the number of subjects immunized was small and further studies with a broader scope need to be done.
As for effectiveness, preclinical and clinical studies have shown mixed results. Some studies have found no change in BP, others a discrete reduction of BP and others a decrease in BP as effective as existing drugs that target RAS. The developed vaccines to date have been meticulously designed to trigger highly specific and strong antibody responses. Selection of carrier protein as well as adjuvant is as important as the main antigen to successfully reduce BP. It is important to notice that all vaccines have shown a decline of the induced antibody titers after achieving peak levels and thus therapeutic vaccines are not curative. Besides BP reduction mediated by the vaccines against RAS, other benefits include the attenuation of Ang II–induced cardiac remodeling and the development of other cardiovascular diseases related to autoantibody production, exerted by molecular markers released from stressed cells [68]. If demonstrated to be effective and safe, vaccination is anticipated to improve adherence of hypertensive patients due to the antibody t1/2 in the range of months.
Besides dealing with the adherence problem, a vaccine against RAS could solve the problems associated with conventional drugs such as undesirable side effects and drug interactions associated with polypharmacy. Additional benefits found in therapeutic vaccines compared with conventional drugs that target RAS are reduction in early-morning BP surge when most cardiovascular events occur and there is no obvious RAS feedback.
Effective immunization against RAS seems like a promising option to target the detrimental effects of RAS overactivation. We are excited to see the transition to clinical trials of the vaccines that have yielded excellent results in animal models. Therapeutic vaccines against RAS could open a new door not only to treatment of hypertension but other chronic diseases.
Acknowledgments
We thank Belinda Carrion for her helpful discussion.
Abbreviations
- ACE
angiotensin-converting enzyme
- ACE2
angiotensin-converting enzyme 2
- ACEi
ACE inhibitors
- AGT
angiotensinogen
- Ang I
angiotensin I
- Ang II
angiotensin II
- Ang-(1–7)
angiotensin-(1–7)
- Ang-(1–9)
angiotensin-(1–9)
- ApoE−/−
apolipoprotein E-null
- ARB
angiotensin receptor blocker
- AT1R
angiotensin II type 1 receptor
- BP
blood pressure
- BSA
bovine serum albumin
- DBP
diastolic blood pressure
- KLH
keyhole limpet hemocyanin
- L-NAME
NG-nitro-l-arginine methyl ester
- MAP
mean arterial pressure
- MHC
major histocompatibility complex
- PspA
pneumococcal surface protein A
- RAS
renin–angiotensin system
- sACE2
soluble angiotensin-converting enzyme 2
- SBP
systolic blood pressure
- SD rats
Sprague–Dawley rats
- SHR
spontaneously hypertensive rats
- TT
tetanus toxoid
- VLP
virus-like particles
- WKY
Wistar-Kyoto rats
Author contributions
N.F.G.G. and C.P.H.F. searched literatures. N.F.G.G. and C.E.G.-B. conceptualized and drafted the manuscript. N.F.G.G., G.G.R., S.L., and C.E.G.-B. revised the manuscript. N.F.G.G., S.L., and C.E.G.-B. discussed the manuscript.
Funding
This work was supported by Cardiovascular Medicine Research Group-Tecnologico de Monterrey 0020CAT131 (to C.E.G.-B.) and by the Agencia Nacional de Investigacion y Desarrollo (ANID), Chile: FONDAP 15130011 (to S.L.).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institute for Health Metrics and Evaluation (IHME) (2018) Findings from the Global Burden of Disease Study 2017. http://www.healthdata.org/policy-report/findings-global-burden-disease-study-2017. Accessed 3 Feb 2020
- 2.Burnier M. Drug adherence in hypertension. Pharmacol Res. 2017;125:142–149. doi: 10.1016/j.phrs.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin–angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buczko W, Hermanowicz JM. Pharmacokinetics and pharmacodynamics of aliskiren, an oral direct renin inhibitor. Pharmacol Rep. 2008;60:623–631. [PubMed] [Google Scholar]
- 5.Xu J, Carretero OA, Liao T, et al. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol. 2010;299:1328–1338. doi: 10.1152/ajpheart.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musini VM, Lawrence KA, Fortin PM, et al. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database Syst Rev. 2017;4:CD007066. doi: 10.1002/14651858.CD007066.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurent S. Antihypertensive drugs. Pharmacol Res. 2017;124:116–125. doi: 10.1016/j.phrs.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Pandey R, Quan WY, Hong F, Li jie S. Vaccine for hypertension: modulating the renin–angiotensin system. Int J Cardiol. 2009;134:160–168. doi: 10.1016/j.ijcard.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Brown MJ. Success and failure of vaccines against renin–angiotensin system components. Nat Rev Cardiol. 2009;6:639–647. doi: 10.1038/nrcardio.2009.156. [DOI] [PubMed] [Google Scholar]
