Abstract
Bipolar disorder (BD) is a chronic mental illness characterized by changes in mood that alternate between mania and hypomania or between depression and mixed states, often associated with functional impairment. Although effective pharmacological and non-pharmacological treatments are available, several patients with BD remain symptomatic. The advance in the understanding of the neurobiology underlying BD could help in the identification of new therapeutic targets as well as biomarkers for early detection, prognosis, and response to treatment in BD. In this review, we discuss genetic, epigenetic, molecular, physiological and neuroimaging findings associated with the neurobiology of BD. Despite the advances in the pathophysiological knowledge of BD, the diagnosis and management of the disease are still essentially clinical. Given the complexity of the brain and the close relationship between environmental exposure and brain function, initiatives that incorporate genetic, epigenetic, molecular, physiological, clinical, environmental data, and brain imaging are necessary to produce information that can be translated into prevention and better outcomes for patients with BD.
Keywords: Bipolar disorder, mania, depression, genetics, epigenetics, neurotrophins, mitochondrial dysfunction, oxidative stress, inflammation, hypothalamic-pituitary-adrenal axis, circadian rhythm, neuroimaging
Introduction
Bipolar disorder (BD) is a severe and chronic psychiatric illness that affects approximately 1-4% of the world population.1 It is characterized by changes in mood that alternate between mania and hypomania or between depression and mixed states, often associated with functional impairment.1-3 Although depressive symptoms typically predominate in individuals with BD, the clinical diagnostic hallmark is the presence of manic or hypomanic episodes.4 During episodes of mania or hypomania, the patient may present symptoms related to elevated mood, including euphoria, feelings of greatness, hyperactivity, increased sexual activity, decreased need for sleep, risky behaviors, irritability, and aggression. Conversely, episodes of depression are characterized by anhedonia, sadness, vegetative symptoms, and psychomotor retardation. Mixed episodes manifest as simultaneous states of mania and depression.4 About 14-59% of the individuals with BD report suicidal ideation5; 25 to 50% attempt suicide, and almost 20% die due to suicide.6 Moreover, BD is also associated with increased risk of mortality by other medical conditions, such as cardiovascular disease, diabetes mellitus, external causes of injuries, and respiratory diseases. Life expectancy is decreased by 9 years on average in individuals with BD as compared with the general population.7
Despite the demonstrated efficacy of several drugs to treat BD, lithium (Li) is the only drug considered as a mood stabilizer by the Food and Drug Administration (FDA). More than 70 years have passed since this salt was first proposed as a mood stabilizer. Li is effectively used in BD for maintenance and treatment of acute mania episodes; however, it has a modest antidepressant action.8 Anticonvulsants such as valproate (VPA) and carbamazepine are also used to treat BD. Additionally, some typical (e.g., haloperidol and chlorpromazine) and atypical antipsychotics (e.g., aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone, and asenapine) have also shown efficacy.9-11 Of note, the treatment of depression in BD is particularly challenging, as several drugs used for treating depressive symptoms, such as selective serotonin reuptake inhibitors (SSRI), can induce a switch to hypomania or mania. Thus, these drugs are usually combined with Li for acute treatment and maintenance of bipolar depression.12 It is noteworthy that patients with BD commonly face residual mood symptoms, cognitive and functional impairment, psychosocial disability, and decreased quality of life even with the best treatment available.13,14
BD has been suggested as a progressive condition, and the delay in diagnosis as well as inappropriate treatment can result in repeated mood episodes, persistent subthreshold symptoms, development of co-morbidities, and progression of the disease with cognitive impairment and functional decline.15-17 The term “neuroprogression” has thus been conceptualized as the pathological rewiring of the brain that takes place in parallel with the functional and clinical deterioration that may occur in the course of BD; according to this concept, different stages of BD are associated with distinct neurobiological underpinnings.18-23 Indeed, studies have shown that structural brain changes and cognitive deficits are not consistently found at illness onset, and appear to become more evident with chronicity and recurring episodes.24-27 Moreover, neuroprogression seems to be related to the cumulative effects of immune dysfunction, enhanced oxidative stress, neurotrophic support breakdown, mitochondrial dysfunction, and impairment of cellular resilience.20,21,28,29 Neuroprogression has been also associated with lower responsiveness to treatment, especially with Li and cognitive behavioral therapy.30-32 In this sense, Post et al.33 have suggested that multiple episodes may lead to permanent alterations in neuronal activity, which may translate into greater liability to relapse and poorer treatment response. Of note, such a progressive feature does not seem to be present across all patients, with some not experiencing as much cognitive or functional impairment as others.34 This poses the particular challenge of identifying specific subgroups of susceptible patients, which may require a deeper understanding of the biological basis of BD.
Although numerous factors and mechanisms have been proposed to explain the pathophysiology of BD, its definitive etiopathology remains unknown. Nonetheless, it is believed that the etiology of BD involves an interaction between multiple genetic, neurochemical, and environmental factors (Figure 1).35 Not only would a deep understanding of the neurobiology of BD support the discovery of new targets for effective pharmacological and non-pharmacological therapies, it would also facilitate the identification of biomarkers for diagnosis, prognosis, and response to specific treatments. The last years have witnessed great progress in the understanding of BD pathophysiology. For instance, several lines of thought have led us to believe that BD is associated with neuroprogression, and that over time recurrence may exacerbate subsequent episodes, which are accompanied by functional and cognitive impairment.20,36-38 An underlying hypothesis is that changes in neuroplasticity, with a decrease in neurotrophic factors combined with mitochondrial dysfunction, oxidative stress, inflammation, circadian rhythm abnormalities, and biological aging acceleration, could be associated with the worsening of mood episodes, refractoriness to treatment, cognitive impairment, and functional disability.20 This article seeks to discuss studies addressing the neurobiological mechanisms of BD, providing an overview of genetic components, major signaling pathways, biochemical changes, and neuroimaging findings associated with this disorder.
Figure 1. Etiology of bipolar disorders. The cause of bipolar disorder (BD) is still unknown. However, it has been established that the dynamic interplay between genetic, neurochemical, and environmental factors plays a role in the onset and progression of BD.
Genetics and epigenetics of bipolar disorder
Evidence suggests that BD presents a very strong genetic component, with twin studies showing heritability rates (i.e., the extent to which the disorder can be explained by genetic factors) as high as 70-80%.39,40 The strength of this genetic component is also supported by the increased risk of BD noted in first-degree relatives of patients, including offspring.41 The risk of BD is significantly higher in children of parents diagnosed with BD vs. offspring of control parents42,43; indeed, a plethora of signs and symptoms may accompany such familial risk even in the absence of a full-blown diagnosis of BD.44-48 This familial aggregation suggests a potential relevance of both inherited genes, which can be tested by molecular genetic analyses, and the “inherited” familial environment, which is also known to be potentially impaired in the face of parental psychopathology.49,50
In the search for relevant BD-related genes, several genome-wide association studies (GWASs) conducted in the past years have identified multiple loci with a small effect that may account for heritability,51-62 including 30 recently discovered loci encoding ion channels, neurotransmitter transporters, and synaptic components.63 None of these single nucleotide polymorphisms (SNPs), however, has been shown to present high penetrance or a large effect size, a finding that is consistent with the complex multifactorial model that is believed to underlie the genetics of BD and other psychiatric disorders. Moreover, the difference in heritability rate calculated through twin studies (70-80%) and that calculated based on molecular genetics findings (approximately 30%) is quite large, suggesting a role of other markers or mechanisms.64 These may include gene-by-gene interactions, rare genetic markers (current GWASs only assess “common” alterations), gene-by-environment interactions, and epigenetic markers.
Epigenetic mechanisms encompass several pathways that can mediate gene-by-environment interactions and modulate gene expression and activity without altering the DNA sequence. These include, among others, DNA methylation, histone modifications, chromatin remodeling, and the actions of noncoding RNAs. Several of these have been suggested to play significant roles in BD pathophysiology, especially DNA methylation.65-67 In addition, several epigenetic alterations that may underlie risk/resilience mechanisms have been described in youth at high risk of BD, such as offspring of BD parents.68,69 Specifically, DNA methylation is a fairly stable epigenetic alteration that has been implicated in BD pathogenesis and progression,69-71 as well as in the mechanisms of action of several drugs used to treat patients.65,72-74 DNA methylation in promoters is typically associated with the repression of gene expression and may underlie at least some of the transcriptomic changes reported for BD in multiple tissues.75-79 Moreover, several preclinical and clinical studies have shown that early-life traumatic experiences can induce stable methylation alterations that persist into adulthood, suggesting epigenetic-based alterations as mediators of the clinical effects of early adversity.80-82
Interestingly, one of the mechanisms by which methylomes have been shown to interfere with BD phenotype is the modulation of biological aging processes. Patients with BD have been shown to present several clinical markers that are suggestive of premature aging, including a higher rate of age-related conditions and faster cognitive decline, with previous reports suggesting shortened telomere length compared to controls.83-85 More recently, a marker of biological aging based solely on DNA methylation levels (“epigenetic age” or “DNA methylation age”)86 has been used to explore the aging processes in BD, and the results suggest accelerated epigenetic aging in the blood and brain of BD patients compared to controls.71,87 Recent evidence that the epigenetic clock can be pharmacologically reversed in humans88 offers an interesting treatment possibility for the modulation of premature aging in BD.
In addition to DNA methylation, noncoding RNAs have also deserved much attention, including gene expression modulators such as microRNAs, long noncoding RNAs, and others.89 Several alterations in the levels of microRNAs and long noncoding RNAs have been detected in BD samples, some of which have been validated by independent studies.90,91 Recent studies have also suggested that noncoding RNAs may function as clinically relevant peripheral biomarkers when assessed from neuron-derived extracellular vesicles, which are currently being investigated in BD patients.92,93 Altogether, these studies suggest an important role for noncoding RNAs in BD pathophysiology, and also their potential as an important diagnostic and prognostic tool.
Changes in neuroplasticity and neurotrophic signaling
Neurotrophic factors comprise a group of proteins responsible for regulating neuronal survival processes, neuronal growth, synaptic formation, and cellular plasticity at the central and peripheral nervous systems. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4) are the most common neurotrophic factors studied in psychiatric disorders. However, BDNF is undoubtedly the most studied neurotrophin in BD. Neurotrophins are a specific type of neurotrophic factor, and members of this family include BDNF, NGF, NT-3, and NT-4. Neurotrophins bind and activate a specific family of tyrosine kinase (Trk) receptors, thus promoting modulation of the central nervous system (CNS). There are three specific Trk receptors: NGF binds to TrkA; BDNF and NT-4 bind to TrkB; and NT-3 binds to the TrkC receptor.94
The strong interest in BDNF and other neurotrophins in BD was triggered by the discovery that antidepressants and mood stabilizers could act on these molecules, modulating their signaling pathways. Preclinical studies have shown that the chronic use of antidepressants and mood stabilizers, such as Li and VPA, can increase NGF, BDNF, and GDNF levels in the rat brain.95-98 Currently, a range of studies show decreased levels of BDNF and its receptor TrkB in both blood and brain of patients with BD.99-103 Additionally, a BDNF gene polymorphism that replaces a valine for a methionine (i.e., valine replacing methionine) at codon 66 (Val66Met) has been repeatedly associated with BD.104 A previous study also demonstrated decreased plasma NGF levels in patients with BD.105 Another clinical study showed that NT-3 and NT-4 are elevated in the depressive phase of BD,106 possibly indicating an attempt by the organism to defend itself against cellular stress. In turn, Barbosa et al.107 demonstrated decreased NT-3 and NT-4 levels in the manic phase of BD. Given that neurotrophins are essential for neuronal function and survival, it is assumed that the viability of nerve cells can be affected by a persistent reduction of these molecules in the nervous system.
As in clinical studies, preclinical research has demonstrated that amphetamine (AMPH) or ouabain-induced manic behaviors in rats decrease BDNF, NGF, and GDNF levels in the brain of animals.95,98 Additionally, decreased levels of BDNF, NGF, and GDNF followed depressive-like behaviors in an animal model of depression induced by maternal deprivation or chronic mild stress.108 A previous preclinical study suggested that neuroadaptations induced by chronic administration of dextroamphetamine (d-AMPH) might mimic neuroprogression in BD, because the brain is primed for both the manic and depressive episodes which are characteristic of BD. In that study, the authors showed that d-AMPH withdrawal induces depressive- and anxious-like behaviors in rats, as well as a sensitization to manic-like behaviors. In line with this, d-AMPH sensitization decreased the levels of BDNF, NGF, and GDNF and increased the levels of NT-3 and NT-4/5 in the brain of rats.109
Together, these clinical and preclinical studies suggest changes in neurotrophic factors as an attractive molecular mechanism to explain the decreased cellular plasticity observed in BD, along with other mechanisms described in detail in the next sections. The hypothesis is that changes in neuroplasticity, including alterations in neurotrophic signaling, could be associated with brain damage, which in turn worsens mood episodes and ultimately induces cognitive and functioning deficits in patients with BD.
