ABSTRACT
Hirschsprung disease (HSCR) is a birth defect with an approximate incidence of 1/5,000 live births, and up to one-third of HSCR patients develop Hirschsprung-associated enterocolitis (HAEC), the leading cause of HSCR-related death. Very little is known about the pathogenesis, prevention, and early diagnosis of HAEC. Here, we used a prospective study to investigate the enteric microbiome composition at the time of surgery as a predictor for developing postoperative HAEC. We identified a microbiome signature containing 21 operational taxonomic units (OTUs) that can potentially predict postoperative HAEC with ~85% accuracy. Furthermore, we identified exclusive breastfeeding as a novel protective factor for total HAEC (i.e., preoperative and postoperative HAEC combined). In addition, we discovered that breastfeeding was associated with a lowered risk for HAEC potentially mediated by modulating the gut microbiome composition characterized by a lower abundance of Gram-negative bacteria and lower LPS concentrations. In conclusion, modulating the gut microbiome by encouraging breastfeeding might prevent HAEC progression in HSCR patients.
KEYWORDS: Hirschsprung disease, Hirschsprung-associated enterocolitis, exclusive breastfeeding, the enteric microbiome
Introduction
Hirschsprung disease (HSCR) is a birth defect caused by a congenital absence of ganglion cells (aganglionosis) in the distal colon, which extends for varying distances into the more proximal colon but rarely involves the small intestine.1–3 Consequently, varying lengths of the distal colon are unable to relax, causing functional colonic obstruction over time. While HSCR can be treated by a pull-through surgery, HSCR patients are at high risk of developing enterocolitis before or after the surgery, with classic manifestations including fever, abdominal distention, diarrhea, and sepsis. Hirschsprung-associated enterocolitis (HAEC) is the leading cause of serious morbidity and mortality in HSCR patients.4
To date, very little is known about pathogenesis, prevention, and early diagnosis of HAEC. Preclinical studies suggest that alterations in the intestinal barrier,5 impaired gastrointestinal mucosal immunity,6 and dysbiosis of the enteric microbiome7 may contribute to the occurrence of HAEC. Observational studies have also correlated altered enteric microbiome with HAEC pathogenesis through comparison of HSCR patients with and without HAEC.8,9 A major drawback of such retrospective studies is that the enteric microbiome was assessed after the occurrence of HAEC, when the microbiome could have been dramatically changed by the enterocolitis.
We conducted a hospital-based prospective nested case–control study of 25 postoperative HAEC cases and 50 control HSCR patients who did not develop postoperative HAEC to investigate the relationship between the enteric microbiome at the time of surgery with risk for developing postoperative HAEC. Breastfeeding is a well-known protective factor for necrotizing enterocolitis,10–13 the most common and serious intestinal disease in premature infants, for which altered enteric microbiome has been proposed as an underlying mechanism.14,15 In the current study, we identified a microbiome signature that might predict the development of postoperative HAEC in HSCR patients and found that exclusive breastfeeding might reduce the risk of HAEC through modulation of the enteric microbiome.
Results
Study population
Cohort study
A total of 363 patients diagnosed with HSCR at the Children’s Hospital of Nanjing Medical University (Nanjing, Jiangsu Province, China) were prospectively recruited from June 2011 to June 2013. Fourteen patients transferred to other hospitals were excluded (Figure 1), and the remaining patients were treated by Soave pull-through procedure. We further excluded patients who had allied disorder of HSCR (n = 55), had uncertain feeding pattern (n = 11), or were lost to follow-up (n = 30), leaving 253 patients for cohort analysis (Figure 1). Of the eligible patients, 156 (62%) were diagnosed with classical segment HSCR and 97 (38%) with long segment HSCR or total colonic aganglionosis. The mean gestational age was 39.2 weeks and the mean birth weight was 3.4 kg (Table 1).
Figure 1.
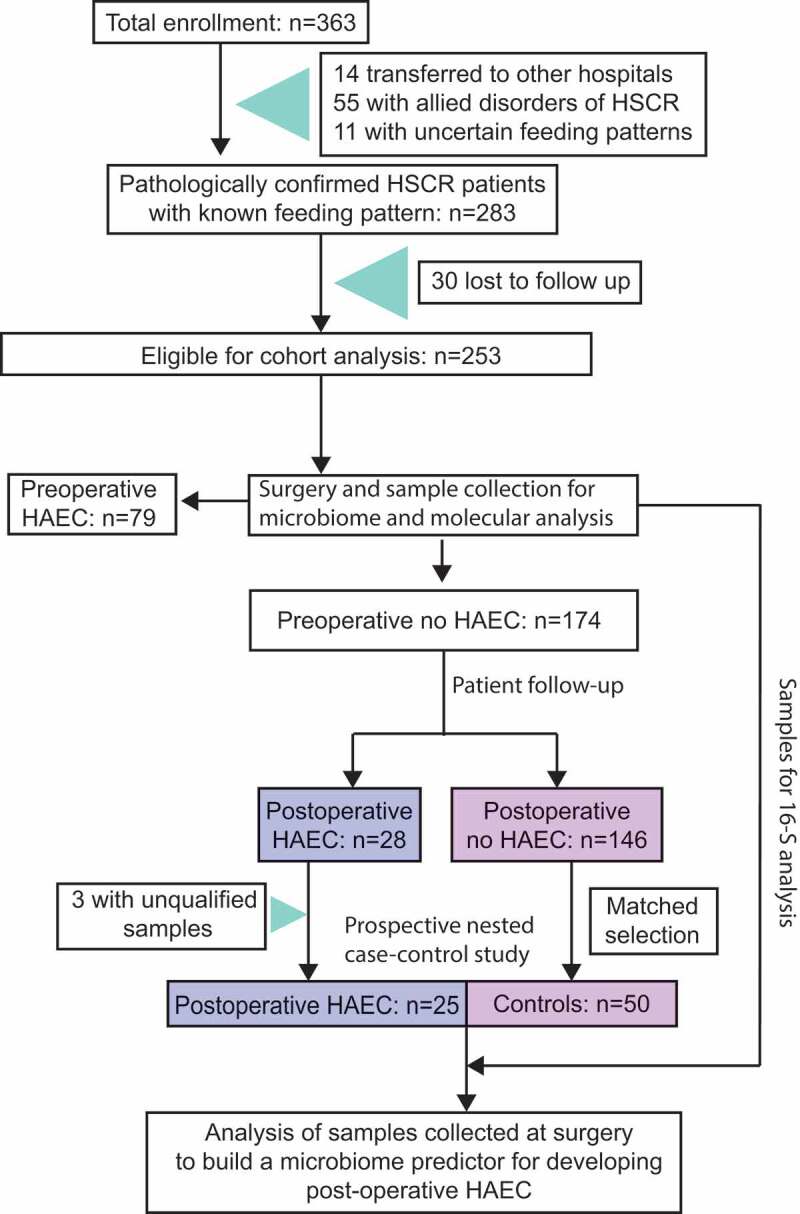
Flow-chart of cohort and prospective nested case–control studies.
