Abstract
Objectives
Use of corticosteroids is common in the treatment of coronavirus disease 2019, but clinical effectiveness is controversial. We aimed to investigate the association of corticosteroids therapy with clinical outcomes of hospitalized COVID-19 patients.
Methods
In this single-centre, retrospective cohort study, adult patients with confirmed coronavirus disease 2019 and dead or discharged between 29 December 2019 and 15 February 2020 were studied; 1:1 propensity score matchings were performed between patients with or without corticosteroid treatment. A multivariable COX proportional hazards model was used to estimate the association between corticosteroid treatment and in-hospital mortality by taking corticosteroids as a time-varying covariate.
Results
Among 646 patients, the in-hospital death rate was higher in 158 patients with corticosteroid administration (72/158, 45.6% vs. 56/488, 11.5%, p < 0.0001). After propensity score matching analysis, no significant differences were observed in in-hospital death between patients with and without corticosteroid treatment (47/124, 37.9% vs. 47/124, 37.9%, p 1.000). When patients received corticosteroids before they required nasal high-flow oxygen therapy or mechanical ventilation, the in-hospital death rate was lower than that in patients who were not administered corticosteroids (17/86, 19.8% vs. 26/86, 30.2%, log rank p 0.0102), whereas the time from admission to clinical improvement was longer (13 (IQR 10–17) days vs. 10 (IQR 8–13) days; p < 0.001). Using the Cox proportional hazards regression model accounting for time varying exposures in matched pairs, corticosteroid therapy was not associated with mortality difference (HR 0.98, 95% CI 0.93–1.03, p 0.4694).
Discussion
Corticosteroids use in COVID-19 patients may not be associated with in-hospital mortality.
Keywords: Corticosteroids, COVID-19, Outcome, SARS-CoV-2 pneumonia, Treatment
Introduction
Since December 2019, the SARS-CoV-2 pandemic has emerged as a major global health threat [1,2]. The characteristics of the viral associated disease coronavirus disease 2019 (COVID-19) have been described [[3], [4], [5], [6]]. In the absence of effective antiviral drugs [7,8], supportive and adjuvant treatments are of importance.
Systemic corticosteroid treatment was commonly used among critically ill patients with viral pneumonia, including severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS), but the effect was controversial. Previous studies showed that systemic corticosteroids did not improve outcome and delayed clearance of viral RNA or increased side effect complications [[9], [10], [11]]. However, a retrospective observational study which enrolled 1278 SARS cases showed that use of corticosteroids in critically ill patients lowered mortality and shortened hospitalization stay [12]. According to studies of influenza, time and dosage of corticosteroid use may affect the efficacy [13,14]. With the COVID-19 pandemic, corticosteroid treatment has received renewed attention. There have been different randomized controlled trials (RCTs) of corticosteroids in which clinical efficacy was controversial [[15], [16], [17]]. Here we describe details in the use of systemic corticosteroids among all eligible patients admitted to Jinyintan Hospital, a designated hospital for COVID-19 in Wuhan, China. We aimed to investigate the association of corticosteroid treatment on COVID-19 outcomes and explore the timing of corticosteroids use in COVID-19.
Material and methods
Study design and data collection
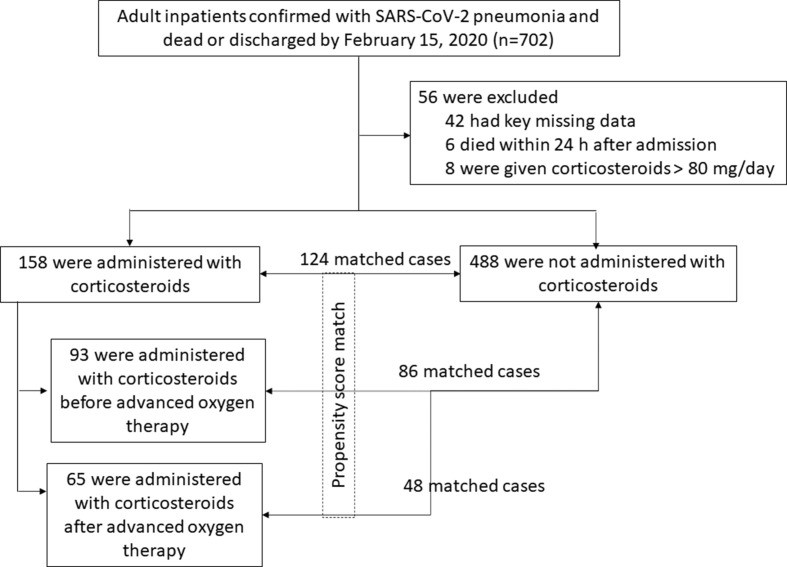
This was a single-centre, retrospective cohort study. Inclusion criteria were adult age (≥18 years old), confirmed SARS-CoV-2 pneumonia and definite outcome (died or discharged) for patients in Jinyintan Hospital (Wuhan, China) between 29 December 2019 and 15 February 2020. The diagnosis was based on World Health Organization interim guidance [18]. Patients who were without available key information, e.g. missing details of corticosteroid use, or dead within 24 hr after admission were excluded. Patients were also excluded if they received corticosteroid dosage higher than recommended [19]. The flow diagram of patient selection is shown in Fig. S1.
