Abstract
Introduction:
The use of multiparametric (mp)MRI to assess prostate cancer (PCa) has increased over the last decade. We aimed to assess if pre-operative mpMRI lesion score, a variable routinely available for men undergoing pre-biopsy MRI, improves the performance of commonly used pre-operative predictive models for PCa recurrence.
Patients and Methods:
We analysed data from 372 PCa patients treated with radical prostatectomy in 2012-2017 and assessed with pre-biopsy mpMRI within 6 months prior to surgery. Suspicious areas for cancer were scored on a standardized 5-point scale. Cox regression was used to assess the association between mpMRI score and the risk of post-operative biochemical recurrence (BCR). Two different models were tested accounting for factors included in the Kattan nomogram and in the D’Amico risk-classification.
Results:
Overall, 53% and 30% of patients were found with a lesion scored 4 or 5 at pre-biopsy mpMRI, respectively. Risk varied widely by mpMRI (29% 2-year risk of BCR for a score 5 versus 5% for 1-2 disease), and mpMRI score was associated with large hazard ratios after adjusting for stage, grade and PSA: 1.66, 1.96 and 2.71 for scores 3, 4 and 5 respectively. However, 95% C.I. were very wide (0.19 to 14.20, 0.26 to 14.65 and 0.36 to 20.55) and included 1.
Conclusions:
Our data did not show that pre-operative models, commonly used to assess PCa risk, were improved after including the pre-biopsy mpMRI score. However, the value of pre-biopsy mpMRI to improve preoperative risk models should be investigated in larger data sets.
Keywords: MRI, prostate cancer, recurrence, PSA, predictive tool
MicroAbstract
We looked at the clinical significance of including the multiparametric (mp)MRI lesion score in common predictive tools for biochemical recurrence (BCR) after surgery for prostate cancer (PCa). Higher mpMRI score were associated with higher risk of BCR, although the association was not statistically significant and the predictive models were not improved by including the mpMRI score. The value of pre-biopsy mpMRI to improve preoperative risk models should be further investigated.
Introduction
The use of multiparametric magnetic resonance imaging (mpMRI) for the detection and assessment of prostate cancer (PCa) has increased in the last few years. 1. As such, mpMRI is currently suggested by clinical guidelines to identify lesions suspicious for cancer that need to be biopsied in men with a previously negative prostate sampling 1,2.
The Prostate Imaging Reporting And Data System (PIRADS) score or a Likert scale are routinely used to define the probability of cancer and its aggression at mpMRI 3,4. Previous data have shown that about 18% of cases with low mpMRI score (e.g. 1-2) would be eventually diagnosed with PCa and about 11% harbor a clinically significant disease 5. Considering that the use of mpMRI as a triage test to decide which patients should forgo or proceed to prostate biopsy is still under investigation 1, the case of a patient referred to biopsy despite a low mpMRI score is a common clinical scenario. When found with PCa, those patients could be eventually counseled for active treatments according to their risk of disease recurrence, which is an indicator of cancer aggression 2.
Factors commonly considered for disease-risk stratification include total serum PSA, clinical stage (cT) and Gleason score (GS) at biopsy 6–8; those factors have been included in preoperative models like the Kattan nomogram 8 and the D’Amico risk classification 7, which are commonly used to stratify patient’s risk before treatment, and have shown high accuracy for predicting biochemical recurrence (BCR) 2,9. Conversely, the accuracy of pre-biopsy mpMRI for predicting disease recurrence has been investigated in only a limited number of studies, with equivocal results 10–14. Patients with a lower pre-operative mpMRI score may harbor less aggressive disease than those with higher scores, regardless of other disease characteristics. If so, mpMRI could be helpful to better stratify the disease-risk and support the physician in counseling the patient regarding treatment. Critically, mpMRI score will be available for many patients and so incorporation into standard risk assessment tools would not require additional procedures or costs.
We evaluated whether adding the pre-biopsy mpMRI score to commonly used preoperative risk-predictive models, such as the Kattan nomogram and the D’Amico risk classification, would improve accuracy for predicting BCR after RP.
