Abstract
Background
The global push for the use of hydroxychloroquine (HCQ) and chloroquine (CQ) against COVID-19 has resulted in an ongoing discussion about the effectivity and toxicity of these drugs. Recent studies report no effect of (H)CQ on 28-day mortality. We investigated the effect of HCQ and CQ in hospitalized patients on the non-ICU COVID-ward.
Methods
A nationwide, observational cohort study was performed in The Netherlands. Hospitals were given the opportunity to decide independently on the use of three different COVID-19 treatment strategies: HCQ, CQ, or no treatment. We compared the outcomes between these groups. The primary outcomes were 1) death on the COVID-19 ward, and 2) transfer to the intensive care unit (ICU).
Results
The analysis included 1064 patients from 14 hospitals: 566 patients received treatment with either HCQ (n = 189) or CQ (n = 377), and 498 patients received no treatment. In a multivariate propensity-matched weighted competing regression analysis, there was no significant effect of (H)CQ on mortality on the COVID ward. However, HCQ was associated with a significantly decreased risk of transfer to the ICU (hazard ratio (HR) = 0.47, 95% CI = 0.27–0.82, p = 0.008) when compared with controls. This effect was not found in the CQ group (HR = 0.80, 95% CI = 0.55–1.15, p = 0.207), and remained significant after competing risk analysis.
Conclusion
The results of this observational study demonstrate a lack of effect of (H)CQ on non-ICU mortality. However, we show that the use of HCQ — but not CQ — is associated with a 53% reduction in risk of transfer of COVID-19 patients from the regular ward to the ICU. Recent prospective studies have reported on 28-day, all-cause mortality only; therefore, additional prospective data on the early effects of HCQ in preventing transfer to the ICU are still needed.
Abbreviations: HCQ, hydroxychloroquine; CQ, chloroquine; AZM, azithromycin; ICU, intensive care unit; ED, emergency department
Keywords: COVID-19, Hydroxychloroquine, Chloroquine, Azithromycin, Clinical course
Introduction
After the emergence of SARS-CoV-2 in December 2019, the new coronavirus spread around the world, resulting in a pandemic. Unfortunately, there is still no proven effective drug or vaccine available against COVID-19, and hospitalized patients with COVID-19 are at high risk for admission to the ICU (10–20%), with 3–10% of patients requiring intubation, and 2–5% of patients dying (Guan et al., 2020a).
Among the drug candidates for treating COVID-19 are hydroxychloroquine (HCQ) and chloroquine (CQ) (Sanders et al., 2020). Insights into the underlying mechanisms of action of HCQ and CQ are still emerging. Both drugs have a large volume of distribution (Zhou et al., 2020, Schrezenmeier and Dörner, 2020). Their molecular structures are comparable, except that HCQ has an extra hydroxyl group. Both interfere with lysosomal activity and decrease membrane stability, reduce signaling pathways for Toll-like-receptors 7 and 9, and impact on transcriptional activity, inhibiting cytokine production (Schrezenmeier and Dörner, 2020).
There are only a few differences between the drugs, of which the most important is drug clearance (Schrezenmeier and Dörner, 2020).
Some observational studies on the efficacy of (H)CQ report clinical benefits and antiviral effects (Gao et al., 2020, Gautret et al., 2020, Arshad et al., 2020, Cortegiani et al., 2020), while others do not (Geleris et al., 2020, Mahevas et al., 2020). A few small, controlled trials have been inconclusive (Tang et al., 2020, Chen et al., 2020). The Recovery study included 176 UK hospitals, comprising 1395 patients receiving high doses of HCQ (9200 mg cumulative dose), and reported no beneficial effects on all-cause mortality at 28 days (26.8% of treated patients versus 25% of controls) (Horby, 2020). The risk of admission to the ICU could not be calculated, since 17–60% of patients were already on (non-invasive) ventilation at randomization. A recent systematic review and meta-analysis, including 11 932 patients on HCQ, found that its use was not associated with reduced mortality (pooled relative risk of RCTs for HCQ use of 1.09) (Fiolet et al., 2020).
Results of other prospective trials are not expected, since the European Discovery and the WHO Solidarity trials have discontinued their HCQ treatment arms because of lack of effect on mortality. Meanwhile, the US FDA and the Infectious Diseases Society of America (IDSA) advise against the use of (H)CQ outside the context of a clinical trial (Swank and McCarten, 2020, Infectious Diseases Society of America Guidelines, 2020).
Based on the available evidence present at the start of the outbreak, a Dutch treatment guideline was developed (RIVM, 2020). Off-label use of both HCQ and CQ was offered as a treatment option; however, the guidelines did not endorse either treatment in particular. Consequently, hospitals decided independently on a treatment protocol with either HCQ or CQ, or to give no treatment. This policy created a unique situation for comparing the efficacy of HCQ and CQ with no treatment in hospitalized non-ICU patients, with a reduction of potential bias by indication.
Methods
Study design
The study was designed as an observational, multicenter, cohort study of hospitalized COVID-19 patients. Before the first patients were admitted, Dutch hospitals independently implemented a treatment protocol with or without (H)CQ. As a consequence, Dutch patients were geographically allocated to their local hospital with or without the intention to treat with (H)CQ. Eligible patients were included retrospectively over the period from February 28 to April 1, 2020. Patients were followed up until they reached one of the clinical endpoints: (1) discharge for cured infection to home or rehabilitation center; (2) transfer from the COVID ward to the intensive care unit (ICU); or (3) death, either during their hospital stay on the ward (non-ICU) or following transfer to a hospice facility. Secondary outcomes were the effects of the use of azithromycin (AZM) and angiotensin-receptor blockers (ARB) on outcome.
Participating hospitals
All hospitals in The Netherlands were considered eligible to participate in the study, including academic hospitals as well as non-teaching hospitals. These hospitals were asked to participate early in the outbreak. All participating hospitals shared their data with the coordinating hospital (Isala, Zwolle), where the statistical analysis was performed. Data-sharing agreements were signed, and the Medical Ethics Review Committee (METC) of Isala approved a waiver for informed consent.