- 10.Maurer P, Bachmann MF. Immunization against angiotensins for the treatment of hypertension. Clin Immunol. 2010;134:89–95. doi: 10.1016/j.clim.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Do TH, Chen Y, Nguyen VT, Phisitkul S. Vaccines in the management of hypertension. Expert Opin Biol Ther. 2010;10:1077–1087. doi: 10.1517/14712598.2010.487060. [DOI] [PubMed] [Google Scholar]
- 12.Bairwa M, Pilania M, Gupta V, Yadav K. Hypertension vaccine may be a boon to millions in developing world. Hum Vaccines Immunother. 2014;10:708–713. doi: 10.4161/hv.27520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagami H, Morishita R. Recent advances in therapeutic vaccines to treat hypertension. Hypertension. 2018;72:1031–1036. doi: 10.1161/HYPERTENSIONAHA.118.11084. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann TA. Immunotherapy: past, present and future. Nat Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The immunological basis of hypertension. Am J Hypertens. 2014;27:1327–1337. doi: 10.1093/ajh/hpu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Z, Chen X, Zhou Y, et al. Therapeutic vaccines against human and rat renin in spontaneously hypertensive rats. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0066420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardiner SM, Auton TR, Downham MR, Sharp HL, Kemp PA, March JE, Martin H, Morgan PJ, Rushton A, Bennett T, Glover JF. Active immunization with angiotensin I peptide analogue vaccines selectively reduces the pressor effects of exogenous angiotensin I in conscious rats. Br J Pharmacol. 2000;129:1178–1182. doi: 10.1038/sj.bjp.0703178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Downham MR, Auton TR, Rosul A, Sharp HL, Sjöström L, Rushton A, Richards JP, Mant TGK, Gardiner SM, Bennett T, Glover JF. Evaluation of two carrier protein–angiotensin I conjugate vaccines to assess their future potential to control high blood pressure (hypertension) in man. Br J Clin Pharmacol. 2003;56:505–512. doi: 10.1046/j.1365-2125.2003.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MJ, Coltart J, Gunewardena K, et al. Randomized double-blind placebo-controlled study of an angiotensin immunotherapeutic vaccine (PMD3117) in hypertensive subjects. Clin Sci. 2004;107:167–173. doi: 10.1042/CS20030381. [DOI] [PubMed] [Google Scholar]
- 20.BTG International Inc (2016) Safety and efficacy study of angiotensin therapeutic vaccine in subjects with mild to moderate hypertension. In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00702221. Accessed 5 April 2020
- 21.Hong F, Quan WY, Pandey R, Yi S, Chi L, Xia LZ, Yuan M, Ming L. A vaccine for hypertension based on peptide AngI-R : a pilot study. Int J Cardiol. 2011;148:76–84. doi: 10.1016/j.ijcard.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Ambühl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, Nief V, Schellekens C, Sladko K, Roubicek K, Pfister T, Rettenbacher M, Volk HD, Wagner F, Müller P, Jennings GT, Bachmann MF. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007;25:63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 23.Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, Volk HD, Stocker H, Müller P, Jennings GT, Wagner F, Bachmann MF. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371:821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 24.Cytos Biotechnology (2009) Cytos Biotechnology reports biochemical findings from phase IIa study with hypertension vaccine CYT006-AngQb. https://www.dgap.de/dgap/News/corporate/cytos-biotechnology-reports-biochemical-findings-from-phase-iia-study-with-hypertension-vaccine-cytangqb/?newsID=539927. Accessed 7 April 2020
- 25.Ou X, Guo L, Wu J, Mi K, Yin N, Zhang G, Li H, Sun M. Construction, expression and immunogenicity of a novel anti-hypertension angiotensin II vaccine based on hepatitis A virus-like particle. Hum Vaccin Immunother. 2013;9:1191–1199. doi: 10.4161/hv.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagami F, Koriyama H, Nakagami H, Osako MK, Shimamura M, Kyutoku M, Miyake T, Katsuya T, Rakugi H, Morishita R (2013) Decrease in blood pressure and regression of cardiovascular complications by angiotensin II vaccine in mice. PLoS One 8 [DOI] [PMC free article] [PubMed]