Data were collected from electronic medical records using a standardized data collection form. All data were checked by two physicians (Z.L. and F.Z.), and a third researcher (G.F.) adjudicated any difference in interpretation between the two primary reviewers.
The study was approved by the Research Ethics Commission of Jinyintan Hospital (KY-2020-01.01) and informed consent was waived by the ethics commission as described previously [6].
Definitions
Disease severity referred to status defined by the Chinese management guideline for COVID-19 (Version 6.0), including non-severe (mild and moderate), severe and critical [21]. Nosocomial infection was diagnosed when the patients had clinical symptoms or signs of pneumonia or bacteraemia and positive culture of pathogens from lower respiratory tract specimens (the sputum, transtracheal aspirates, bronchoalveolar lavage fluid) and/or from blood samples taken 48 hr after admission [22]. Simple oxygen therapy refers to nasal cannula oxygen support or mask oxygen inhalation; advanced oxygen therapy included nasal high-flow oxygen therapy (NHFOT), non-invasive mechanical ventilation (NIMV) and/or invasive mechanical ventilation (IMV), or extracorporeal membrane oxygenation (ECMO). Deterioration was defined as patients with simple oxygen therapy requiring advanced oxygen therapy during the disease course.
Outcomes
The primary clinical outcome was in-hospital death. Secondary clinical outcome was requiring advanced oxygen therapy. Other admission data included the time to undetectable SAS-CoV-2 RNA from admission, Intensive Care Unit (ICU) admission, ICU length of stay (days), hospital length of stay (days), nosocomial infections and duration of detectable RNA after illness onset (days). All patients received nucleic acid tests (NATs) at admission. Subsequently, NATs were conducted when the symptoms improved. In the patients without symptom improvement, NATs were conducted in the week after admission according to routine practice.
Main exposure
Systemic corticosteroid treatment was defined as use of corticosteroids (any of methylprednisolone, prednisolone, dexamethasone and/or hydrocortisone), via oral or intravenous route, and at least one dose in hospital. According to the expert consensus statement in China [19], the recommended dose of corticosteroid was low to moderate (≤0.5–1 mg/kg per day methylprednisolone or equivalent). Low-to-moderate dose was defined as ≤80 mg per day methylprednisolone or equivalent. High-dose was defined as >80 mg per day methylprednisolone or equivalent.
Statistical analysis
Continuous variables were expressed as median (interquartile range [IQR]) and categorical variables as number (proportion). Two-group comparisons (with corticosteroids vs. without corticosteroids) were conducted with Mann–Whitney U test or chi-squared/Fisher exact test, where appropriate.
A propensity score was estimated by logistic regression to determine the probability of corticosteroids treatment of each patient conditionally on observed covariates. Based on the propensity score, we performed a 1:1 match to match patients who were not administered corticosteroids to those who were, on a range ±0.0001 to ±0.1. The match started with a range of ±0.0001, and those who were matched were extracted from the database and excluded from the following ranges. If more than two patients who were not administered corticosteroids were detected, only one of them was selected randomly. Variables involved in the propensity score estimation included age, gender, lymphocyte count, disease severity status and antiviral treatment. As sensitivity analysis, we performed propensity score (PS) matched analysis in two subgroups: patients not requiring advanced oxygen therapy, and patients requiring advanced oxygen therapy.