Methods
After institutional review board approval, we analyzed data from 402 patients who underwent a pre-biopsy mpMRI and were treated with RP at our institute between January 2012 and June 2017.
All included patients underwent the mpMRI assessment within 6 months prior to surgery. Images were acquired under a magnetic field of 1.5-T with endorectal coil or 3-T without endorectal coil. The mpMRI was performed either at our institution (N=187) or in outside centers (N=215); outside studies were internally reviewed. MRI systems (GE Healthcare, Wisconsin USA) and multichannel phased-array coils were used. Sequences acquired included T1-weighted images, T2-weighted images, diffusion-weighted sequences and parametric maps of apparent diffusion coefficients, and dynamic contrast-enhanced sequence. The imaging results were assessed by one of six experienced members of our institution’s genitourinary radiology section. Regions-of Interest (ROI) at mpMRI were scored on a 5-point Likert scale as previously published 15,16. This scale was developed in our institute using whole-mount prostatectomy specimens as reference and has previously shown equivalency with the original version of the PIRADS score17,18.
Patients with identified ROI (Likert≥3) at mpMRI, underwent a transrectal targeted biopsy under visual or software registration, followed by a transrectal ultrasound guided systematic biopsy. In the absence of ROI (Likert score <3), only systematic biopsies were performed. Biopsy specimens were reviewed by dedicated genitourinary pathologists. Given that we were interested in assessing the ability of the mpMRI score in predicting cancer outcomes after accounting for other predictors such as GS, we excluded from the analysis patients presenting biopsy cores with the highest GS in regions outside the identified ROI at mpMRI (N=22). As a sensitivity analysis, we included all patients with available biopsy data.
All patients were treated with RP with or without pelvic lymph node dissection according to pre-operative risk. Post-operative surveillance was based on serum PSA and physical examination performed at 6 weeks after surgery and every 6 months for the first 5 years. Patients were considered to have BCR if PSA was ≥0.1 ng/mL and remained ≥0.1 ng/mL on repeat assessment.
Patients missing pre-operative or pathological data were not considered for the analyses (N=3), likewise, those submitted to adjuvant treatments after surgery were excluded (N=5).
Statistical analyses
The aim of the study was to assess if the pre-biopsy mpMRI score was able to predict BCR after surgery and improve the accuracy of two pre-operative models: the Kattan nomogram (including total PSA, biopsy primary and secondary GS and clinical stage [cT]) and the D’Amico risk classification (including total PSA, biopsy GS sum and clinical stage [cT]). Cox regression analysis was used to test the association between predictors and post-operative BCR. Given the low number of events, the number of covariates that we could include was limited, and we therefore calculated a linear predictor for the Kattan and D’Amico models. The linear predictor provides a way to control for the effects of these covariates in all models regardless of the number of events, using only a single covariate (the linear predictor). The coefficients for the Kattan nomogram were obtained from https://www.mskcc.org/nomograms/prostate/pre_op/coefficients in January 2018. Kaplan-Meier analyses were used to estimate the disease-free survival probability after surgery according the pre-biopsy mpMRI score. Statistical analyses were conducted using Stata 15.0 (StataCorp, College Station, TX, USA).
Results
Table 1 reports pre- and post-operative characteristics of the entire cohort (N=372). Most patients had a suspected lesion scored 4 (53%) or 5 (30%) at the pre-biopsy mpMRI. After biopsy, half of the patients was diagnosed with a GS 3+4 PCa (50%) and 24% had a GS 4+3 disease. A total of 66% of patients were considered at intermediate risk of recurrence after treatment according the pre-operative D’Amico risk classification. Likewise, at the pathological evaluation after surgery, the majority of patients was diagnosed with a GS 3+4 (57%) and 4+3 (26%) disease. Lymph node dissection was performed in nearly all cases (97.6%), with positive lymph nodes found in 11%.
