Abstract
During this COVID-19 pandemic, patients with symptoms such as fever, cough, sore throat, and coryza were advised to have RT-PCR testing for SARS-CoV-2 infection. We described here an elderly female with chronic lymphocytic leukemia, who presented with atypical symptoms that were not directly attributable to COVID-19. This patient was admitted to the non-COVID-19 ward for supportive care. Later, her chest x-ray revealed pneumonia that was confirmed to be COVID-19 by RT-PCR testing several days later. In resource-poor settings where molecular testing results suffered from delays or were altogether unavailable, the use of diagnostic imaging such as a chest x-ray could serve as a quick guide in the assessment and management of these patients especially if the imaging results suggest COVID-19 infection.
Keywords: Screening, Cancer patients, Covid-19, Diagnostic imaging
Introduction
One of the recommendations by the World Health Organization in the screening and triage of patients for potential SARS-CoV-2 infection is the interrogation for associated symptoms such as fever, cough, sore throat, anosmia, and coryza (1,2). However, symptoms and clinical presentation may vary among patients and other objective parameters may assist in effectively screening patients for potential COVID-19 infection.
Case
A 63-year-old female with Chronic Lymphocytic Leukemia (CLL) presented at our hospital for blood transfusion. At the time of her admission at the medicine ward, she had minimal non-productive cough which the patient claimed had been present for several months already. Other respiratory symptoms, fever, and diarrhea were all absent. This patient had no history of recent international travel, contact with someone coming from overseas, nor with a known case of COVID-19. Physical examination revealed tachycardia and fine bi-basal rales. Complete blood count on admission showed hypochromic anemia with anisocytosis, thrombocytopenia, and leukocytosis with lymphocytic predominance (Table 1 .). A chest radiograph was done for further evaluation.
Table 1.
Patient's complete blood count at admission showed marked anemia, decreased platelets, and lymphocytic leukocytosis consistent with chronic lymphocytic leukemia.
| Result | Unit | Reference Range | |
|---|---|---|---|
| WBC Count | 90.80 | x109/L | 4.50–11.0 |
| RBC Count | 1.40 | x1012/L | 4.2–5.4 |
| Hemoglobin | 35 | g/L | 120–160 |
| Hematocrit | 0.13 | 0.38–0.47 | |
| Platelet | 88 | x109/L | 150–450 |
| Differential Count | |||
| Neutrophils | 0.08 | 0.50–0.70 | |
| Lymphocytes | 0.90 | 0.20–0.50 | |
| Monocytes | 0.02 | 0.02–0.09 | |
| Eosinophils | 0.00 | 0.00–0.06 | |
| Basophils | 0.00 | 0.00–0.02 | |
| No Toxic Granules Seen | |||
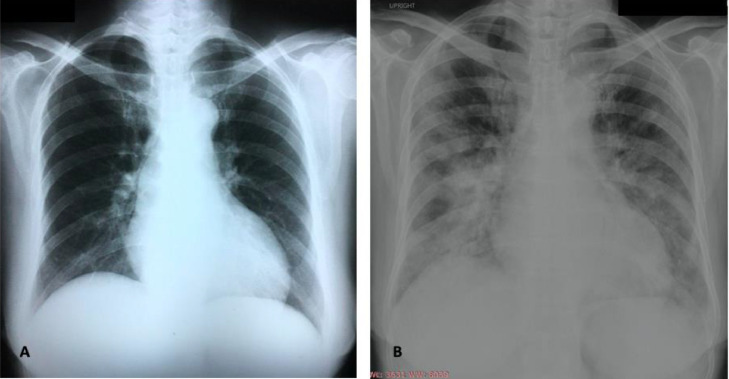
The chest radiograph (Fig. 1 ), showed interval development of patchy inhomogeneous opacities in both lungs with confluence in the mid to lower lung zones suggestive of pneumonia. This change was noted in comparison to a radiograph taken during a prior admission four weeks earlier during which time her CLL was diagnosed.