Kaplan–Meier curves and log-rank tests were used to display and test the differences of in-hospital death rates between groups with or without corticosteroid treatment. In multivariable COX proportional hazard model, only variables with a p < 0.05 in univariable analysis or a presumptive association with the event were included to avoid overfitting. Moreover, for patients were treated with corticosteroids at different times after admission, the treatment was regarded as a time-varying covariate.
A two-sided α < 0.05 was considered statistically significant. Statistical analyses were conducted using SAS software, version 9.4 (SAS Institute).
Results
During 29 December 2019 to 15 February 2020, 702 patients with confirmed SARS-CoV-2 pneumonia and definite outcome (discharged or died) were admitted to Jinyintan Hospital (Fig. 1 ). We excluded 48 inpatients without available key information (details of corticosteroid use) in their medical records (n = 42) or dead within 24 hr after admission (n = 6). Eight patients received high-dose corticosteroid and they were excluded. A total 646 patients were included, and 158 were administered with low-to-moderate dose corticosteroids and 488 were free of corticosteroids (Table 1 ).
Fig. 1.
Schematic of patients' selection in the study. Advanced oxygen therapy included nasal high-flow oxygen therapy (NHFOT), non-invasive mechanical ventilation (NIMV) and/or invasive mechanical ventilation (IMV), or extracorporeal membrane oxygenation (ECMO).
Table 1.
Characteristics of inpatients before and after propensity score match
| Characteristics | Before propensity score match |
After propensity score match |
||||||
|---|---|---|---|---|---|---|---|---|
| Total N = 646 |
Without corticosteroids N = 488 |
Corticosteroids N = 158 |
p | Total N = 248 |
Without corticosteroids N = 124 |
Corticosteroids N = 124 |
p | |
| Age (years) | 57.0 (47.0, 67.0) | 56.5 (46.0, 66.0) | 59.5 (48.0, 69.0) | 0.0313 | 60.0 (48.0, 70.0) | 58.5 (48.0, 69.5) | 61.0 (49.0, 70.0) | 0.4749 |
| Male | 321 (49.7) | 227 (46.5) | 94 (59.5) | 0.0046 | 139 (56.0) | 69 (55.6) | 70 (56.5) | 0.8982 |
| Time from illness onset to admission | 11.0 (8.0, 15.0) | 12.0 (8.0, 15.0) | 11.0 (8.0, 13.0) | 0.1999 | 11.0 (8.0, 14.0) | 11.0 (8.0, 15.0) | 11.0 (8.0, 13.0) | 0.9815 |
| Hospital length of stay (days) | 11.0 (8.0, 14.0) | 10.0 (7.0, 14.0) | 12.0 (9.0, 16.0) | 0.0002 | 11.0 (8.0, 15.0) | 11.0 (8.0, 14.0) | 12.0 (9.0, 16.0) | 0.0916 |
| Comorbidity | 292 (45.2) | 220 (45.1) | 72 (45.6) | 0.9148 | 127 (51.2) | 71 (57.3) | 56 (45.2) | 0.0567 |
| Hypertension | 185 (28.6) | 142 (29.1) | 43 (27.2) | 0.6490 | 77 (31.0) | 45 (36.3) | 32 (25.8) | 0.0744 |
| Diabetes | 90 (13.9) | 70 (14.3) | 20 (12.7) | 0.5948 | 41 (16.5) | 26 (21.0) | 15 (12.1) | 0.0601 |
| Coronary heart disease | 33 (5.1) | 26 (5.3) | 7 (4.4) | 0.6561 | 15 (6.0) | 9 (7.3) | 6 (4.8) | 0.4242 |