Table 1 –
Pre- and post-operative patients’ characteristics (N=372)
| Pre-operative characteristics | |
|---|---|
| Biopsy-naïve patients | 283 (76%) |
| Pre-biopsy mpMRI | |
| Likert 1 – 2 | 24 (6.5%) |
| Likert 3 | 39 (10%) |
| Likert 4 | 199 (53%) |
| Likert 5 | 110 (30%) |
| Age median (IQR) | 64 (59, 69) |
| PSA ng/mL median (IQR) | 6.6 (4.9, 9.8) |
| Clinical stage (cT) | |
| T1c -T2a | 310 (83%) |
| T2b- T2c | 50 (13%) |
| >=T3 | 12 (3.2%) |
| Pca Gleason score at biopsy | |
| 3+3 | 22 (5.9%) |
| 3+4 | 186 (50%) |
| 4+3 | 88 (24%) |
| 4+4 | 44 (12%) |
| 9-10 | 32 (8.6%) |
| PCa risk category* | |
| low risk | 17 (4.6%) |
| intermediate risk | 245 (66%) |
| high risk | 110 (30%) |
| Post-operative characteristics | |
| Pca Gleason score after surgery | |
| 3+3 | 16 (4.3%) |
| 3+4 | 211 (57%) |
| 4+3 | 96 (26%) |
| 4+4 | 23 (6.2%) |
| 9-10 | 26 (7.0%) |
| Lymph node invasion | 41 (11%) |
| pN stage | |
| N0 | 322 (87%) |
| N1 | 41 (11%) |
| Nx | 9 (2.4%) |
| pT stage | |
| 2 | 174 (47%) |
| ≥3 | 198 (53%) |
D’Amico risk classification
Keys: mpMRI score= multiparametric magnetic resonance imaging: PCa= prostate cancer
The median (IQR) post-operative follow-up of disease-free patients was 17 (11-27) months and the overall risk of BCR at 2-year from surgery was 21% (95% CI: 17-27; Table 2). Figure 1 shows the Kaplan-Meier analysis estimating post-operative disease-free survival stratified according to the pre-biopsy mpMRI score. Patients with a pre-operative mpMRI score from 3 to 5 appear to have lower disease-free survival rates after surgery as compared to score 1-2 disease. The estimated risk of BCR for a Likert score 5 disease was 29% (95% CI: 20-41) at 2-year as compared to 5% (95% CI: 1-28) for a Likert score 1-2 disease (Table 2).
Table 2 –
Estimated risk of BCR (95% CI) according to mpMRI score
| Time from surgery | Overall | score 1-2 | score 3 | score 4 | score 5 |
|---|---|---|---|---|---|
| 6 mos | 8% (6, 12) | 5% (1, 28) | 0% (-) | 9% (5, 14) | 12% (7, 20) |
| 12 mos | 13% (10, 17) | 5% (1, 28) | 7% (2, 24) | 13% (9, 19) | 18% (11, 26) |
| 24 mos | 21% (17, 27) | 5% (1, 28) | 20% (9, 43) | 18% (13, 25) | 29% (20, 41) |
Keys: mpMRI= multiparametric magnetic resonance imaging
Figure 1-.
