Abstract
Immune cells can sense and respond to biophysical cues — from dynamic forces to spatial features — during their development, activation, differentiation and expansion. These biophysical signals regulate a variety of immune cell functions such as leukocyte extravasation, macrophage polarization, T cell selection and T cell activation. Recent studies have advanced our understanding on immune responses to biophysical cues and the underlying mechanisms of mechanotransduction, which provides rational basis for the design and development of immune-modulatory therapeutics. This review discusses the recent progress in mechanosensing and mechanotransduction of immune cells, particularly monocytes/macrophages and T lymphocytes, and features new biomaterial designs and biomedical devices that translate these findings into biomedical applications.
Current Opinion in Biotechnology 2020, 66:236–245
This review comes from a themed issue on Tissue, cell and pathway engineering
Edited by Li Tang, Peng Xu and Haoran Zhang
For a complete overview see the Issue and the Editorial
Available online 30th September 2020
https://doi.org/10.1016/j.copbio.2020.09.004
0958-1669/© 2020 Elsevier Ltd. All rights reserved.
Introduction
Immune cells employ numerous strategies to combat infections and maintain tissue homeostasis. They originate from hematopoietic stem cells and differentiate into myeloid (i.e. basophils, neutrophils, eosinophils, monocytes and mast cells) and lymphoid (i.e. T cells and B cells) lineages, which have distinct roles in the innate and adaptive immune systems respectively. Sensing and responding to mechanical cues is critical for immune cells to interact with their neighboring cells, surrounding extracellular matrix (ECM), and other cells for immunosurveillance and effector functions [for reviews, see Refs. 1, 2, 3].
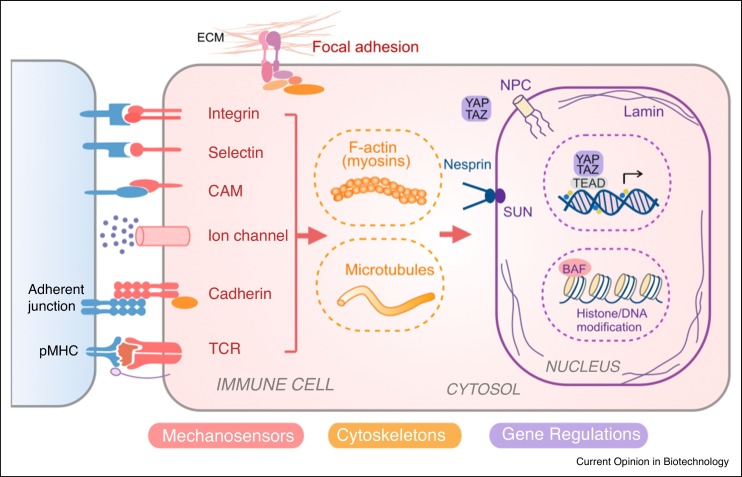
Accumulative evidence suggests that biophysical cues, in addition to biochemical signals, play an important role in modulating immune cells functions. Through the process of mechanotransduction, cells can convert external biophysical stimuli into intracellular biochemical signals [2], which may lead to cytoskeletal re-organization, gene regulation and/or epigenetic modification of chromatin [3] (Figure 1 ). Many cell adhesion molecules (CAMs) involved in cell-cell communication can serve as mechanosensors, including integrins, selectins and cadherins [for review, see Ref. 4]. Integrins and focal adhesion complexes are also important for cell-matrix interaction. Another category of mechanosensor includes ion channels for gating soluble ions such as Ca2+, Na+ and K+. For example, a mechanosensitive ion channel, Piezo1 has been shown to sense mechanical cues in both macrophages and T cells [5••,6]. Furthermore, in lymphocytes, T cell receptors (TCRs) and B cell receptors (BCRs) are mechanically essential for recognizing antigens and activating effector functions. Lymphocyte function-associated antigen 1 (LFA-1) is an integrin that not only functions as a receptor for cell adhesion during migration but also mediates T cell activation as the secondary signal (Signal 2) where TCR and peptide-major histocompatibility complexes (pMHC) interaction provides the primary signal (Signal 1). Both integrin-ligand interaction and TCR-pMHC interaction can form catch bond that exhibits extended bond life upon tangential forces [2].
Figure 1.