Patients
Inclusion and exclusion criteria were designed to select a study sample of hospitalized patients with moderate to severe COVID-19. New confirmed COVID-19 cases were included if they were aged > 18 years and if they were admitted to the emergency department (ED) and subsequently hospitalized on the non-ICU hospital COVID-19 ward. Exclusion criteria were age < 18 years, admission to the ICU, or death within 24 h after presentation at the ED. Patients transferred between Dutch hospitals, for example due to capacity issues, were also excluded. Confirmed COVID-19 infection was defined as either positive SARS-CoV-2 real-time reverse transcriptase polymerase chain reaction (PCR) on swab material, sputum, or bronchoalveolar lavage samples (Corman et al., 2020), or typical findings on chest computed tomography (CT). Typical CT findings were defined as CO-RAD 4–5, using the CO-RAD classification system (COVID-19 Reporting and Data System, developed by the Dutch Radiology Society to describe levels of suspicion for COVID-19 infection) (Prokop et al., 2020). Routine blood tests were carried out for hematological and biochemical analysis, according to standard hospital laboratory techniques. Since the use of (H)CQ for COVID-19 was off-label, patients were started on (H)CQ only after giving informed consent.
Data collection
Data were extracted from Electronic Health Records (EHR) in all participating hospitals by medical students and/or infectious disease (ID) physicians. Data were collected on site using a standardized data-collection form on a secured website of the coordinating hospital. Patient data were immediately anonymized and encoded upon entry into the online research manager program. Collected data included patient characteristics, such as comorbidities, registered ICU-restrictive policy by treating physician, routine laboratory results, SARS-Cov2-PCR and chest CT-scan results, medical treatment before admission, and antibiotic treatment during hospitalization.
Statistical analysis
Differences between HCQ and CQ users (cases) and non-users (controls) were compared using χ2 statistics or the Fisher exact test for categorical variables, and the independent t-test or Mann-Whitney U test for continuous variables. The data were analyzed within a Cox proportional hazard regression framework. Follow-up commenced from the date of hospital admission and ended on the dates of death or ICU admission, and patients were censored at the time they were discharged from hospital. Hazard ratios were calculated for (H)CQ use in relation to the primary endpoints of death and ICU admission, or a combination of these endpoints denoted as a composite adverse endpoint. Death and ICU admission are competing risk events; therefore, competing risk regression analysis was conducted for these two endpoints according to the method developed by Fine and Gray (1999). Instead of KM survival curves, survival data were summarized using the cumulative incidence function (CIF) or cumulative risks of an event, which indicate the probability of the event at a given time. The proportional hazards assumption was confirmed by Schoenfeld’s global test and inspection of log (―log [survival]) curves. Propensity score (PS) matching was used for making causal inferences for the treatment on the clinical outcome. A set of pre-test covariates that were associated with the treatment was selected and PS scores were estimated using logistic regression, with treatment as the outcome measure. Separate PS-matched Cox regression models with and without adjustment for potential confounders were used (see Appendix), but only the results of the overall and inverse-probability-of-treatment-weighted (IPTW) Cox regression analysis are shown. Analyses were adjusted for gender, age, comorbidity CVA, comorbidity diabetes, comorbidity asthma/COPD, use of broad-spectrum antibiotics, therapeutic anticoagulation, prophylactic anticoagulation, first day at ED, and ICU restriction. The combined endpoint risk regression analyses were stratified by ICU restriction, because of the distinctive patient characteristics in this group. For PS estimation and matching the PS matching R package in SPSS and the PSMATCH2 package in Stata were used. All tests were two-sided and p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 24.0 and the STATA version 14 statistical package (Stata Corporation, College Station, TX).
Results
Inclusion and baseline characteristics
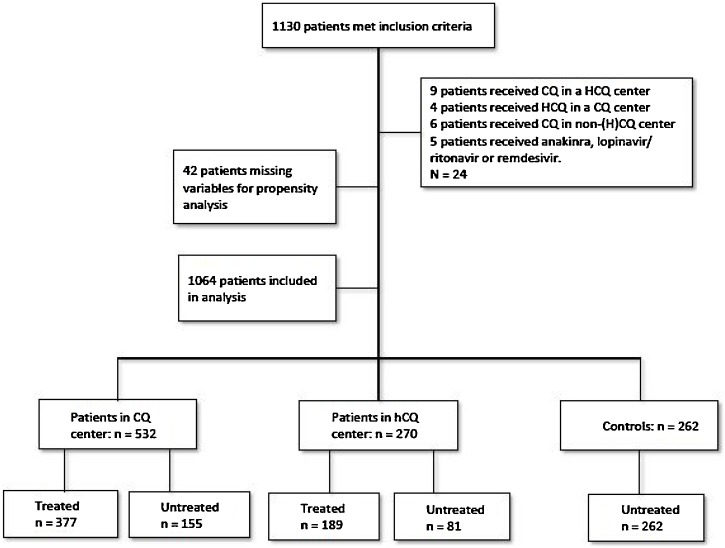
Between February 28 and April 1, 2020, 1130 patients admitted to the 14 participating hospitals in The Netherlands met the inclusion criteria; 1106 patients were eligible for inclusion. After propensity score matching the analytic cohort consisted of 1064 patients, comprising 566 (53.2%) treated patients, both with HCQ (N = 189; 17.8%) and CQ (N = 377; 35.4%), and 498 (46.8%) untreated controls (see Figure 1 ).
Figure 1.
Number of included COVID-19 patients.
HCQ = hydroxychloroquine, CQ = chloroquine.
