Abstract
An unbridled host immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to underlie severe cases of the disease and has been labeled a ‘cytokine storm syndrome’ (CSS). Here, we emphasize that categorization of syndromes triggered by a completely novel pathogen based on other seemingly similar, but potentially distinct, known entities is an inherently risky endeavor.
Keywords: severe acute respiratory syndrome coronavirus 2, COVID-19;cytokine storm syndrome, multisystem inflammatory syndrome in children, inflammation, virology
When You Hear Hoofbeats, Think Horses not Zebras
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of coronavirus disease 2019 (COVID-19), can lead to severe pneumonia, multiorgan failure, and death. An overexuberant immune response may contribute to severe COVID-19, which has been likened to other cytokine storm syndromes (CSSs). While the clinical manifestations of SARS-CoV-2 infection range from asymptomatic to fatal disease, it remains less clear how dysregulated cytokine responses and viral immune evasion contribute to this spectrum of clinical findings. Among the myriad clinical syndromes identified to date is the multisystem inflammatory syndrome in children (MIS-C), which is temporally associated with the SARS-CoV-2 pandemic. Whether the SARS-CoV-2-associated CSS (termed S-CSS for purposes of this review) and MIS-C fall within a spectrum of disorders familiar to us or represent groups of novel clinical entities (a herd of zebras, in essence) remains undetermined. However, new evidence in this rapidly moving area indicates that SARS-CoV-2-associated inflammatory disorders may be unique entities triggered by this completely novel pathogen. Here, we review the proposed pathogenesis of MIS-C within the context of S-CSS and highlight the need to gain further understanding of these disorders to define optimal therapeutic targets.
Moving Target: When the Frame of Reference Is a Zoo of Different Beasts
CSSs involve life-threatening immune activation triggered by genetic, infectious, or iatrogenic causes, with associated hypercytokinemia, hemodynamic compromise, and multiorgan dysfunction. While different forms of CSS share these features, varying underlying host risk factors and inciting triggers have important implications for the pathogenesis of the disease. CSS in general, with reference to COVID-19, has recently been reviewed [1]. Briefly, not only viral, but also other infections may trigger primary (inherited) and secondary (acquired) hyperinflammatory syndromes, including hemophagocytic lymphohistiocytosis (HLH) [2]. Severe respiratory viral infections, such as influenza, in otherwise healthy hosts can induce a distinct form of CSS (reviewed in [3]). The heterogeneity of CSS disorders is exemplified by the fact that selective neutralization of a single cytokine IL-6 dramatically reverses the course of cytokine release syndrome (CRS), a syndrome related to antitumor immunotherapies [4], while cure of some patients with HLH may require bone marrow transplantation [5]. While targeted therapies based on understanding of the pathogenesis of unique CSS are emerging [e.g., neutralization of IL-6 for CRS, or interferon (IFN)-γ-neutralizing antibodies for pediatric HLH [6]), and there may be overlap in the pathogenesis and therapy, it is clear that a ‘one-size fits all’ approach does not work.
Cause or Consequence of the Chaos? Hypercytokinemia in Patients with S-CSS and MIS-C
Initial reports of adult patients with COVID-19 described hemodynamic compromise, endovascular lesions, multiorgan dysfunction, and elevated markers of inflammation that could not be ascribed entirely to respiratory failure from viral pneumonia [7]. Biphasic onset of fever and clinical worsening after an initial period of stability invoke the ‘cytokine storm’ (Figure 1 ). Commonly involved systems in S-CSS include severe pulmonary disease with acute respiratory distress syndrome (ARDS), cardiac impairment, liver dysfunction, acute kidney injury, and coagulopathy. Markers of more severe disease include lymphopenia [8], and elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), and ferritin, which are nonspecific indicators of inflammation and cellular injury [9., 10., 11.]. Elevated D-dimer levels and thrombocytopenia portend worse outcomes and reflect an associated coagulopathy [11,12]. Large cohorts of pediatric patients with severe COVID-19 and associated laboratory findings are uncommon. Although detailed descriptions of pediatric S-CSS are few, the available data suggest elevated ferritin and CRP and endovascular damage with elevated D-dimers are also common in these patients [13].