Mitochondrial dysfunction and oxidative stress
Mitochondria are organelles that are responsible, both directly and indirectly, for cellular functions such as energy production; they also function as sources of cellular growth substrates and play crucial roles in oxidative/nitrosative stress, cell resilience and cell death.110-112 In the brain, mitochondria are critical for the modulation of neuronal activity, short- and long-term neuronal plasticity, cellular resilience, and behavioral adaptations, mainly through actions on long-term potentiation, the hallmark process of learning and memory.112-115 For more than 50 years, multiple studies have highlighted mitochondrial dysfunction as a common pathway in BD pathophysiology, triggered by mechanisms such as impaired oxidative phosphorylation, shift to glycolytic production of energy, general decrease in energy, and abnormalities in the morphology and intracellular distribution of mitochondria.116 The “mitochondrial hypothesis” suggests that BD is triggered, at least in part, by mitochondrial dysfunction, which can be intimately linked to a wide range of processes associated with treatment outcomes and disease progression or severity, including inflammation, oxidative stress, stress response systems, and accelerated aging.
A considerable number of studies in the literature has shown an increase in lactate levels and a reduction in intracellular pH (ipH) in the brain of patients with BD, possibly indicating altered mitochondrial function and a glycolytic shift consistent with impaired mitochondrial metabolism in BD.116-120 Glycolysis-only adenosine triphosphate (ATP) production has been shown to be powerless to maintain normal levels of Na+/K+-ATPase activity in neurons, inducing a large calcium influx into neurons, followed by glutamate excitotoxicity and neuronal apoptosis, both of which play a central role in neurodegeneration.121 Mallakh et al.122 propose that both manic and depressed states in BD could be caused by Na+/K+-ATPase dysfunction – a small reduction in Na+/K+-ATPase activity in the brain would lead to a hyperexcitable state (mania) by bringing the resting potential of neurons closer to the threshold for activation and increasing the duration of neurotransmitter release; in turn, a further reduction in Na+/K+-ATPase function would bring the resting potential even closer to the threshold for activation, decreasing the amplitude of the action potential and resulting in inhibition of neurotransmitter release (depression).122
An additional link between mitochondrial dysfunction and BD is supported by data demonstrating that patients with BD present significantly lower levels of phosphocreatine (PCr) (a high-energy compound) and adenosine diphosphate (ADP) and decreased PCr peak area percentage, suggesting regional hypometabolism in all three mood states.123 Additionally, the PCr peak area percentage was observed to be decreased in the left frontal lobe of patients during the depressive state, as well as in the right frontal lobe during manic/euthymic states.124,125 Moreover, previous studies have shown that patients with BD present lower levels of N-acetyl-aspartate (NAA), which is thought to represent a surrogate marker for impaired mitochondria, along with a negative correlation between NAA/creatine + PCr or NAA levels and illness duration.126-129 Taken together, these studies provide indirect evidence that mitochondrial dysfunction may play a role in illness progression.20
Several clinical and animal studies have reported alterations in various energetic metabolism components, including downregulation of nuclear mRNA molecules and proteins involved in the Krebs cycle, electron transport chain (ETC) I-IV complexes, and creatine kinase, as well as a marked decrease in the activity of ETC complexes and Krebs cycle enzymes.130-143 Together, these studies suggest a reduced ability to oxidize NADH and FADH and transfer electrons to ubiquinone in BD, resulting in reactive oxygen species (ROS) production through an increase in the rate of electron leakage by ETC complexes.
More recent attention has focused on the maintenance of a healthy mitochondrial pool, which is critically regulated by the dynamics and turnover of the mitochondrial population. A damaged mitochondrion may segregate its damaged components into subcompartments and divide, whereas a healthy mitochondrion with a potential healthier membrane will continue to participate in fusion and fission cycles. Depolarized damaged mitochondria are often degraded through mitophagy via the PTEN-induced kinase 1 (PINK1)-Parkin pathway.144 Thus, these dynamic processes of mitochondrial fusion, fission, transport, and turnover enable recruitment of healthy mitochondria to subcellular compartments with high demands for ATP; disruptions in any of these processes will lead to mitochondrial pathology, cellular dysfunction, and neurological defects.145 Previous research findings into mitochondrial morphology have shown that prefrontal cortex neurons of the postmortem brain from patients with BD and peripheral cells from living BD patients display morphological abnormalities (more mitochondria of smaller size) and an abnormal pattern of clumping and marginalization in the intracellular distribution of mitochondria.146 By drawing on the concept of mitochondrial quality control, recent studies have demonstrated that patients with BD present an imbalance in mitochondrial fission and fusion toward fission, followed by a decrease in the levels of mitophagy proteins and increase in caspase-3 protein levels in peripheral blood mononuclear cells, suggesting that, in patients with BD, the number of damaged mitochondria exceeds the capacity of mitophagy, and apoptosis becomes the dominant pathway to minimize tissue damage.147,148
Moreover, numerous studies have provided evidence of increased ROS production and oxidative stress in patients with BD. Replicated evidence has documented alterations in multiple aspects of oxidative stress regulation, including the production of ROS and reduced antioxidant capacity. Meta-analyses have shown significantly greater levels of lipid peroxidation markers, DNA/RNA damage, and nitric oxide in BD.149,150 However, mixed results regarding two of the primary antioxidant enzymes, glutathione peroxidase (GPx) and superoxide dismutase (SOD), have been reported in BD, followed by a meta-analysis that did not reach statistical significance for the comparison of GPx and SOD activity levels in individuals with BD vs. controls.149 Conversely, a recent study by Das et al.151 showed that, although glutathione levels did not differ in BD and controls, there was a positive, BD-specific correlation between lactate and glutathione levels, indicating a physiological association between the antioxidant system and mitochondrial dysfunction.
Several investigators have reported that excessive oxidative stress in pathological conditions induces point mutations and may result in large deletions of mitochondrial DNA (mtDNA) due to restricted DNA repair ability and the absence of histones in mitochondria. Regarding BD, there is an inconsistency in mtDNA copy number (mtDNAcn) studies that could be attributed to the diversity in clinical features, tissue types, and ethnicity.71,152-155 A recent meta-analysis identified no significant differences in mtDNAcn exhibited by BD patients or controls, while an Asian-specific meta-analysis for BD-mtDNAcn studies revealed significantly lower mtDNAcn in patients with BD, with a low level of heterogeneity.155 Moreover, mtDNAcn was inversely correlated with the number of relapses of manic episodes.154 A possible mechanism for the lower levels of mtDNAcn could be related to DNA polymerase gamma (POLG) dysfunction due to oxidative stress. In fact, recent studies have demonstrated that individuals with BD present downregulation of POLG, the replicative polymerase responsible for maintaining mtDNA, as well as downregulation of the DNA repair enzyme, 8-oxoguanine-DNA glycosylase 1 (OGG1).156,157
The accumulating evidence reviewed above delineates multifaceted mitochondrial dysfunction as a pathological factor in BD. Moreover, mitochondrial dysfunction, apoptosis, and non-methylated mtDNA can activate Toll-like receptors and lead to spontaneous inflammasome activation, stimulating cytokine production and inducing rapid activation of the immune system.
Immune-inflammatory imbalance and kynurenine pathway
Immune dysfunction in BD is supported by pre-clinical and clinical evidence showing elevated levels of pro-inflammatory cytokines, including interleukin-4 (IL-4), interleukin-1beta (IL-1β), interleukin-6 (IL-6), tumor necrosis factor (TNF)-alpha, soluble interleukin-2 receptor (sIL-2R), and soluble receptor of TNF-type 1 (STNFR1), among others, in patients compared to controls.158-164 A recent systematic review has suggested an important role for acute inflammatory response during mania and depression, with the elevation in proinflammatory cytokines seemingly restored after remission of symptoms.118 In addition, further findings in BD have also described significant negative associations between inflammatory markers and general cognitive abilities, as well as neuroanatomical alterations.165-169 However, another systematic review claims that an absolute conclusion cannot be reached regarding the presence of neuroinflammation in BD, since the findings are not consistent.170
Several different mechanisms have been proposed to explain the role of immune dysfunction in BD,171 including changes in blood-brain barrier, cell death-induced release of damage-associate molecular patterns with consequent immune activation, genetic mechanisms, dysfunction of the gut-brain axis, and a role of the kynurenine pathway. Activation of the kynurenine pathway by cytokines such as interferon-gamma (IFN-γ) and TNF-α is described as one of several contributors to psychiatric pathogeneses. Kynurenine metabolites mediate immune-inflammation and neurodegeneration and can lead to neurotoxicity and impaired neurotransmission. The enzyme indoleamine 2,3-dioxygenase (IDO-1) converts tryptophan into kynurenine and is activated by inflammatory cytokines. Kynurenine is then metabolized into hydroxykynurenine and quinolinic acid in microglia, and into kynurenic acid (KYNA) in astrocytes. Hydroxykynurenine and quinolinic acid are N-methyl-D-aspartic acid (NDMA) agonists and increase the production of free radicals, while KYNA, an N-methyl-D-aspartate (NMDA) receptor antagonist, has neuroprotective effects but also leads to cognitive deficits and psychosis when in higher levels.172-174
A previous study showed an imbalance toward the neurotoxicity path derived from kynurenine metabolism, as evidenced by decreased levels of KYNA, in addition to increased hydroxykynurenine/kynurenine ratio in BD compared with healthy controls.175 The higher hydroxykynurenine levels may correlate to cognitive dysfunction not only in the acute phases of BD, but also in euthymic periods.27 Recent evidence shows that KYNA levels in cerebrospinal fluid represent a biomarker for psychotic episodes in BD. This higher risk of developing psychosis may be explained by enhanced dopaminergic transmission due to increased brain KYNA concentrations.176 Conversely, decreased peripheral KYNA and unchanged KYNA levels were observed in the CNS in BD patients during the depressive phase.177
Abnormalities in kynurenine metabolism resulting from the inflammatory response displayed in mood disorders may also contribute to the structural (volume reduction) and functional alterations observed in the hippocampus and amygdala.175,178 One possible explanation for this process is dendritic atrophy in the context of the neurotoxicity occurring in unmedicated patients with BD.178
Hypothalamic-pituitary-adrenal axis
In addition to the genetic component involved in the pathogenesis and pathophysiology of BD, it is known that nongenetic factors, such as psychosocial stress, can trigger the development of mood disorders.179 The hypothalamic-pituitary-adrenal (HPA) axis is the primary mediator of the biological response to stress. Abnormalities in this system have been associated with the clinical course of BD180 and may contribute to increased risk of clinical relapse following an intense psychosocially stressful event,181 although the underlying mechanisms involved in these associations remain essentially unknown.180
A meta-analysis has demonstrated that BD is related to a more prominent activity level of the HPA axis, as evidenced by increased basal cortisol, postdexamethasone (PDEX) cortisol, and adrenocorticotropic hormone (ACTH) levels, along with a higher response to the dexamethasone (DEX)/corticotropin releasing hormone (CRH) test.182 The alterations described in HPA axis activity vary according to the clinical phase of BD. Although the hyperactive HPA axis seems more prominent during the manic phase, it may persist during clinical remission because euthymic BD patients also display higher levels of cortisol.182 The clinical heterogeneity of the depressive phase may explain the conflicting findings related to HPA axis activity, with high cortisol levels found in melancholic depression and normal or low cortisol levels associated with atypical depression.183,184
Particularly interesting is the study by Fries et al.70 showing that patients with BD, particularly at a late stage of illness, presented increased salivary PDEX cortisol levels followed by reduced ex vivo glucocorticoid receptor (GR) responsiveness and increased basal protein levels of FK506-binding protein 51, a cochaperone known to desensitize GR, in peripheral blood mononuclear cells. Moreover, individuals with BD presented increased methylation at the FK506-binding protein 5 (FKBP5) gene, suggesting a dysfunctional negative feedback of the HPA axis and impaired GR responsiveness due to increased FKBP51 levels and increased FKBP5 intronic methylation. In this same study, the authors suggest that, as BD progresses, there is decreased resilience to stress and a higher risk of new mood episodes, since stress resilience and coping mechanisms are primarily mediated by the HPA axis. High levels of PDEX cortisol may also be involved in the mechanism of increased late-stage BD recurrence. Therefore, DEX suppression test results and HPA axis hyperactivity may have prognostic value in BD.70
Furthermore, the hyperactive HPA axis correlates with an increased risk of cognitive dysfunction185 and is known to have neurotoxic effects on the hippocampus that can later predispose to dementia.186 Lee et al.187 described that patients with BD with poorer cognitive performance had higher levels of serum cortisol, as well as a significant correlation between cognitive function after 24 weeks of standard treatment and longitudinal changes in cortisol levels, reinforcing the hypothesis that serum cortisol may be involved in the psychopathological mechanisms of cognitive decline in BD. Moreover, some evidence suggests that an abnormal HPA axis may explain the increased number and size of corticotrophs, leading to the larger pituitary volume found in neuroimaging studies of patients with BD vs. healthy controls.188 Interestingly, some HPA alterations seem to be a trait predisposing to mood disorders – it was demonstrated that first-degree relatives of subjects with BD had elevated baseline cortisol levels and alterations in the response to the DEX/CRH test.189-191 Accordingly, a previous study with offspring of parents with BD showed that these at-risk children have epigenetic alterations that can modulate GR responsiveness and the HPA axis,69 ultimately suggesting a biological mechanism by which the stress axis may be compromised in BD and in at-risk subjects.