| Chronic obstructive lung disease | 8 (1.2) | 5 (1.0) | 3 (1.9) | 0.4102 | 8 (3.2) | 5 (4.0) | 3 (2.4) | 0.4700 |
| Carcinoma | 15 (2.3) | 11 (2.3) | 4 (2.5) | 0.8419 | 3 (1.2) | 1 (0.8) | 2 (1.6) | 0.5576 |
| Chronic kidney disease | 14 (2.2) | 11 (2.3) | 3 (1.9) | 0.7868 | 4 (1.6) | 2 (1.6) | 2 (1.6) | 1.0000 |
| Others | 97 (15.0) | 70 (14.3) | 27 (17.1) | 0.4013 | 38 (15.3) | 19 (15.3) | 19 (15.3) | 1.0000 |
| Temperature ≥37.3 °C | 576 (89.2) | 427 (87.5) | 149 (94.3) | 0.0168 | 231 (93.1) | 115 (92.7) | 116 (93.5) | 0.8016 |
| Cough | 536 (83.0) | 400 (82.0) | 136 (86.1) | 0.2324 | 206 (83.1) | 102 (82.3) | 104 (83.9) | 0.7349 |
| Sputum | 148/645 (22.9) | 98/487 (20.1) | 50/158 (31.6) | 0.0028 | 74/247 (30.0) | 33/123 (26.8) | 41/124 (33.1) | 0.2848 |
| Dyspnoea | 362 (56.0) | 238 (48.8) | 124 (78.5) | <.0001 | 175 (70.6) | 82 (66.1) | 93 (75.0) | 0.1254 |
| White blood cell count ( × 109/L) | 6.1 (4.5, 8.4) | 5.7 (4.4, 7.8) | 7.5 (4.9, 11.2) | <.0001 | 6.8 (4.6, 9.5) | 6.8 (4.4, 9.3) | 6.8 (4.7, 10.5) | 0.6658 |
| Lymphocyte count ( × 109/L) | 1.0 (0.7, 1.4) | 1.1 (0.8, 1.5) | 0.7 (0.5, 0.9) | <.0001 | 0.7 (0.6, 1.0) | 0.8 (0.6, 1.0) | 0.7 (0.5, 1.0) | 0.1725 |
| Creatinine (μmol/L) | 67.3 (55.8, 81.6) | 66.6 (55.1, 80.1) | 70.0 (58.4, 89.3) | 0.0196 | 70.2 (58.4, 86.6) | 71.0 (59.1, 85.5) | 69.4 (57.5, 89.1) | 0.7838 |
| Consolidation | 354 (54.8) | 265 (54.3) | 89 (56.3) | 0.6565 | 133 (53.6) | 69 (55.6) | 64 (51.6) | 0.5243 |
| Ground-glass opacity | 483 (74.8) | 361 (74.0) | 122 (77.2) | 0.4151 | 184 (74.2) | 91 (73.4) | 93 (75.0) | 0.7716 |
| Disease severity status | <.0001 | 0.9221 | ||||||
| General | 409 (63.3) | 367 (75.2) | 42 (26.6) | 81 (32.7) | 41 (33.1) | 40 (32.3) | ||
| Severe | 104 (16.1) | 68 (13.9) | 36 (22.8) | 64 (25.8) | 33 (26.6) | 31 (25.0) | ||
| Critically ill | 133 (20.6) | 53 (10.9) | 80 (50.6) | 103 (41.5) | 50 (40.3) | 53 (42.7) | ||
| Antibiotic | 598 (92.6) | 441 (90.4) | 157 (99.4) | 0.0002 | 239 (96.4) | 116 (93.5) | 123 (99.2) | 0.0114 |
| Antiviral | 93 (14.4) | 57 (11.7) | 36 (22.8) | 0.0005 | 37 (14.9) | 19 (15.3) | 18 (14.5) | 0.8585 |
| Oxygen therapy or mechanical ventilation on admission | ||||||||
| High-flow nasal cannula oxygen therapy | 112 (17.3) | 48 (9.8) | 64 (40.5) | <0.0001 | 84 (33.9) | 36 (29.0) | 48 (38.7) | 0.1074 |
| Non-invasive mechanical ventilation | 65 (10.1) | 23 (4.7) | 42 (26.6) | <0.0001 | 44 (17.7) | 20 (16.1) | 24 (19.4) | 0.5061 |
| Invasive mechanical ventilation | 52 (8.0) | 21 (4.3) | 31 (19.6) | <0.0001 | 38 (15.3) | 20 (16.1) | 18 (14.5) | 0.7244 |
| ECMO | 2 (0.3) | 1 (0.2) | 1 (0.6) | 0.4296 | 2 (0.8) | 1 (0.8) | 1 (0.8) | 1.0000 |
Data are median (IQR) or n (%). p values were calculated by Mann–Whitney U test, χ2 test, or Fisher's exact test, as appropriate. ECMO, extracorporeal membrane oxygenation.