Table 1.
Characteristics of 253 Hirschsprung disease patients in the cohort study by exclusive breastfeeding status.
| Characteristic | Exclusive breastfeeding |
||
|---|---|---|---|
| No(n = 142) | Yes(n = 111) | P | |
| Gestational age, weeks, mean (SD) | 39.3 (1.4) | 39.2 (1.3) | 0.83a |
| Birth weight, kg, mean (SD) | 3.5 (0.4) | 3.4 (0.5) | 0.77a |
| Gender, No. (%) | 0.82b | ||
| Female | 24 (16.9) | 20 (18.0) | |
| Male | 118 (83.1) | 91 (82.0) | |
| Mode of delivery, No. (%) | 0.03b | ||
| Cesarean | 86 (60.6) | 52 (46.8) | |
| Vaginal | 56 (39.4) | 59 (53.2) | |
| HSCR classificationc, No. (%) | 0.01b | ||
| Classical segment HSCR | 78 (54.9) | 78 (70.3) | |
| Long segment HSCR or total colonic aganglionosis | 64 (45.1) | 33 (29.7) | |
| Ostomy, No. (%) | 0.01b | ||
| No | 113 (79.6) | 101 (91.0) | |
| Yes | 29 (20.4) | 10 (9.0) | |
| Age at surgery, No. (%) | 0.58b | ||
| ≤180 days | 122 (85.9) | 98 (88.3) | |
| >180 days | 20 (14.1) | 13 (11.7) | |
| Surgical approach, No. (%) | 0.04b | ||
| Open pull-through | 29 (20.4) | 10 (9.0) | |
| Totally transanal endorectal pull-through | 70 (49.3) | 62 (55.9) | |
| Laparoscopic-assisted transanal pull-through | 43 (30.3) | 39 (35.1) | |
aCalculated using t-test.
bCalculated using χ2 test.
cHSCR is classified as classical segment HSCR when the aganglionic segment does not extend beyond the upper sigmoid, long-segment HSCR when the aganglionic segment extends to the splenic flexure or transverse colon, and total colonic aganglionosis when the aganglionic segment extends to the colon and a short segment of the terminal ileum.
Abbreviations: HSCR, Hirschsprung disease; SD, standard deviation.
Prospective nested case–control study
During 3 years of follow-up, we observed 107 incident cases of HAEC, of which 28 were postoperative. Using tissue samples (i.e., the cutting edge of the dilated segment) collected at the pull-through surgery, we conducted a prospective nested case–control study of 25 postoperative HAEC cases and 50 controls who were randomly selected from the 146 HSCR patients who never had HAEC during follow-up (Figure 1). We compared clinical information of the selected controls and the 146 HSCR patients without enterocolitis, and did not observe any appreciable differences (Table S1). Postoperative HAEC cases (n = 25) and controls (n = 50) also have similar characteristics (Table 2). Before the pull-through surgery, all patients received mechanical bowel preparation, without the use of oral or intravenous antibiotics.
Table 2.
Characteristics of 75 Hirschsprung disease patients in the case–control study.
| Characteristic | Postoperative HAEC cases (n = 25) | Controls (n = 50) | P |
|---|---|---|---|
| Gestational age, weeks, mean (SD) | 39.2 (1.4) | 39.4 (1.1) | 0.55a |
| Birth weight, kg, mean (SD) | 3.5 (0.5) | 3.5 (0.5) | 0.87a |
| Gender, No. (%) | 0.07b | ||
| Female | 2 (8.0) | 13 (26.0) | |
| Male | 23 (92.0) | 37 (74.0) | |
| Mode of delivery, No. (%) | 0.19b | ||
| Cesarean | 16 (64.0) | 24 (48.0) | |
| Vaginal | 9 (36.0) | 26 (52.0) | |
| HSCR classificationc, No. (%) | 0.87b | ||
| Classical segment HSCR | 15 (60.0) | 29 (58.0) | |
| Long segment HSCR or total colonic aganglionosis | 10 (40.0) | 21 (42.0) | |
| Ostomy, No. (%) | 1.00d | ||
| No | 22 (88.0) | 43 (86.0) | |
| Yes | 3 (12.0) | 7 (14.0) | |
| Age at pull-through procedure, No. (%) | 0.47d | ||
| ≤180 days | 21 (84.0) | 45 (90.0) | |
| >180 days | 4 (16.0) | 5 (10.0) | |
| Surgical approach, No. (%) | 0.97b | ||
| Open pull-through | 3 (12.0) | 7 (14.0) | |
| Totally transanal endorectal pull-through | 13 (52.0) | 25 (50.0) | |
| Laparoscopic-assisted transanal pull-through | 9 (36.0) | 18 (36.0) |
aCalculated using t-test.
bCalculated using χ2 test.
cHSCR is classified as classical segment HSCR when the aganglionic segment does not extend beyond the upper sigmoid, long-segment HSCR when the aganglionic segment extends to the splenic flexure or transverse colon, and total colonic aganglionosis when the aganglionic segment extends to the colon and a short segment of the terminal ileum.
dCalculated using Fisher’s exact test.
Abbreviations: HAEC, Hirschsprung-associated enterocolitis; HSCR, Hirschsprung disease; SD, standard deviation.