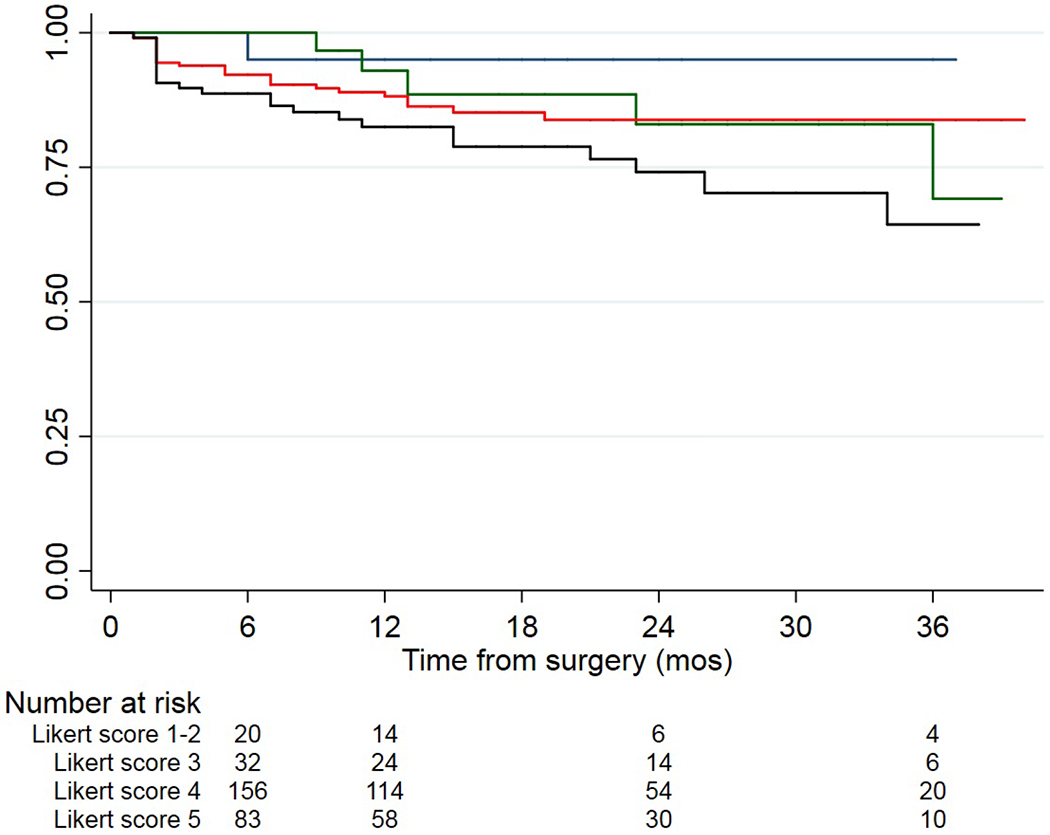
Kaplan-Meier curves showing disease-free survival stratified according to mpMRI score; the blue line represents patients with Likert 1-2 lesion; the green line represents patients with Likert 3 lesion; the red line represents patients with Likert 4 lesion; the black line represents patients with Likert 5 lesion.
Table 3 shows the multivariable Cox regression testing the association between mpMRI score and the risk of post-operative BCR. Higher mpMRI scores were associated with increasing hazard ratios (HRs) of recurrence: score 5 disease was associated with an HR of 2.71 and 5.05 as compared to a score 1-2 disease, after accounting either the Kattan nomogram (model 1) and the D’Amico risk classification (model 2), respectively. However, confidence intervals were wide and the association between the mpMRI score and BCR did not reach statistical significance (p>0.3). As expected, the accuracy of the Kattan nomogram (c-index: 0.724) and the D’Amico risk classification (c-index: 0.651) was not significantly improved by adding the mpMRI score (Model 1- c-index: 0.725; Model 2- c-index: 0.674).
Table 3 –
Pre-operative Cox-regression model predicting biochemical recurrence after surgery adjusting for factors included in the Kattan nomogram (Model 1: clinical stage, PSA, primary and secondary Gleason at biopsy) and in the D’Amico risk classification (Model 2: clinical stage, PSA, Gleason sum at biopsy)
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| mpMRI score | ||||||
| Likert 1-2 vs. 3 | 1.66 | 0.19, 14.20 | 0.4 | 3.61 | 0.43, 30.03 | 0.3 |
| Likert 1-2 vs. 4 | 1.96 | 0.26, 14.65 | 3.60 | 0.49, 26.35 | ||
| Likert 1-2 vs. 5 | 2.71 | 0.36, 20.55 | 5.05 | 0.68, 37.30 | ||
Keys: mpMRI score= multiparametric magnetic resonance imaging
Similarly, at the sensitivity analysis including all patients with available biopsy data (N=394), the mpMRI score was not significantly associated with post-operative BCR when included in the multivariable Cox regression model (Supplementary table 1).
Discussion
We found large differences between the central estimates of recurrence for different mpMRI scores, with a higher risk of recurrence for patients with high score lesions. However, our findings were associated with wide confidence intervals and were not statistically significant. Given the ready availability of mpMRI score in an increasing number of patients, our results warrant further research to better define the association between mpMRI findings and the risk of PCa recurrence after surgery.