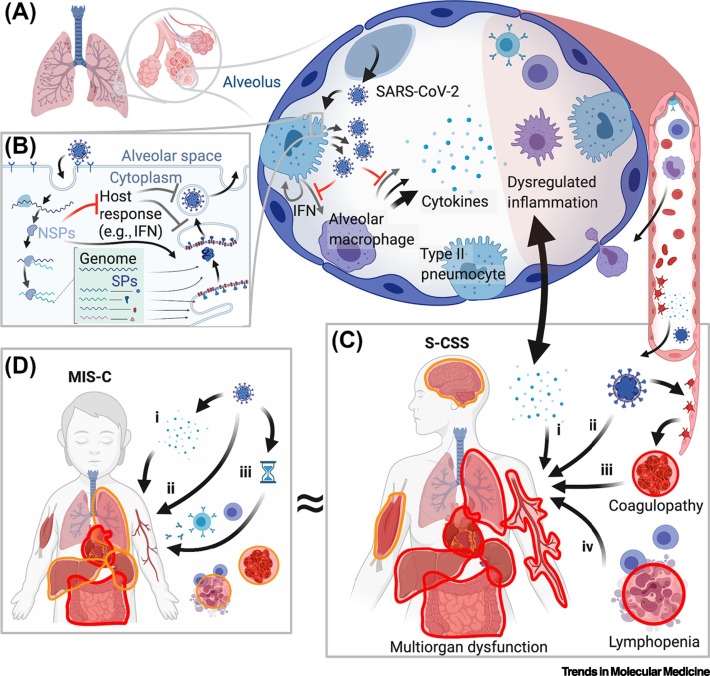
Figure 1.
Schematic Overview of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Associated Cytokine Storm Syndrome (S-CSS) and Multisystem Inflammatory Syndrome in Children (MIS-C).
(A) SARS-CoV-2 infects Type II pneumocytes initially in airways and alveoli, resulting in activation of alveolar macrophages, cytokine production, and additional inflammatory infiltration. (B) Simplified SARS-CoV-2 viral lifecycle indicating production of non-structural proteins (NSPs) that suppress an initial interferon (IFN) response in infected cells that ordinarily restricts viral replication. This virulence mechanism may contribute proximally to a dysregulated inflammatory response. (C) S-CSS occurs primarily in adults and involves multiple organ systems, most prominently the lung, heart, liver, gastrointestinal, renal, vasculature, coagulation, and lymphoid populations. Lung injury and multiorgan dysfunction may also contribute to each other in a feed-forward manner (double-headed arrow). Proposed mechanisms of S-CSS include: (i) cytokine hyperproduction; (ii) cellular injury to directly infected cells; (iii) coagulopathy due to endothelial injury; and/or (iv) depletion of lymphocyte populations. (D) MIS-C occurs primarily in children with distinct findings, including prominent cardiac and gastrointestinal involvement, and more infrequent or less severe involvement of other systems. It remains to be determined whether MIS-C is a direct consequence of cytokines produced by acute or persistent infection (i); direct infection of involved tissues (ii); or (iii) represents a delayed para-infectious autoinflammatory complication of SARS-CoV-2 exposure (indicated by hourglass symbol). ≈, reflects the uncertainty over whether MIS-C is a distinct syndrome or exists along the spectrum of disease seen in S-CSS. In (C) and (D), more severe or frequently involved systems are indicated by red outlines, less severe or more infrequent involvement by orange outlines.