Patients characteristics
Among 646 patients included, the median age was 57 (IQR, 47–67) years, and 49.7% (321/646) were male. Severe or critically ill patients (116/158, 73.4%) received corticosteroid treatment more frequently (Table 1).
After the propensity score match, a total of 124 propensity score-matched pairs were selected. No significant differences were observed after match and characteristics for patients with or without corticosteroids were well balanced. Other details of patient characteristics are shown in Table 1.
Corticosteroids treatment
Of 158/646 (24.5%) patients administered systemic low-to-moderate dose corticosteroids, 149 patients received methylprednisolone, eight received prednisolone and one received dexamethasone. The median of the initial daily methylprednisolone-equivalent dose was 80 mg (IQR 40–80 mg) (Table 2 ). Corticosteroid therapy was started at a median of 13 (IQR 11–17) days after illness onset and 3 (IQR 2–5) days after admission, with a median duration of 5 (IQR 3–8) days.
Table 2.
Corticosteroids treatment and outcomes of inpatients before and after propensity score match
| Corticosteroids therapy outcomes | Before propensity score match |
After propensity score match |
||||||
|---|---|---|---|---|---|---|---|---|
| Total N = 646 |
Without corticosteroids N = 488 |
Corticosteroids N = 158 |
p | Total N = 248 |
Without corticosteroids N = 124 |
Corticosteroids N = 124 |
p | |
| Corticosteroids administered | ||||||||
| Methylprednisolone | 149/158 (94.3) | — | 149/158 (94.3) | 115/124 (92.7) | — | 115 (92.7) | ||
| Prednisolone | 8/158 (5.1) | — | 8/158 (5.1) | 8/124 (6.5) | — | 8 (6.5) | ||
| Dexamethasone | 1/158 (0.6) | — | 1/158 (0.6) | 1/124 (0.8) | — | 1 (0.8) | ||
| Duration of corticosteroids treatment (days) | — | — | 5 (3, 8) | — | — | 5 (3, 7) | ||
| Initial dose (equivalent methylprednisolone, mg/day) | — | — | 80 (40, 80) | — | — | 80 (40, 80) | ||
| Time from illness onset to corticosteroids treatment (days) | — | — | 13 (11, 17) | — | — | 14 (11, 17) | ||
| Time from admission to corticosteroids treatment (days) | — | — | 3 (2, 5) | — | — | 3 (2, 5) | ||
| Clinical outcomes | 518 (80.2) | 432 (88.5) | 86 (54.4) | <.0001 | 154 (62.1) | 77 (62.1) | 77 (62.1) | 1.0000 |
| ICU admission | 82 (12.7) | 38 (7.8) | 44 (27.8) | <.0001 | 58 (23.4) | 32 (25.8) | 26 (21.0) | 0.3681 |
| ICU length of stay (days) | 8 (4, 11) | 7 (4, 11) | 8 (4, 11) | 0.5272 | 8 (4, 10) | 7 (4, 11) | 8 (2, 10) | 0.9551 |
| Hospital length of stay (days) | 11 (8, 14) | 10 (7, 14) | 12 (9, 16) | 0.0002 | 11 (8, 15) | 11 (8, 14) | 12 (9, 16) | 0.0916 |
| Death | 128 (19.8) | 56 (11.5) | 72 (45.6) | <.0001 | 94 (37.9) | 47 (37.9) | 47 (37.9) | 1.0000 |
| Duration of detectable RNA after illness onset (days)a | 19 (15, 23) | 19 (14, 23) | 19 (16, 23) | 0.3984 | 19 (16, 23) | 20 (16, 23) | 19 (16, 23) | 0.7644 |
| Duration of detectable RNA after admission (days)a | 8 (4, 11) | 7 (4, 11) | 9 (6, 13) | 0.0063 | 9 (6, 12) | 9 (6, 12) | 9 (6, 13) | 0.8041 |
| Nosocomial infection | 25 (3.9) | 14 (2.9) | 11 (7.0) | 0.0204 | 18 (7.3) | 11 (8.9) | 7 (5.6) | 0.3276 |
Data are median (IQR) or n (%). p values were calculated by Mann–Whitney U test, χ2 test, or Fisher's exact test, as appropriate. ICU, intensive care unit; IMV, invasive mechanical ventilation.
The date of viral negative was available in survival patients, because the virus was continuously detectable until death in non-survivors.