Fig. 1.
(A) Chest radiograph taken February 2020 which shows no infiltrates compared to (B) taken March 2020 showing interval development of patchy inhomogeneous opacities in both lungs with confluence in the mid to lower lung zones suggestive of pneumonia.
A decision was made to treat her pneumonia with antibiotics. As the COVID-19 pandemic had just been declared, testing for SARS-CoV-2 infection was likewise done. Due to concerns that the patient could have COVID-19 she was promptly transferred to a separate unit for isolation. All exposed staff were immediately advised to self-quarantine. Other patients in proximity to the case were monitored closely for signs and symptoms of COVID-19.
Five days later, her reverse transcription – polymerase chain reaction (RT-PCR) results came out positive for COVID-19 and the patient was immediately transferred to the COVID-19 dedicated unit. Fortunately, at the end of the prescribed quarantine period no personnel nor additional patients developed COVID-19 infection due to this exposure. The patient also recovered from COVID-19 after an eight-week admission.
Discussion
Patients with hematologic malignancies are at an increased risk for infection arising from their malignancy and from the interventions being given for their treatment. CLL is a profoundly immunosuppressed state. Defective T-cell function in CLL increase susceptibility to viral infections such as COVID-19. ([3], [4], [5])
The clinical course of SARS-CoV-2 infection for immunosuppressed patients may also vary from mild to critical disease. (6) These observations are also apparent in our case presented here. Patients who present atypically or with no symptoms are the most challenging to screen. Their evaluation is further confounded by symptoms arising from their malignancy that mirror those of COVID-19. All these highlight the importance of having a high index of suspicion for COVID-19 in the evaluation of patients that present atypically such as individuals with malignancies.
What clued us into the possibility of COVID-19 in this patient was the result of her chest radiograph, which showed bilateral infiltrates characteristic of this disease. Chest x-rays, particularly bedside radiographs were indispensable in the management of COVID-19 because these allowed clinicians to monitor the progress of patients particularly those with severe and critical illness (7). Moreover, chest x-rays could also be used to help in the diagnosis of this disease. A study published by Ippolito et al. showed that sensitivity and specificity of chest x-ray in detecting COVID-19 were 57% (95% CI: 47–67) and 89% (95% CI: 83–94), respectively. Characteristic manifestations of the SARS-CoV-2 related pneumonia on chest radiographs were like those of common viral pneumonia but also had the presence of diffuse interstitial and alveolar opacities. The sensitivity of a chest x-ray was said to increase if the symptoms manifested > 5 days prior to the radiograph and in those >50 years, however this came at the expense of lower specificity. (8) Another study by Wong et al. reported the sensitivity of chest x-rays to be 69% (95% CI: 56–80). A criticism of x-rays was its lack of sensitivity needed to detect ground-glass opacities (GGOs) that were early manifestations of COVID-19 on imaging (9). In addition, imaging results also needed to be correlated with clinical findings as the absence of typical findings on chest x-ray would not preclude the diagnosis of COVID-19.
Computed tomography (CT) scans of the chest may be better at detecting GGOs (9). A recent systemic review by Xu et al. included sixteen studies and reported a pooled sensitivity 92% (95% CI = 86–96). The meta-analysis had significant heterogeneity and sensitivity was highest for the three studies that were conducted in Wuhan, China (97, 96 and 99%,respectively). For the studies done outside Wuhan, sensitivity ranged from 62 to 98%. Specificity was reported by only two studies at 25% (95% CI: 22–30) and 33% (95% CI: 23–44), respectively. GGOs and consolidative opacities are among the most common CT findings associated with COVID-19 (10). Concerns with the use of chest CT for the screening of potential COVID-19 cases is that this procedure can be logistically difficult and more expensive than a chest x-ray. Moreover, dedicated machines for screening potential COVID-19 patients are not always available especially in resource-constrained settings. Lastly, the CT scanners and the staff manning these can become vectors of infection particularly to vulnerable patients (11,12).