A dysregulated immune response with suppression of IFN responses and concurrent hyperproduction of other cytokines [e.g., IL-6 and tumor necrosis factor (TNF)] has been proposed to give rise to S-CSS [14,15]. Cytokines correlating to disease severity and poor survival include IL-6, IL-8, and IL-10, among others [8., 9., 10.,12,16]. A recent study found elevated markers of apoptosis on lymphocytes profiled from patients with severe COVID-19, indicating a potential route to their depletion [15]. Nevertheless, it remains undetermined whether hyperproduction of cytokines in S-CSS is a cause or a consequence of the observed organ injury and immune cell abnormalities. Trials with therapies that inhibit specific cytokines (e.g., IL-6 and IL-1) and more indiscriminate immunosuppressants (e.g., steroids) are underway and may provide further insights.
Cohorts of pediatric patients with MIS-C reveal overlapping but distinct pictures from S-CSS with respect to their clinical and laboratory profile (Table 1 ). The syndrome is defined by fever, multiorgan dysfunction, and laboratory evidence of inflammation (Table 2 ). Abdominal pain, vomiting and diarrhea, mucocutaneous findings (conjunctivitis and rash), and shock with cardiac involvement are more common in MIS-C than in S-CSS [17,18]. Respiratory symptoms, while often present, are mild, and musculoskeletal and neurological findings are rare. The inflammatory response is evident by prolonged fever and elevated CRP in all patients. In individual studies, IL-6 and ferritin levels have been increased in some patients, with additional findings similar to S-CSS of lymphopenia and mild thrombocytopenia [19,20]. To date, almost all patients with MIS-C have been treated similarly to patients with Kawasaki Disease (KD), with intravenous immunoglobulin and occasionally corticosteroids, which nonspecifically suppress inflammation. A smaller number of patients have also been given IL-6 and IL-1β-neutralizing therapies for refractory disease, although studies comparing the efficacy of individual treatments have not been reported. Out of 570 patients with MIS-C in the USA reported to the US Centers for Disease Control (CDC), there were ten fatalities (1.8%) [with only one fatality in Group 1 patients, who had extensive multisystem involvement and positive serology more often, and with features less consistent with acute respiratory COVID-19 (Group 2) or KD (Group 3)] [17], which is a lower mortality rate than adults with severe COVID-19.
Table 1.
Review of MIS-C Cohortsa
| Cases (N) | Ages (med, mean) (range, IQR, or [SD]) | Involved systems [N (%)] |
Other (fatalities, other cytokine abnormalities) | Laboratory findings [med (range or IQR) or mean [SD])] |
SARS-CoV-2 test + [N (% of tested)] |
Refs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNS b | Mucosal membranec | Respiratory | Cardiacd | Gastrointestinale | Rash | Joint | Renalf | Peripheral blood counts (×109/l) | CRP (mg/l) | IL-6 (pg/ml) | Ferritin (ug/l) | SARS-CoV-2 RT-PCR | SARS-CoV-2 IgG | ||||