Mechanotransduction network in immune cells. Immune cells can sense the biophysical cues by receptors including integrins (i.e. LFA-1, Mac-1, VLA-4), selectins (i.e. P-selectin, L-selectin), cell adhesion molecules (CAMs) (i.e. ICAM-1, VCAM), ion channels (i.e. Piezo1), cadherins and T cell receptor (TCR). These signals can be transduced through cytoskeleton (actin, microtubule) and nucleoskeleton (e.g. lamin), and/or converted into biochemical signaling events. The mechanotransduction process not only affects the mechanical structure and property of cell and nucleus, but also regulates immune cell phenotypes and functions via transcriptional factors (e.g. YAP/TAZ) and epigenetic modifications.
*Abbreviation: LFA: Lymphocyte Function-associated Antigen; Mac1: Macrophage-1 Antigen; VLA-4: Integrin α4β1 (very late antigen-4); ICAM: Intercellular Adhesion Molecule 1; VCAM: Vascular Cell Adhesion Protein 1.
Mechanical signals on cell surface can further propagate via intracellular structures such as cytoskeleton and nucleoskeleton and induce the reorganization of these structures. In addition, signaling molecules (e.g. Rho GTPases) and transcription factors may serve as mechanotransducers to relay signals and regulate cytoskeleton dynamics and gene expression. For instance, yes-associated protein 1 (YAP1) emerges as an important mechanotransducer for sensing a wide range of environmental factors such as ECM rigidity, cell density, cell shape, stretching and shear stress [for review, see Ref. 7].
In this review, we summarize recent findings on mechanotransduction in innate immunity and adaptive immunity with a focus on macrophages and T lymphocytes. We then discuss how fundamental insights into the mechanobiology of immune cells can inspire the design of engineering tools, biomaterials, and drug delivery platforms for tissue regeneration and cancer immunotherapy.
Mechano-regulation of innate immunity
Innate immunity consists of first-line responses against pathogens. Macrophages fill a crucial role in the host defense to clear pathogens and maintain tissue homeostasis, which are evidently regulated by both biochemical and biomechanical cues [for reviews, see Refs. 8, 9, 10, 11]. Firstly, like other immune cells, monocytes need to migrate to inflamed sites. Mechanotransduction during cell trafficking will be briefly discussed here as it has been widely studied regardless of cell types and functions [for reviews, see Ref. 12,13]. Subsequently, biophysical cues in the extracellular environment such as interstitial flow, cyclical forces, stretching, spatial confinement as well as matrix stiffness, can influence the activation and polarization of macrophages, which will be the focus in this review.
Cell trafficking
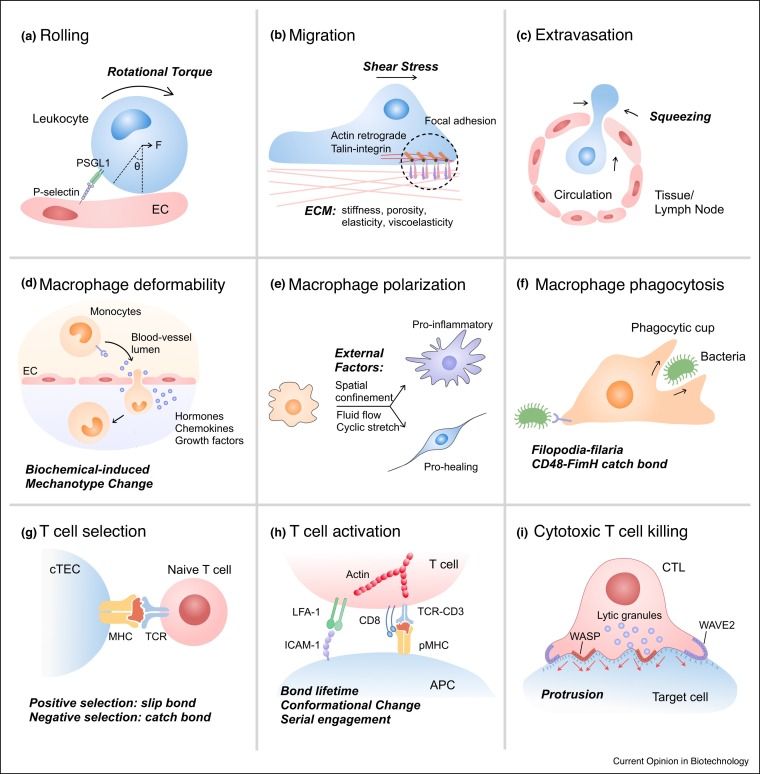
Throughout their lifetime, immune cells exert and receive mechanical stresses as they travel through the circulation, attach to the blood vessel wall, and extravasate into local tissue niches (Figure 2 a–c, see figure legends for detailed description) [2]. Immune cells express integrins, selectins and chemokine receptors to interact with the ligands on endothelial cells (ECs) or respond to a chemical gradient for adhesion and transmigration. Mechanotransduction enables the process of monocytes extravasation and lymphocytes homing. Additionally, cytoskeletal proteins such as actin and non-muscle myosin II mediate rapid changes in cytoskeletal architecture on the timescale of seconds, a response rate essential for the motility of immune cells. While macrophages mainly adapt mesenchymal migration mediated through specific cell-ECM adhesion, amoeboid migration that lacks substantial cell-ECM attachment is an alternative migration mode in three-dimensional (3D) ECM [13].