Lung ultrasound is emerging as a promising point-of-care tool to diagnose COVID-19 and efforts to standardize the use of this modality have come forth (13). Although it has several advantages compared to CT scans including the absence of radiation, lower cost, portability, and lower contamination risk, perhaps the most significant challenge with the use of lung ultrasound remains to be its potential for subjectivity and its reliance on the experience and expertise of the sonographer (14,15). In resource-constrained settings where trained personnel are not readily available, treatment decision-making and resource allocation may need to rely on more objective bases for the diagnosis of COVID-19.
Considering the strengths of each modality and the availability of resources to us, the Department of Medicine at our Center included chest radiography as part of the initial diagnostics for patients scheduled for admission to its non-COVID-19 wards. This included our cancer patients who would receive treatment or supportive care. Early in the pandemic turn-around times for RT-PCR testing were at least 5 days, which meant significant delays in the provision of care to cancer patients who needed it sooner. The use of chest x-rays complemented careful anamnesis and physical examination in the immediate triage of patients. Following the uptake in use of RT-PCR due to increased testing capacity, the chest x-ray remained an invaluable part of the screening for our patients. This was because although RT-PCR was the gold standard for the diagnosis of COVID-19, this test had a reported false negative rate of 2–29%. This was quite significant considering the consequences of mistakes in patient triage. (16) Radiologic findings like bilateral opacities and lower lobe consolidations alerted the admitting physician regarding the possibility of COVID-19 even if a patient had a negative swab. The diagnostic performance of chest x-rays in the detection of COVID-19 was bolstered by the higher pre-test probability of the disease due to the continuing high rates of community transmission of COVID-19 in our locality.
As quarantine measures are eased the challenge of resuming patient services is one that requires much thought. Point of care (POC) antibody tests may have value in identifying patients with cancer who have recovered from COVID-19. Unfortunately, data shows that individuals with cancer exhibit lower rates of seroconversion compared to those without malignancy. Moreover, it appears that prior cytotoxic or surgical therapy within four weeks of SARS-CoV-2 infection may affect seroconversion in patients with cancer (17,18). More studies are needed to elucidate the effect of cancer and its treatment on the immune response to COVID-19; and as a corollary, how serologic testing can guide screening and treatment decision-making in the clinic. It remains unlikely from available data that these POC tests will supplant RT-PCR in the diagnosis of COVID-19. The goal is to ensure that patients are given appropriate care while mitigating the risk of acquiring COVID-19.
Conclusion
In a resource-poor setting where results for COVID-19 RT-PCR tests are delayed or where it is unavailable, the use of diagnostic imaging such as a chest x-ray may be a quick guide in the assessment and management of patients especially if the imaging results are suggestive of COVID-19 infection. Further study into the value of diagnostic imaging and serologic testing are needed for high-risk patients with cancer since symptomatic triaging for COVID-19 may be too challenging.
Ethical considerations
The authors have obtained informed consent from the patient in this report. All identifying information have been anonymized.
Author roles
FT and DS contributed equally to this work
FT: Conceptualization; Methodology; Investigation; Resources; Formal Analysis; Writing, OD/R&E; Project Administration
DS: Conceptualization; Methodology; Investigation; Formal Analysis; Writing OD/R&E; Supervision; Project Administration
JC: Resources; Formal Analysis; Writing OD
MP: Resources; Formal Analysis; Writing R&E
CJ: Formal Analysis; Writing OD/R&E; Supervision
Funding
This paper did not receive any funding.
Declaration of Competing Interest
All authors have no conflicts of interest.
References
- 1.World Health Organization. Maintaining essential health services: operational guidance for the COVID-19 context. 2020;(June):55.