Table 1 shows the characteristics of the study population. The distribution of patients over the three hospital groups was as follows: 270 patients (25.4%) were admitted to an HCQ hospital, 532 (50%) to a CQ hospital, and 262 (24.6%) to a hospital with a protocol of no additional treatment. In both HCQ and CQ hospitals at least 70% of patients received treatment. Median time from admission to receipt of treatment was short: 1 day in both groups (HCQ 1.00, SD 1.5 days; CQ 1.00, SD 1.19 days). Most patients were male (60%) and body mass index (BMI) was 28 in all three groups. Comorbidities were comparable, except for cardiac disease, which saw a higher incidence in the non-treated group. Some patients had an ICU-restrictive policy, for instance due to comorbidity or high age: in the HCQ group 36% of patients had an ICU restriction (68/189), in the CQ group 30.5% (115/377), and 48.5% of patients without treatment (242/498) were not considered eligible for admission to the ICU. During follow-up, 191 patients (18%) died, 147 (13.8%) were admitted to the ICU, and 726 (68.2%) were discharged from the hospital upon recovery.
Table 1.
Characteristics of the study population.
| Total (N = 1064) | Chloroquine centers (N = 532) |
Hydroxychloroquine centers (N = 270) |
No therapy centers (N = 262) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Treated | No treatment | Missing | p | Treated | No treatment | Missing | p | All | Missing | |||||
| Total: N, % | 377 | 35.4 | 155 | 14.6 | 189 | 17.8 | 81 | 7.6 | 262 | 24.6 | |||||
| Gender (male): N, % | 244 | 64.7 | 78 | 50.3 | 0 | 0.002* | 123 | 65.1 | 43 | 53.1 | 0 | 0.063* | 156 | 59.5 | 0 |
| Age: M, SD | 66.4 | 13.5 | 71.8 | 15.3 | 0 | 0.000‡ | 64.7 | 14.5 | 63.9 | 17.2 | 0 | 0.944‡ | 68.8 | 14.8 | 0 |
| BMI: M, SD | 28.2 | 4.9 | 28.1 | 5.3 | 98 | 0.996‡ | 27.5 | 4.1 | 28.5 | 6.2 | 147 | 0.537‡ | 27.7 | 5.4 | 69 |
| ICU restriction: N, % | 115 | 30.8 | 86 | 55.5 | 0 | 0.000* | 68 | 36 | 29 | 36 | 0 | 0.978* | 127 | 48.5 | 0 |
| Comorbidities: N, % | |||||||||||||||
| Hypertension | 133 | 35.3 | 65 | 41.9 | 0 | 0.149* | 62 | 32.8 | 25 | 30.9 | 0 | 0.755* | 103 | 39.3 | 0 |
| Heart failure | 15 | 4 | 24 | 15.5 | 0 | 0.000* | 12 | 6.3 | 11 | 13.6 | 0 | 0.051* | 36 | 13.7 | 0 |
| Myocardial infarction | 29 | 7.7 | 16 | 10.3 | 0 | 0.322* | 6 | 3.2 | 7 | 8.6 | 0 | 0.054* | 27 | 10.3 | 0 |
| Atrial fibrillation | 43 | 11.4 | 41 | 26.5 | 0 | 0.000* | 22 | 11.6 | 13 | 16 | 0 | 0.323* | 34 | 13 | 0 |
| CVA | 31 | 8.2 | 22 | 14.2 | 0 | 0.037* | 10 | 5.3 | 3 | 3.7 | 0 | 0.577* | 20 | 7.6 | 0 |
| Diabetes type 1 or 2 | 69 | 18.3 | 46 | 29.7 | 0 | 0.004* | 47 | 24.9 | 17 | 21 | 0 | 0.492* | 49 | 18.7 | 0 |
| Asthma or COPD | 80 | 21.2 | 35 | 22.6 | 0 | 0.729* | 21 | 11.1 | 17 | 21 | 0 | 0.032* | 54 | 20.6 | 0 |
| OSAS | 24 | 6.4 | 6 | 3.9 | 0 | 0.261* | 9 | 4.8 | 2 | 2.5 | 0 | 0.382* | 18 | 6.9 | 0 |
| Chronic kidney disease (creat. > 150 µmol/L) | 14 | 3.7 | 7 | 4.5 | 1 | 0.670* | 12 | 6.3 | 5 | 6.2 | 0 | 0.956* | 20 | 7.6 | 1 |
| Active malignancy | 29 | 7.7 | 12 | 7.7 | 0 | 0.984 | 14 | 7.4 | 6 | 7.4 | 0 | 1* | 17 | 6.5 | 0 |
| Muscle disease | 5 | 1.3 | 1 | 0.6 | 0 | 0.499* | 1 | 0.5 | 1 | 1.2 | 0 | 0.536* | 6 | 2.3 | 1 |
| History of DVT/LE | 23 | 6.1 | 8 | 5.2 | 0 | 0.686* | 11 | 5.8 | 5 | 6.2 | 0 | 0.91* | 23 | 8.8 | 0 |
| Immunosuppressive | 23 | 6.1 | 8 | 5.2 | 0 | 0.674* | 8 | 4.2 | 1 | 1.2 | 0 | 0.208* | 32 | 12.2 | 0 |
| Diagnosis based on…: N, % | |||||||||||||||