Circadian rhythm abnormalities
Sleep disturbances commonly occur in BD and are part of the diagnostic criteria for BD. Normally, there is reduced sleep during manic episodes, and insomnia or hypersomnia during depressive episodes.4,192 Dampened and shifted circadian rhythms may explain some of the sleep disturbances frequently reported by patients with BD.193-195 Indeed, circadian dysregulation is associated with BD both during acute episodes and during inter-episode periods, indicating that these disruptions in circadian rhythms may represent biological markers of the BD.196 Actigraphy studies have demonstrated that individuals at risk for BD exhibit a lower relative amplitude of the rest-activity cycle, and more sleep irregularity than controls.197 Notably, variability in the sleep-wake cycle has been shown to predict the onset of depressive episodes among people with inter-episode BD.198 Similarly, patients with BD have been shown to present lower activity levels, also in addition to sleeping longer, taking longer to fall asleep, spending more time in bed and awake in the middle of the night, and showing a more variable pattern of sleep-wake cycles. Data from several studies have suggested that patients also exhibit more considerable intra-daily variability and lower inter-daily stability, as well as relative amplitude of the rest-activity cycle relative to controls.199
Additionally, one of the most commonly reported rhythm-related findings in BD is a significantly higher prevalence of evening chronotypes.200-204 In individuals with BD, eveningness has also been associated with earlier age of onset, rapid-cycling course, and other factors such as reduction in the peak of the melatonin secretion at night.202 Mondin et al.205 showed that patients with BD with evening preference had lower levels of IL-6, TNF-α and thiobarbituric acid reactive substance (TBARS), suggesting that chronotype may affect interleukin and oxidative stress levels in BD. Furthermore, population-based studies have demonstrated that the eveningness chronotype is more common in cyclothymic individuals, especially those with at least one prior episode of depression, and some, but not all, temperament studies that are putatively linked to risk of BD (hyperthymic temperament) show a higher prevalence of this chronotype in at-risk populations.206
Moreover, the secretion of melatonin and cortisol also follows a circadian pattern. Evidence has indicated that people with BD exhibit melatonin secretion abnormalities, suggesting that differences in the amount and timing of melatonin secretion may reflect different mood states. During the manic episode, high levels of melatonin were observed during the day, with an advanced nighttime peak,207 whereas reduced levels and a later onset of melatonin secretion have been reported in bipolar depression compared with unipolar depression, and in euthymic phases of BD compared with matched controls.208,209 Similarly, 24-h cortisol secretion is significantly higher in patients with BD than in controls, independent of the clinical phase (manic, depressive, or euthymic).210 These findings are consistent with the observation of increased GR mRNA in the hippocampus and amygdala in patients with BD compared with those in controls.211
In the past few decades, several genetic association studies have demonstrated a link between multiple circadian genes and BD, such as CLOCK, ARTNL1, CSNK1ɛ, PER3, NPAS2, NR1D1, TIMELESS, RORA, RORB, and GSK3β. All had modest associations with BD, supporting a polygenic heritability through which multiple genes additively contribute to the risk of BD.212-220 Although GSK3β is considered a likely candidate gene, no association has been reported between BD and the GSK3β gene polymorphism. However, the frequency of a copy number variant (CNV) at the GSK3β locus was higher in individuals with BD than in controls.221,222
In BD, the circadian genes PER3, REV-ERBα, and GSK3β were associated with an early age of disease onset, and the CRY2, CLOCK, ARNTL2, TIMELESS, and CSNK1ɛ were related to rapid BD cycling and/or high disease recurrence.223-226 These findings are consistent with the observation that an early age of onset is associated with more severe circadian disruptions, such as eveningness and sleep quality. Thus, specific circadian genes may be significant in a particular BD subgroup (early onset). Furthermore, these findings suggest that variations in some circadian genes may explain the high sensitivity to rhythm changes observed in BD and may be associated with disease onset or relapse.196 Additionally, chronotype and genotype association studies in BD showed that the 3111T/C CLOCK variant is associated with an extreme night chronotype, and one nonsynonymous PER3 coding SNP and two CSNK1ɛ intronic SNPs were associated with the night chronotype in BD patients.212,220
Circadian genes have also been implicated in bipolar-like behaviors in animal models. The most notable example is the behavior of mice with an exon 19 exclusion in the CLOCK gene, which represents a valuable model of mania.227 These ClockΔ19 mice display hyperactivity, increased exploratory drive, lowered depression-like behavior, higher impulsivity, abnormal sleep/wake cycles, and increased reward response. ClockΔ19 mutant mice show a craving for rewarding stimuli similar to patients with BD in the manic state.228,229 The manic behavior of CLOCK mutant mice can be reversed by treatment with Li or by restoring a functional CLOCK gene in the ventral tegmental area.230 Another mouse model involving circadian genes includes transgenic mice that overexpress GSK3β and show a manic-like phenotype.231
Neuroimaging findings in bipolar disorder
The first studies using computed tomography (CT) have shown structural abnormalities as significantly larger ventricular-to-brain ratios in groups of individuals with BD compared with controls.232 Following the pioneering study (among others) of Pearlson & Veroff,232 cumulative evidence produced by CT and magnetic resonance imaging (MRI) have shown changes in neuroanatomic structures associated with BD. In one of the first reviews concerning volumetric structural neuroimaging in BD, Soares and Mann described a larger third ventricle and a smaller cerebellum, as well as periventricular hyperintensities in BD patients, the latter also found in the elderly with unipolar depression.233 More recently, the ENIGMA Working Group, which includes 28 international groups, showed reduced bilateral cortical thickness in the frontal, temporal, and parietal regions of patients with BD, especially the left rostral middle frontal cortex, left fusiform gyrus, and left pars opercularis. The authors also found an association between the duration of illness with cortical thickness, and increased cortical thickness with the use of Li. A history of psychosis in patients with BD was associated with reduced surface area in the right caudal anterior cingulate cortex and left inferior temporal gyrus compared with patients without psychosis.234 Regarding the volume of brain regions associated with mood regulation and reward, some studies showed smaller amygdala and hippocampus and a larger striatum, although contrasting findings have also been found.235-238 The integration of MRI with machine learning methods to distinguish between patients with BD and healthy controls, as well as for clinical stratification, was investigated by Mwangi et al., showing that the algorithm had up to 70% of accuracy and higher probability scores for those in the late-stage category (more than 10 total lifetime manic episodes including hospitalizations).239 This potential impact of machine learning techniques in the evaluation of individuals with BD was extensively explored in a systematic review by Librenza-Garcia et al.240 Of 51 studies included, 38 applied machine learning to discriminate between BD and healthy controls or other psychiatric disorders, especially with neuroimaging data.240 For instance, Fung et al.241 investigated psychiatric diagnosis accuracy using a support vector machine (SVM) algorithm with brain cortical thickness and surface area data. The authors found that structural brain differences between individuals with BD and major depressive disorder were able to discriminate these psychiatric disorders with 74.3% (adequate) accuracy (sensitivity: 62.5%; specificity: 84.2%).241 More recently, a Big Data Task Force from the International Society for Bipolar Disorders has expanded this literature review and discussed issues to be addressed in machine learning-based studies, including the main barriers for applying these techniques and strategies to approach them.242
Since human behavior, including cognition, emotion, and social interaction, reflects complex neural circuit communication,243 the signs and symptoms we observe in individuals with psychiatric disorders could be understood as the manifestation of different brain circuitry dysfunction.244 Diffusion tensor imaging (DTI) is a neuroimaging technique used to evaluate white matter fiber tract connectivity between different regions, both proximal and distal.245 A review246 of DTI studies in subjects with BD showed a consistent decrease in fractional anisotropy (FA) values in patients, especially in the fronto-limbic tracts and corpus callosum. As part of the ENIGMA network, Favre et al.247 investigated white matter abnormalities in patients with BD compared to healthy controls. The authors found white matter microstructure alterations principally within the cingulum, the main pathway in the limbic system, and in interhemispheric connectivity by the corpus callosum.247 Thus, DTI studies have shown consistent abnormalities in regions associated with emotional regulation as well as in structures that integrate these regions interhemispherically.
Foley et al.,248 based on previous studies from the literature, have investigated whether fractional anisotropy in the uncinate fasciculus distinguished participants with BD-I from those with BD-II and healthy controls. The results showed significantly decreased FA in the uncinate fasciculus in patients with BD-I compared with both healthy controls and BD-II patients, suggesting a distinction in the pathophysiology of BD subtypes. Mahon et al.249 used DTI to identify white matter biomarkers of genetic risk. To achieve their aim, the authors evaluated participants with BD, unaffected siblings of individuals with BD, and healthy controls. The results showed that FA within the right temporal lobe of unaffected siblings was significantly different, and intermediate in relation to participants with BD and healthy controls, suggesting a biomarker for genetic risk of BD.249
Using specific cognitive and emotional tasks, functional MRI (fMRI) methods allow the investigation of neural circuitry associated with distinct behaviors. Using a cognitive control task, Smucny et al.250 evaluated whether a continuum exists in the underlying neural circuitry across participants with a diagnosis of schizophrenia and BD and healthy controls. The authors found a linear trend in the task-associated dorsolateral prefrontal cortex (DLPFC) and superior parietal cortex (SPC) response among the three groups, with participants with schizophrenia showing more severe dysfunction and those with BD an intermediate pattern. These findings show that, despite the different categorical diagnoses, individuals with schizophrenia and BDs may share some neurobiological characteristics.250
Functional neuroimaging studies with BD patients have found connectivity dysfunctions in neural circuits associated with emotion processing, emotion regulation and reward processing.251 Patterns of amygdala activation and connectivity during emotion processing tasks were compared between individuals with BD and unipolar depression by Korgaonkar et al., with both groups in remission.252 The findings demonstrated lower connectivity of the amygdala with the insula and hippocampus and of the amygdala with the medial orbitofrontal cortex in patients with BD.252 Individuals with BD also presented a lower modulatory effect of the DLPFC on the amygdala during emotion regulation tasks compared with healthy controls.253 In a review, Nusslock & Alloy suggest that hypersensitivity to reward-relevant cues is related to hypo/manic symptoms, especially excessive approach motivation and psychomotor hyperactivation, representing a risk trait for BD.254 Acuff et al.255 compared reward processing circuitry among offspring of bipolar parents, offspring of parents with non-BD disorder, and offspring of healthy parents during a reward processing task using fMRI. The results showed that offspring of parents with BD had lower functional connectivity between the right ventral striatum-left caudal anterior cingulate in response to loss; and higher functional connectivity between the right pars orbitalis-left and right orbitofrontal cortex in response to reward, indicating potential neural markers for the risk of BD.255
Despite the impressive advances in technology and interesting neuroimaging findings associated with a psychiatric diagnosis,247 as well as the response to treatment and risk identification,255,256 there is still no direct clinical application of brain imaging at this moment in psychiatry.