Exclusive breastfeeding was associated with lower risk of HAEC (cohort study)
HSCR patients who were exclusively breastfed (n = 111) had significantly lower risk of total HAEC (i.e., preoperative and postoperative HAEC combined), compared to those without exclusive breastfeeding (n = 142), with an adjusted RR of 0.61 (95% CI, 0.44–0.85; P = .003; Table 3), whereas formula feeding and mixed feeding resulted in similar risk of HAEC (P = .95; Table S2). In 174 HSCR patients without preoperative HAEC, the association between exclusive breastfeeding and risk of postoperative HAEC did not reach statistical significance, with an adjusted RR of 0.51 (95% CI, 0.24–1.08; P = .08; Table 3).
Table 3.
Association between exclusive breastfeeding and risk of Hirschsprung-associated enterocolitis in the cohort study.
| Exclusive breastfeeding |
|||
|---|---|---|---|
| Yes |
|||
| Outcome | No | RR (95% CI) | P |
| Overall Hirschsprung-associated enterocolitis | |||
| No. of HSCR patients | 142 | 111 | |
| No. of enterocolitis | 73 | 34 | |
| Unadjusted model | Reference | 0.60 (0.43–0.82) | 0.002 |
| Multivariable-adjusted modela | Reference | 0.61 (0.44–0.85) | 0.003 |
| Postoperative Hirschsprung-associated enterocolitis | |||
| No. of HSCR patients without preoperative enterocolitis | 88 | 86 | |
| No. of postoperative enterocolitis | 19 | 9 | |
| Unadjusted model | Reference | 0.48 (0.23–1.01) | 0.05 |
| Multivariable-adjusted modela | Reference | 0.52 (0.25–1.08) | 0.08 |
| Multivariable-adjusted modelb | Reference | 0.51 (0.24–1.08) | 0.08 |
aAdjusted for gestational age (continuous), gender (female, male), mode of delivery (cesarean, vaginal), birth weight (continuous), and classical segment HSCR (yes, no).
bAdditionally adjusted for ostomy (yes, no), age at pull-through surgery (≤180 or > 180 days), and surgery approach (open pull-through, totally transanal endorectal pull-through, Laparoscopic-assisted transanal pull-through).
Abbreviations: CI; confidence interval; HSCR, Hirschsprung disease; RR, relative risk.
An enteric microbiome signature can potentially predict postoperative HAEC occurrence (case–control study)
In the 25 postoperative HAEC cases and 50 control HSCR patients who did not develop postoperative HAEC, we sequenced the microbiome in the enteric mucosa of the cutting edge of the dilated segment that was collected at the time of surgery when HAEC had not occurred. The cutting edge of the dilated segment is adjacent to normal tissue and thus may best represent the characteristics of normal colonic tissues and anastomotic stoma after surgery. Postoperative HAEC cases and controls had a substantially different enteric microbiome composition. We note a significant decrease in α diversity (Figure 2(a); all P≤ 0.0074) and a significant difference in β diversity (Fig. S1A and B; P = .001) in postoperative HAEC cases vs. controls. In postoperative HAEC cases, we did not observe a significant increase in the abundance of Gram-negative bacteria (Fig. S1C; P = .07), and no significant difference in the ratio of Gram-negative to Gram-positive bacteria (Fig. S1D; P = .15). However, KEGG pathway analysis showed significant enrichment for LPS biosynthesis proteins (P = .04) and secretion system (P = .03) in postoperative HAEC cases (Figure 2(b)).
Figure 2.
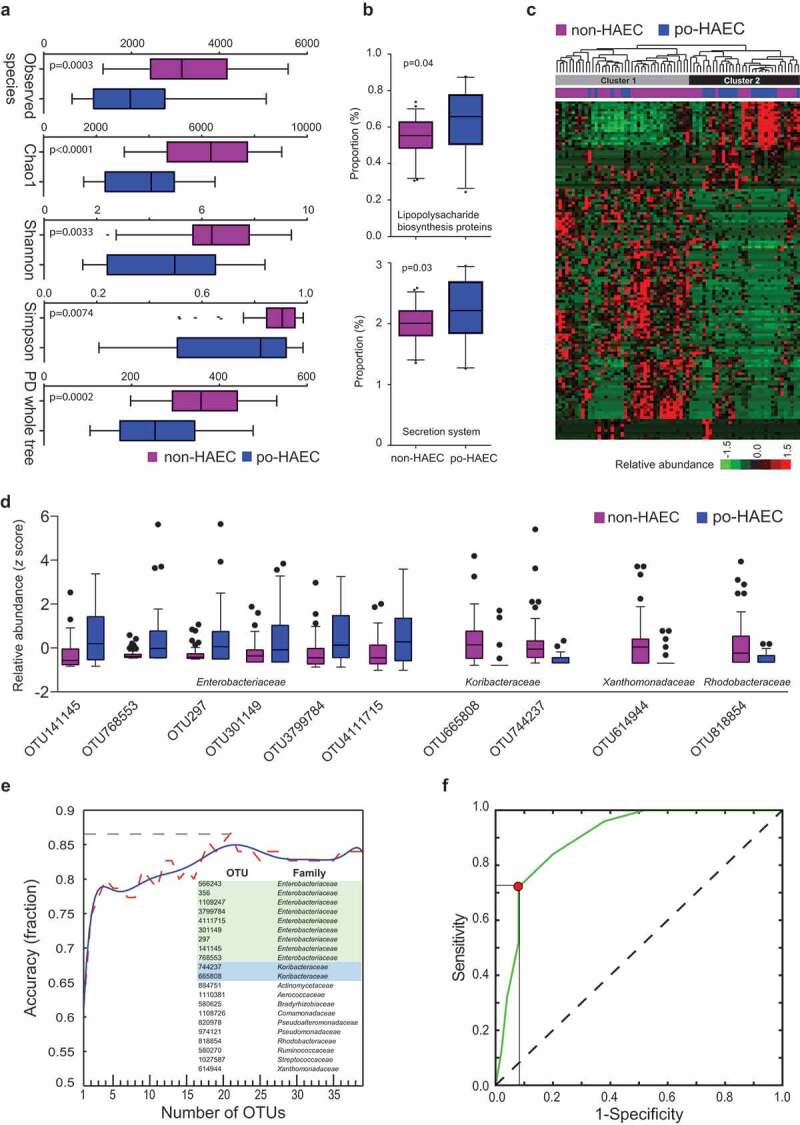
The enteric microbiome predicted postoperative Hirschsprung-associated enterocolitis occurrence. (a) The α diversity in po-HAEC cases vs. controls. (b) Kyoto Encyclopedia of Genes and Genomes predicted the relative frequency of lipopolysaccharide biosynthesis proteins and secretion system in po-HAEC cases vs. controls. (c) Unsupervised hierarchical clustering of 131 OTUs with significantly different abundance between po-HAEC cases and controls. (d) Selective OTUs with significantly different abundance between po-HAEC cases and controls. (e) A 21-OTU signature that predicted po-HAEC occurrence. (f) Receiver operating characteristic curve for the 21-OTU signature.