| 10 | 7.25 (2.9–16) | 4 (40) | 9 (80) | n.r. | 9 (90) | 6 (60) | 9 (80) | n.r. | n.r. | 7/10 neutrophiliag, ALC 0.8 (0.4–1.9), Plt 121 (66–192) | 242 (9–525) | 177 [137] | 893 (199–3213) | 2 (20) | 8 (80) | [24] | |
| 16 | 10 (4.7–12.5) | 3 (18) | 15 (94) | 2 (12) | 11 (69) | 13 (81) | 13 (81) | 1 (6) | 9 (56) | Elevated TNF and IL-1 in 1 patient, normal in 3 patients | WBC 11.5 (9–14), ANC 9.2 (7.6–10.7), ALC 1.2 (0.8–1.7), Plt 188 (164–244) | 207 (162–236) | 270 (136–526) | 1067 (272–1709) | 11 (69) | 7 (87) | [25] |
| 21 | 7.9 (3.7–16.6) | 6 (29) | 17 (81) | n.r. | 17 (81) | 21 (100) | 16 (76) | 2 (10) | 11 (52) | WBC 17.4 (5.4–42.8), ANC 13.6 (3.3–36.4), ALC 1.1 (0.4–5.6), Plt 499 (78–838) | 253 (89–363) | 170 (4–1366) | n.r. | 8 (38) | 19 (90) | [26] | |
| 156 | 8 (5–11) | n.r. | 24 (22) | n.r. | 79 (73) | n.r. | n.r. | n.r. | n.r. | 1 fatality | n.r. | n.r. | n.r. | n.r. | 79 (73) (type n.r.) | [27] | |
| 58h | 9 (5.7–14) | 5 (9) | 26 (45) | 12 (21) | 29 (50) | 30 (52) | 30 (52) | n.r. | 13 (22) | 1 fatality | WBC 17 (12–22), ANC 13 (10–19), ALC 0.8 (0.5–1.5), Plt 151 (104–210) | 229 (156–338) | n.r. | 610 (359–1280) | 15 (26) | 40 (87) | [28] |
| 33 | 10 (6–13) | 4 (12) | 12 (36) | 11 (33) | 21 (66) | 23 (69) | 14 (42) | n.r. | n.r. | 1 fatality | WBC 11.0 (8.5–14.4), ALC 1.1 (0.6–1.3), Plt 176 (131–282) | 255 (181–310) | 200 (56–330) | 568 (340–954) | 11 (33) | 27 (81) | [29] |
| 15 | 12 (3–20) | n.r. | 4 (27) | 3 (20) | 13 (87) | 13 (87) | 7 (47) | n.r. | n.r. | 1 fatality; IL-8 elevated in all patients; TNF elevated in 12/15 patients; IL-1 normal in all | 13/15 lymphopenicg, Plt 198 (42–516) | 249 (47–390) | 253 (31–504) | 628 (264–10170) | 8 (53) | 15 (100) | [30] |
| 99 | 31% (0–5 y) 42% (6–12 y) 26% (13–20 y) | 2 (2) | 60 (61) | 40 (40) | 59 (60) | 79 (80) | 61 (62) | 4 (4) | 10 (10) | Two fatalities | WBC 10.4 (6.7–14.5), 58/89 lymphopenicg; 0/89 neutropenicg, Plt 155 (105–233) | 219 (150–300) | 116 (37–315) | 522 (305–820) | 50 (51) | 76 (99) | [31] |
| 186 | 8.3 (3.3–12.5) | 12 (6) | 137 (74)i | 131 (70) | 149 (80) | 171 (92) | i | 4 (2) | 15 (8) | Four fatalities | 147/184 lymphopenicg, 126/184 neutrophiliag, Plt 133 (88, 235) | 178 (128–259) | n.r. | 639 (333–1178) | 43 (59) | 36 (62) | [32] |
| 18 | 7.7 [7.0] | 0 | n.r. | 4 (22) | n.r. | 5 (29) | 5 (28) | 3 (16) | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | 2 (11) | 8 (47) | [18] |
Abbreviations: ANC, absolute neutrophil count; ALC, absolute lymphocyte count; IQR, interquartile range; n.r., not reported; Plt, platelets; SD, standard deviation; WBC, white blood cell count.
Does not include headache or meningeal signs.
Includes conjunctivitis.
Includes echocardiographic findings, need for resuscitative medications, and elevated cardiac markers (troponin and brain natriuretic protein; BNP).
Does not include isolated abdominal pain, which was uncommonly reported as an isolated symptom among studies but is present in up to 62% of reported patients [17].
Includes acute kidney injury.
Absolute counts not reported.
Patients from initial cohort recognizing the disorder [33] were included in a larger cohort [28] listed in the table.
Mucocutaneous (mucous membrane and cutaneous not differentiated).