Figure 2.
Mechanical factors regulate immune cell functions. (a) Because of the flow of body fluids, cell experiences rolling and steering. (b) Actin polymerization happens at the leading edge of the cell; constant retrograde flow of actin and myosin contraction propels the cell for migration. Interaction of integrins and formation of focal adhesion are important characteristics for adhesion-dependent mesenchymal migration. Some well-known receptor-ligand pairs involved in the adhesion on endothelial cells (ECs) include LFA-ICAM-1, Mac1-multiple ligands, VLA-4-VCAM, P-selection-PSGL-1, CCR2-CCL2, CCR5-CCL5. (c) Neutrophils and monocytes navigate via blood circulation and extravasate through the capillary epithelium to a specific inflammatory site for clearance of pathogen. T lymphocytes are also squeezed through cell-cell junctions when trafficking through the high epithelial venules. (d–f) Examples of mechanotransduction involved in innate immunity such as (d) macrophage deformability, (e) macrophage polarization and (f) macrophage phagocytosis. (g–i) Example of mechanotransduction in adaptive immunity such as (g) T cell selection in the thymus, (h) T cell activation and (i) Cytotoxic T cell killing.
*Abbreviation: PSGL-1: P-selectin glycoprotein ligand-1; CCR: C-C chemokine receptor; CCL: Chemokine (C-C motif) ligand.
Macrophage deformability
Soluble biochemical signals such as endogenous hormones and foreign pathogens are capable of triggering mechanical changes and influencing cellular behaviors ( Figure 2d). For example, stress hormones — epinephrine and norepinephrine — govern fight-or-flight responses by modulating muscle contraction [14]. Stress hormone activation of the β-adrenergic receptor (β-AR) signaling pathway further impacts macrophage mechanobiology, decreasing the deformability of macrophage cells through the rearrangement of branched filamentous actin in the cortical region [15]. Such a decrease in cell deformability induced by β-AR activation is also associated with an increase in macrophage phagocytosis and chemotaxis [15]. Moreover, environmental pathogens can change host cell mechanotypes. For example, the activation of nucleotide-binding oligomerization domain-like receptors (NLRs) by Salmonella infection, especially NLRC4, results in reduced cell deformability of mouse bone marrow-derived macrophages as determined using an optical stretcher [16]. The resultant cell stiffening in response to NLRC4 inflammasome activation prevents further uptake and bacterial dissemination [17]. Taken together, these studies suggest that the modulation of cell mechanotypes by soluble external cues from the host or pathogens can regulate macrophage functions during physiological and pathological conditions.
Macrophage polarization
Guided by chemokines and cytokines, macrophages can polarize into different phenotypes such as pro-inflammatory (M1) or pro-healing (M2) (Figure 2e). Physiologically relevant forces due to interstitial flow and hydrostatic pressure may regulate the polarization of macrophages. In most solid tumors, tumor-residing macrophages experience elevated levels of interstitial fluid flow compared to normal tissue [16]. Interstitial flow polarizes mouse bone marrow-derived macrophages towards a M2-like phenotype via integrin/Src-mediated signaling [18]. Mimicking mechanical cues that are experienced by macrophages in the lung, cyclical hydrostatic pressure initiates an inflammatory response via the mechanically activated ion channel Piezo1 [5••, for additional stretch effects on macrophages, see Ref. 10].