A total of 131 OTUs had significantly different abundance between postoperative HAEC cases and controls (Table S3). Using these OTUs, postoperative HAEC cases and controls could be successfully differentiated by unsupervised hierarchical clustering. As shown in Figure 2(c), Cluster 1 contained 35 of 50 controls (70%), while Cluster 2 contained 19 of 25 cases (76%) (P for χ2 test = 0.0002). Notably, of the 10 most significant OTUs, six belong to Enterobacteriaceae, with higher abundance in cases than controls (Table S3; Figure 2(d)).
To predict postoperative HAEC occurrence, we used stepwise random forest classification to identify a 21-OTU signature (Figure 2(e)), of which nine belong to Enterobacteriaceae. The signature could classify samples into cases and controls with ~85% accuracy (Figure 2(e)), with sensitivity and specificity optimized to be 72% and 92%, respectively (area under the ROC curve = 0.90; Figure 2(f)).
Exclusive breastfeeding was associated with an enteric microbiome and with lower occurrence of postoperative HAEC (case–control study)
We next investigated whether the protective effect of exclusive breastfeeding on postoperative HAEC was associated with modulation of the enteric microbiome. We compared the enteric microbiome at the time of surgery among HSCR patients, who did not previously develop HAEC, with and without exclusive breastfeeding. First, we did not observe a difference in α diversity (Figure 3(a); 0.066 ≤ P ≤ 0.16); however, we found a significant difference in β diversity (Fig. S2A and B; P = .003) in exclusively breastfed patients compared to non-exclusively breastfed patients. Second, we observed lower abundance of Gram-negative bacteria (Fig. S2C; P = .02), as well as a decrease in the ratio of Gram-negative to Gram-positive bacteria (Fig. S2D; P = .03), in exclusively breastfed patients. Third, KEGG pathway analysis showed significant enrichment for LPS biosynthesis proteins (P = .0050) and secretion system (P = .0042) in patients without exclusive breastfeeding (Figure 3(b)).
Figure 3.
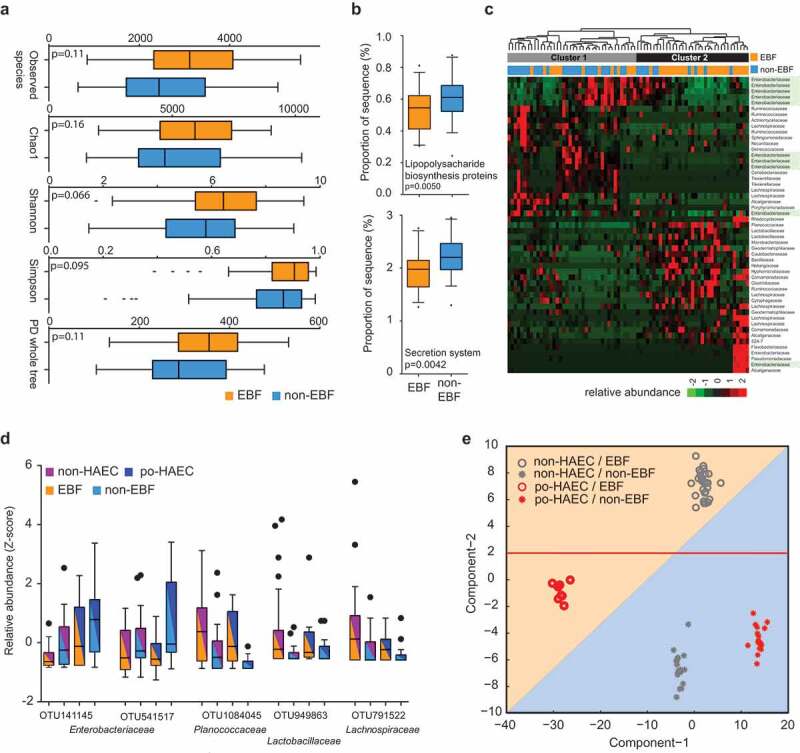
Exclusive breastfeeding associated with an enteric microbiome and with lower occurrence of postoperative Hirschsprung-associated enterocolitis. (a) The α diversity in EBF vs. non-EBF patients. (b) Kyoto Encyclopedia of Genes and Genomes predicted the relative frequency of lipopolysaccharide biosynthesis proteins and secretion system in EBF vs. non-EBF patients. (c) Unsupervised hierarchical clustering of 51 OTUs with significantly different abundance between EBF and non-EBF patients. (d) Selective OTUs with significantly different abundance between EBF and non-EBF patients. (e) Linear discriminant analysis of the 51 OTUs separating exclusively breastfed non-HAEC subjects from the rest.
The abundance of a total of 51 OTUs was significantly different between patients with and without exclusive breastfeeding (Table S4; Figure 3(c,d)). As shown in Figure 3(c), Cluster 1 contained 27 of 37 patients without exclusive breastfeeding (73%), while Cluster 2 contained 25 of 38 exclusively breastfed patients (66%) (P for χ2 test = 0.0008). We examined whether the 51 OTUs can distinguish postoperative HAEC cases and controls using linear discriminant analysis (LDA), which revealed four non-overlapping clusters (Figure 3(e)). Component-1 and component-2, which represent the top two microbiome feature transformation coefficients that maximize the separability of classes in the new feature space, provide clear separability among the four classes. Interestingly, component-1 maximizes the separability between po-HAEC EBF/non-EBF patients, while component-2 maximizes the separability between non-HAEC EBF/non-EBF patients. These results suggest that the protective effect of exclusive breastfeeding on postoperative HAEC might be mediated through modulating the abundance of these OTUs.