Conclusion, challenges, and perspectives
The use of tools and technologies that can positively affect mental health parameters using translational approaches still faces several challenges, particularly in the case of BD. First, there is no biomarker with significant biological and clinical validation in BD, which complicates diagnosis and hinders the development of new drugs. Second, the current literature highlights an important limitation of the existing evidence – most studies are still cross-sectional, and thus capable of identifying associations but not causal relationships or longitudinal patterns of development. Third, BD involves heterogeneous symptomatology, various comorbidities, and cognitive impairment, as well as a wide range of genetic and environmental factors. Similarly to other psychiatric disorders, BD is diagnosed based on purely clinical criteria and history taking, interviewing, and behavioral observation; and overlaps pathophysiologically with numerous other disorders.64,257-260 In this context, the translation of biological findings in BD to the clinical setting is still highly complex, and there is still a long way between translating these initial findings into approved therapies for patients.261
As discussed in the present article, brain-imaging studies have shown evidence of change in regional activity, functional connectivity, neuronal activity, and bioenergetics associated with BD, while past research on animals and human models have added mechanistic evidence on bioenergetic dysfunction, inflammation, oxidative stress, as well as abnormalities in signaling networks, HPA axis, and circadian rhythm (Figure 2). In addition to its complexity, brain function is constantly influenced by environmental exposure, which can be associated not only with the risk of mental illness but also resilience and protection.262 It is not surprising that all of these changes are involved in the neurobiology of BD, a disease with a complicated clinical course involving manic, depressive, mixed, and euthymic episodes.
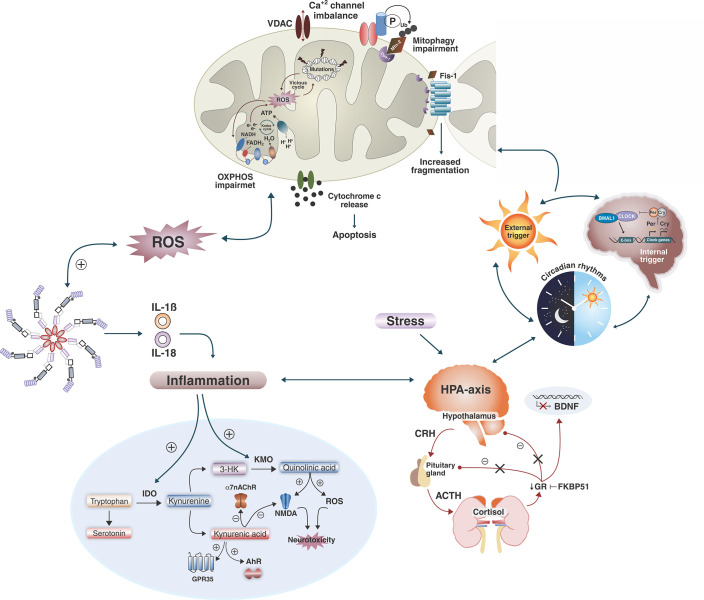
Figure 2. Summary of recent research on neurobiological mechanisms of BD. Multiple biochemical pathways, not all of which are shown here, interact simultaneously to cause cellular damage. Mitochondrial dysfunction in BD pathophysiology is based on changes affecting oxidative phosphorylation, energy production, increased formation of ROS, mitochondrial DNA damage, membrane permeability, Ca+2 imbalance, and impairment in mitochondrial dynamics and mitophagy. These alterations can lead to increased apoptosis and NLRP3-inflammasome activation. However, this relationship may be bidirectional, wherein mitochondrial dysfunction can increase inflammatory factors, and inflammation can induce ROS production and mitochondrial dysfunction. Inflammation, also reported in BD, is responsible for the activation of enzymes indoleamine 2,3-dioxygenase and kynurenine 3-monooxygenase (KMO), leading to the skewing of the kynurenine metabolic balance toward increased neurotoxicity. Moreover, inflammatory mediators and stress mechanisms activate the HPA axis resulting in secretion of corticosteroids from the adrenal cortex. In BD, the negative feedback of cortisol to the hypothalamus and pituitary components is thought to be impaired, leading to continual activation of the HPA axis and excess cortisol release. Cortisol receptors become desensitized, leading to increased activity of pro-inflammatory immune mediators and downregulation of neurotrophic factors such as the brain-derived neurotrophic factor. Besides, corticosteroids are secreted rhythmically, displaying ultradian and circadian patterns, and CLOCK-related genes directly regulate glucocorticoid receptor expression. Circadian rhythms also play a role in mitochondrial functioning by regulating biogenesis, fission/fusion, and mitophagy. These alterations could initiate a vicious cycle where multiple systems and mechanisms exacerbate and accelerate cellular damage, synaptic dysfunction, and impaired neurogenesis, resulting in progressive structural brain changes and cognitive decline thought to contribute to the neuroprogression of BD. 3-HK = 3-hydroxykynurenine; ACTH = adrenocorticotropic hormone; BD = bipolar disorder; BDNF = brain-derived neurotrophic factor; Ca = calcium; CRH = corticotropin releasing hormone; Fis-1 = mitochondrial fission 1 protein; FKBP51 = FK506-binding protein 51; GR = glucocorticoid receptor; HPA = hypothalamic-pituitary-adrenal; IDO = indoleamine 2,3-dioxygenase; IL = interleukin; KMO = kynurenine 3-monooxygenase; NMDA = N-methyl-D-aspartate; OXPHOS = mitochondrial oxidative phosphorylation; P = phosphorus; ROS = reactive oxygen species.
In addition, studies providing insights into how particular brain abnormalities lead to one, not another specific clinical presentation, and the interaction between immune and neurochemical alterations and cognition are needed for a better understanding of the neurobiology of BD. The step towards accurate biomarker identification in psychiatry, including those for BD, will probably require the integration of different sources of evidence. Multidisciplinary research on a single cohort of patients will have more power to increase understanding of BD biology than independent genomic, cell biology, brain imaging, and clinical studies. Moreover, the improvement of technologies related to neuroimaging is undoubtedly a promising measure for advancing the study of neural circuits involved in BD. Finally, longitudinal cohort studies using multimodal and standardized techniques are essential to increase the understanding of BD.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
The Translational Psychiatric Program (USA) is funded by the Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth). The Laboratory of Neurosciences (Brazil) is one of the centers of the Instituto Nacional de Ciência e Tecnologia de Medicina Molecular (INCT-MM) and one of the members of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). This research was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (SSV and JQ), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (JQ), Instituto Cérebro e Mente (JQ), and Universidade do Extremo Sul Catarinense (UNESC) (SSV and JQ). SSV and JQ are CNPq Research Fellows.
Footnotes
How to cite this article: Scaini G, Valvassori SS, Diaz AP, Lima CN, Benevenuto D, Fries GR, et al. Neurobiology of bipolar disorders: a review of genetic components, signaling pathways, biochemical changes, and neuroimaging findings. Braz J Psychiatry. 2020;42:536-551. http://dx.doi.org/10.1590/1516-4446-2019-0732
References
- 1.Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387:1561–72. doi: 10.1016/S0140-6736(15)00241-X. [DOI] [PubMed] [Google Scholar]
- 2.Cotrena C, Branco LD, Kochhann R, Shansis FM, Fonseca RP. Quality of life, functioning and cognition in bipolar disorder and major depression: a latent profile analysis. Psychiatry Res. 2016;241:289–96. doi: 10.1016/j.psychres.2016.04.102. [DOI] [PubMed] [Google Scholar]
- 3.Zarate CA, Jr, Tohen M, Land M, Cavanagh S. Functional impairment and cognition in bipolar disorder. Psychiatr Q. 2000;71:309–29. doi: 10.1023/a:1004632206684. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 5.Gonda X, Pompili M, Serafini G, Montebovi F, Campi S, Dome P, et al. Suicidal behavior in bipolar disorder: epidemiology, characteristics and major risk factors. J Affect Disord. 2012;143:16–26. doi: 10.1016/j.jad.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Latalova K, Kamaradova D, Prasko J. Suicide in bipolar disorder: a review. Psychiatr Danub. 2014;26:108–14. [PubMed] [Google Scholar]
- 7.Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70:931–9. doi: 10.1001/jamapsychiatry.2013.1394. [DOI] [PubMed] [Google Scholar]
- 8.Andrade C. Lithium levels and treatment efficacy. Bipolar Disord. 2020;22:89–90. doi: 10.1111/bdi.12836. [DOI] [PubMed] [Google Scholar]
- 9.Moreno RA, Moreno DH, Soares MB, Ratzke R. [Anticonvulsants and antipsychotics in the treatment of bipolar disorder] Braz J Psychiatry. 2004;26(Suppl 3):37–43. doi: 10.1590/s1516-44462004000700009. [DOI] [PubMed] [Google Scholar]
- 10.Licht RW. Typical and atypical antipsychotics in bipolar disorder. Acta Neuropsychiatr. 2000;12:115–9. doi: 10.1017/S0924270800035559. [DOI] [PubMed] [Google Scholar]
- 11.Derry S, Moore RA. Atypical antipsychotics in bipolar disorder: systematic review of randomised trials. BMC Psychiatry. 2007;7:40. doi: 10.1186/1471-244X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato T. Current understanding of bipolar disorder: toward integration of biological basis and treatment strategies. Psychiatry Clin Neurosci. 2019;73:526–40. doi: 10.1111/pcn.12852. [DOI] [PubMed] [Google Scholar]
- 13.Bortolato B, Miskowiak KW, Kohler CA, Vieta E, Carvalho AF. Cognitive dysfunction in bipolar disorder and schizophrenia: a systematic review of meta-analyses. Neuropsychiatr Dis Treat. 2015;11:3111–25. doi: 10.2147/NDT.S76700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bo Q, Tian L, Li F, Mao Z, Wang Z, Ma X, et al. Quality of life in euthymic patients with unipolar major depressive disorder and bipolar disorder. Neuropsychiatr Dis Treat. 2019;15:1649–57. doi: 10.2147/NDT.S201567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leboyer M, Kupfer DJ. Bipolar disorder: new perspectives in health care and prevention. J Clin Psychiatry. 2010;71:1689–95. doi: 10.4088/JCP.10m06347yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knezevic V, Nedic A. Influence of misdiagnosis on the course of bipolar disorder. Eur Rev Med Pharmacol Sci. 2013;17:1542–5. [PubMed] [Google Scholar]
- 17.Muneer A. Staging models in bipolar disorder: a systematic review of the literature. Clin Psychopharmacol Neurosci. 2016;14:117–30. doi: 10.9758/cpn.2016.14.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. 2009;12:441–5. doi: 10.1017/S1461145708009498. [DOI] [PubMed] [Google Scholar]
- 19.Berk M, Berk L, Dodd S, Cotton S, Macneil C, Daglas R, et al. Stage managing bipolar disorder. Bipolar Disord. 2014;16:471–7. doi: 10.1111/bdi.12099. [DOI] [PubMed] [Google Scholar]
- 20.Fries GR, Pfaffenseller B, Stertz L, Paz AV, Dargél AA, Kunz M, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14:667–75. doi: 10.1007/s11920-012-0319-2. [DOI] [PubMed] [Google Scholar]
- 21.Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–17. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Gama CS, Kunz M, Magalhaes PV, Kapczinski F. Staging and neuroprogression in bipolar disorder: a systematic review of the literature. Braz J Psychiatry. 2013;35:70–4. doi: 10.1016/j.rbp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Schneider MR, DelBello MP, McNamara RK, Strakowski SM, Adler CM. Neuroprogression in bipolar disorder. Bipolar Disord. 2012;14:356–74. doi: 10.1111/j.1399-5618.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 24.Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 25.Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, et al. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am J Psychiatry. 2002;159:1841–7. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- 26.El-Badri SM, Ashton CH, Moore PB, Marsh VR, Ferrier IN. Electrophysiological and cognitive function in young euthymic patients with bipolar affective disorder. Bipolar Disord. 2001;3:79–87. doi: 10.1034/j.1399-5618.2001.030206.x. [DOI] [PubMed] [Google Scholar]
- 27.Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8:103–16. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 28.Post RM, Fleming J, Kapczinski F. Neurobiological correlates of illness progression in the recurrent affective disorders. J Psychiatr Res. 2012;46:561–73. doi: 10.1016/j.jpsychires.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev. 2008;32:675–92. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, et al. Cognitive-behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial. Br J Psychiatry. 2006;188:313–20. doi: 10.1192/bjp.188.4.313. [DOI] [PubMed] [Google Scholar]
- 31.Kessing LV, Hansen HV, Christensen EM, Dam H, Gluud C, Wetterslev J, et al. Do young adults with bipolar disorder benefit from early intervention? J Affect Disord. 2014;152-154:403–8. doi: 10.1016/j.jad.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Swann AC, Bowden CL, Calabrese JR, Dilsaver SC, Morris DD. Differential effect of number of previous episodes of affective disorder on response to lithium or divalproex in acute mania. Am J Psychiatry. 1999;156:1264–6. doi: 10.1176/ajp.156.8.1264. [DOI] [PubMed] [Google Scholar]
- 33.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 34.Passos IC, Mwangi B, Vieta E, Berk M, Kapczinski F. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134:91–103. doi: 10.1111/acps.12581. [DOI] [PubMed] [Google Scholar]