Table 2.
| CSS | MIS-C (CDC) | KD (AHA) | TSS (CDC) |
|---|---|---|---|
| Age | <21 | Typically <5 y | Any |
| AND | |||
| Fever | >38.0°C ≥24 h | ≥5d | (i) >38.9°C |
| AND | AND | AND/OR (4 of 5) | |
| Clinical features | Severe disease requiring hospitalization with multisystem (≥2) involvement (cardiac, renal, respiratory, hematological, GI, dermatological, CNS) | ≥4 of following: (i) mucocutaneous signs; (ii) bilateral nonexudative conjunctival injection; (iii) rash; (iv) extremity changes; (v) cervical lymphadenopathy (≥1.5 cm). KDSS = KD + hypotension |
(ii) Rash; (iii) desquamation; (iv) hypotension; (v) multisystem involvement (GI, muscular, mucous membrane including conjunctival injection, renal, hepatic, low platelets, CNS) |
| AND | OR (with 2–3 clinical features) | AND | |
| Laboratory | (i) One or more inflammatory marker (CRP, ESR, fibrinogen, procalcitonin, D-dimer, ferritin, LDH, or IL-6, elevated PMN, lymphocytopenia, hypoalbuminemia); AND (ii) SARS-CoV-2 RT-PCR, antibody, or antigen positive (or exposure within 4 weeks) AND no other microbiological diagnosis |
Supportive of incomplete KD: (i) elevated CRP and/or ESR, AND (A) Three or more of: anemia, platelets ≥450 000, albumin ≤3.0, elevated ALT, WBC ≥15 000/ml, urine ≥10 WBC, OR (B) positive echocardiogram AND No other microbiological diagnosis |
Negative blood or CSF culture (blood may be positive for Staphylococcus aureus) AND negative serologies for Rocky Mountain spotted fever, leptospirosis, or measles virus |
Advising professional/public health agency in parentheses.
Abbreviations: AHA, American Heart Association; ALT, alanine aminotransferase; CDC, US Center for Disease Control; CRP, C-reactive protein; CSF, cerebrospinal fluid; ESR, erythrocyte sedimentation rate; GI, gastrointestinal; LDH, lactate dehydrogenase; PMN, polymorphonuclear cell/ neutrophil; WBC, white blood cell count.
Zebras or Unicorns at Center Ring?
Given the clinical and laboratory features, it is reasonable to consider MIS-C as a separate but related entity to the severe multiorgan dysfunction observed in patients with S-CSS (Figure 1). For example, patients with MIS-C less commonly have severe respiratory, renal, and hepatic involvement and more often have cardiac dysfunction. Lymphopenia and thrombocytopenia are observed in some MIS-C patients (40% and 41%, respectively, in Group 1 patients reported in [17]) and, while lower levels are associated with more severe COVID-19 [21,22], an association for these factors in outcomes in MIS-C remains to be determined.
There are features of MIS-C that separate it from two other forms of CSS that exhibit similar features but are unrelated to SARS-CoV-2: KD shock syndrome (KDSS) and toxic shock syndrome (TSS) (Table 2). Cardiac involvement is characteristic for KDSS and gastrointestinal (GI) involvement more common with TSS, while both coincide in MIS-C. The prominence of GI symptoms raises the possibility that SARS-CoV-2 infection of GI tissue or autoinflammatory involvement of this site contributes to MIS-C. Additionally, cardiac involvement with MIS-C most typically presents as ventricular dysfunction, with only a small percentage having coronary artery dilation [17]. The pathogenesis of KD itself remains enigmatic. KD is a medium vessel vasculitis that may involve antibodies to a persistent, as of yet unidentified, viral pathogen. By contrast, TSS results from superantigens elaborated by bacteria that lead to indiscriminate cytokine release from lymphocytes. Thus, MIS-C exhibits features of two entities with patently distinct disease mechanisms. In addition, typical KD, TSS, and other similar disorders could occur in patients testing positive for acute or recent SARS-CoV-2 infection, and diagnostic inertia should be avoided so as not to detract from their appropriate diagnosis and management.