Enteric LPS concentrations in relation to exclusive breastfeeding and postoperative HAEC occurrence (case–control study)
KEGG pathway analysis suggested that HSCR patients without exclusive breastfeeding had an enteric microbiome enriched for LPS biosynthesis proteins, which was subsequently associated with postoperative HAEC occurrence. To confirm this finding, we measured LPS concentrations in the homogenate of the same enteric tissue in the cutting edge of the dilated segment as used for microbiome analysis and examined their relationships with exclusive breastfeeding and postoperative HAEC.
Consistent with the KEGG predictions, we observed that 20 of 131 OTUs (Table S3) were significantly correlated with LPS levels, eight were positively correlated and 12 were negatively correlated (P < .05; Table S5). Moreover, we observed lower LPS concentrations (1) in exclusively breastfed patients vs. those without exclusive breastfeeding (Figure 4(a); P = .046) and (2) in non-postoperative HAEC controls vs. postoperative HAEC cases (Figure 4(b); P = .019). The adjusted OR for postoperative HAEC associated with a 5-ng/mL increase in LPS concentration was 1.14 (95% CI, 1.03–1.26). While controlling for the abundance of Gram-negative bacteria, we continued to observe a significant association between LPS concentrations and odds of postoperative HAEC (OR, 1.14; 95% CI, 1.03–1.27), indicating that LPS is a potential key mediator in the pathway. Interestingly, the difference in LPS concentrations between cases and controls appeared to be greater in exclusively breastfed patients than those without exclusive breastfeeding (Figure 4(c)).
Figure 4.
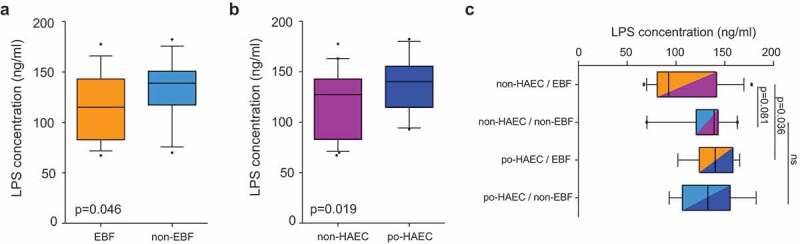
Enteric lipopolysaccharide concentrations in relation to exclusive breastfeeding and postoperative Hirschsprung-associated enterocolitis status. (a) LPS concentrations in EBF vs. non-EBF patients. (b) LPS concentrations in po-HAEC cases vs. controls. (c) LPS concentrations by combined categories of EBF and po-HAEC status.
Discussion
In a cohort of 253 HSCR patients, we identified exclusive breastfeeding as a novel protective factor for total HAEC (i.e., preoperative and postoperative HAEC combined). Using prospectively collected tissue samples from 25 postoperative HAEC cases and their controls, we identified a 21-OTU signature that can potentially be used to predict postoperative HAEC occurrence and further demonstrated that exclusive breastfeeding was associated with an enteric microbiome and with a lower occurrence of postoperative HAEC. Taken together, these data suggest that exclusive breastfeeding might reduce the risk of HAEC through modulation of the enteric microbiome.
Breastfeeding is an important factor in the development of the enteric microbiome,16–18 and breast milk ingestion may facilitate the enrichment of microbes during the acquisition of the enteric microbiome.15 Gram-negative bacteria, a major cause of enteric infection, can activate mucosal inflammation by binding LPS, a component of the outer membrane, to enteric toll-like receptor 4,19,20 and human milk can attenuate LPS-induced enteric inflammation by modulating CD14 expression in human enterocytes.21 In this study, we showed that HSCR patients who were exclusively breastfed tended to have a lower abundance of Gram-negative bacteria, particularly Enterobacteriaceae, thereby being less prone to postoperative HAEC. Taken together, we hypothesize that exclusive breastfeeding might decrease the biosynthesis and release of LPS, thus reducing the occurrence of postoperative HAEC.
Several small case–control studies have been conducted to retrospectively compare the enteric microbiome of HSCR patients with different HAEC status, reporting mixed findings.8,9,22,23 Two studies, including a total of 17 HSCR patients, observed increased Proteobacteria and decreased Bacteroidetes in HAEC cases vs. controls.8,9 Another study of 18 HSCR patients observed increased Proteobacteria and Bacteroidetes as well as decreased Firmicutes, comparing patients who had a history of HAEC to those who did not.22 It has also been reported that patients with a history of recurrent HAEC had increased Proteobacteria and Bacteroidetes, compared to those without a history.23 However, in all these studies, the enteric microbiome was measured after the occurrence of HAEC and could have been dramatically altered by the enterocolitis. In contrast, a prospective study design, such as that in our analyses, limits the likelihood of reverse causation by collecting tissue samples before the occurrence of HAEC, lending additional validity to the results.
Abundant evidence indicates that probiotics support gut health and may decrease the incidence of necrotizing enterocolitis in premature infants.24 Similarly, probiotic use has been proposed as a potential prophylaxis for HAEC. Lactobacilli and Bifidobacteria are the two most common genera of probiotics; a retrospective case–control study found a marked decrease in bifidobacterial but no difference in Lactobacilli, comparing HSCR patients with HAEC to those without HAEC.25 Several studies have examined probiotic use in relation to HAEC incidence with mixed findings: one multicenter randomized clinical trial found that orally administered probiotics (Bifidobacterium, Lactobacillus, and Enterococcus) significantly decreased the incidence and severity of postoperative HAEC,26 whereas another randomized trial and two observational studies reported null results.22,27,28 A meta-analysis including these studies reported an OR of 0.72 (95% CI, 0.37–1.39; P = .33) for the effect of probiotic use on HAEC incidence.24 In the current study, we did not find adequate evidence supporting the use of probiotics in HSCR patients. Although we observed a higher abundance of Lactobacillus in the enteric microbiome of exclusively breastfed patients, no significant difference was observed between postoperative HAEC cases and controls.