| PCR | 359 | 95.2 | 145 | 93.5 | 0 | 0.431* | 180 | 95.2 | 79 | 96.3 | 0 | 0.699* | 252 | 96.2 | 0 |
| CT | 16 | 4.2 | 8 | 5.2 | 0 | 0.643* | 9 | 4.8 | 2 | 2.5 | 0 | 0.382* | 9 | 3.4 | 0 |
| Clinical judgement | 2 | 0.5 | 2 | 1.3 | 0 | 0.357* | 0 | 0 | 0 | 0 | 0 | N/A | 1 | 0.4 | 0 |
| Vitals and laboratory results at presentation: M (N), SD | |||||||||||||||
| Temperature | 38.1 | 1.0 | 37.9 | 1.0 | 1 | 0.009§ | 38.1 | 1.0 | 38.0 | 1.0 | 1 | 0.476‡ | 38.0 | 1.05 | 1 |
| Oxygen needed: N, % | 326 | 86.5 | 93 | 60 | 0 | 0.000* | 167 | 88.4 | 56 | 69.1 | 0 | 0.000* | 163 | 62.2 | 0 |
| CRP | 97 | 72.9 | 83.1 | 75.8 | 2 | 0.003‡ | 105.3 | 76.9 | 64.1 | 48.5 | 28 | 0.0000‡ | 88.3 | 74.9 | 3 |
| Leucocytes | 7.0. | 3.1 | 6.9 | 3.4 | 6 | 0.313‡ | 7.0 | 5.1 | 7.3 | 4.0 | 29 | 0.524‡ | 7.0 | 3.0 | 3 |
| Lymphocytes | 1.0 | 1.4 | 1.0 | 1.0 | 20 | 0.901‡ | 1.3 | 4.4 | 1.3 | 1.1 | 63 | 0.006‡ | 1.1 | 1.0 | 37 |
| Platelets | 207.9 | 83.5 | 204.9 | 81.2 | 11 | 0.443‡ | 205.6 | 95.68 | 177.6 | 107.4 | 67 | 0.357‡ | 203.3 | 86.2 | 6 |
| Creatinine | 93.1 | 44.7 | 106 | 68.1 | 3 | 0.090‡ | 92.8 | 73.5 | 103.0 | 112.6 | 29 | 0.096‡ | 107.9 | 107.6 | 4 |
| LDH at presentation | 356.2 | 142.3 | 312.2 | 118.5 | 40 | 0.000‡ | 346.7 | 148.1 | 340.1 | 140.1 | 54 | 0.692‡ | 347.2 | 143.6 | 22 |
| Pre-hospital medication: N, % | |||||||||||||||
| ACE inhibitors | 55 | 14.6 | 34 | 22.1 | 2 | 0.037* | 30 | 16.0 | 15 | 18.8 | 0 | 0.588* | 52 | 20.1 | 3 |
| Angiotensine-2 receptor antagonists | 48 | 12.8 | 24 | 15.6 | 2 | 0.390* | 25 | 13.4 | 9 | 11.3 | 4 | 0.624* | 27 | 10.5 | 4 |
| Therapeutic anticoag. | 50 | 13.3 | 37 | 24 | 2 | 0.002* | 29 | 15.8 | 17 | 21.5 | 7 | 0.26* | 51 | 19.9 | 6 |
| In-hospital medication | |||||||||||||||
| Broad-spectrum antibiotics: N, % | 327 | 86.7 | 99 | 63.9 | 0 | 0.000* | 185 | 97.9 | 71 | 87.7 | 0 | 0.0010* | 196 | 74.8 | 0 |
| Azithromycin: N, % | 31 | 8.2 | 33 | 21.3 | 0 | 0.000* | 48 | 25.4 | 45 | 55.6 | 0 | 0.0000* | 53 | 20.2 | 0 |
| Cumulative dosage AZM: M (N), SD | 833.3 | 461.1 | 1241.9 | 560.8 | 3 | 0.001‡ | 2020.8 | 1115.5 | 1661.1 | 834.5 | 0 | 0.137‡ | 2264.4 | 925.4 | 1 |
| Cumulative dosage CQ/HCQ: M (N), SD | 2179.5 | 897.6 | N/A | N/A | 1 | N/A | 1823.5 | 636.1 | N/A | N/A | 19 | N/A | N/A | N/A | N/A |
| Therapeutic anticoag.: N, % | 66 | 17.5 | 51 | 32.9 | 0 | 0.000* | 38 | 20.1 | 19 | 23.5 | 0 | 0.536* | 56 | 21.4 | 0 |
| Prophylactic anticoag.: N, % | 318 | 84.4 | 99 | 63.9 | 0 | 0.000* | 161 | 85.2 | 57 | 70.4 | 0 | 0.005* | 148 | 56.5 | 0 |
| Deep venous thrombosis: N, % | 1 | 0.3 | 0 | 0 | 3 | 0.519* | 0 | 0 | 0 | 0 | 1 | N/A | 2 | 0.8 | 3 |
| Pulmonary embolism: N, % | 6 | 2.1 | 1 | 0.8 | 115 | 0.355* | 3 | 1.6 | 0 | 0 | 4 | 0.253* | 4 | 1.5 | 3 |
| Endpoints | |||||||||||||||
| Discharged for cured infection: N, % | 245 | 65.0 | 107 | 69.0 | 0 | 0.370* | 139 | 73.5 | 58 | 71.6 | 0 | 0.742* | 177 | 67.6 | 0 |
| ICU admission: N, % | 72 | 19.1 | 10 | 6.5 | 0 | 0.000* | 20 | 10.6 | 3 | 3.7 | 0 | 0.064* | 42 | 16.4 | 0 |
| Death or hospice: N, % | 60 | 15.9 | 38 | 24.5 | 0 | 0.020* | 30 | 15.9 | 20 | 24.7 | 0 | 0.087* | 43 | 16.0 | 0 |
M = mean, SD = standard deviation, *= χ2 test, † = Fisher exact test, § = independent t-test, ‡ = non-parametric Mann-Whitney test, HCQ = hydroxychloroquine, CQ = chloroquine, AZM = azithromycin, BMI = body mass index, ICU = intensive care unit, CVA = cerebrovascular accident, OSAS = obstructive sleep apnea syndrome.