In addition to dynamic forces, spatial confinement modulates the polarization and function of macrophages. Macrophages adopt different geometries in vivo [19]. The confinement of macrophages using cell crowding, 3D microwells, or 2D micropatterning to limit their spreading results in the suppression of pro-inflammatory gene expression profile by reducing actin polymerization, lowering the nuclear translocation of myocardin-related transcription factor-A (MRTF-A), and modulating epigenetic modification and chromatin compaction via upregulation of histone deacetylase-3 (HDAC-3) [20••]. Using a similar 2D micropatterning approach to control macrophage cell shape and elongation without exogenous cytokines leads to the expression of M2 phenotype markers [21•]. Such shape-induced polarization is abrogated when actin and actomyosin contractility are inhibited by pharmacological agents, suggesting a role of the cytoskeleton and/or cell geometry in regulating macrophage polarization [22].
The mechanical environment — including the stiffness of the surrounding tissue or matrix — is another source of biophysical cues that modulate immune responses. The elastic modulus in different tissues can vary from a few kilopascals (kPa) in bone marrow and brain to several megapascals (MPa) in cartilages and bones [23]. On stiff polyacrylamide hydrogels (323 kPa), THP-1 derived macrophages are primed towards a pro-inflammatory phenotype with impaired phagocytosis, while soft (11 kPa) and medium (88 kPa) stiffness prime cells towards an anti-inflammatory, highly phagocytic phenotype [23]. Soft substrates (4 kPa) result in suppressed expression of M1-related genes while increasing selected M2-related genes in THP-1 cells compared to stiff substrate (>10 MPa) [24]. Interestingly, substrate stiffness can influence M2 polarization indirectly via modulating paracrine function of mesenchymal stem cells (MSCs). Human MSCs (hMSCs) cultured on soft substrates produced significantly higher levels of immunomodulatory and trophic factors compared to hMSCs cultured on rigid substrates, which result in M2 polarization of macrophages [25].
Phagocytosis
As professional phagocytes, macrophages must identify, catch and engulf pathogens. Stiffness, shape and size of the prey can all influence the formation of the phagocytic cup. Generally, increasing the size of preys is the most effective way to catch macrophages’ attention [8]. Stiffer preys can more efficiently initiate phagocytosis, and such phenomenon is more significant in rod-shaped rather than spherical particles [8]. These trends may relate to how easy to establish receptor-ligand interaction between the macrophage and the invader and how well the macrophage can exert forces through the actin filaments and microtubules. Once the macrophage catches a surface-bound pathogen, they exploit single fimbrium-filopodium contacts to anchor pathogens via catch bonds, helping them extend the bond life and hold onto the prey (Figure 2f) [8].
Mechano-regulation of adaptive immunity
Adaptive immune responses — mediated by lymphocytes — are also modified and potentiated by mechanical cues at the molecular, cellular, and tissue level. Because of the highly mechano-sensitive interactions of TCRs with pMHC on the surface of antigen presenting cells (APCs), mechanotransduction is crucial during T cell selection, development, activation, and, effector function [for reviews, see Refs. 26, 27, 28]. Advances in single-cell experimental tools have aided greatly in expanding our understanding of TCR mechanotransduction [for reviews, Refs. 1,29]. Here, we highlight recent advances in our understanding of mechanobiology at different stages of a T cell’s life cycle.
T cell selection
Unlike innate immune cells that express a fixed set of pattern recognition receptors, the T cell population has a diverse set of TCRs, allowing for recognition of foreign peptide antigens from pathogens. After rearrangement of TCRs via V(D)J recombination, positive and negative selection in the thymus interacting with cortical thymic epithelial cell (cTEC) ensures that T cells possess TCRs that can interact with the specific MHC molecules with appropriate force-dependent binding affinity. Interestingly, the mechanotransduction mechanisms mediating positive and negative selection are distinct, with slip bonds dominating during positive selection and catch bonds induced during negative selection (Figure 1g) [30••].
T cell activation
T cell activation requires the recognition of cognate pMHC on the surface of APCs by TCRs, generating cytoskeleton tension through nucleation of actin foci via Wiskott–Aldrich syndrome protein (WASP) and contraction of the resultant actin filaments by myosin II and leading to formation of immune synapses (Figure 1h) [31•].