- 35.Manji HK, Henter ID, Zarate CA., Jr Bipolar disorder: a neurobiological synthesis. Curr Top Behav Neurosci. 2011;5:331–40. doi: 10.1007/7854_2010_98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elshahawi HH, Essawi H, Rabie MA, Mansour M, Beshry ZA, Mansour AN. Cognitive functions among euthymic bipolar I patients after a single manic episode versus recurrent episodes. J Affect Disord. 2011;130:180–91. doi: 10.1016/j.jad.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Rosa AR, Gonzalez-Ortega I, Gonzalez-Pinto A, Echeburúa E, Comes M, Martínez-Àran A, et al. One-year psychosocial functioning in patients in the early vs. late stage of bipolar disorder. Acta Psychiatr Scand. 2012;125:335–41. doi: 10.1111/j.1600-0447.2011.01830.x. [DOI] [PubMed] [Google Scholar]
- 38.Buoli M, Caldiroli A, Caletti E, Zugno E, Altamura AC. The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry. 2014;55:1561–6. doi: 10.1016/j.comppsych.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 39.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 40.Edvardsen J, Torgersen S, Roysamb E, Lygren S, Skre I, Onstad S, et al. Heritability of bipolar spectrum disorders. Unity or heterogeneity? J Affect Disord. 2008;106:229–40. doi: 10.1016/j.jad.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Gottesman II, Laursen TM, Bertelsen A, Mortensen PB. Severe mental disorders in offspring with 2 psychiatrically ill parents. Arch Gen Psychiatry. 2010;67:252–7. doi: 10.1001/archgenpsychiatry.2010.1. [DOI] [PubMed] [Google Scholar]
- 42.Duffy A, Goodday S, Keown-Stoneman C, Grof P. The emergent course of bipolar disorder: observations over two decades from the Canadian high-risk offspring cohort. Am J Psychiatry. 2019;176:720–9. doi: 10.1176/appi.ajp.2018.18040461. [DOI] [PubMed] [Google Scholar]
- 43.Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull. 2014;40:28–38. doi: 10.1093/schbul/sbt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diler RS, Goldstein TR, Hafeman D, Rooks BT, Sakolsky D, Goldstein BI, et al. Characteristics of depression among offspring at high and low familial risk of bipolar disorder. Bipolar Disord. 2017;19:344–52. doi: 10.1111/bdi.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levenson JC, Axelson DA, Merranko J, Angulo M, Goldstein TR, Mullin BC, et al. Differences in sleep disturbances among offspring of parents with and without bipolar disorder: association with conversion to bipolar disorder. Bipolar Disord. 2015;17:836–48. doi: 10.1111/bdi.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, et al. Altered functioning of reward circuitry in youth offspring of parents with bipolar disorder. Psychol Med. 2016;46:197–208. doi: 10.1017/S003329171500166X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Axelson D, Goldstein B, Goldstein T, Monk K, Yu H, Hickey MB, et al. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: a longitudinal study. Am J Psychiatry. 2015;172:638–46. doi: 10.1176/appi.ajp.2014.14010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong R, Stefan G, Horrocks J, Goodday SM, Duffy A. Investigating the association between anxiety symptoms and mood disorder in high-risk offspring of bipolar parents: a comparison of Joint and Cox models. Int J Bipolar Disord. 2019;7:22. doi: 10.1186/s40345-019-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shalev A, Merranko J, Goldstein T, Miklowitz DJ, Axelson D, Goldstein BI, et al. A longitudinal study of family functioning in offspring of parents diagnosed with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2019;58:961–70. doi: 10.1016/j.jaac.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freed RD, Tompson MC, Wang CH, Otto MW, Hirshfeld-Becker DR, Nierenberg AA, et al. Family functioning in the context of parental bipolar disorder: associations with offspring age, sex, and psychopathology. J Fam Psychol. 2015;29:108–18. doi: 10.1037/fam0000048. [DOI] [PubMed] [Google Scholar]
- 51.Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–83. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen DT, Jiang X, Akula N, Shugart YY, Wendland JR, Steele CJM, et al. Genome-wide association study meta-analysis of European and Asian-ancestry samples identifies three novel loci associated with bipolar disorder. Mol Psychiatry. 2013;18:195–205. doi: 10.1038/mp.2011.157. [DOI] [PubMed] [Google Scholar]
- 54.Cichon S, Muhleisen TW, Degenhardt FA, Mattheisen M, Miró X, Strohmaier J, et al. Genome-wide association study identifies genetic variation in neurocan as a susceptibility factor for bipolar disorder. Am J Hum Genet. 2011;88:372–81. doi: 10.1016/j.ajhg.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–8. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green EK, Hamshere M, Forty L, Gordon-Smith K, Fraser c, Russell E, et al. Replication of bipolar disorder susceptibility alleles and identification of two novel genome-wide significant associations in a new bipolar disorder case-control sample. Mol Psychiatry. 2013;18:1302–7. doi: 10.1038/mp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou L, Bergen SE, Akula N, Song J, Hultman CM, Landén M, et al. Genome-wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder. Hum Mol Genet. 2016;25:3383–94. doi: 10.1093/hmg/ddw181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muhleisen TW, Leber M, Schulze TG, Strohmaier J, Degenhardt F, Treutlein J, et al. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat Commun. 2014;5:3339. doi: 10.1038/ncomms4339. [DOI] [PubMed] [Google Scholar]
- 59.Scott LJ, Muglia P, Kong XQ, Guan W, Flickinger M, Upmanyu R, et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci U S A. 2009;106:7501–6. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–69. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–63. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007:CD005594. doi: 10.1002/14651858.CD005594.pub2. [DOI] [PubMed] [Google Scholar]
- 63.Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cross-Disorder Group of the Psychiatric Genomics Consortium. Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fries GR, Li Q, McAlpin B, Rein T, Walss-Bass C, Soares JC, et al. The role of DNA methylation in the pathophysiology and treatment of bipolar disorder. Neurosci Biobehav Rev. 2016;68:474–88. doi: 10.1016/j.neubiorev.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ludwig B, Dwivedi Y. Dissecting bipolar disorder complexity through epigenomic approach. Mol Psychiatry. 2016;21:1490–8. doi: 10.1038/mp.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaine ME, Seifuddin F, Sabunciyan S, Lee RS, Benke KS, Monson ET, et al. Differentially methylated regions in bipolar disorder and suicide. Am J Med Genet B Neuropsychiatr Genet. 2019;180:496–507. doi: 10.1002/ajmg.b.32754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duffy A, Goodday SM, Keown-Stoneman C, Scotti M, Maitra M, Nagy C, et al. Epigenetic markers in inflammation-related genes associated with mood disorder: a cross-sectional and longitudinal study in high-risk offspring of bipolar parents. Int J Bipolar Disord. 2019;7:17. doi: 10.1186/s40345-019-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fries GR, Quevedo J, Zeni CP, Kazimi IF, Zunta-Soares G, Spiker DE, et al. Integrated transcriptome and methylome analysis in youth at high risk for bipolar disorder: a preliminary analysis. Transl Psychiatry. 2017;7:e1059. doi: 10.1038/tp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fries GR, Vasconcelos-Moreno MP, Gubert C, dos Santos BT, Sartori J, Eisele B, et al. Hypothalamic-pituitary-adrenal axis dysfunction and illness progression in bipolar disorder. Int J Neuropsychopharmacol. 2014;18(1) doi: 10.1093/ijnp/pyu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fries GR, Bauer IE, Scaini G, Wu MJ, Kazimi IF, Valvassori SS, et al. Accelerated epigenetic aging and mitochondrial DNA copy number in bipolar disorder. Transl Psychiatry. 2017;7:1283. doi: 10.1038/s41398-017-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burghardt KJ, Goodrich JM, Dolinoy DC, Ellingrod VL. DNA methylation, insulin resistance and second-generation antipsychotics in bipolar disorder. Epigenomics. 2015;7:343–52. doi: 10.2217/epi.15.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Houtepen LC, van Bergen AH, Vinkers CH, Boks MP. DNA methylation signatures of mood stabilizers and antipsychotics in bipolar disorder. Epigenomics. 2016;8:197–208. doi: 10.2217/epi.15.98. [DOI] [PubMed] [Google Scholar]
- 74.Pisanu C, Katsila T, Patrinos GP, Squassina A. Recent trends on the role of epigenomics, metabolomics and noncoding RNAs in rationalizing mood stabilizing treatment. Pharmacogenomics. 2018;19:129–43. doi: 10.2217/pgs-2017-0111. [DOI] [PubMed] [Google Scholar]
- 75.Schubeler D. Function and information content of DNA methylation. Nature. 2015;517:321–6. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 76.Hess JL, Tylee DS, Barve R, de Jong S, Ophoff RA, Kumarasinghe N, et al. Transcriptomic abnormalities in peripheral blood in bipolar disorder, and discrimination of the major psychoses. Schizophr Res. 2020;217:124–35. doi: 10.1016/j.schres.2019.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee YC, Chao YL, Chang CE, Hsieh MH, Liu KT, Chen HC, et al. Transcriptome changes in relation to manic episode. Front Psychiatry. 2019;10:280. doi: 10.3389/fpsyt.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gamazon ER, Zwinderman AH, Cox NJ, Denys D, Derks EM. Multi-tissue transcriptome analyses identify genetic mechanisms underlying neuropsychiatric traits. Nat Genet. 2019;51:933–40. doi: 10.1038/s41588-019-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362(6420) doi: 10.1126/science.aat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weaver IC. Shaping adult phenotypes through early life environments. Birth Defects Res C Embryo Today. 2009;87:314–26. doi: 10.1002/bdrc.20164. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell C, Schneper LM, Notterman DA. DNA methylation, early life environment, and health outcomes. Pediatr Res. 2016;79:212–9. doi: 10.1038/pr.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rizzo LB, Costa LG, Mansur RB, Swardfager W, Belangero SI, Grassi-Oliveira R, et al. The theory of bipolar disorder as an illness of accelerated aging: implications for clinical care and research. Neurosci Biobehav Rev. 2014;42:157–69. doi: 10.1016/j.neubiorev.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Seelye A, Thuras P, Doane B, Clason C, VanVoorst W, Urosevic S. Steeper aging-related declines in cognitive control processes among adults with bipolar disorders. J Affect Disord. 2019;246:595–602. doi: 10.1016/j.jad.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 85.Huang YC, Wang LJ, Tseng PT, Hung CF, Lin PY. Leukocyte telomere length in patients with bipolar disorder: an updated meta-analysis and subgroup analysis by mood status. Psychiatry Res. 2018;270:41–9. doi: 10.1016/j.psychres.2018.09.035. [DOI] [PubMed] [Google Scholar]
- 86.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fries GR, Bauer IE, Scaini G, Valvassori SS, Walss-Bass c, Soares JC, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Disord. 2019 doi: 10.1111/bdi.12876. doi: http://10.1111/bdi.12876. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Fahy GM, Brooke RT, Watson JP, Good z, Vasanawala SS, Maecker H, et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell. 2019;18:e13028. doi: 10.1111/acel.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen J, Xue Y. Emerging roles of non-coding RNAs in epigenetic regulation. Sci China Life Sci. 2016;59:227–35. doi: 10.1007/s11427-016-5010-0. [DOI] [PubMed] [Google Scholar]
- 90.Fries GR, Carvalho AF, Quevedo J. The miRNome of bipolar disorder. J Affect Disord. 2018;233:110–6. doi: 10.1016/j.jad.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 91.Sayad A, Taheri M, Omrani MD, Fallah H, Oskooei VK, Ghafouri-Fard S. Peripheral expression of long non-coding RNAs in bipolar patients. J Affect Disord. 2019;249:169–74. doi: 10.1016/j.jad.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 92.Fries GR, Quevedo J. Exosomal microRNAs as potential biomarkers in neuropsychiatric disorders. Methods Mol Biol. 2018;1733:79–85. doi: 10.1007/978-1-4939-7601-0_6. [DOI] [PubMed] [Google Scholar]