Previous studies have investigated the link between MRI score and risk of post-treatment BCR. Park et al. assessed pre-surgical MRI findings in 282 patients with PCa 11and showed that the apparent tumor presence on MRI, as scored with a 5-point scale, was independently associated with post-operative BCR after accounting for cT, PSA and biopsy Gleason. Likewise, in a study including 421 PCa patients assessed with mpMRI before RP, the authors showed that a three-level PCa suspicion score based on MRI findings was independently associated with BCR after accounting for common pre-operative risk factors 10. Conversely, Tan et al. recently reported data from 255 patients who underwent a prostate mpMRI before RP, showing that the updated version of the PIRADS score (PIRADS v2) was not independently associated with the risk of post-operative BCR 13. Likewise, we observed a higher probability of recurrence associated with worse imaging tumor characteristics, as scored with a Likert scale that is consistent with the original PIRADS 17,18, although it was not statistically significant. It could be the case that the number of events and the rate of patients with lower mpMRI scores were not large enough to detect a significant difference between patients with different scores, thus hampering our ability to draw a final conclusion on the association between pre-operative mpMRI and the risk of PCa recurrence.
Our results suggest that further research should be conducted on mpMRI score to determine whether it improves the accuracy of commonly used preoperative predictive tools. Given the widespread use of mpMRI to improve PCa detection at biopsy, the mpMRI score would be easily available at the time of pre-operative assessment. As such, integrating this score in risk-stratification models could be valuable even for a small improvement in predictive accuracy.
Other MRI features, which are not routinely integrated in the PIRADS score, could help in predicting oncological outcomes, but may be less readily available in the everyday practice. Nonetheless, they deserve to be investigated in future studies. MR spectroscopic imaging (MRSI), for instance, has been previously recognized as a useful tool for assessing the metabolic characteristics of the prostatic lesions, allowing for the discrimination of high-grade tumors 19 and has shown to improve the accuracy of the PIRADS v2 for predicting Gleason pattern ≥ 4 20. Reisaeter et al. showed that the performance of both the University of California San Francisco Cancer of the Prostate Risk Assessment (CAPRA) tool and the D’Amico pre-operative risk-stratification, could be increased by including the tumor apparent diffusion coefficient (ADC) and the radiological extraprostatic extension assessed with the mpMRI 12. Similarly, Zhang et al. 14 reported that implementing the CAPRA and D’Amico models with multiple mpMRI features, such as the PIRADS score, the tumor ADC and the MRI-local staging, led to an increased accuracy in predicting 3-years BCR. Such research can be run in parallel with that examining routinely reported MRI features.
Our study has some limitations; the median patient follow-up was only 17 months, with 75% of patients being followed for at least 2 years. This relatively short follow-up is in part responsible for the low number of recurrences observed in our cohort. Longer follow-up is needed for a better assessment of predictors of PCa recurrence. The targeted biopsies were not always conducted under software registration, therefore, the correspondence between imaging and histology after biopsy could be questioned. Finally, more than half of the mpMRI assessments were conducted in outside institutions. That said, all studies were internally reviewed by experienced radiologists in our center, assigning an independent Likert score to the detected lesion, thus partially reducing the limitation of outside MRIs.
Conclusions
We observed a higher, although non-significant, estimated risk of recurrence of patients with high mpMRI scores. These findings suggest that routinely available MRI information is a potential marker to add to pre-operative prediction models to stratify patient’s risk and inform treatment planning. However, larger studies are needed to confirm value.
Supplementary Material
Clinical practice points.
