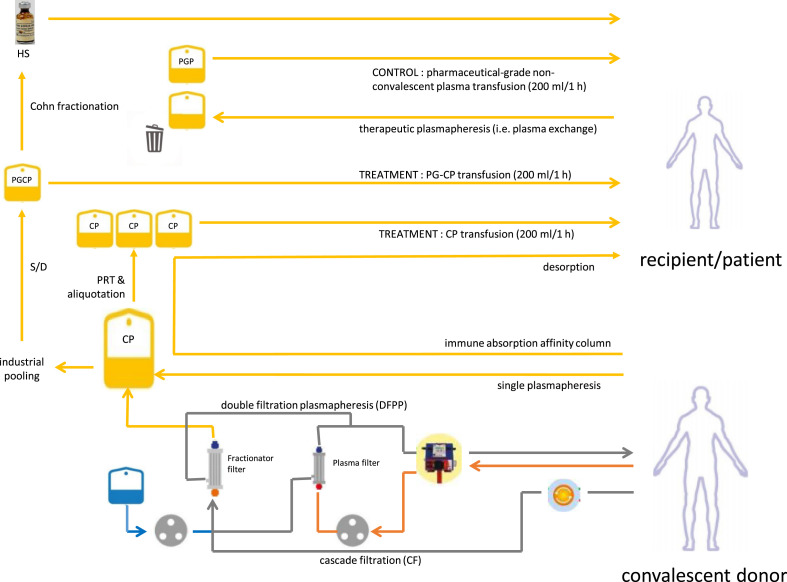
Signals from recent several publications have suggested convalescent plasma (CP) as an effective treatment for non-mechanically ventilated individuals with coronavirus disease 2019 (COVID-19) [1], despite other trials being inconclusive or negative [2,3]. Health systems should choose: is it better to directly bank CP from recovered donors or instead to ship CP to industries for transformation into pharmaceutical-grade CP or fractionation into hyperimmune serum? Until now, hyperimmune serum has been typically used for post-exposure prophylaxis (for wxample, in tetanus, diphtheria or rabies) for infectious diseases that can be prevented by vaccines and treated by antimicrobials, leaving no room for deployment of alternative therapeutic CP programmes. Today, a unique landscape is provided by advances in plasma apheresis and plasma fractionation procedures on the one side, and the occurrence of a respiratory virus pandemic for which no curative antiviral is available on the other.
Although there are now more than 80 clinical trials investigating CP therapy for COVID-19 worldwide [1] and more than 90 000 patients have been treated to date with CP in the USA alone under expanded access programmes, the vast majority of health policy-makers are generally assuming this approach to be a transient stage. Fig. 1 summarizes the possible routes from CP donors towards plasma-derived (combination) therapies. Hyperimmune serum is often considered a superior, pharmaceutical-grade product than freshly collected CP: as such, it is often believed that CP should be replaced by hyperimmune serum as soon as it is available. In the mean time, several countries are considering solvent/detergent-inactivated pharmaceutical-grade convalescent plasma as an intermediate stage, which could facilitate logistics and benefit assessment in randomized clinical trials, especially when pharmaceutical-grade non-convalescent plasma is used as a control. Scalability (i.e. the availability of therapeutic doses for large numbers of patients) for hyperimmune serum and CP is similar, given that CP is the source material for hyperimmune serum. Nevertheless, several additional points show differential features, and they should be carefully analysed before drawing conclusions.
Fig. 1.
Routes from convalescent plasma donation to plasma-derived medicinal products. CP, convalescent plasma; HS, hyperimmune serum; PGP, pharmaceutical-grade plasma; PGCP, pharmaceutical-grade convalescent plasma; PRT, pathogen reduction technologies; S/D, solvent/detergent.
A first consideration is speed of access, i.e. manufacturing turn-around time. Collection of CP can be implemented very early during the course of a pandemic (as soon as a single fit donor is judged fully recovered and has relevant titres of neutralizing antibodies), whereas hyperimmune serum usually requires several months to adjust fractionation plants according to good manufacturing production regulations because severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a recent virus. Its diversity is much lower than for other viruses, but with the rapid massive infection of diverse human populations, major genetic variation is becoming increasingly likely. To date, mutations within the receptor-binding domain of the Spike protein impacting on antibody neutralization are accumulating. If the dominant strain changes later in the pandemics (e.g. in successive epidemic waves), the existing hyperimmune sera could be ineffective, whereas CP collection can be restarted immediately and result in an effective new product. If no dominant strain emerges but rather different strains circulate in different areas of the world at the same time (as is still currently the case with SARS-CoV-2 [4]), hyperimmune serum manufactured from donations collected in one continent could prove ineffective in a different continent. Even if hyperimmune serum pools CP units from different countries, the dilution factor is likely to make the most useful antibody specificities available at useless titres within a single hyperimmune serum dose.