It is well known that lipopolysaccharide is produced by Gram-negative bacteria.29,30 We performed KEGG pathway analysis and found significant enrichment for LPS biosynthesis proteins and secretion system in postoperative HAEC cases. Significant enrichment for LPS biosynthesis proteins and secretion system was also observed in patients without exclusive breastfeeding. ELISA with the homogenate of the enteric tissue in the cutting edge of the dilated segment confirmed that the level of LPS was increased in postoperative HAEC cases and decreased in the exclusive-breastfeeding group. We found that Gram-negative bacteria belonging to the family Caulobacteraceae were most positively correlated with LPS levels. These data indicate that breastfeeding might decrease the abundance of microbes that produce LPS and potentially lower the risk of postoperative HAEC. A multi-center study to further validate this finding and explore the underlying mechanism is granted.
The current study has several strengths. We established a large cohort of HSCR patients, collected detailed information on clinical characteristics, and followed the patients for up to 3 years. To our knowledge, this is the first study to prospectively examine the enteric microbiome in relation to HAEC. Nonetheless, this work has potential limitations. The enteric microbiome varies substantially by race and geography.31 All participants in our study are Han Chinese, and validation studies in other populations are warranted. Since our tissue samples were collected at the time of surgery, our microbiome signature mostly represents the mucosa-associated microbiome and could only be used to predict postoperative HAEC. Furthermore, the time between surgery and the development of HAEC varies and the microbes identified in our biomarker panel may not be causative for the development of post-operative HAEC. In the future, using noninvasive sample collections (for example, stool samples), we might be able to develop a microbiome signature that can predict the onset and progression of HAEC in HSCR patients. This signature could also serve as a biomarker panel to monitor HSCR patients at risk for developing HAEC.
In conclusion, we demonstrated a link between exclusive breastfeeding, the enteric microbiome and enteric LPS levels and HAEC and identified a microbiome signature that might predict the occurrence of postoperative HAEC. Our study suggests that modulating the gut microbiome by encouraging breastfeeding might prevent HAEC progression in HSCR patients.
Materials and methods
Ethical approval
The study was approved by the Medicine Ethics Committee at Children’s Hospital of Nanjing Medical University and the Human Subjects Committee at the Lawrence Berkeley National Laboratory, and all participants provided informed consent.
Assessment of infant feeding pattern
When HSCR patients were 6 months old, parents were asked by a physician (W.T.) the following two questions regarding their feeding pattern: (1) What was the primary feeding pattern during the past 6 months: exclusive breastfeeding, formula feeding, or mixed feeding? (2) Had the primary feeding pattern ever been changed?
Identification of HAEC
At the Children’s Hospital of Nanjing Medical University, diagnosis of HAEC was based on the criteria published by Pastor et al.,32 and documented by a physician (W.T.). A summary of the HAEC scoring system is presented in Table S6, and a score of 10 or greater resulted in the diagnosis of HAEC. Moreover, HSCR patients were asked to have follow-up visits in 3 months, 6 months, 1 year, 2 years, and 3 years after surgery. On each visit, the physician asked if the patient had ever been diagnosed with HAEC by any hospital.
Microbiome sequencing
At the pull-through surgery, the cutting edge of dilated segments of HSCR patients was collected and immediately stored at −80°C. Our standard of excision is to find mature ganglion cells on the cutting edge of the dilated segment based on fast pathology during surgery. Generally, the distance between the cutting edge of the dilated segment and the transitional segment is more than 10 cm. For classical segment HSCR, the cutting edge was usually located in the proximal sigmoid colon or the descending colon. For long-segment HSCR, the cutting edge reached the descending colon, the transverse colon, and sometimes the ascending colon. In the case of total colonic aganglionosis, the cutting edge reached the small intestine. We collected the enteric mucosa in the cutting edge of the dilated segment for sequencing, which is adjacent to normal tissue and thus better represents the characteristics of normal enteric tissues and anastomotic stoma after surgery. DNA in the enteric mucosa was extracted and purified by the QIAamp DNA Mini Kit (QIAGEN). The 16S rRNA gene was amplified by the polymerase chain reaction (PCR), using primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), which target the V4 hypervariable region. PCR products were purified by the GeneJET Gel Extraction Kit (Thermo Scientific). Libraries were prepared by the TruSeq DNA PCR-Free Sample Preparation Kit. The library quality was assessed by the Qubit 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. PCR products were sequenced by the Illumina HiSeq 2500 System, generating 250-bp paired-end reads. All sequences are available under the NCBI Sequence Read Archive BioProject ID PRJNA578412.
Sequence data analysis
The sequence reads were quality-filtered using QIIME (Quantitative Insights Into Microbial Ecology, V1.9.1). Filtered reads were clustered into OTUs, using an open-reference picking process with a threshold of 97% similarity to the reference database (Greengenes OTUs (16S) v13_8). After each sample was rarefied to 35,186 reads (originally 35,186 ~ 84,996 reads/sample), we used QIIME to calculate α diversity indices, including observed species, Chao1, Shannon, Simpson, and phylogenetic diversity whole tree, as well as unweighted UniFrac distance matrices (β diversity). In the enteric microbiome, Proteobacteria and Bacteriodetes are the two most abundant phyla of Gram-negative bacteria, while Firmicutes and Actinobacteria are the two most abundant phyla of Gram-positive bacteria. In our samples, these four phyla accounted for 96% (median) of the enteric microbiome. Thus, we summed the relative abundance (henceforth referred to as “abundance”) of Proteobacteria and Bacteriodetes to represent Gram-negative bacteria and calculated to represent the ratio of Gram-negative to Gram-positive bacteria. The software package PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) was used to perform metagenomic and functional analyses according to KEGG (Kyoto Encyclopedia of Genes and Genomes) Orthology.
Lipopolysaccharide (LPS) assay
LPS was measured in the same tissues used for microbiome analysis. We used the cutting edge of dilated segments adjacent to normal tissue, which was made into homogenate by diluting 1 mg enteric mucosa with 9 μL phosphate-buffered saline (Beyotime, Nantong, China). The cutting edge of the dilated segment is adjacent to normal tissue and thus may best represent the characteristics of normal colonic tissues after surgery. After centrifugation for 5 min at 4°C and 15,700 × g, we obtained supernatants and measured LPS by an enzyme-linked immunosorbent assay (Xin Yu Biotech, Shanghai, China), using TECAN infinite M200 multimode microplate reader at 450 nm (Tecan, Mechelen, Belgium).33 To investigate the relationship between specific microbes and LPS levels we performed Spearman rank correlation analysis between LPS levels and 131 OTUs that were significantly different between postoperative HAEC cases and controls in 75 cases and controls.