T cells must maintain a low enough activation threshold to rapidly scan secondary lymphoid organs for scant, low-affinity foreign antigens, yet high enough to prevent autoimmune responses to self-antigens. The magnitude, duration, frequency, and timing of triggering forces on TCRs all contribute to a ligand-discrimination process that determines whether T cell activation will occur. TCR triggering is recognized as a nonequilibrium process that requires energy [32]. More details on the role of physical forces on T cell triggering have recently been revealed, as factors such as types of bond (i.e. catch, ideal versus slip [28]) and types of motion (i.e. stable synapses versus motile kinapses [33]). Stimulatory and non-stimulatory ligands can bind to TCRs with similar affinity, but only stimulatory ligands form catch bonds, which strengthen during disengagement and couple binding to signaling [34]. At the level of a single TCR-pMHC interaction, agonist, but not antagonist, ligands can sustain mechanical force to generate a conformational change of the heavy chain of MHC class I [35]. Such mechano-chemical coupling on molecular level provides a mechanism for TCR-pMHC catch bond formation [36••]. Triggered by antigen, Further, serial engagement with repeated pulling on TCRs is suggested to be required for T cell activation [36••; a review on serial engagement, see Ref. 32]. Taken together, bond lifetime, conformational changes and serial engagement all play important roles in TCR antigen recognition and T cell activation, but the detailed mechanism still needs further investigation.
Moreover, the flexibility of T cells — which reflects the stiffness of their cortical cytoskeleton — governs signaling differences in naïve versus effector T cells. Activated CD4+ helper T cells exhibit increased deformability of their cortical cytoskeleton, which allows larger immune synapses to be formed with APCs compared to naïve T cells. The stiffer actin cortex in naïve cells is associated with greater basal activity in the RhoA-ROCK (Rho-activated kinase)-LIMK (LIM kinase) axis, resulting in decreased activity of the actin-severing enzyme cofilin [37]. In addition to cell rigidity, cytoskeletal dynamics alter TCR binding during activation. The velocity of retrograde actin flow during immune synapse formation was shown to be correlated with TCR binding kinetics [38].
At the cellular and tissue level, T cells can sense the mechanical properties of their environment. Stiffer (40 kPa) 3D hydrogel matrices augment CD4+ T cell proliferation, activation and migration compared to softer matrices (4 kPa) [39]. Mechanistically, on stiffer substrates, YAP mediates enhanced translocation of nuclear factor of activated T cells (NFAT) into the nucleus, resulting in increased T cell activation and effector function [40]. Interestingly, simply applying mechanical forces via oscillation on a shaker leads to greater T cell expansion and activation, compared to static conditions [41], which may due to more effective encountering and contact between T cells and engineered APCs at the optimal oscillation frequency.
Besides mechanical force and environmental factors, the spatial distribution of ligands also affects how T cells perceive activation signals. Micropillar arrays embedded with anti-CD3 (activation signal) and anti-CD28 (co-stimulator) are useful platforms to investigate T cell mechanosensing on a single cell level. Microtubule organization at the cell-array interface shows the local contraction and expansion of CD4+ T cells. Varying pillar height and shape induces changes in T cell cytoskeletal organization and interferon-γ secretion, which is associated with the effector functions [42•]. Artificial antigen presenting surfaces consisting of patterned micro-dot arrays surrounded by a fluid supported lipid bilayer are also useful tools for mimicking pMHC nanoclusters. TCR, zeta-chain-associated protein kinase (ZAP-70) and actin are colocalized with the ligand-dots in absence of intercellular adhesion molecule 1 (ICAM-1) — the ligand for LFA-1. However, in presence of ICAM-1 in the patterned array, actin shows global changes and accumulates peripherally, and the synapse area increases significantly. Such enhancing effects of TCR clustering on actin organization and cell spreading are exclusive to LFA-1 as the costimulatory molecule CD28 does not have the same impact [43].
Force enhances T cell cytotoxicity
When killing target cells, forces exerted by cytotoxic T cells on target cell membranes enhance the formation of perforin pores (Figure 1i) [44]. Also, perforin release occurs at the base of actin-rich protrusions and is regulated by the cytoskeletal regulator WASP and the Arp2/3 actin nucleation complex [45]. The coordination of perforin release and assembly by distinct actin cytoskeletal structure underscores the importance of mechanical events in the function of cytotoxic T lymphocytes (CTLs).