- 93.Fries GR, Lima CN, Valvassori SS, Zunta-Soares G, Soares JC, Quevedo J. Preliminary investigation of peripheral extracellular vesicles' microRNAs in bipolar disorder. J Affect Disord. 2019;255:10–4. doi: 10.1016/j.jad.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 94.Kalb R. The protean actions of neurotrophins and their receptors on the life and death of neurons. Trends Neurosci. 2005;28:5–11. doi: 10.1016/j.tins.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Frey BN, Andreazza AC, Cereser KM, Martins MR, Valvassori SS, Réus GZ, et al. Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci. 2006;79:281–6. doi: 10.1016/j.lfs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 96.Banasr M, Duman RS. Keeping ‘trk' of antidepressant actions. Neuron. 2008;59:349–51. doi: 10.1016/j.neuron.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 97.Varela RB, Valvassori SS, Lopes-Borges J, Mariot E, Dal-Pont GC, Amboni RT, et al. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of wistar rats. J Psychiatr Res. 2015;61:114–21. doi: 10.1016/j.jpsychires.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 98.Jornada LK, Moretti M, Valvassori SS, Ferreira CL, Padilha PT, Arent CO, et al. Effects of mood stabilizers on hippocampus and amygdala BDNF levels in an animal model of mania induced by ouabain. J Psychiatr Res. 2010;44:506–10. doi: 10.1016/j.jpsychires.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 99.de Oliveira GS, Cereser KM, Fernandes BS, Kauer-Sant'Anna M, Fries GR, Stertz L, et al. Decreased brain-derived neurotrophic factor in medicated and drug-free bipolar patients. J Psychiatr Res. 2009;43:1171–4. doi: 10.1016/j.jpsychires.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 100.Kapczinski F, Dias VV, Frey BN, Kauer-Sant'Anna M. Brain-derived neurotrophic factor in bipolar disorder: beyond trait and state: comment on ‘Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders'. Bipolar Disord. 2009;11:221–2. doi: 10.1111/j.1399-5618.2008.00616.x. author reply 222-3. [DOI] [PubMed] [Google Scholar]
- 101.Soontornniyomkij B, Everall IP, Chana G, Tsuang MT, Achim CL, Soontornniyomkij V. Tyrosine kinase B protein expression is reduced in the cerebellum of patients with bipolar disorder. J Affect Disord. 2011;133:646–54. doi: 10.1016/j.jad.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ray MT, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang TL, Hung YY, Lee CT, Chen RF. Serum protein levels of brain-derived neurotrophic factor and tropomyosin-related kinase B in bipolar disorder: effects of mood stabilizers. Neuropsychobiology. 2012;65:65–9. doi: 10.1159/000328991. [DOI] [PubMed] [Google Scholar]
- 104.Craddock N, O'Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genet. 2005;42:193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barbosa IG, Huguet RB, Neves FS, Reis HJ, Bauer ME, Janka Z, et al. Impaired nerve growth factor homeostasis in patients with bipolar disorder. World J Biol Psychiatry. 2011;12:228–32. doi: 10.3109/15622975.2010.518629. [DOI] [PubMed] [Google Scholar]
- 106.Loch AA, Zanetti MV, de Sousa RT, Chaim TM, Serpa MH, Gattaz WF, et al. Elevated neurotrophin-3 and neurotrophin 4/5 levels in unmedicated bipolar depression and the effects of lithium. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:243–6. doi: 10.1016/j.pnpbp.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 107.Barbosa IG, Morato IB, Huguet RB, Rocha FL, Machado-Vieira R, Teixeira AL. Decreased plasma neurotrophin-4/5 levels in bipolar disorder patients in mania. Braz J Psychiatry. 2014;36:340–3. doi: 10.1590/1516-4446-2014-1380. [DOI] [PubMed] [Google Scholar]
- 108.Valvassori SS, Resende WR, Budni J, Dal-Pont GC, Bavaresco DV, Réus GZ, et al. Sodium butyrate, a histone deacetylase inhibitor, reverses behavioral and mitochondrial alterations in animal models of depression induced by early- or late-life stress. Curr Neurovasc Res. 2015;12:312–20. doi: 10.2174/1567202612666150728121121. [DOI] [PubMed] [Google Scholar]
- 109.Valvassori SS, Mariot E, Varela RB, Bavaresco DV, Dal-Pont GC, Ferreira CL, et al. The role of neurotrophic factors in manic-, anxious- and depressive-like behaviors induced by amphetamine sensitization: Implications to the animal model of bipolar disorder. J Affect Disord. 2019;245:1106–13. doi: 10.1016/j.jad.2018.10.370. [DOI] [PubMed] [Google Scholar]
- 110.Chakrabarty S, Kabekkodu SP, Singh RP, Thangaraj K, Singh KK, Satyamoorthy K. Mitochondria in health and disease. Mitochondrion. 2018;43:25–9. doi: 10.1016/j.mito.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 111.Kadenbach B, Ramzan R, Wen L, Vogt S. New extension of the Mitchell Theory for oxidative phosphorylation in mitochondria of living organisms. Biochim Biophys Acta. 2010;1800:205–12. doi: 10.1016/j.bbagen.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 112.Budd SL, Nicholls DG. Mitochondria in the life and death of neurons. Essays Biochem. 1998;33:43–52. doi: 10.1042/bse0330043. [DOI] [PubMed] [Google Scholar]
- 113.Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–6. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- 114.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–77. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 115.Todorova V, Blokland A. Mitochondria and synaptic plasticity in the mature and aging nervous system. Curr Neuropharmacol. 2017;15:166–73. doi: 10.2174/1570159X14666160414111821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scaini G, Rezin GT, Carvalho AF, Streck EL, Berk M, Quevedo J. Mitochondrial dysfunction in bipolar disorder: evidence, pathophysiology and translational implications. Neurosci Biobehav Rev. 2016;68:694–713. doi: 10.1016/j.neubiorev.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 117.Clausen T, Zauner A, Levasseur JE, Rice AC, Bullock R. Induced mitochondrial failure in the feline brain: implications for understanding acute post-traumatic metabolic events. Brain Res. 2001;908:35–48. doi: 10.1016/s0006-8993(01)02566-5. [DOI] [PubMed] [Google Scholar]
- 118.Rudkin TM, Arnold DL. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol. 1999;56:919–26. doi: 10.1001/archneur.56.8.919. [DOI] [PubMed] [Google Scholar]
- 119.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–8. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 120.Weber WA, Dudley J, Lee JH, Strakowski SM, Adler CM, DelBello MP. A pilot study of alterations in high energy phosphoryl compounds and intracellular pH in unmedicated adolescents with bipolar disorder. J Affect Disord. 2013;150:1109–13. doi: 10.1016/j.jad.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 121.Silver IA, Deas J, Erecinska M. Ion homeostasis in brain cells: differences in intracellular ion responses to energy limitation between cultured neurons and glial cells. Neuroscience. 1997;78:589–601. doi: 10.1016/s0306-4522(96)00600-8. [DOI] [PubMed] [Google Scholar]
- 122.el-Mallakh RS, Wyatt RJ. The Na,K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37:235–44. doi: 10.1016/0006-3223(94)00201-D. [DOI] [PubMed] [Google Scholar]
- 123.Kato T, Shioiri T, Murashita J, Hamakawa H, Inubushi T, Takahashi S. Phosphorus-31 magnetic resonance spectroscopy and ventricular enlargement in bipolar disorder. Psychiatry Res. 1994;55:41–50. doi: 10.1016/0925-4927(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 124.Modica-Napolitano JS, Lagace CJ, Brennan WA, Aprille JR. Differential effects of typical and atypical neuroleptics on mitochondrial function in vitro. Arch Pharm Res. 2003;26:951–9. doi: 10.1007/BF02980205. [DOI] [PubMed] [Google Scholar]
- 125.Wyss M, Schulze A. Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience. 2002;112:243–60. doi: 10.1016/s0306-4522(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 126.Chang K, Adleman N, Dienes K, Barnea-Goraly N, Reiss A, Ketter T. Decreased N-acetylaspartate in children with familial bipolar disorder. Biol Psychiatry. 2003;53:1059–65. doi: 10.1016/s0006-3223(02)01744-4. [DOI] [PubMed] [Google Scholar]
- 127.Deicken RF, Pegues MP, Anzalone S, Feiwell R, Soher B. Lower concentration of hippocampal N-acetylaspartate in familial bipolar I disorder. Am J Psychiatry. 2003;160:873–82. doi: 10.1176/appi.ajp.160.5.873. [DOI] [PubMed] [Google Scholar]
- 128.Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biol Psychiatry. 2000;47:475–81. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- 129.Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–6. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- 130.Valvassori SS, Bavaresco DV, Feier G, Cechinel-Recco K, Steckert AV, Varela RB, et al. Increased oxidative stress in the mitochondria isolated from lymphocytes of bipolar disorder patients during depressive episodes. Psychiatry Res. 2018;264:192–201. doi: 10.1016/j.psychres.2018.03.089. [DOI] [PubMed] [Google Scholar]
- 131.Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–8. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- 132.Gubert C, Stertz L, Pfaffenseller B, Panizzutti BS, Rezin GT, Massuda R, et al. Mitochondrial activity and oxidative stress markers in peripheral blood mononuclear cells of patients with bipolar disorder, schizophrenia, and healthy subjects. J Psychiatr Res. 2013;47:1396–402. doi: 10.1016/j.jpsychires.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 133.Ben-Shachar D, Zuk R, Gazawi H, Reshef A, Sheinkman A, Klein E. Increased mitochondrial complex I activity in platelets of schizophrenic patients. Int J Neuropsychopharmacol. 1999;2:245–53. doi: 10.1017/S1461145799001649. [DOI] [PubMed] [Google Scholar]
- 134.Correa C, Amboni G, Assis LC, Martins MR, Kapczinski F, Streck EL, et al. Effects of lithium and valproate on hippocampus citrate synthase activity in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:887–91. doi: 10.1016/j.pnpbp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 135.Feier G, Valvassori SS, Varela RB, Resende WR, Bavaresco DV, Morais MO, et al. Lithium and valproate modulate energy metabolism in an animal model of mania induced by methamphetamine. Pharmacol Biochem Behav. 2013;103:589–96. doi: 10.1016/j.pbb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 136.Rezin GT, Furlanetto CB, Scaini G, Valvassori SS, Gonçalves CL, Ferreira GK, et al. Fenproporex increases locomotor activity and alters energy metabolism, and mood stabilizers reverse these changes: a proposal for a new animal model of mania. Mol Neurobiol. 2014;49:877–92. doi: 10.1007/s12035-013-8566-8. [DOI] [PubMed] [Google Scholar]
- 137.Streck EL, Amboni G, Scaini G, Di-Pietro PB, Rezin GT, Valvassori SS, et al. Brain creatine kinase activity in an animal model of mania. Life Sci. 2008;82:424–9. doi: 10.1016/j.lfs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 138.Valvassori SS, Rezin GT, Ferreira CL, Moretti M, Gonçalves CL, Cardoso MR, et al. Effects of mood stabilizers on mitochondrial respiratory chain activity in brain of rats treated with d-amphetamine. J Psychiatr Res. 2010;44:903–9. doi: 10.1016/j.jpsychires.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 139.Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–8. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 140.Iwamoto K, Kakiuchi C, Bundo M, Ikeda K, Kato T. Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders. Mol Psychiatry. 2004;9:406–16. doi: 10.1038/sj.mp.4001437. [DOI] [PubMed] [Google Scholar]
- 141.MacDonald ML, Naydenov A, Chu M, Matzilevich D, Konradi C. Decrease in creatine kinase messenger RNA expression in the hippocampus and dorsolateral prefrontal cortex in bipolar disorder. Bipolar Disord. 2006;8:255–64. doi: 10.1111/j.1399-5618.2006.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun X, Wang JF, Tseng M, Young LT. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31:189–96. [PMC free article] [PubMed] [Google Scholar]