When patients received corticosteroids, 17/158 (10.8%) did not require supplemental oxygen, 76/158 (48.1%) required simple oxygen therapy, 49/158 (31.0%) required NHFOT and/or NIMV and 16/158 (10.1%) required IMV and/or ECMO.
Effects of corticosteroids on in-hospital death
In the entire cohort, patients who received corticosteroid treatment had a higher mortality rate than those who did not (72/158, 45.6% vs. 56/488, 11.5%, p < 0.0001).
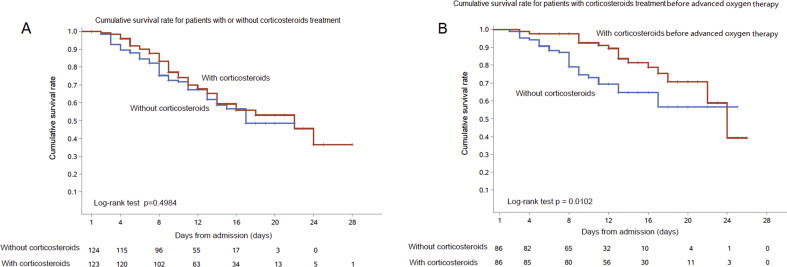
Among 124 propensity score-matched pairs, there was no difference of in-hospital mortality (Table 2 and Fig. 2 A). ICU admission and median ICU length of stay were similar in the two matched groups (Table 2).
Fig. 2.
Kaplan–Meier curves of cumulative death rate for patients administered with corticosteroids at any category (A) and before advanced oxygen therapy (B).
Among patients administered with corticosteroid treatment before advanced oxygen therapy, 93 patients started corticosteroids (Fig. 1). Eighty-six propensity score-matched pairs were generated and the baseline characteristics are described in Table S1. Patients who received corticosteroids showed a lower in-hospital death rate (17/86, 19.8% vs. 26/86, 30.2%, log rank test p 0.0102). ICU length of stay was similar in matched pairs. ICU admission rate was less in patients who received corticosteroids, but without significant difference (9/86, 10.5% vs. 18/86, 20.9%, p 0.0592) (Table S2).
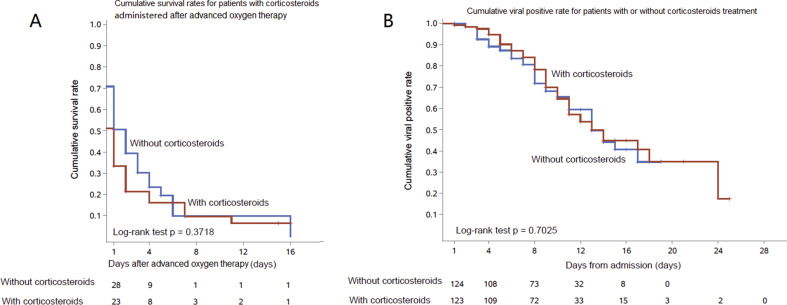
In the patients initiating corticosteroids after they required advanced oxygen therapy, a total of 48 propensity score-matched pairs were generated (Table S1). There were no significant differences in ICU admission, ICU length of stay, in-hospital mortality, hospital length of stay (Table S2) and cumulative survival rate (Fig. S1A) between matched pairs.
In univariable analysis, HR of in-hospital death was 0.46 (95% CI 0.26–0.82) in patients with corticosteroids initiation before advanced oxygen support in 86 propensity score-matched pairs (p 0.0087). Age, female, diabetes and hypertension were associated with higher risk of death (Table S3). After adjusting for the time-varying exposures and variables listed in Table 3 , corticosteroid treatment was not associated with mortality in 124 matched pairs (adjusted HR (aHR) 0.92; 95% CI 0.56–1.49; p 0.7234) or those administered with corticosteroids before advanced oxygen therapy (aHR, 0.57; 95% CI 0.24–1.32, p 0.1875) (Table 3).
Table 3.