Applications: engineering immune cell functions
Unravelling the basic mechanobiology on molecular, cellular, and tissue levels inspires novel biomedical applications, especially in the fields of tissue regeneration and cancer immunotherapy. Novel approaches with bench-to-bed translational potentials such as patient cell mechanics measurements, T cell expansion ex vivo, immunoengineering in vivo, and genetic engineering are discussed and also summarized in Table 1 .
Table 1.
Engineered biophysical factors to modulate immune cells
| Biophysical factors | Cell types | Application summary | Ref. |
|---|---|---|---|
| Cell mechanotyping | All | Single-cell mechanotyping enables the characterization of diverse sets of specialized immune cells such as peripheral blood mononuclear cells (PBMCs) and stress-induced macrophages. | [15, 46, 61] |
| ECM stiffness | Macrophage | Human macrophages exhibit a wound healing phenotype on stiffer 3D fibrillar native matrices – collagen I, glycosaminoglycans (GAGs) | [62] |
| Macrophage | Compared to unmodified fibrin gel, photoinitiated dityrosine-crosslinked fibrin gel increases cell spreading and motility and enhances inflammatory activation. | [51] | |
| T lymphocyte | Protein-coated beads made from a soft elastomer - polydimethylsiloxane (PDMS) enhance T cell expansion. | [49] | |
| T lymphocyte | 0.5 kPa–100 kPa poly-acrylamide hydrogels: stiffer gel increases cytokine production, T cell metabolism and cell cycle progression. | [50] | |
| T lymphocyte | 4 kPa–40 kPa RGD-modified alginate hydrogel: stiffer gel augments T-cell activation as compared to the softer material or 2D culture. | [39] | |
| T lymphocyte | An artificial T-cell stimulating matrix is engineered using hyaluronic acid-based hydrogel with optimized combination of the ECM environment and conjugated stimulatory signals for antigen-specific CD8+ T cell activation ex vivo. | [53] | |
| Oscillatory forces | Macrophage | Cyclic mechanical compression achieved by biphasic ferrogels reduces fibrosis, M1 macrophage presence and inflammation in severe skeletal muscle injuries. | [55] |
| T lymphocyte | Compared to static culture, an oscillatory mechanoenvironment doubles antigenic signal strength for CD8+ T cell expansion. | [41] | |
| Squeezing | T lymphocyte | Squeezing cells through a microfluidic device mechanically disrupts cell membrane for drug delivery and results in minimal aberrant transcriptional responses. | [54] |
| Microstructure confinement | Macrophage | Spatial confinement downsizes the inflammatory response of macrophages. | [23] |
| Macrophage | Gelatin-based gels with smaller (30 μm) and softer (20 kPa) pores induce proinflammatory macrophages, while larger (80 μm) and stiffer pores (190 kPa) induce anti-inflammatory macrophages. | [51] | |
| Ligand presentation |
Macrophage | Fibrin matrices induce anti-inflammatory macrophages, but the soluble precursor fibrinogen stimulates inflammatory responses. Presence of both abrogate inflammation. | [58] |
| T lymphocyte | Mesoporous silica micro-rods wrapped in lipid bilayers to present membrane-bounded T cell activation and co-stimulation signals. | [56•,57] | |
| T lymphocyte | Stimulatory signals conjugated to the engineered matrix can successfully activate CD8+ T cell, whereas soluble signals have much less effects. | [53] | |
| Mechanogenetics | T lymphocyte | By engineering the genetic circuits with a mechanosensor Piezo1 ion channel, T cells are modified to be remotely activated by the mechanical perturbance from ultrasound waves and transduce into transcriptional activation for CAR expression. | [59•] |
| T lymphocyte | CAR responsiveness to soluble ligands can be fine-tuned by adjusting the mechanical coupling between the CAR’s ligand-binding and signaling domains | [60] |
Mechanotyping
Cellular deformability is an essential, physiologically relevant feature of immune cells. Mechanotype measurements can now be achieved with single-cell resolution and throughputs of up to 2000 cells/second by flowing a suspension of cells through microfluidic channels, squeezing the cells, and using automated image analysis to quantify metrics such as circularity and deformation timescale [46, 47, 48, 49]. Importantly, cellular mechanotype data can provide valuable information on the status of a patient’s immune system. For example, immune activation can be detected by measuring the size and deformability of cells from pleural effusions of patients. Cells from these inflamed patient pleural fluids are more deformable and larger in cell size compared to native population of leukocytes [47]. Cellular mechanotyping has potential to predict the degree of inflammation and progression of tumors in individual patients.