Primary outcomes
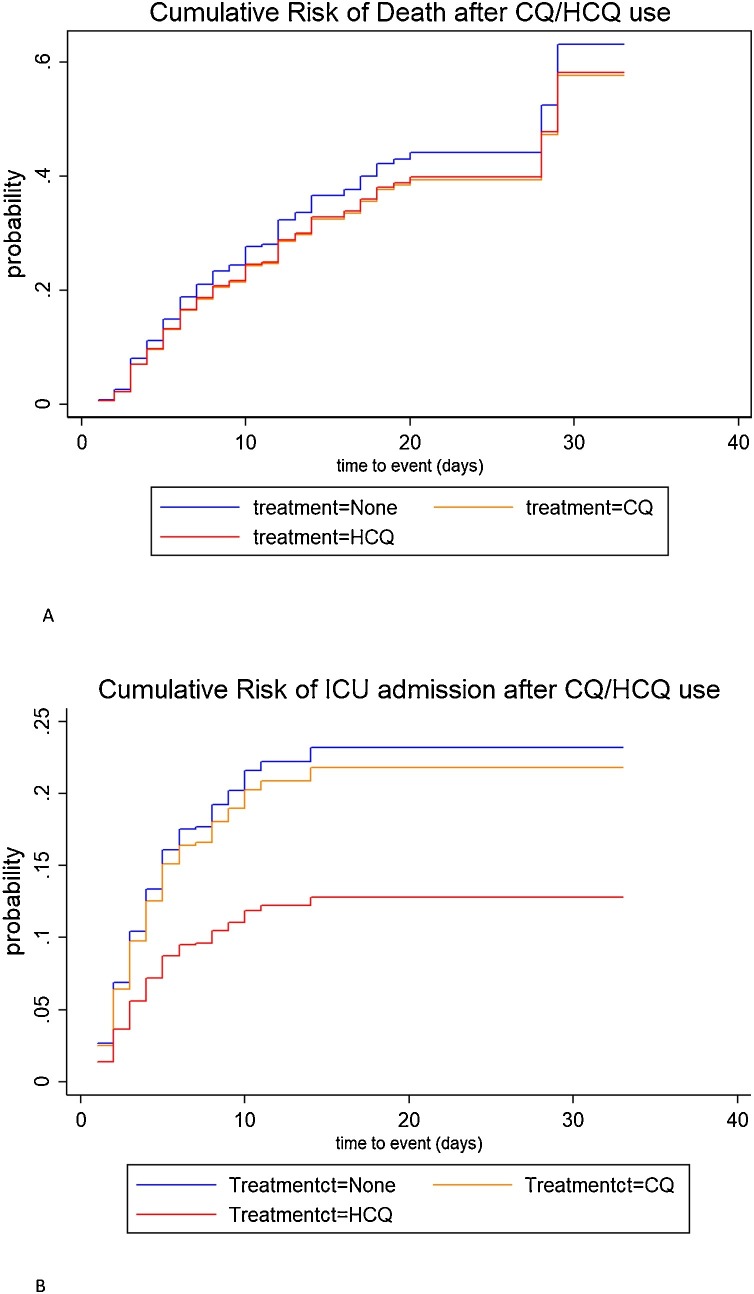
Table 2 shows the results of the unadjusted and adjusted overall and weighted competing risk analyses for the different endpoints by type of medication. Figure 2 A and B show the corresponding cumulative incidence functions (CIF). Multivariate analysis proves that both CQ and HCQ use were not statistically associated with a risk of death on the non-ICU COVID ward (for CQ, hazard ratio (HR) = 0.99, 95% CI = 0.70–1.43; for HCQ, HR = 0.96, 95% CI = 0.63–1.45). However, HCQ use was associated with a statistically significant decreased risk of transfer to the ICU (HR = 0.47, 95% CI = 0.27–0.82, p = 0.008) when compared with controls. This effect was not found in the CQ group (HR = 0.80; 95% CI = 0.55–1.15, p = 0.207). In addition, for the composite adverse endpoint, a significantly decreased risk was observed for HCQ (HR = 0.68, 95% CI = 0.49–0.95, p = 0.024) but not for CQ use (HR = 0.85, 95% CI = 0.66–1.10, p = 0.224).
Table 2.
Clinical outcome hazard ratio (HR) estimates for HCQ and CQ use among COVID19 patients under separate risk models.
|
N = 1012* |
Endpoint: death |
Endpoint: ICU admission |
Combined endpoint |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted3 |
Unadjusted |
Adjusted4 |
Unadjusted |
Adjusted5* |
||||||||||||||
| Model | Drug use | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Overall1 | None (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| CQ | 0.64 | 0.47–0.88 | 0.007 | 1.01 | 0.71–1.44 | 0.937 | 1.50 | 1.05–2.13 | 0.024 | 0.91 | 0.63–1.31 | 0.619 | 0.94 | 0.74–1.18 | 0.590 | 0.97 | 0.75–1.24 | 0.795 | |
| HCQ | 0.62 | 0.41–0.93 | 0.020 | 0.92 | 0.58–1.46 | 0.736 | 0.82 | 0.49–1.37 | 0.453 | 0.56 | 0.33–0.95 | 0.031 | 0.69 | 0.50–0.95 | 0.023 | 0.73 | 0.51–1.02 | 0.068 | |
| Weighted Competing risk2 | None (ref) | 1.0 | 1.0 | 1.0 | |||||||||||||||
| CQ | 0.86 | 0.61–1.21 | 0.392 | 0.99 | 0.70–1.43 | 0.991 | 0.93 | 0.64–1.35 | 0.708 | 0.80 | 0.55–1.15 | 0.207 | 0.85 | 0.67–1.10 | 0.205 | 0.85 | 0.66–1.10 | 0.224 | |
| HCQ | 0.87 | 0.58–1.32 | 0.518 | 0.96 | 0.63–1.45 | 0.681 | 0.52 | 0.30–0.89 | 0.017 | 0.47 | 0.27–0.82 | 0.008 | 0.66 | 0.48–0.91 | 0.011 | 0.68 | 0.49–0.95 | 0.024 | |
1Cox regression model without propensity score (PS) adjustment and competing regression analysis; 2competing risk regression with weighted PS adjustment (see statistical method section for explanation of the different models); HR = hazard ratio; CI = confidence interval; CQ = chloroquine; HCQ = hydroxychloroquine; *total number of patients in the analysis; 3,4,5adjusted for gender, age, comorbidity CVA, comorbidity diabetes, comorbidity asthma/COPD, use of broad-spectrum antibiotics, therapeutic anticoagulation, prophylactic anticoagulation, first day in ED, ICU restriction.