Statistical analyses
Log-binomial regression models were used to calculate relative risks (RRs) and 95% confidence intervals (CIs) of HAEC associated with exclusive breastfeeding (SAS “PROC GENMOD”).34 Logistic regression models were used to estimate odds ratios (ORs) of postoperative HAEC for a 5-ng/mL increase in LPS concentration (SAS “PROC LOGISTIC”). In multivariable analyses, we adjusted for gestational age (as a continuous variable), gender (female, male), mode of delivery (cesarean, vaginal), birth weight (as a continuous variable), and HSCR classification (classical segment HSCR, long-segment HSCR or total colonic aganglionosis). When we examined postoperative HAEC occurrence, we additionally adjusted for ostomy (yes, no), age at pull-through procedure (≤180 or >180 days), and surgical approach (open pull-through, totally transanal endorectal pull-through, laparoscopic-assisted transanal pull-through).
We compared the enteric microbiome between groups: (1) postoperative HAEC cases and controls; (2) HSCR patients with and without exclusive breastfeeding. The α diversity indices, abundance of Gram-negative bacteria, ratio of Gram-negative to Gram-positive bacteria, abundance of KEGG pathways, and LPS concentrations were compared by Wilcoxon rank-sum test. The β diversity, measured by unweighted UniFrac distance matrices, was compared by permutational multivariate analysis of variance (R package “vegan”) and visualized by principal coordinate analysis (R package “ape”).
We excluded operational taxonomic units (OTUs) if (1) they had less than 100 reads across all samples or (2) their family was unknown or uncertain, resulting in a final set of 1,213 OTUs. The OTU abundance (z-score) was compared by t-test, and the significant OTUs were visualized by heatmap using unsupervised hierarchical clustering (Gene Cluster 3.0). We examined whether the OTUs, which had significantly different abundance between patients with and without exclusive breastfeeding, can distinguish postoperative HAEC cases and controls, using linear discriminant analysis (MATLAB).
To identify a microbiome signature that predicted postoperative HAEC occurrence, we employed stepwise random forest classification (forward selection), coupled with leave-one-out cross validation, to select OTUs from those with significantly different abundance between postoperative HAEC cases and controls at P < .01 (MATLAB). To evaluate the performance of the identified signature, we calculated classification accuracy and generated a receiver operating characteristic (ROC) curve, defining the optimal cut point as the point maximizing the sum of sensitivity and specificity. All statistical tests were two-sided, and a P-value of <0.05 was considered statistically significant unless specified otherwise.
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of China [NSFC 81570467]; Key Research and Development Program of Jiangsu Province [BE2017609]; Nanjing Medical Science and Technique Development Foundation [ZKX17039]; and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We also thank Qiming Gen, Huan Chen, Changgui Lu, Weiwei Jiang, Wei Li, and Jie Tang at the Children’s Hospital of Nanjing Medical University for sample collection.
Funding Statement
This work was supported by the Jiangsu Provincial Key Research and Development Program [BE2017609];National Natural Science Foundation of China [NSFC 81570467];Nanjing Medical Science and Technique Development Foundation [ZKX17039];Priority Academic Program Development of Jiangsu Higher Education Institutions [PAPD].
Abbreviations
- HSCR
Hirschsprung disease
- HAEC
Hirschsprung-associated enterocolitis
- po-HAEC
postoperative HAEC
- PD
phylogenetic diversity
- OTU
operational taxonomic unit
- EBF
exclusive breastfed
- LPS
lipopolysaccharide
Author contributions
W.T., Y.S., A.S., and Y.X. conceived the study. Y.S. and L.Z. provided data. Y.S., C.Y., Y.Z., H.C., J.M., and A.S. performed the data analysis; Y.S., C.Y., and A.S. drafted the manuscript. A.S. and Y.X supervised this study. All authors read and approved the final manuscript.
COI-statement
The authors declare no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Baxter KJ, Bhatia AM.. Hirschsprung’s disease in the preterm infant: implications for diagnosis and outcome. Am Surg. 2013;79:734–738. [PubMed] [Google Scholar]
- 2.Escobar MA, Grosfeld JL, West KW, Scherer LR, Rouse TM, Engum SA, Rescorla FJ. Long-term outcomes in total colonic aganglionosis: a 32-year experience. J Pediatr Surg. 2005;40:955–961. doi: 10.1016/j.jpedsurg.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Heuckeroth RO. Hirschsprung disease - integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol. 2018;15:152–167. doi: 10.1038/nrgastro.2017.149. [DOI] [PubMed] [Google Scholar]
- 4.Frykman PK, Short SS. Hirschsprung-associated enterocolitis: prevention and therapy. Semin Pediatr Surg. 2012;21:328–335. doi: 10.1053/j.sempedsurg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiagarajah JR, Yildiz H, Carlson T, Thomas AR, Steiger C, Pieretti A, Zukerberg LR, Carrier RL, Goldstein AM.. Altered goblet cell differentiation and surface mucus properties in Hirschsprung disease. PLoS One. 2014;9:e99944. doi: 10.1371/journal.pone.0099944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gosain A, Barlow-Anacker AJ, Erickson CS, Pierre JF, Heneghan AF, Epstein ML, Kudsk KA.. Impaired cellular immunity in the murine neural crest conditional deletion of endothelin receptor-B model of Hirschsprung’s disease. PLoS One. 2015;10:e0128822. doi: 10.1371/journal.pone.0128822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward NL, Pieretti A, Dowd SE, Cox SB, Goldstein AM. Intestinal aganglionosis is associated with early and sustained disruption of the colonic microbiome. Neurogastroenterol Motil. 2012;24:874–e400. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Poroyko V, Yan Z, Pan L, Feng Y, Zhao P, Xie Z, Hong L. Characterization of intestinal microbiomes of Hirschsprung’s disease patients with or without enterocolitis using Illumina-MiSeq high-throughput sequencing. PLoS One. 2016;11:e0162079. doi: 10.1371/journal.pone.0162079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Z, Poroyko V, Gu S, Zhang Z, Pan L, Wang J, Bao N, Hong L. Characterization of the intestinal microbiome of Hirschsprung’s disease with and without enterocolitis. Biochem Biophys Res Commun. 2014;445:269–274. doi: 10.1016/j.bbrc.2014.01.104. [DOI] [PubMed] [Google Scholar]