- 143.Yoshimi N, Futamura T, Bergen SE, Iwayama Y, Ishima T, Sellgren C, et al. Cerebrospinal fluid metabolomics identifies a key role of isocitrate dehydrogenase in bipolar disorder: evidence in support of mitochondrial dysfunction hypothesis. Mol Psychiatry. 2016;21:1504–10. doi: 10.1038/mp.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kornmann B. Quality control in mitochondria: use it, break it, fix it, trash it. F1000Prime Rep. 2014;6:15. doi: 10.12703/P6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Z, Okamoto KI, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 146.Cataldo AM, McPhie DL, Lange NT, Punzell S, Elmiligy S, Ye NZ, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol. 2010;177:575–85. doi: 10.2353/ajpath.2010.081068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scaini G, Barichello T, Fries GR, Kennon EA, Andrews T, Nix BR, et al. TSPO upregulation in bipolar disorder and concomitant downregulation of mitophagic proteins and NLRP3 inflammasome activation. Neuropsychopharmacology. 2019;44:1291–9. doi: 10.1038/s41386-018-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scaini G, Fries GR, Valvassori SS, Zeni CP, Zunta-Soares G, Berk M, et al. Perturbations in the apoptotic pathway and mitochondrial network dynamics in peripheral blood mononuclear cells from bipolar disorder patients. Transl Psychiatry. 2017;7:e1111. doi: 10.1038/tp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–8. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 150.Andreazza AC, Kauer-Sant'anna M, Frey BN, Bond DJ, Kapczinski F, Trevor Young L, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–44. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 151.Das TK, Javadzadeh A, Dey A, Sabesan P, Théberge J, Radua J, et al. Antioxidant defense in schizophrenia and bipolar disorder: A meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:94–102. doi: 10.1016/j.pnpbp.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 152.de Sousa RT, Uno M, Zanetti MV, Shinjo SM, Busatto GF, Gattaz WF, et al. Leukocyte mitochondrial DNA copy number in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:32–5. doi: 10.1016/j.pnpbp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 153.Tsujii N, Otsuka I, Okazaki S, Yanagi M, Numata S, Yamaki N, et al. Mitochondrial DNA copy number raises the potential of left frontopolar hemodynamic response as a diagnostic marker for distinguishing bipolar disorder from major depressive disorder. Front Psychiatry. 2019;10:312. doi: 10.3389/fpsyt.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang D, Li Z, Liu W, Zhou J, Ma X, Tang J, et al. Differential mitochondrial DNA copy number in three mood states of bipolar disorder. BMC Psychiatry. 2018;18:149. doi: 10.1186/s12888-018-1717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamaki N, Otsuka I, Numata S, Yanagi M, Mouri K, Okazaki S, et al. Mitochondrial DNA copy number of peripheral blood in bipolar disorder: the present study and a meta-analysis. Psychiatry Res. 2018;269:115–7. doi: 10.1016/j.psychres.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 156.Ceylan D, Tuna G, Kirkali G, Tunca z, Can G, Arat HE, et al. Oxidatively-induced DNA damage and base excision repair in euthymic patients with bipolar disorder. DNA Repair (Amst) 2018;65:64–72. doi: 10.1016/j.dnarep.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Munkholm K, Peijs L, Vinberg M, Kessing LV. A composite peripheral blood gene expression measure as a potential diagnostic biomarker in bipolar disorder. Transl Psychiatry. 2015;5:e614. doi: 10.1038/tp.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barbosa IG, Bauer ME, Machado-Vieira R, Teixeira AL. Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plast. 2014;2014:360481. doi: 10.1155/2014/360481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rosenblat JD, McIntyre RS. Bipolar disorder and immune dysfunction: epidemiological findings, proposed pathophysiology and clinical implications. Brain Sci. 2017;7(11) doi: 10.3390/brainsci7110144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sayana P, Colpo GD, Simoes LR, Giridharan VV, Teixeira AL, Quevedo J, et al. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res. 2017;92:160–82. doi: 10.1016/j.jpsychires.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 161.Tonin PT, Valvassori SS, Lopes-Borges J, Mariot E, Varela RB, Teixeira AL, et al. Effects of ouabain on cytokine/chemokine levels in an animal model of mania. J Neuroimmunol. 2014;276:236–9. doi: 10.1016/j.jneuroim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 162.Valvassori SS, Dal-Pont GC, Tonin PT, Varela RB, Ferreira CL, Gava FF, et al. Coadministration of lithium and celecoxib attenuates the behavioral alterations and inflammatory processes induced by amphetamine in an animal model of mania. Pharmacol Biochem Behav. 2019;183:56–63. doi: 10.1016/j.pbb.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 163.Valvassori SS, Resende WR, Dal-Pont G, Sangaletti-Pereira H, Gava FF, Peterle BR, et al. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord. 2017;19:246–58. doi: 10.1111/bdi.12503. [DOI] [PubMed] [Google Scholar]
- 164.Valvassori SS, Tonin PT, Varela RB, Carvalho AF, Mariot E, Amboni RT, et al. Lithium modulates the production of peripheral and cerebral cytokines in an animal model of mania induced by dextroamphetamine. Bipolar Disord. 2015;17:507–17. doi: 10.1111/bdi.12299. [DOI] [PubMed] [Google Scholar]
- 165.Magioncalda P, Martino M, Tardito S, Sterlini B, Conio B, Marozzi V, et al. White matter microstructure alterations correlate with terminally differentiated CD8+ effector T cell depletion in the peripheral blood in mania: combined DTI and immunological investigation in the different phases of bipolar disorder. Brain Behav Immun. 2018;73:192–204. doi: 10.1016/j.bbi.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 166.Barbosa IG, Rocha NP, Huguet RB, Ferreira RA, Salgado JV, Carvalho LA, et al. Executive dysfunction in euthymic bipolar disorder patients and its association with plasma biomarkers. J Affect Disord. 2012;137:151–5. doi: 10.1016/j.jad.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 167.Hope S, Hoseth E, Dieset I, Mørch RH, Aas M, Aukrust P, et al. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr Res. 2015;165:188–94. doi: 10.1016/j.schres.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 168.Bauer IE, Pascoe MC, Wollenhaupt-Aguiar B, Kapczinski F, Soares JC. Inflammatory mediators of cognitive impairment in bipolar disorder. J Psychiatr Res. 2014;56:18–27. doi: 10.1016/j.jpsychires.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rosenblat JD, Brietzke E, Mansur RB, Maruschak NA, Lee Y, McIntyre RS. Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: Evidence, pathophysiology and treatment implications. J Affect Disord. 2015;188:149–59. doi: 10.1016/j.jad.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 170.Giridharan VV, Sayana P, Pinjari OF, Ahmad N, da Rosa MI, Quevedo J, et al. Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol Psychiatry. 2020;25:94–113. doi: 10.1038/s41380-019-0448-7. [DOI] [PubMed] [Google Scholar]
- 171.Fries GR, Walss-Bass C, Bauer ME, Teixeira AL. Revisiting inflammation in bipolar disorder. Pharmacol Biochem Behav. 2019;177:12–9. doi: 10.1016/j.pbb.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 172.van den Ameele S, van Nuijs AL, Lai FY, Schuermans J, Verkerk R, van Diermen L, et al. A mood state-specific interaction between kynurenine metabolism and inflammation is present in bipolar disorder. Bipolar Disord. 2020;22:59–69. doi: 10.1111/bdi.12814. [DOI] [PubMed] [Google Scholar]
- 173.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Tumour necrosis factor-alpha and lipopolysaccharide enhance interferon-induced tryptophan degradation and pteridine synthesis in human cells. Biol Chem Hoppe Seyler. 1989;370:1063–9. doi: 10.1515/bchm3.1989.370.2.1063. [DOI] [PubMed] [Google Scholar]
- 174.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Birner A, Platzer M, Bengesser SA, Dalkner N, Fellendorf FT, Queissner R, et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PloS One. 2017;12:e0172699. doi: 10.1371/journal.pone.0172699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Olsson SK, Sellgren C, Engberg G, Landen M, Erhardt S. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar Disord. 2012;14:719–26. doi: 10.1111/bdi.12009. [DOI] [PubMed] [Google Scholar]
- 177.Sellgren CM, Gracias J, Jungholm O, Perlis RH, Engberg G, Schwieler L, et al. Peripheral and central levels of kynurenic acid in bipolar disorder subjects and healthy controls. Transl Psychiatry. 2019;9:37. doi: 10.1038/s41398-019-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J, et al. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology. 2015;52:200–11. doi: 10.1016/j.psyneuen.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gershon A, Johnson SL, Miller I. Chronic stressors and trauma: prospective influences on the course of bipolar disorder. Psychol Med. 2013;43:2583–92. doi: 10.1017/S0033291713000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin North Am. 2005;28:469–80. doi: 10.1016/j.psc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 181.Weiss RB, Stange JP, Boland EM, Black SK, LaBelle DR, Abramson LY, et al. Kindling of life stress in bipolar disorder: comparison of sensitization and autonomy models. J Abnorm Psychol. 2015;124:4–16. doi: 10.1037/abn0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Murri MB, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–42. doi: 10.1016/j.psyneuen.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 183.Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18:692–9. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- 184.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–26. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 185.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65:209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 186.Lupien SJ, Nair NP, Briere S, Maheu F, Tu MT, Lemay M, et al. Increased cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev Neurosci. 1999;10:117–39. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- 187.Lee HH, Chang CH, Wang LJ, Wu CC, Chen HL, Lu T, et al. The correlation between longitudinal changes in hypothalamic-pituitary-adrenal (HPA)-axis activity and changes in neurocognitive function in mixed-state bipolar II disorder. Neuropsychiatr Dis Treat. 2018;14:2703–13. doi: 10.2147/NDT.S173616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Takahashi T, Malhi GS, Wood SJ, Walterfang M, Yücel M, Lorenzetti V, et al. Increased pituitary volume in patients with established bipolar affective disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1245–9. doi: 10.1016/j.pnpbp.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 189.Deshauer D, Grof E, Alda M, Grof P. Patterns of DST positivity in remitted affective disorders. Biol Psychiatry. 1999;45):1023–9. doi: 10.1016/s0006-3223(98)00334-5. [DOI] [PubMed] [Google Scholar]
- 190.Ellenbogen MA, Hodgins S, Walker CD, Couture S, Adam S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrinology. 2006;31:1164–80. doi: 10.1016/j.psyneuen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 191.Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–7. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- 192.Steinan MK, Scott J, Lagerberg TV, Melle I, Andreassen OA, Vaaler AE, et al. Sleep problems in bipolar disorders: more than just insomnia. Acta Psychiatr Scand. 2016;133:368–77. doi: 10.1111/acps.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165:830–43. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- 194.Bradley AJ, Webb-Mitchell R, Hazu A, Slater N, Middleton B, Gallagher P, et al. Sleep and circadian rhythm disturbance in bipolar disorder. Psychol Med. 2017;47:1678–89. doi: 10.1017/S0033291717000186. [DOI] [PubMed] [Google Scholar]
- 195.Scott J. Clinical parameters of circadian rhythms in affective disorders. Eur Neuropsychopharmacol. 2011;21(Suppl 4):S671–5. doi: 10.1016/j.euroneuro.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 196.Milhiet V, Boudebesse C, Bellivier F, Drouot X, Henry C, Leboyer M, et al. Circadian abnormalities as markers of susceptibility in bipolar disorders. Front Biosci (Schol Ed) 2014;6:120–37. doi: 10.2741/s419. [DOI] [PubMed] [Google Scholar]
- 197.Ng TH, Chung KF, Ho FY, Yeung WF, Yung KP, Lam TH. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep Med Rev. 2015;20:46–58. doi: 10.1016/j.smrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 198.Ng TH, Chung KF, Ng TK, Lee CT, Chan MS. Correlates and prognostic relevance of sleep irregularity in inter-episode bipolar disorder. Compr Psychiatry. 2016;69:155–62. doi: 10.1016/j.comppsych.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 199.Faedda GL, Ohashi K, Hernandez M, McGreenery CE, Grant MC, Baroni A, et al. Actigraph measures discriminate pediatric bipolar disorder from attention-deficit/hyperactivity disorder and typically developing controls. J Child Psychol Psychiatry. 2016;57:706–16. doi: 10.1111/jcpp.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chung JK, Lee KY, Kim SH, Kim EJ, Jeong SH, Jung HY, et al. Circadian rhythm characteristics in mood disorders: comparison among bipolar I disorder, bipolar II disorder and recurrent major depressive disorder. Clin Psychopharmacol Neurosci. 2012;10:110–6. doi: 10.9758/cpn.2012.10.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Boudebesse C, Lajnef M, Geoffroy PA, Bellivier F, Nieto I, Gard S, et al. Chronotypes of bipolar patients in remission: validation of the French version of the circadian type inventory in the FACE-BD sample. Chronobiol Int. 2013;30:1042–9. doi: 10.3109/07420528.2013.798330. [DOI] [PubMed] [Google Scholar]