One critical question is the mechanism by which SARS-CoV-2 infection contributes to MIS-C. In the absence of data on the community prevalence of SARS-CoV-2 infection (i.e., the denominator), it is not yet possible to determine the relative risk of developing MIS-C in children exposed to SARS-CoV-2, or the baseline probability of testing positive for the virus in the general population. Mild disease observed in children with COVID-19, along with known asymptomatic carriage of the virus, make these analyses imperative and inclusion of pediatric patients in serological surveys is prudent. Nevertheless, given the high positive rate of SARS-CoV-2 testing in MIS-C cases, and close temporal association with the pandemic, the disorder is likely due to either a direct consequence of acute viral infection or a delayed para-infectious immune-mediated disease. While initial studies suggested a delay in presentations of MIS-C after peak SARS-CoV-2 activity in affected communities, a recent retrospective analysis from the UK showed that some MIS-C cases overlapped with the SARS-CoV-2 peak [23]. Most patients with MIS-C lack severe respiratory symptoms, although, in some MIS-C series, respiratory symptoms and pulmonary radiographic findings have been reported in up to 70% of cases, suggesting that a subgroup has active infection. Importantly, the relative mild respiratory component in MIS-C points away from an aggressive immune response to primary pulmonary disease akin to S-CSS, and more towards a secondary para-infectious process. Higher SARS-CoV-2 qPCR cycle threshold (Ct) values in patients with MIS-C suggest a lower viral load and also point towards a para-infectious etiology [19]. Interestingly, even within patients with MIS-C, there are suggestions of different subtypes, with younger non-white patients presenting with conjunctivitis and abdominal pain having positive antibodies (subacute infection). This contrasts with a group of patients with MIS-C who presented with respiratory symptoms and positive PCR testing (acute infection) [23]. It is also important to consider that initial criteria for MIS-C case identification were relatively broad to capture many cases, and it remains possible that patients meeting current criteria with primarily respiratory disease could represent an entity that is distinct from MIS-C.
Two recent studies provide immunological data suggesting that MIS-C and severe COVID-19 are distinct entities. Systematic profiling of serum cytokines showed distinct inflammatory profiles in adults with S-CSS versus children with MIS-C and KD, with elevated IL-8 and IL-7 defining the S-CSS group, and MIS-C appearing more similar to KD in this comparison [20]. Comparing groups of healthy children with those with SARS-CoV-2, MIS-C, or KD, IL-6 and IL-17A were significantly higher only in the KD group [20]. A second study showed that pediatric patients with MIS-C exhibited distinct cytokine profiles from those with severe SARS-CoV-2 respiratory disease, exhibiting higher IL-10 and TNF [19]. It is not clear whether the differences observed in the cytokines that define these groups are a result of different patient populations, testing strategies, or in the groups used for comparisons. As with S-CSS, additional study and identification of effective (and ineffective) therapies will help in the ‘taxonomic’ classification of MIS-C and potential subgroups.
The Last Act: Juggling Torches on a Tightrope
One lesson that will surely emerge from the COVID-19 pandemic is that the necessity to intervene in the setting of immense devastation must be tempered by a core principle of medical ethics: first, do no harm. Empiric treatments of inflammatory entities related to a novel disease with an incomplete molecular understanding of the virulence factors and host response, increase the risk of violating this maxim. The risk increases further when demand for individual therapies exceeds supply and additional alternatives are sought. In this setting, we face even higher chances of getting ‘burned’ and are forced to walk a narrower tightrope.