A second point is safety. Several countries have mandated pathogen reduction technologies and additional molecular disease screening on every CP donation; although these rules make CP far more expensive, CP is today at least as safe as hyperimmune serum in terms of transfusion-transmitted infections, and exposes the recipient to a single donor instead of thousands of donors pooled. ABO blood group incompatibility and haemolysis due to natural isoagglutinins is also a common adverse effect from hyperimmune serum, which does not occur in ABO-matched CP transfusions. The risk of transfusion-related acute lung injury in nations where previously gravid females are prevented from donations is close to zero, and hence the risk from CP and hyperimmune serum (which also contains anti-HLA IgG) is probably comparable (anti-HLA antibodies not being among the current mandatory release tests for hyperimmune serum). The final reinfusion volume is hardly a great advantage, given that the 200-mL therapeutic dose of CP is not enough to cause circulatory overload in patients [5].
A third point is potency, i.e. efficacy at delivering clinical benefit. Hyperimmune serum is definitively standardized to a specified neutralizing IgG content per volume, as measured by a viral neutralization test. Nevertheless, there is currently no clearly defined threshold for neutralizing antibody content in hyperimmune serum stocks for COVID-19. Although hyperimmune serum, because of its pooling nature, obviously contains more antibody specificities than a single unit of CP, the dose does not necessarily represent the best possible dose, because it reflects dilutions of a few very high-titre donations into thousands of low-titre donations within the pool. Dilution invariably happens in pharmaceutical-grade convalescent plasma manufacturing because of pooling. Vendors have an obvious interest at maximizing product volume to increase incomes: this could lead to inclusion of donations with very low antibody titres. In the last pandemic it has been commonly observed that only a small fraction of donors develops high titres of neutralizing antibodies. In other words, a single CP unit could theoretically have a higher titre of neutralizing IgG than a standardized hyperimmune serum dose, and this is especially relevant in COVID-19, where there is a risk for so-called antibody-dependent enhancement of infection [6]. CP, but not hyperimmune serum, contains immunoglobulins of classes other than IgG, and IgA could be especially useful against SARS-CoV-2.
Most importantly, very few studies on hyperimmune serum efficacy in the treatment of respiratory infections have been run to date. In the setting of influenza, one randomized controlled trial of hyperimmune serum has shown efficacy only in a subcohort treated within 5 days of symptom onset [7]. Although more effective plasma fractionation technologies are under development, the currently used, old-fashioned process causes loss of about half the protein content [8]. Most of the neutralizing antibody responses in the IgG class have been shown to be associated with the IgG1 and IgG3 subclasses for SARS-CoV-2 [9]; unfortunately, the IgG3 fraction is often depleted during industrial fractionation [10], and its impact on plaque reduction neutralization test titres should be carefully evaluated. Additionally, CP can include different soluble factors expected to be beneficial such as anti-inflammatory cytokines or, in ABO-matched units, anti-A isoagglutinins (expected to inhibit SARS-CoV-2 entry [11]), which do not occur at all (or, in the case of isoagglutinins, occur at lower concentrations) in hyperimmune serum. Clotting factors contained in CP, but not in hyperimmune serum, can be useful in haemorrhagic infections (such as in Ebola virus) or potentially detrimental in prothrombotic infections (such as COVID-19).
A fourth, relevant point is cost. Under the safest and most expensive scenario for CP collection to date [12], the overall cost per patient of CP is probably lower than that of hyperimmune serum. This assumes that the cost of three 200-mL therapeutic doses of CP equals the cost of a single non-convalescent apheresis unit plus the cost of a pathogen reduction kit plus the cost of additional molecular disease screening (hepatitis A virus, hepatitis E virus and parvovirus B19) plus the cost of the viral neutralization test. On the other side of the coin, the cost of hyperimmune serum also includes the cost of additional shipping to distant locations, pooling and fractionation steps, and manufacturer's profit. A careful analysis should nevertheless include the benefit from additional plasma derivatives (other than hyperimmune serum) that can be achieved from industrial CP fractionation, unless CP is collected under waivers (as often happens under emergency settings). Even a small difference in cost between a single therapeutic dose of CP and a single therapeutic dose of hyperimmune serum could prove significant when the number of patients is extremely high, such as in a pandemic. Donors from countries where remunerated donation is forbidden could be reluctant to donate for creating private profits, and this phenomenon could impact CP availability. Nevertheless, both hyperimmune serum and CP will presumably cost less than the vast majority of drugs currently under clinical trials for COVID-19 (with differences likely to be smaller for small chemicals and higher for monoclonal antibodies or cell therapies).