All analyses except the competing risks regression were stratified by ICU restriction to reflect underlying potential differences in adverse incidences and risk factor prevalences.
Figure 2.
Cumulative incidence functions (CIF) by type of medication. A. Cumulative risk of death. B. Cumulative risk of transfer to ICU.
Secondary outcomes
Since the use of azithromycin (AZM) and angiotensin receptor blockers (ARB) has been postulated to have an effect on COVID-19, we additionally analyzed the effect of this treatment on outcome; 210 patients were started on AZM therapy on admission, while 854 patients did not receive AZM. In the KM analysis there was no significant difference between these two groups in reaching the composite adverse endpoint (P log rank = 0.071) and no significant interaction effect was found for H(CQ) combined with AZM use (p = 0.2195).
In total, 180 patients were using angiotensin-II receptor antagonists (ARB, n = 70) or angiotensin-converting enzyme inhibitors (ACEi, n = 110), and continued treatment during admission. There was no difference in outcome for the composite adverse endpoint for continued ACEi use (HR = 1.21; 95% CI = 0.78–1.90, p = 0.397) nor for continued ARB use (HR = 1.21; 95% CI = 0.70–2.10, p = 0.498), as compared with no therapy.
Discussion
This study demonstrated a new and clinically important finding: the use of HCQ on the COVID-19 ward is associated with a decreased risk of transfer to the ICU. After competing risk analysis, the risk of admission to the ICU was reduced by 53%. This finding suggests that starting early treatment with HCQ (within 1 day of admission) on the regular COVID ward might prevent progression to critical respiratory illness. This is consistent with the suggestion that HCQ treatment reduces the risk of disease progression more effectively earlier in the course of the disease (Kachuri et al., 2015, Wang et al., 2020a). This holds true for many other viral infections, such as influenza and herpes simplex, where treatment must be initiated soon after onset of symptoms in order to confer benefit. However, treatment with HCQ before onset of symptoms did not prevent COVID-19, as was demonstrated in a randomized controlled trial investigating postexposure use of HCQ (Boulware et al., 2020).
Second, we could not demonstrate a significant effect of treatment with HCQ or CQ on on-ward mortality. One of the strengths of our study was that we selected a clearly defined cohort of patients on the regular non-ICU COVID-ward, thus our results reflected mortality before transfer to the ICU only. In recent literature, evidence is accumulating that there is no beneficial effect of HCQ on mortality. Mortality numbers in systematic reviews and in prospective HCQ studies, such as the Recovery trial, are frequently reported in terms of 28-day all-cause mortality, and do not differentiate between on-ward mortality and mortality after transfer to the ICU (Horby, 2020, Fiolet et al., 2020).
In our study, there was no significant difference in outcome between patients treated with AZM, nor in patients on ACEi or ARB therapy. It has been suggested that ARB therapy increases susceptibility to COVID-19, but other studies report conflicting results (Zhang et al., 2020, Fosbøl et al., 2020). Our data confirm the lack of effect on outcome, both for pre-hospital use as well as for in-hospital continuation of ACEi or ARB therapy.
Surprisingly, we found a differential effect of HCQ and CQ on COVID-19, while in the literature these drugs are frequently reported in terms of a composite outcome. There are several possible explanations for this differential effect. The first explanation is a possible difference in pharmacokinetics between both drugs. There is a substantial difference in renal drug-clearance – 51% in CQ and 21% in HCQ (Schrezenmeier and Dörner, 2020). Furthermore, the distribution volumes of HCQ and CQ are different; HCQ has a volume of distribution of 5522 liters (whole blood), as compared with 14 000–56 000 liters for CQ (Drugbank, 2020a, Furst, 1996, Drugbank, 2020b). It is still a matter of debate whether the 4-aminoquinoline drugs have anti-viral activity or immunomodulating properties (Gautret et al., 2020, Maisonnasse et al., 2020). The immunomodulating effect of HCQ in has been reported in rheumatology literature (Schrezenmeier and Dörner, 2020). In clinical practice, patients with rheumatoid disease are treated with HCQ but not CQ as anti-inflammatory therapy, according to clinical guidelines (Arayssi et al., 2018). It is conceivable that the beneficial effect of early HCQ in COVID-19 lies in the reduction of localized inflammation in the lung. This is supported by the results of a recent observational study, which indicated that the use of moderate-dose systemic corticosteroids on the general ward lowered the hazard of ICU transfer (Majmundar et al., 2020).
Another important strength of our study was the random distribution of patients between hospitals with different treatment protocols. Unintentionally, three groups of patients were created, almost as in prospective research. We were able to investigate the differences between patients on or off treatment with a reduced risk of bias by indication.
This study had some limitations. First, all observational cohort studies are prone to bias by confounding. We used weighted propensity scores to adjust optimally for differences between treated patients and controls. However, randomized studies are needed to confirm our data. Another limitation of this study was a lack of data on the adverse effects of (H)CQ. There is ongoing global discussion about possible drug toxicity in COVID-19 patients and increased mortality associated with HCQ treatment (Borba et al., 2020, Magagnoli et al., 2020). Since HCQ and CQ are FDA- and EMA-approved drugs, the adverse effects are well documented (Fosbøl et al., 2020). Yet these adverse effects are similar to the commonly reported COVID-19 symptoms (fever, fatigue, dry cough, dyspnea, myalgia), while nausea and diarrhea are also frequently observed (Guan et al., 2020b, Wang et al., 2020b). Older patients are more likely to have abdominal complaints as presenting symptoms of COVID-19 (Godaert et al., 2020). Because of the difficulty in distinguishing symptoms of COVID-19 from adverse effects of (H)CQ treatment we decided to refrain from collecting patient-reported symptoms retrospectively.