- 202.Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, Kupfer DJ, et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22:571–84. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- 203.Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disord. 2008;10:271–5. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 204.Wood J, Birmaher B, Axelson D, Ehmann M, Kalas C, Monk K, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009;166:201–9. doi: 10.1016/j.psychres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mondin TC, de Azevedo Cardoso T, Moreira FP, Wiener C, Oses JP, Souza LD, et al. Circadian preferences, oxidative stress and inflammatory cytokines in bipolar disorder: a community study. J Neuroimmunol. 2016;301:23–9. doi: 10.1016/j.jneuroim.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 206.Ottoni GL, Antoniolli E, Lara DR. Circadian preference is associated with emotional and affective temperaments. Chronobiol Int. 2012;29:786–93. doi: 10.3109/07420528.2012.679329. [DOI] [PubMed] [Google Scholar]
- 207.Novakova M, Prasko J, Latalova K, Sladek M, Sumova A. The circadian system of patients with bipolar disorder differs in episodes of mania and depression. Bipolar Disord. 2015;17:303–14. doi: 10.1111/bdi.12270. [DOI] [PubMed] [Google Scholar]
- 208.Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TK, Hermens DF, et al. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: comparison of unipolar and bipolar phenotypes. Eur Psychiatry. 2013;28:412–6. doi: 10.1016/j.eurpsy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 209.Dallaspezia S, Benedetti F. Melatonin, circadian rhythms, and the clock genes in bipolar disorder. Curr Psychiatry Rep. 2009;11:488–93. doi: 10.1007/s11920-009-0074-1. [DOI] [PubMed] [Google Scholar]
- 210.Deshauer D, Duffy A, Alda M, Grof E, Albuquerque J, Grof P. The cortisol awakening response in bipolar illness: a pilot study. Can J Psychiatry. 2003;48:462–6. doi: 10.1177/070674370304800706. [DOI] [PubMed] [Google Scholar]
- 211.Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJ. Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28:121–37. doi: 10.1016/s0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 212.Lee KY, Song JY, Kim SH, Kim SC, Joo EJ, Ahn YM, et al. Association between CLOCK 3111T/C and preferred circadian phase in Korean patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1196–201. doi: 10.1016/j.pnpbp.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 213.Shi J, Wittke-Thompson JK, Badner JA, Hattori E, Potash JB, Willour VL, et al. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1047–55. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Dessa Sadovnick A, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:234–41. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, et al. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:23–6. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- 216.Michelon L, Meira-Lima I, Cordeiro Q, Miguita K, Breen G, Collier D, et al. Association study of the INPP1, 5HTT, BDNF, AP-2beta and GSK-3beta GENE variants and restrospectively scored response to lithium prophylaxis in bipolar disorder. Neurosci Lett. 2006;403:288–93. doi: 10.1016/j.neulet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 217.McCarthy MJ, Nievergelt CM, Shekhtman T, Kripke DF, Welsh DK, Kelsoe JR. Functional genetic variation in the Rev-Erbα pathway and lithium response in the treatment of bipolar disorder. Genes Brain Behav. 2011;10:852–61. doi: 10.1111/j.1601-183X.2011.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rybakowski JK, Dmitrzak-Weglar M, Kliwicki S, Hauser J. Polymorphism of circadian clock genes and prophylactic lithium response. Bipolar Disord. 2014;16:151–8. doi: 10.1111/bdi.12136. [DOI] [PubMed] [Google Scholar]
- 219.Lai YC, Kao CF, Lu ML, Chen HC, Chen PY, Chen CH, et al. Investigation of associations between NR1D1, RORA and RORB genes and bipolar disorder. PloS One. 2015;10:e0121245. doi: 10.1371/journal.pone.0121245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee KY, Ahn YM, Joo EJ, Jeong SH, Chang JS, Kim SC, et al. No association of two common SNPs at position -1727 A/T, -50 C/T of GSK-3 beta polymorphisms with schizophrenia and bipolar disorder of Korean population. Neurosci Lett. 2006;395:175–8. doi: 10.1016/j.neulet.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 222.Lachman HM, Pedrosa E, Petruolo OA, Cockerham M, Papolos A, Novak T, et al. Increase in GSK3beta gene copy number variation in bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:259–65. doi: 10.1002/ajmg.b.30498. [DOI] [PubMed] [Google Scholar]
- 223.Benedetti F, Serretti A, Colombo C, Lorenzi C, Tubazio V, Smeraldi E. A glycogen synthase kinase 3-β promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci Lett. 2004;368:123–6. doi: 10.1016/j.neulet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 224.Benedetti F, Bernasconi A, Lorenzi C, Pontiggia A, Serretti A, Colombo C, et al. A single nucleotide polymorphism in glycogen synthase kinase 3-β promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci Lett. 2004;355:37–40. doi: 10.1016/j.neulet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 225.Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci Lett. 2008;445:184–7. doi: 10.1016/j.neulet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 226.Severino G, Manchia M, Contu P, Squassina A, Lampus S, Ardau R, et al. Association study in a Sardinian sample between bipolar disorder and the nuclear receptor REV-ERBalpha gene, a critical component of the circadian clock system. Bipolar Disord. 2009;11:215–20. doi: 10.1111/j.1399-5618.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 227.Coyle JT. What can a clock mutation in mice tell us about bipolar disorder? Proc Natl Acad Sci U S A. 2007;104:6097–8. doi: 10.1073/pnas.0701491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–9. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 229.van Enkhuizen J, Minassian A, Young JW. Further evidence for ClockDelta19 mice as a model for bipolar disorder mania using cross-species tests of exploration and sensorimotor gating. Behav Brain Res. 2013;249:44–54. doi: 10.1016/j.bbr.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pearlson GD, Veroff AE. Computerised tomographic scan changes in manic-depressive illness. Lancet. 1981;2:470. doi: 10.1016/s0140-6736(81)90798-4. [DOI] [PubMed] [Google Scholar]
- 233.Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 234.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CR, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23:932–42. doi: 10.1038/mp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ho NF, Chong PL, Lee DR, Chew QH, Chen G, Sim K. The amygdala in schizophrenia and bipolar disorder: a synthesis of structural MRI, diffusion tensor imaging, and resting-state functional connectivity findings. Harv Rev Psychiatry. 2019;27:150–64. doi: 10.1097/HRP.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 236.Niida R, Yamagata B, Matsuda H, Niida A, Uechi A, Kito S, et al. Regional brain volume reductions in major depressive disorder and bipolar disorder: an analysis by voxel-based morphometry. Int J Geriatr Psychiatry. 2019;34:186–92. doi: 10.1002/gps.5009. [DOI] [PubMed] [Google Scholar]
- 237.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170:533–41. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ong D, Walterfang M, Malhi GS, Styner M, Velakoulis D, Pantelis C. Size and shape of the caudate nucleus in individuals with bipolar affective disorder. Aust N Z J Psychiatry. 2012;46:340–51. doi: 10.1177/0004867412440191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mwangi B, Wu MJ, Cao B, Passos IC, Lavagnino L, Keser Z, et al. Individualized prediction and clinical staging of bipolar disorders using neuroanatomical biomarkers. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:186–94. doi: 10.1016/j.bpsc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Librenza-Garcia D, Kotzian BJ, Yang J, Mwangi B, Cao B, Lima LN, et al. The impact of machine learning techniques in the study of bipolar disorder: a systematic review. Neurosci Biobehav Rev. 2017;80:538–54. doi: 10.1016/j.neubiorev.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 241.Fung G, Deng Y, Zhao Q, Li Z, Qu m, Li K, et al. Distinguishing bipolar and major depressive disorders by brain structural morphometry: a pilot study. BMC Psychiatry. 2015;15:298. doi: 10.1186/s12888-015-0685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Passos IC, Ballester PL, Barros RC, Librenza-Garcia D, Mwangi B, Birmaher B, et al. Machine learning and big data analytics in bipolar disorder: a position paper from the International Society for Bipolar Disorders Big Data Task Force. Bipolar Disord. 2019;21:582–94. doi: 10.1111/bdi.12828. [DOI] [PubMed] [Google Scholar]
- 243.Insel TR, Cuthbert BN. Medicine. Brain disorders? Precisely. Science. 2015;348:499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- 244.Deisseroth K. Optogenetics and psychiatry: applications, challenges, and opportunities. Biol Psychiatry. 2012;71:1030–2. doi: 10.1016/j.biopsych.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 245.Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Duarte JA, de Araujo E Silva JQ, Goldani AA, Massuda R, Gama CS. Neurobiological underpinnings of bipolar disorder focusing on findings of diffusion tensor imaging: a systematic review. Braz J Psychiatry. 2016;38:167–75. doi: 10.1590/1516-4446-2015-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abé C, et al. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology. 2019;44:2285–93. doi: 10.1038/s41386-019-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Foley SF, Bracher-Smith M, Tansey KE, Harrison JR, Parker GD, Caseras X. Fractional anisotropy of the uncinate fasciculus and cingulum in bipolar disorder type I, type II, unaffected siblings and healthy controls. Br J Psychiatry. 2018;213:48–54. doi: 10.1192/bjp.2018.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mahon K, Burdick KE, Ikuta T, Braga RJ, Gruner P, Malhotra AK, et al. Abnormal temporal lobe white matter as a biomarker for genetic risk of bipolar disorder. Biol Psychiatry. 2013;73:177–82. doi: 10.1016/j.biopsych.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Smucny J, Lesh TA, Newton K, Niendam TA, Ragland JD, Carter CS. Levels of cognitive control: a functional magnetic resonance imaging-based test of an RDoC domain across bipolar disorder and schizophrenia. Neuropsychopharmacology. 2018;43:598–606. doi: 10.1038/npp.2017.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–43. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Korgaonkar MS, Erlinger M, Breukelaar IA, Boyce P, Hazell P, Antees C, et al. Amygdala activation and connectivity to emotional processing distinguishes asymptomatic patients with bipolar disorders and unipolar depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:361–70. doi: 10.1016/j.bpsc.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 253.Zhang L, Opmeer EM, van der Meer L, Aleman A, Curcic-Blake B, Ruhe HG. Altered frontal-amygdala effective connectivity during effortful emotion regulation in bipolar disorder. Bipolar Disord. 2018;20:349–58. doi: 10.1111/bdi.12611. [DOI] [PubMed] [Google Scholar]
- 254.Nusslock R, Alloy LB. Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J Affect Disord. 2017;216:3–16. doi: 10.1016/j.jad.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Acuff HE, Versace A, Bertocci MA, Ladouceur CD, Hanford LC, Manelis A, et al. Baseline and follow-up activity and functional connectivity in reward neural circuitries in offspring at risk for bipolar disorder. Neuropsychopharmacology. 2019;44:1570–8. doi: 10.1038/s41386-019-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Webb CA, Olson EA, Killgore WD, Pizzagalli DA, Rauch SL, Rosso IM. Rostral anterior cingulate cortex morphology predicts treatment response to internet-based cognitive behavioral therapy for depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:255–62. doi: 10.1016/j.bpsc.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bellivier F, Geoffroy PA, Scott J, Schurhoff F, Leboyer M, Etain B. Biomarkers of bipolar disorder: specific or shared with schizophrenia? Front Biosci (Elite Ed) 2013;5:845–63. doi: 10.2741/e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Neale BM, Sklar P. Genetic analysis of schizophrenia and bipolar disorder reveals polygenicity but also suggests new directions for molecular interrogation. Curr Opin Neurobiol. 2015;30:131–8. doi: 10.1016/j.conb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 259.Cardno AG, Owen MJ. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr Bull. 2014;40:504–15. doi: 10.1093/schbul/sbu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Machado-Vieira R. Tracking the impact of translational research in psychiatry: state of the art and perspectives. J Transl Med. 2012;10:175. doi: 10.1186/1479-5876-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tost H, Champagne FA, Meyer-Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci. 2015;18:1421–31. doi: 10.1038/nn.4108. [DOI] [PubMed] [Google Scholar]