- 2.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambertini M., Toss A., Passaro A., Criscitiello C., Cremolini C., Cardone C. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists’ perspective. ESMO Open [Internet] 2020 Mar 1;5(2) doi: 10.1136/esmoopen-2020-000759. http://esmoopen.bmj.com/content/5/2/e000759.abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye X., Xiao X., Li B., Zhu W., Li Y., Wu J. Low Humoral Immune Response and Ineffective Clearance of SARS-Cov-2 in a COVID-19 Patient With CLL During a 69-Day Follow-Up [Internet] Front Oncol. 2020;10:1272. doi: 10.3389/fonc.2020.01272. https://www.frontiersin.org/article/10.3389/fonc.2020.01272 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee L.Y.W., Cazier J.-.B., Starkey T., Briggs S.E.W., Arnold R., Bisht V. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol [Internet] 2020;2045(20):1–8. doi: 10.1016/S1470-2045(20)30442-3. https://linkinghub.elsevier.com/retrieve/pii/S1470204520304423 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillen E., Pineiro G.J., Revuelta I., Rodriguez D., Bodro M., Moreno A. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20(7):1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan L., Li D., Xue H., Zhang L., Liu Z., Zhang B. Progress and prospect on imaging diagnosis of COVID-19. Chinese J Acad Radiol [Internet] 2020;3(1):4–13. doi: 10.1007/s42058-020-00031-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ippolito D., Pecorelli A., Maino C., Capodaglio C., Mariani I., Giandola T. Diagnostic impact of bedside chest X-ray features of 2019 novel coronavirus in the routine admission at the emergency department: case series from Lombardy region. Eur J Radiol [Internet] 2020;129(April) doi: 10.1016/j.ejrad.2020.109092. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan L., Li D., Xue H., Zhang L., Liu Z., Zhang B. Progress and prospect on imaging diagnosis of COVID-19. Chinese J Acad Radiol [Internet] 2020;3(1):4–13. doi: 10.1007/s42058-020-00031-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu B., Xing Y., Peng J., Zheng Z., Tang W., Sun Y., et al. Chest CT for Detecting COVID-19: a Systematic Review and Meta-Analysis of Diagnostic Accuracy. 2020;(866). [DOI] [PMC free article] [PubMed]
- 11.Hope M.D., Raptis C.A., Shah A., Hammer M.M., Henry T.S. A role for CT in COVID-19? What data really tell us so far. Lancet. 2020;395(10231):1189–1190. doi: 10.1016/S0140-6736(20)30728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naguib M., Moustafa F., Salman M.T., Saeed N.K., Al-Qahtani M. The use of radiological imaging alongside reverse transcriptase PCR in diagnosing novel coronavirus disease 2019: a narrative review. Future Microbio. 2020;15:897–903. doi: 10.2217/fmb-2020-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonsenso D., Pata D., Chiaretti A. COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med. 2020;8(5):e27. doi: 10.1016/S2213-2600(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buda N., Segura-Grau E., Cylwik J., Wełnicki M. Lung ultrasound in the diagnosis of COVID-19 infection - A case series and review of the literature. Adv Med Sci. 2020;65(2):378–385. doi: 10.1016/j.advms.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargani L., Soliman-Aboumarie H., Volpicelli G., Corradi F., Pastore M.C., Cameli M. Why, when, and how to use lung ultrasound during the COVID-19 pandemic: enthusiasm and caution. Eur Hear J - Cardiovasc Imaging. 2020;21(9):941–948. doi: 10.1093/ehjci/jeaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engla Lurie N. Journal - 2010 - New engla nd journal. N Engl J Med [Internet] 2020;38(1):1969–1973. Available from: nejm.org. [Google Scholar]
- 17.Liu T., Zeng G., Tao H., Shi Y., Group C-19 in CPR. Wang T. Low prevalence of IgG antibodies to SARS-CoV-2 in cancer patients with COVID-19. Int J Cancer [Internet] 2020 Jun 11:n/a(n/a). doi: 10.1002/ijc.33148. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solodky M.L., Galvez C., Russias B., Detourbet P., N'Guyen-Bonin V., Herr A.-.L. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol [Internet] 2020 Aug 1;31(8):1087–1088. doi: 10.1016/j.annonc.2020.04.475. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]