Lower mpMRI scores have been associated with less aggressive histology, suggesting that pre-operative mpMRI could be useful in pre-operative disease-risk stratification and treatment planning
We observed increasing risk of PCa recurrence associated with higher mpMRI score, although the association did not reach significance
Including mpMRI score in preoperative predictive model did not increase the accuracy for predicting post-operative recurrence. However, the relatively small number of patients included may have affected the results
The mpMRI score is a routinely available information at the time of pre-operative risk assessment which may be clinically useful for treatment planning. However larger studies should investigate the association between mpMRI score and the risk of PCa recurrence
Acknowledgements:
Funding sources: This work was supported in part by the Sidney Kimmel Center for Prostate and Urologic Cancers, P50-CA92629 SPORE grant from the US National Cancer Institute, and the P30-CA008748 Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Moldovan PC, Van den Broeck T, Sylvester R, et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017;72(2):250–266. doi: 10.1016/j.eururo.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 3.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64(4):544–552. doi: 10.1016/j.eururo.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 4.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–757. doi: 10.1007/s00330-011-2377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multiparametric MRI and TRUS biopsy in prostate cancer (PROMTS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–822. doi: 10.1016/S0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 6.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. https://www.ncbi.nlm.nih.gov/pubmed/9749478. [DOI] [PubMed] [Google Scholar]
- 8.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90(10):766–771. https://www.ncbi.nlm.nih.gov/pubmed/9605647. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JM, Raymond E, O’Callaghan ME, et al. Optimum Tools for Predicting Clinical Outcomes in Prostate Cancer Patients Undergoing Radical Prostatectomy: A Systematic Review of Prognostic Accuracy and Validity. Clin Genitourin Cancer. 2017;15(5):e827–e834. doi: 10.1016/j.clgc.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 10.Ho R, Siddiqui MM, George AK, et al. Preoperative Multiparametric Magnetic Resonance Imaging Predicts Biochemical Recurrence in Prostate Cancer after Radical Prostatectomy. PLoS One. 2016;11(6):e0157313. doi: 10.1371/journal.pone.0157313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JJ, Kim CK, Park SY, Park BK, Lee HM, Cho SW. Prostate cancer: role of pretreatment multiparametric 3-T MRI in predicting biochemical recurrence after radical prostatectomy. AJR Am J Roentgenol. 2014;202(5): W459–65. doi: 10.2214/AJR.13.11381 [DOI] [PubMed] [Google Scholar]
- 12.Reisaeter LAR, Futterer JJ, Losnegard A, et al. Optimising preoperative risk stratification tools for prostate cancer using mpMRI. Eur Radiol. 2018;28(3):1016–1026. doi: 10.1007/s00330-017-5031-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan N, Shen L, Khoshnoodi P, et al. Pathological and 3 Tesla Volumetric Magnetic Resonance Imaging Predictors of Biochemical Recurrence after Robotic Assisted Radical Prostatectomy: Correlation with Whole Mount Histopathology. J Urol. 2017. doi: 10.1016/j.juro.2017.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YD, Wu CJ, Bao ML, et al. MR-based prognostic nomogram for prostate cancer after radical prostatectomy. J Magn Reson Imaging. 2017;45(2):586–596. doi: 10.1002/jmri.25441 [DOI] [PubMed] [Google Scholar]
- 15.Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011. ;259(3):775–784. doi: 10.1148/radiol.11102066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargas HA, Akin O, Shukla-Dave A, et al. Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology. 2012;265(2):478–487. doi: 10.1148/radiol.12120041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenkrantz AB, Kim S, Lim RP, et al. Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology. 2013;269(2):482–492. doi: 10.1148/radiol.13122233 [DOI] [PubMed] [Google Scholar]
- 18.Rosenkrantz AB, Lim RP, Haghighi M, Somberg MB, Babb JS, Taneja SS. Comparison of interreader reproducibility of the prostate imaging reporting and data system and likert scales for evaluation of multiparametric prostate MRI. AJR Am J Roentgenol. 2013;201(4):W612–8. doi: 10.2214/AJR.12.10173 [DOI] [PubMed] [Google Scholar]
- 19.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190(5):1721–1727. doi: 10.1016/j.juro.2013.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leapman MS, Wang ZJ, Behr SC, et al. Impact of the integration of proton magnetic resonance imaging spectroscopy to PI-RADS 2 for prediction of high grade and high stage prostate cancer. Radiol Bras. 2017;50(5):299–307. doi: 10.1590/0100-3984.2016.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