- 10.Schanler RJ. [In time: human milk is the feeding strategy to prevent necrotizing enterocolitis]. Rev Paul Pediatr. 2015;33:131–133. doi: 10.1016/j.rpped.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamkin DH. Mother’s milk, feeding strategies, and lactoferrin to prevent necrotizing enterocolitis. JPEN J Parenter Enteral Nutr. 2012;36:25S–9S. doi: 10.1177/0148607111420158. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez NA, Caplan MS. Oropharyngeal administration of mother’s milk to prevent necrotizing enterocolitis in extremely low-birth-weight infants: theoretical perspectives. J Perinat Neonatal Nurs. 2015;29:81–90. doi: 10.1097/JPN.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 13.Alshaikh B, Kostecky L, Blachly N, Yee W. Effect of a quality improvement project to use exclusive mother’s own milk on rate of necrotizing enterocolitis in preterm infants. Breastfeed Med. 2015;10:355–361. doi: 10.1089/bfm.2015.0042. [DOI] [PubMed] [Google Scholar]
- 14.Brower-Sinning R, Zhong D, Good M, Firek B, Baker R, Sodhi CP, Hackam DJ, Morowitz MJ. Mucosa-associated bacterial diversity in necrotizing enterocolitis. PLoS One. 2014;9:e105046. doi: 10.1371/journal.pone.0105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory KE, Samuel BS, Houghteling P, Shan G, Ausubel FM, Sadreyev RI, Walker WA.. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016;4:68. doi: 10.1186/s40168-016-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GA, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumann-Dudenhoeffer AM, D’Souza AW, Tarr PI, Warner BB, Dantas G. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med. 2018;24:1822–1829. doi: 10.1038/s41591-018-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown BP, Jaspan HB. Compositional analyses reveal correlations between taxon-level gut bacterial abundance and peripheral T cell marker expression in African infants. Gut Microbes. 2019;1–8. doi: 10.1080/19490976.2019.1643673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–110. doi: 10.1016/S0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 21.He Y, Liu S, Kling DE, Leone S, Lawlor NT, Huang Y, Feinberg SB, Hill DR, Newburg DS. The human milk oligosaccharide 2ʹ-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut. 2016;65:33–46. doi: 10.1136/gutjnl-2014-307544. [DOI] [PubMed] [Google Scholar]
- 22.Frykman PK, Nordenskjold A, Kawaguchi A, Hui TT, Granstrom AL, Cheng Z, Tang J, Underhill DM, Iliev I, Funari VA, et al. Characterization of bacterial and fungal microbiome in children with Hirschsprung disease with and without a history of enterocolitis: A multicenter study. PLoS One. 2015;10:e0124172. doi: 10.1371/journal.pone.0124172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuvonen MI, Korpela K, Kyrklund K, Salonen A, de Vos W, Rintala RJ, Pakarinen MP. Intestinal microbiota in Hirschsprung Disease. J Pediatr Gastroenterol Nutr. 2018;67:594–600. doi: 10.1097/MPG.0000000000001999. [DOI] [PubMed] [Google Scholar]
- 24.Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Semin Pediatr Surg. 2018;27:39–46. doi: 10.1053/j.sempedsurg.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen DH, Shi CR, Chen JJ, Yu SY, Wu Y, Yan WB. Detection of intestinal bifidobacteria and lactobacilli in patients with Hirschsprung’s disease associated enterocolitis. World J Pediatr. 2009;5:201–205. doi: 10.1007/s12519-009-0038-x. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Li Z, Xu Z, Wang Z, Feng J. Probiotics prevent Hirschsprung’s disease-associated enterocolitis: a prospective multicenter randomized controlled trial. Int J Colorectal Dis. 2015;30:105–110. doi: 10.1007/s00384-014-2054-0. [DOI] [PubMed] [Google Scholar]
- 27.El-Sawaf M, Siddiqui S, Mahmoud M, Drongowski R, Teitelbaum DH. Probiotic prophylaxis after pullthrough for Hirschsprung disease to reduce incidence of enterocolitis: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. J Pediatr Surg. 2013;48:111–117. doi: 10.1016/j.jpedsurg.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Demehri FR, Frykman PK, Cheng Z, Ruan C, Wester T, Nordenskjold A, Kawaguchi A, Hui TT, Granström AL, Funari V, et al. Altered fecal short chain fatty acid composition in children with a history of Hirschsprung-associated enterocolitis. J Pediatr Surg. 2016;51:81–86. doi: 10.1016/j.jpedsurg.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronco C. Lipopolysaccharide (LPS) from the cellular wall of Gram-negative bacteria, also known as endotoxin, is a key molecule in the pathogenesis of sepsis and septic shock. Preface Blood Purif. 2014;37(Suppl 1):1. doi: 10.1159/000357412. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney RP, Lowary TL. New insights into lipopolysaccharide assembly and export. Curr Opin Chem Biol. 2019;53:37–43. doi: 10.1016/j.cbpa.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol. 2017;8:1162. doi: 10.3389/fmicb.2017.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastor AC, Osman F, Teitelbaum DH, Caty MG, Langer JC. Development of a standardized definition for Hirschsprung’s-associated enterocolitis: a Delphi analysis. J Pediatr Surg. 2009;44:251–256. doi: 10.1016/j.jpedsurg.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 33.Zhu-Shimoni J, Yu C, Nishihara J, Wong RM, Gunawan F, Lin M, Krawitz D, Liu P, Sandoval W, Vanderlaan M. Host cell protein testing by ELISAs and the use of orthogonal methods. Biotechnol Bioeng. 2014;111:2367–2379. doi: 10.1002/bit.25327. [DOI] [PubMed] [Google Scholar]
- 34.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