It is postulated that the pathophysiology of COVID-19 is characterized by three phases of illness (Siddiqi and Mehra, 2020). In the initial viral stage (phases 1–2) patients are moderately affected, viral replication and localized inflammation in the lung cause hypoxemia and lymphopenia, and patients are admitted to the hospital cohort ward. This is followed by systemic hyperinflammation (phase 3) and severe disease, where patients are potentially admitted to the ICU for invasive mechanical ventilation. In this phase, the use of stronger immunomodulating agents, such as hydrocortisone, dexamethasone, anakira, and tocilizumab, can be considered (Horby, 2020, van de Veerdonk et al., 2020).
In conclusion, our observational study demonstrates that the early clinical use of HCQ – but not CQ – in hospitalized non-ICU COVID-19 patients is associated with a decreased risk of transfer to the ICU. Once patients are critically ill, the process of hyperinflammation and hypercoagulation is probably not influenced by HCQ, and treatment with strong immunosuppressants and anticoagulant therapy are more important for the survival of patients with severe COVID-19.
Cinflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
There were no funding sources or study sponsors involved in this study.
Ethical approval
The Medical Ethics Review Committee waived ethical approval for this study.
Acknowledgements
We acknowledge the dedication, commitment, and sacrifices of all personnel in our hospitals through the COVID-19 outbreak. We would like to thank the staff from the Pulmonary Department as well as those from the LMMI. From the Isala Academy, Saskia Abbes, Machteld van der Weg, and Joep Dille helped us greatly with the research manager and import of data files from other hospitals. We would also like to thank the medical students and others who helped in entering all data in the research manager. Finally, we acknowledge the suffering and loss experienced by our patients and their families.
Appendix A.
This appendix contains a complete description of the statistical analysis that was performed.
Statistical analysis
Differences between HCQ and CQ and controls were compared using χ2 statistics or the Fisher exact test for categorical variables and the independent t-test or Mann-Whitney U test for continuous variables. HCQ and CQ use was related to the primary endpoints ‘death’ and ‘ICU admission’ using univariate and multivariable analyses within a Cox proportional hazard regression framework. Follow-up commenced on the date of hospital admission and ended on the date of death, date of ICU admission, or date of discharge – whichever came first. The proportional hazards assumption was confirmed by Schoenfeld’s global test and inspection of log (−log [survival]) curves. Kaplan-Meier estimates were used to construct survival curves, and the log-rank χ2 was used to compare the curves. For correction of the nonrandomized observational design of the study, propensity score (PS) matching was used for making causal inferences of the treatment on the clinical outcome. A set of pre-test covariates that were associated with the treatment was selected, based on the results of univariate and stepwise regression analysis, together with clinical relevance. PS scores were estimated for each patient using logistic regression, with the treatment assignment HCQ or CQ as the outcome measure. The balance on the covariates throughout the matching procedure was checked by comparing the treatment and control groups before and after matching, using the standardized mean difference of covariates. The treatment effects were estimated by calculating the hazard ratio for HCQ and CQ use in relation the primary endpoints ‘death’, ‘ICU admission’, or the combination of these endpoints, denoted as a ‘composite adverse endpoint’. Separate Cox regression models with and without adjustment for potential confounders were used to evaluate the effect of treatment on the different endpoints (see Table A1 ). The first model is an overall Cox model without propensity score matching. Three PS-matched methods were used: (1) PS pair matching, e.g. k-nearest neighbors matching – for each treated patient, selecting k controls with closest propensity scores. The Cox model was run on only those patients and their matched controls. (2) PS stratification – grouping individuals in five groups (quintiles) with similar PS values. (3) Inverse probability of treatment weighting (IPTW) – by computing the inverse PS followed by weighing the patients accordingly, with heavy weights excluded by skimming. As a result, an artificial sample is created in which the distribution of covariates is equal among treatment groups. In addition to the PS-matched models, regression adjustment using PS as a covariate was performed. All analyses were stratified by ICU restriction, because of the distinct patient characteristics of this group. Appropriate sensitivity analyses were performed to determine the validity of the regression analyses. For PS estimation and matching, the PS-matching R package in SPSS and the PSMATCH2 package in Stata were used. All tests were two-sided and p < 0.05 was considered statistically significant. All statistical analyses were performed using the STATA version 14 statistical package (Stata Corporation, College Station, TX).
Table A1.
Clinical outcome hazard ratio estimates for HCQ and CQ use among COVID19 patients under separate propensity risk models.
|
N = 1012* |
Endpoint: death |
Endpoint: ICU admission |
Combined endpoint |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted3 |
Unadjusted |
Adjusted3 |
Unadjusted |
Adjusted4 |
||||||||||||||
| Model | Drug use | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Overall1 | None (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| CQ | 0.64 | 0.47–0.88 | 0.007 | 1.01 | 0.71–1.44 | 0.937 | 1.50 | 1.05–2.13 | 0.024 | 0.91 | 0.63–1.31 | 0.619 | 0.94 | 0.74–1.18 | 0.590 | 0.96 | 0.75–1.24 | 0.772 | |
| HCQ | 0.62 | 0.41–0.93 | 0.020 | 0.92 | 0.58–1.46 | 0.736 | 0.82 | 0.49–1.37 | 0.453 | 0.56 | 0.33–0.95 | 0.031 | 0.69 | 0.50–0.95 | 0.023 | 0.71 | 0.51–1.01 | 0.054 | |
| Matched paired2 | None (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| CQ | 0.66 | 0.38–1.13 | 0.130 | 0.59 | 0.23–1.52 | 0.272 | 0.85 | 0.53–1.38 | 0.523 | 0.82 | 0.41–1.64 | 0.567 | 0.77 | 0.53–1.10 | 0.144 | 0.85 | 0.50–1.43 | 0.535 | |
| HCQ | 0.66 | 0.34–1.26 | 0.207 | 0.35 | 0.12–1.03 | 0.057 | 0.41 | 0.22–0.78 | 0.007 | 0.48 | 0.21–1.08 | 0.075 | 0.51 | 0.32–0.81 | 0.004 | 0.50 | 0.28–0.91 | 0.023 | |