- 27.Watanabe R, Suzuki J, Wakayama K et al (2017) A peptide vaccine targeting angiotensin II attenuates the cardiac dysfunction induced by myocardial infarction. Sci Rep:1–13 [DOI] [PMC free article] [PubMed]
- 28.Zhu F, Liao Y, Li L, et al. Target organ protection from a novel angiotensin II receptor (AT1) vaccine ATR12181 in spontaneously hypertensive rats. Cell Mol Immunol. 2006;3:107–114. [PubMed] [Google Scholar]
- 29.Li L, Tian M, Liao Y, Zhou ZH, Wei F, Zhu F, Wang M, Wang B, Wei YM. Effect of active immunization against angiotensin II type 1 (AT1) receptor on hypertension & arterial remodelling in spontaneously hypertensive rats (SHR) Indian J Med Res. 2014;139:619–624. [PMC free article] [PubMed] [Google Scholar]
- 30.Azegami T, Sasamura H, Hayashi K, Itoh H. Vaccination against the angiotensin type 1 receptor for the prevention of L-NAME-induced nephropathy. Hypertens Res. 2012;35:492–499. doi: 10.1038/hr.2011.212. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Qiu Z, Yang S, Ding D, Chen F, Zhou Y, Wang M, Lin J, Yu X, Zhou Z, Liao Y. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals. Hypertension. 2013;61:408–416. doi: 10.1161/HYPERTENSIONAHA.112.201020. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Wang S, Qiu Z, Song X, Pan Y, Hu X, Zhang H, Deng Y, Ding D, Wu H, Yang S, Wang M, Zhou Z, Liao Y, Chen X. ATRQb-001vaccine prevents atherosclerosis in apolipoprotein E-null mice. J Hypertens. 2016;34:474–485. doi: 10.1097/HJH.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 33.Pan Y, Zhou Z, Zhang H, et al. The ATRQβ-001 vaccine improves cardiac function and prevents postinfarction cardiac remodeling in mice. Hypertens Res. 2018;42:329–340. doi: 10.1038/s41440-018-0185-3. [DOI] [PubMed] [Google Scholar]
- 34.Hu X, Deng Y, Chen X et al (2017) Immune response of a novel ATR-AP205-001 conjugate anti-hypertensive vaccine. Sci Rep:1–13 [DOI] [PMC free article] [PubMed]
- 35.Azegami T, Yuki Y, Hayashi K, et al. Intranasal vaccination against angiotensin II type 1 receptor and pneumococcal surface protein A attenuates hypertension and pneumococcal infection in rodents. J Hypertens. 2017;36:387–394. doi: 10.1097/HJH.0000000000001519. [DOI] [PubMed] [Google Scholar]
- 36.Danser AHJ. Renin and prorenin as biomarkers in hypertension. Curr Opin Nephrol Hypertens. 2012;21:508–514. doi: 10.1097/MNH.0b013e32835623aa. [DOI] [PubMed] [Google Scholar]
- 37.Volpe M, Battistoni A, Chin D, Rubattu S, Tocci G. Renin as a biomarker of cardiovascular disease in clinical practice. Nutr Metab Cardiovasc Dis. 2012;22:312–317. doi: 10.1016/j.numecd.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Goldblatt H, Haas E, Lamfrom H. Antirenin in man and animals. Trans Assoc Am Phys. 1951;64:122–125. [PubMed] [Google Scholar]
- 39.Michel J, Guettiert C, Philippe M, et al. Active immunization against renin in normotensive marmoset. Proc Natl Acad Sci U S A. 1987;84:4346–4350. doi: 10.1073/pnas.84.12.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel J, Sayah S, Guettier C, et al. Physiological and Immunopathological consequences of active immunization of spontaneously hypertensive and normotensive rats against murine renin. Circulation. 1990;81:1899–1910. doi: 10.1161/01.cir.81.6.1899. [DOI] [PubMed] [Google Scholar]
- 41.Schutze MP, Leclerc C, Jolivet M, et al. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985;135:2319–2322. [PubMed] [Google Scholar]
- 42.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, Dubin G. Comparison of the immunogenicity and safety of CervarixTM and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 43.Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. doi: 10.1161/HYPERTENSIONAHA.110.157149. [DOI] [PubMed] [Google Scholar]
- 44.Kawai T, Forrester SJ, O’Brien S, Baggett A, Rizzo V, Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res. 2017;125:4–13. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaudhary M, Chaudhary S. Unravelling the lesser known facets of angiotensin II type 1 receptor. Curr Hypertens Rep. 2017;19:1–10. doi: 10.1007/s11906-017-0699-0. [DOI] [PubMed] [Google Scholar]
- 46.Feng Z, Yu-Hua L, Liudong L, et al. Abstract 2753: Observation of long-term efficacy and safety of an ATR12181 vaccine against hypertension in SHR. Circulation. 2006;114:II_575–II_575. [Google Scholar]
- 47.Singh BM, Mehta JL. Interactions between the renin–angiotensin system and dyslipidemia. Arch Intern Med. 2003;163:1296–1304. doi: 10.1001/archinte.163.11.1296. [DOI] [PubMed] [Google Scholar]