A last point is the logistics of storage, distribution and administration. Shelf-life is very similar (2 years for CP versus 2–3 years for hyperimmune serum), but storage temperature under current regulations is easier to achieve for hyperimmune serum (2°C–8°C) than for plasma (below –25°C). Nevertheless, these regulations are designed for labile clotting factor preservation and poorly apply to preservation of neutralizing antibodies, especially as the product has to be reinfused within a few days and has been treated with pathogen reduction technologies. Delivery route also favours hyperimmune serum: while CP can be administered only intravenously, hyperimmune serum can also be delivered intramuscularly.
Searching the databases of published research, we were not able to find any pharmaco-economics or efficacy studies comparing hyperimmune serum with CP for any pathogen. We feel that rigorous analysis accounting for the five above-mentioned points, and eventually a randomized trial comparing hyperimmune serum with CP, should be run before health authorities endorse industry support and decide which fraction of CP should be addressed to plasma fractionators.
Transparency declaration
We declare that we have no conflicts of interest related to this manuscript.
Funding
We declare that no external funding was received.
Authors' contributions
DF and MT revised the literature and designed the viewpoint structure. DF wrote the first draft and FM and GA critically revised the manuscript. All the authors approved the final version.
Editor: L. Leibovici
References
- 1.Focosi D., Anderson A.O., Tang J.W., Tuccori M. Convalescent plasma therapy for COVID-19: state of the art. Clin Microbiol Rev. 2020;33 doi: 10.1128/CMR.00072-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. medrXiv preprints; 2020. Convalescent plasma in the management of moderate COVID-19 in India: an open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial) 2020.09.03.20187252. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharbharan A., Jordans C.C.E., GeurtsvanKessel C., den Hollander J.G., Karim F., Mollema F.P.N. medrXiv preprints; 2020. Convalescent Plasma for COVID-19. A randomized clinical trial. 2020.07.01.20139857. Ahead of print. [Google Scholar]
- 4.Koyama T., Weeraratne D., Snowdon J.L., Parida L. Emergence of drift variants that may affect COVID-19 vaccine development and antibody treatment. Pathogens (Basel, Switzerland) 2020;9 doi: 10.3390/pathogens9050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyner M.J., Bruno K.A., Klassen S.A., Kunze K.L., Johnson P.W., Lesser E.R. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95 doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0321-6. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung I.F.N., To K.K.W., Lee C.K., Lee K.L., Yan W.W., Chan K. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144:464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 8.Oncley J.L., Melin M., Richert D.A., Cameron J.W., Gross P.M. The separation of the antibodies, isoagglutinins, prothrombin, plasminogen and beta1-lipoprotein into subfractions of human plasma. J Am Chem Soc. 1949;71:541–550. doi: 10.1021/ja01170a048. [DOI] [PubMed] [Google Scholar]
- 9.Suthar M.S., Zimmerman M., Kauffman R., Mantus G., Linderman S., Vanderheiden A. Rapid generation of neutralizing antibody responses in COVID-19 patients. medRxiv. 2020 doi: 10.1016/j.xcrm.2020.100040. 2020.05.03.20084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Audet S., Virata-Theimer M.L., Beeler J.A., Scott D.E., Frazier D.J., Mikolajczyk M.G. Measles-virus-neutralizing antibodies in intravenous immunoglobulins. J Infect Dis. 2006;194:781–789. doi: 10.1086/506363. [DOI] [PubMed] [Google Scholar]
- 11.Focosi D. Anti-A Isohemagglutinin titers and SARS-CoV2 neutralization: implications for children and convalescent plasma selection. Br J Haematol. 2020 doi: 10.1111/bjh.16932. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchini M., Marano G., Velati C., Pati I., Pupella S., Liumbruno G.M. Vox Sanguinis; 2020. Operational protocol for donation of anti-COVID-19 convalescent plasma in Italy. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]