Adjusted hazard ratios of risk factors associated with death for matched cases administered with corticosteroids
| Risk factors | All matched cases | p | Corticosteroids treatment before advanced oxygen therapy | p | |
|---|---|---|---|---|---|
| Corticosteroids | No | Ref | Ref | ||
| Yes | 0.92 (0.56-1.49) | 0.7234 | 0.57 (0.24–1.32) | 0.1875 | |
| Age (years) | 1.08 (1.06-1.09) | <0.0001 | 1.09 (1.06–1.12) | <0.0001 | |
| Diabetes | No | Ref | Ref | ||
| Yes | 1.45 (0.93-2.28) | 0.1043 | 1.85 (1.05–3.26) | 0.0331 | |
| Hypertension | No | Ref | Ref | ||
| Yes | 1.00 (0.65-1.53) | 0.9939 | 0.64 (0.33–1.25) | 0.1907 | |
| Antiviral | No | Ref | Ref | ||
| Yes | 0.93 (0.49-1.76) | 0.8193 | 0.90 (0.44–1.86) | 0.7792 | |
| Corticosteroids∗ time from admission to corticosteroids treatment | 0.98 (0.93-1.03) | 0.4694 | 0.87 (0.73–1.03) | 0.1122 |
Adjusted hazard ratios were expressed as adjusted hazard ratios (95% confidence intervals), estimated by Cox proportional hazard model.
Corticosteroids was taken as time-varying covariate.
In the patients who were administered corticosteroids before advanced oxygen therapy (86 matched pairs), corticosteroid treatment did not reduce mortality (aHR, 0.87; 95% CI 0.73–1.03, p 0.1122) (Table 3) or deterioration (aHR, 0.99; 95% CI 0.81–1.21, p 0.9195) (Table S4).
Effects of low-to-moderate dose corticosteroids on duration of detectable SARS-CoV-2 RNA and nosocomial infections among matched groups.
Among all 646 patients, the median duration of detectable SARS-CoV-2 RNA after illness onset was 20 (IQR 15–24) days and 19 (IQR 16–24) days in patients who received corticosteroids. In 124 matched pairs, there was no significant difference (Table S1, Table 2 and Fig. S1B).
The analysis of 124 propensity score-matched pairs showed a similar rate of nosocomial infections in patients with corticosteroids use and without (7/124, 5.6% vs. 11/124, 8.9%, p 0.3276).
Discussion
This study investigated the impact of corticosteroids on clinical outcomes of patients with COVID-19. The propensity score-based matching analysis showed that corticosteroid use in COVID-19 patients was not associated with a difference in mortality after adjustment for time-varying confounders.
There are controversies of corticosteroid use among patients with acute respiratory infections. In patients with influenza, mortality and nosocomial infections may be increased after corticosteroid treatment [13]. In a retrospective study of MERS patients, the authors found no association between corticosteroids administration and mortality, but prolonged duration of detectable MERS virus RNA [9]. The recent RECOVERY study showed the use of dexamethasone decreased 28-day mortality in severe or critical COVID-19 patients. The differences in the effect of corticosteroids among different clinical studies may be due to different timing and dosages. In a recent meta-analysis [17], the authors showed the benefit associated with corticosteroids appeared greater in critically ill patients with COVID-19 who were not receiving invasive mechanical ventilation at randomization, although this comparison was based on only four trials and 144 patients, of whom 42 died.
In influenza pneumonia and MERS pneumonia, a high dose of corticosteroids prolonged viral shedding and increased the rate of secondary infection [9,13,23,24]. There is no evidence that a higher dose of corticosteroids is better than lower dose.
After the release of the RECOVERY trial, corticosteroids were soon accepted as the ‘standard’ therapy for severe and critical COVID-19, including recent WHO guidance [25]. However, there are still uncertainties, including generalizability of the RECOVERY findings to settings with lower baseline mortality; superinfections and other potential risks of corticosteroids; and long-term prognosis of corticosteroids users. Implications of the evidence from both our study and the RECOVERY study provided clues to design RCTs following PICO criteria in future. The populations should be restricted to certain age groups and oxygen therapy status (corticosteroids should be started before advanced oxygen therapy is needed); mortality as the primary outcome is undoubtably right, but virological outcome and adverse effects of corticosteroids should also be taken as essential outcomes.
There were some limitations in the study. Firstly, as an observational study, baseline characteristics differed greatly between patients who received corticosteroids and who did not. Propensity score-matched analysis was conducted to remedy the imbalance but with exclusion of a quite large proportion (about 20%) of patients. The sample size was small, and the lack of difference might be due to limited power. Secondly, the conclusion of the study is based on retrospective data. As there was no placebo, we could not match for time of corticosteroid use when calculating the propensity score because the time point of corticosteroids initiation did not exist in patients without corticosteroid administration; in fact, time-varying adjustment was conducted to deal with this. Thirdly, some parameters, for example hospital length of stay, are only described here, since as outcome measures they would be affected by survivor bias. As a retrospective study, side effects of corticosteroids, such as hypertension and hyperglycaemia, were missed. The timing of viral RNA sampling was sparse and not accurate, we report it here for completeness but the evidence is not conclusive.