- 48.Morawietz H, Rueckschloss U, Niemann B, Duerrschmidt N, Galle J, Hakim K, Zerkowski HR, Sawamura T, Holtz J. Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation. 1999;100:899–902. doi: 10.1161/01.cir.100.9.899. [DOI] [PubMed] [Google Scholar]
- 49.Epelman S, Shrestha K, Troughton RW, Francis GS, Sen S, Klein AL, Wilson Tang WH. Soluble angiotensin converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail. 2009;15:565–571. doi: 10.1016/j.cardfail.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Úri K, Fagyas M, Mányiné Siket I, Kertész A, Csanádi Z, Sándorfi G, Clemens M, Fedor R, Papp Z, Édes I, Tóth A, Lizanecz E. New perspectives in the renin–angiotensin–aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS One. 2014;9:e87845. doi: 10.1371/journal.pone.0087845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shere A, Eletta O, Goyal H (2017) Circulating blood biomarkers in essential hypertension: a literature review. J Lab Precis Med 2
- 52.Úri K, Fagyas M, Kertész A et al (2016) Circulating ACE2 activity correlates with cardiovascular disease development. J Renin-Angiotensin-Aldosterone Syst 17 [DOI] [PMC free article] [PubMed]
- 53.Patel VB, Zhong J-C, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ocaranza MP, Riquelme JA, García L, et al. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos RAS, Ferreira AJ. Angiotensin-(1–7) and the renin–angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122–128. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 56.Moriguchi A, Tallant EA, Matsumura K, Reilly TM, Walton H, Ganten D, Ferrario CM. Opposing actions of angiotensin-(1–7) and angiotensin II in the brain of transgenic hypertensive rats. Hypertension. 1995;25:1260–1265. doi: 10.1161/01.hyp.25.6.1260. [DOI] [PubMed] [Google Scholar]
- 57.Ding D, Du Y, Qiu Z, et al. Vaccination against type 1 angiotensin receptor prevents streptozotocin-induced diabetic nephropathy. J Mol Med. 2015;94:207–218. doi: 10.1007/s00109-015-1343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drummer OH, Kourtis S, Johnson H. Effect of chronic enalapril treatment on enzymes responsible for the catabolism of angiotensin I and formation of angiotensin II. Biochem Pharmacol. 1990;39:513–518. doi: 10.1016/0006-2952(90)90058-s. [DOI] [PubMed] [Google Scholar]
- 59.Rajan TV. The Gell–Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol. 2003;24:376–379. doi: 10.1016/s1471-4906(03)00142-x. [DOI] [PubMed] [Google Scholar]
- 60.Wood RA. Allergic reactions to vaccines. Pediatr Allergy Immunol. 2013;24:521–526. doi: 10.1111/pai.12102. [DOI] [PubMed] [Google Scholar]
- 61.Fritsche PJ, Helbling A, Ballmer-Weber BK. Vaccine hypersensitivity – update and overview. Swiss Med Wkly. 2010;140:238–246. doi: 10.4414/smw.2010.12980. [DOI] [PubMed] [Google Scholar]
- 62.Cabral-Marques O, Marques A, Giil LM, de Vito R, Rademacher J, Günther J, Lange T, Humrich JY, Klapa S, Schinke S, Schimke LF, Marschner G, Pitann S, Adler S, Dechend R, Müller DN, Braicu I, Sehouli J, Schulze-Forster K, Trippel T, Scheibenbogen C, Staff A, Mertens PR, Löbel M, Mastroianni J, Plattfaut C, Gieseler F, Dragun D, Engelhardt BE, Fernandez-Cabezudo MJ, Ochs HD, al-Ramadi BK, Lamprecht P, Mueller A, Heidecke H, Riemekasten G. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat Commun. 2018;9:5224. doi: 10.1038/s41467-018-07598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meffre E, Wardemann H. B-Cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Cabral-Marques O, Carvalho-Marques AH, Schimke LF, et al. Loss of balance in normal GPCR-mediated cell trafficking. Front Biosci (Landmark Ed) 2019;24:18–34. doi: 10.2741/4707. [DOI] [PubMed] [Google Scholar]
- 65.Xia Y, Kellems RE. Is preeclampsia an autoimmune disease? Clin Immunol. 2009;133:1–12. doi: 10.1016/j.clim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cabral-Marques O, Riemekasten G. Functional autoantibodies targeting G protein-coupled receptors in rheumatic diseases. Nat Rev Rheumatol. 2017;13:648–656. doi: 10.1038/nrrheum.2017.134. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi Y, Haga S, Ishizaka Y, Mimori A. Autoantibodies to angiotensin-converting enzyme 2 in patients with connective tissue diseases. Arthritis Res Ther. 2010;12:R85. doi: 10.1186/ar3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krishnan-Sivadoss I, Mijares-Rojas IA, Villarreal-Leal RA, Torre-Amione G, Knowlton AA, Guerrero-Beltrán CE (2020) Heat shock protein 60 and cardiovascular diseases: an intricate love-hate story. Med Res Rev. 10.1002/med.21723 [DOI] [PMC free article] [PubMed]