| Stratified quintiles2 | None (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| CQ | 0.77 | 0.54–1.08 | 0.124 | 0.98 | 0.68–1.41 | 0.919 | 0.97 | 0.67–1.40 | 0.863 | 0.79 | 0.54–1.14 | 0.204 | 0.86 | 0.67–1.11 | 0.242 | 0.89 | 0.69–1.15 | 0.373 | |
| HCQ | 0.84 | 0.55–1.30 | 0.431 | 0.86 | 0.54–1.39 | 0.547 | 0.48 | 0.29–0.82 | 0.007 | 0.50 | 0.29–0.84 | 0.010 | 0.65 | 0.46–0.91 | 0.011 | 0.66 | 0.46–0.94 | 0.020 | |
| Weighted2 | None (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| CQ | 0.81 | 0.58–1.15 | 0.243 | 0.94 | 0.65–1.35 | 0.732 | 0.91 | 0.63–1.32 | 0.615 | 0.79 | 0.55–1.15 | 0.217 | 0.85 | 0.67–1.10 | 0.205 | 0.85 | 0.66–1.10 | 0.228 | |
| HCQ | 0.78 | 0.52–1.18 | 0.244 | 0.93 | 0.61–1.41 | 0.734 | 0.50 | 0.29–0.85 | 0.011 | 0.47 | 0.27–0.82 | 0.008 | 0.66 | 0.48–0.91 | 0.011 | 0.68 | 0.49–0.95 | 0.022 | |
1Cox regression model without propensity score (PS) adjustment; 2PS adjustment – see statistical method section for explanation of the different models; HR = hazard ratio, CI = confidence interval; CQ = chloroquine, HCQ = hydroxychloroquine; 3adjusted for gender, age, comorbidity CVA, comorbidity diabetes, comorbidity asthma/COPD, use of of broad-spectrum antibiotics, therapeutic anticoagulation, prophylactic anticoagulation, first day in ED; 4adjusted for gender, age, comorbidity asthma/COPD, use of broad-spectrum antibiotics, prophylactic anticoagulation, first day in ED; *all analyses were stratified by ICU restriction to reflect underlying potential differences in adverse incidences and risk factor prevalences.
References
- Arayssi T., Harfouche M., Darzi A. Recommendations for the management of rheumatoid arthritis in the Eastern Mediterranean region: an adolopment of the 2015 American College of Rheumatology guidelines. Clin Rheumatol. 2018;37(11):2947–2959. doi: 10.1007/s10067-018-4245-5. [DOI] [PubMed] [Google Scholar]
- Arshad S., Kilgore P., Chaudhry Z.S. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;0(0) doi: 10.1016/j.ijid.2020.06.099. https://linkinghub.elsevier.com/retrieve/pii/S1201971220305348 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borba M.G.S., Val F.F.A., Sampaio V.S. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- Boulware D.R., Pullen M.F., Bangdiwala A.S. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020:1–9. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Hu J., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020;7 2020.03.22.20040758. [Google Scholar]
- Corman V.M., Landt O., Kaiser M. Detection of 2019 -nCoV by RT-PCR. Euro Surveill. 2020;25(3):1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani A., Ippolito M., Ingoglia G., Iozzo P., Giarratano A., Einav S. Update I. A systematic review on the efficacy and safety of chloroquine/hydroxychloroquine for COVID-19. J Crit Care. 2020;59:176–190. doi: 10.1016/j.jcrc.2020.06.019. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugbank Drugbank.ca; 2020. Hydroxychloroquine. https://www.drugbank.ca/drugs/DB01611 [cited 2020 Jul 2];Available from:
- Drugbank Drugbank.ca; 2020. Chloroquine. https://www.drugbank.ca/drugs/DB00608#reference-A191676 [cited 2020 Jul 2];Available from:
- Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- Fiolet Thibault, Guihur Anthony, Rebeaud Mathieu, Mulot Matthieu, Peiffer-Smadja Nathan, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosbøl E.L., Butt J.H., Østergaard L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020:1–10. doi: 10.1001/jama.2020.11301. http://www.ncbi.nlm.nih.gov/pubmed/32558877 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst D.E. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;5(Suppl. 1):11–15. [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godaert L., Emeline P., Demoustier-Tampere D., Souleymane Coulibaly P., Hequet F., Dramé M. 2020. Letters to editors: clinical characteristics of older patients: the experience of a geriatric short-stay unit dedicated to patients with COVID-19 in France. January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y. Clinical Characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P.L.M. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. 2020. https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-co 5 June [cited 2020 Jun 5];Available from:
- Infectious Diseases Society of America Guidelines Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. 2020. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ Available from: [DOI] [PMC free article] [PubMed]
- Kachuri L., Amos C.I., Mckay J.D. 2015. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magagnoli J., Narendran S., Pereira F. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med. 2020 doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahevas M., Tran V.-T., Roumier M. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv. 2020;2020 04.10.20060699. [Google Scholar]
- Maisonnasse P., Guedj J., Contreras V. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020 doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- Majmundar M., Kansara T., Lenik J. Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York Metropolitan region. PLoS One. 2020;2:1–14. doi: 10.1371/journal.pone.0238827. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop M., van Everdingen W., van Rees Vellinga T. CO-RADS – A categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020;(1) doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVM Coronavirus disease (COVID-19) 2020. https://www.rivm.nl/en/novel-coronavirus-covid-19/coronavirus-disease-covid-19 3 March. Available from:
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19). A review. JAMA. 2020;2019 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. Available from: [DOI] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Hear Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank Kim, McCarten K. Hydroxychloroquine and chloroquine: all adverse events in the setting of COVID-19. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/OSE_Review_Hydroxychloroquine-Cholorquine-19May2020_Redacted.pdf Available from:
- Tang W., Cao Z., Han M. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ. 2020;369(April):1–11. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk F., Netea M.G., van Deuren M. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. Preprints. 2020;(October):1–29. https://www.preprints.org/manuscript/202004.0023/v1 Available from: [Google Scholar]
- Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhu L., Cai J. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Dai S.M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;(February):1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]