Concluding Remarks
As we gain a deeper understanding of the pathogenesis of S-CSS and MIS-C, and the results of randomized trials are published, a clearer picture will emerge, and targeted therapies may be identified (see Outstanding Questions). Knowledge in this area is keeping pace with the spread of the virus, with new insights arising on a continual basis. Indeed, while this manuscript was in the proof stage, the CDC reported a series of adult patients with presentations resembling MIS-C, termed multisystem inflammatory syndrome in adults (MIS-A) (www.cdc.gov/mmwr/volumes/69/wr/mm6940e1.htm?s_cid=mm6940e1_w). Ultimately, we may find that manifestations of S-CSS represent a circus of unique zebras in which the virus is orchestrating the chaos at center ring. As the tent over this complex host–pathogen interaction comes down, rigorous studies will surely tip the scales in our favor and lessons learned may improve our readiness for when the next circus inevitably comes to town.
Outstanding Questions.
What is the mechanism by which SARS-CoV-2 infection contributes to multisystem inflammation?
Is the hyperproduction of cytokines in S-CSS a cause or a consequence of the observed organ injury and immune cell abnormalities? Trials with therapies that inhibit specific cytokines (e.g., IL-6 and IL-1) and more indiscriminate immunosuppressants (e.g., steroids) should provide further insights.
Is MIS-C a distinct syndrome or does it exist along the spectrum of disease seen in S-CSS? In the absence of data on the community prevalence of SARS-CoV-2 infection (i.e., the denominator), it is not yet possible to determine the relative risk of developing MIS-C in children exposed to SARS-CoV-2, or the baseline probability of testing positive for the virus in the general population.
What are the optimal clinical characteristics and biomarkers to identify and classify cases of MIS-C, S-CSS, and other diseases associated with CSS? Precise disease classification may help to guide cohort selection for trials to identify optimal therapies.
Do we need to reconsider the initial criteria for MIS-C case identification that were set relatively broad to capture many cases? This could help to better evaluate and treat patients.
Alt-text: Outstanding Questions
Acknowledgments
Acknowledgements
We thank Melanie Yarbrough for helpful comments on the manuscript.
References
- 1.Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinn I.K., et al. Genetic and mechanistic diversity in pediatric hemophagocytic lymphohistiocytosis. Blood. 2018;132:89–100. doi: 10.1182/blood-2017-11-814244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q., et al. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald J.C., et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit. Care Med. 2017;45:e124–e131. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henter J-I., et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F., et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N. Engl. J. Med. 2020;382:1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- 7.Chen J., et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry B.M., et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 10.Liu F., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan F., et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int. J. Med. Sci. 2020;17:1281–1292. doi: 10.7150/ijms.46614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings M.J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Dambrauskas S., et al. Pediatric critical care and COVID19. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1766. [DOI] [PubMed] [Google Scholar]
- 14.Blanco-Melo D., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadjadj J., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;31 doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han H., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfred-Cato S., et al. COVID-19-associated Multisystem Inflammatory Syndrome in Children — United States, March–July 2020. Morb. Mortal. Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonker L.M., et al. Pediatric SARS-CoV-2: clinical presentation, infectivity, and immune responses. J. Pediatr. 2020 doi: 10.1016/j.jpeds.2020.08.037. Published online August 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diorio C., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Invest. 2020 doi: 10.1172/JCI140970. Published online July 30, 2020. http://dx.doi.org.10.1172/jci140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Consiglio C.R., et al. The immunology of Multisystem Inflammatory Syndrome in children with COVID-19. Cell. 2020;383:334–346. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J. Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippi G., et al. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swann O.V., et al. Clinical characteristics of children and young people admitted to hospital with Covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdoni L., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pouletty M., et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann. Rheum. Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toubiana J., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belot A., et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Eurosurveillance. 2020;25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittaker E., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaushik S., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): A multi-institutional study from New York City. J. Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riollano-Cruz M., et al. Multisystem inflammatory syndrome in children (MIS-C) elated to COVID-19: A New York City experience. J. Med. Virol. 2020 doi: 10.1002/jmv.26224. Published online June 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dufort E.M., et al. Multisystem inflammatory syndrome in children in New York State. N. Engl. J. Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldstein L.R., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riphagen S., et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]