In conclusion, corticosteroids use in COVID-19 patients was not associated with a difference in mortality after adjustment for time-varying confounders. With several open questions, more investigations are still needed.
Author contribution
BC, ZHH, ZBL, XL, GHF, FZ and conceived of the project and drafted the paper. BC, ZBL, GHF, FZ, YMW performed the analysis, and all authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published. ZBL, GHF, FZ, LXH, JPY, LNY, LHS, KX, JYX, ZSH collected the data. All authors agree to be accountable for the all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. None of the material has been published or is under consideration elsewhere, including the Internet. BC and ZHH take full responsibility for the data and analysis.
Transparency declaration
Major Projects of National Science and Technology on New Drug Creation and Development (2020ZX09201001) and (2020ZX09201012); Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003; 2020-I2M-CoV19-005); Natural Science Foundation of China (82041011/H0104). All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and no conflicts of interest are reported.
Acknowledgements
We acknowledge all healthcare workers involved in the diagnosis and treatment of patients in Wuhan. All authors declare that they have no competing interests. We thank Dr Nelson Lee (Division of Infectious Diseases, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta) for review of the manuscript.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.09.045.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in WUHAN, China: challenges for global health governance. JAMA. 2020;323:709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. Severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the coronavirus study group. bioRxiv. 2020 2020.2002.2007.937862. [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:407–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with covid-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeming Wang D.Z., Du Guanghua, Du Ronghui, Zhao Jianping, Jin Yang, Fu Shouzhi. Remdesivir in adults with severe covid-19: results of a randomized, double-blind, placebo-controlled, multicenter trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Resp Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 10.Lee N., Allen Chan K.C., Hui D.S., Ng E.K., Wu A., Chiu R.W. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao J.Z., Ma L., Gao J., Yang Z.J., Xing X.Y., Zhao H.C. Glucocorticoid-induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy. Zhonghua Nei Ke Za Zhi. 2004;43:179–182. [PubMed] [Google Scholar]
- 12.Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao B., Gao H., Zhou B., Deng X., Hu C., Deng C. Adjuvant corticosteroid treatment in adults with influenza a (h7n9) viral pneumonia. Crit Care Med. 2016;44:e318–e328. doi: 10.1097/CCM.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Yang S.G., Gu L., Zhang Y., Yan X.X., Liang Z.A. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza a(h1n1)pdm09 viral pneumonia. Influenza Other Respir Virus. 2017;11:345–354. doi: 10.1111/irv.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group R.C., Horby P., Lim W.S., Emberson R.J., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020 [Google Scholar]
- 16.Jeronimo C.M.P., Farias M.E.L., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C. Methylprednisolone as adjunctive therapy for patients hospitalized with covid-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Group TWREAfC-TRW Association between administration of systemic corticosteroids and mortality among critically ill patients with covid-1: a meta-analysis. JAMA. 2020 doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . WHO; Geneva: 2020. Clinical management of severe acute respiratory infection when novel coronavirus. [Google Scholar]
- 19.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-ncov pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NHCotpsRo China. Chinese management guideline for covid-19. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf (version 6.0)
- 22.Lee N., Leo Y.S., Cao B., Chan P.K., Kyaw W.M., Uyeki T.M. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur Resp J. 2015;45:1642–1652. doi: 10.1183/09031936.00169714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamontagne F., Briel M., Guyatt G.H., Cook D.J., Bhatnagar N., Meade M. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care. 2010;25:420–435. doi: 10.1016/j.jcrc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Hui D.S. Systemic corticosteroid therapy may delay viral clearance in patients with middle east respiratory syndrome coronavirus infection. Am J Resp Crit Care Med. 2018;197:700–771. doi: 10.1164/rccm.201712-2371ED. [DOI] [PubMed] [Google Scholar]
- 25.WHO/2019-nCoV/Corticosteroids/2020.1 . 2020. Corticosteroids for covid-19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.