Materials stiffness
Immune cell responses could also be modulated by tuning the physical properties of matrix, specifically substrate stiffness. Scaffolds of both natural and synthetic biomaterials have been optimized for immune cell cultivation in 2D and 3D. Generally, a stiffer scaffold promotes T lymphocytes cell growth and activation ex vivo [49,50]. However, macrophages show less consistent responses to scaffold stiffness across studies due to different macrophage sources and different substrate materials and stiffness [23,24,51]. A potentially confounding factor is that scaffold mechanical properties are tightly linked to pore size and thus spatial confinement, which may also contribute to macrophage polarization [52]. A new approach using the cryoprotectant dimethyl sulfoxide (DMSO) allows the control of scaffold pore size independent of stiffness, enabling researchers to evaluate individual effects of stiffness and spatial confinement on immune cell phenotype and function in porous biomaterial scaffolds [52]. Understanding the effects of material stiffness and porosity on immune cells will provide a rational basis for the design of artificial matrix to stimulate immune cell expansion ex vivo and modulate immune cell functions in situ by mimicking APCs and/or constructing an artificial niche [39,50,53].
Dynamic environment
Besides changing the surrounding environment, externally exerting forces on immune cells can increase the efficiency of drug delivery, in vitro cell expansion, and in vivo cell function. Squeezing cells through a narrow microfluidic channel is one way to mechanically disrupt the cell membrane for effective gene delivery. Unlike electroporation, which disrupts the expression profile of human T cells, squeezing results in minimal aberrant transcriptional responses [54]. Moreover, an oscillatory mechanoenvironment shows better antigenic signal strength, compared to conventional, static culture for CD8+ T cell expansion [41]. Thus, novel engineering tools and materials can be developed and tailored to control immune cell functions. For example, biphasic ferrogels, which have the mechanical capacity for large, fatigue-resistant deformation, are used for massage-like muscle stimulation [55]. In the model of severe skeletal muscle injuries in mice, macrophages subjected to cyclic mechanical compression show reduction in fibrosis, M1 macrophage and inflammation [55].
Ligand presentation
Engineering the spatial arrangement and 3D geometry of ligands is an alternative strategy to enhance immune cell activation. How a soluble ligand spatially binds to a T cell affects the efficacy of immune response. T cell activation has greater preference towards ligands physical bound to a surface, such as on lipid membranes or conjugated surfaces. Therefore, engineered scaffolds with bounded T cell activation signals is a potential strategy for in vivo local T cell recruitment and expansion [53,56•,57]. The difference in a free-floating ligand and physically bound ligand also affects macrophages. Whereas fibrin-gel induces anti-inflammatory macrophages secreting cytokines such as interleukin-10, the soluble precursor fibrinogen stimulates inflammatory responses [58]. Therefore, designing the presentation of chemical molecules on scaffolds can be a strategy for biomaterial innovation.
Genetic engineering
Mechanical modulation of immune cell responses could additionally be achieved by modulating mechanosensing gene circuits using systems and synthetic biology tools. In recent years, engineered T cells with chimeric antigen receptors (CAR), known as CAR-T cell therapy, have emerged as an effective, FDA-approved immunotherapy for various liquid cancers. For targeted and controlled delivery, mechanosensitive CAR T cells are constructed by harnessing the mechanosensing power of Piezo1 ion channel, so that the mechanical perturbance from ultrasonic waves can activate the CART T-cell at a desired location [59•]. In another study, TGF-β is rewired from an immune suppressive molecule to a CAR T-cell growth factor, thus achieving the first T cell response upon soluble ligand [60]. This actuation process can be precisely regulated by mechanical forces: 1) CAR receptor dimerization is required to bring the signaling domains of the CAR in proximity; 2) CAR itself needs to be mechanically rigid enough for mechanotransmission across the membrane. Such mechanical coupling can be used for fine tuning of T cell responses via extracellular spacer insertion to loosen the coupling [60].
Conclusions and future perspectives
Mechanotransduction in immune cells is dynamic, complex, and essential for the immune responses. Integrins, ion channels, TCRs, cytoskeletal proteins, and transcription factors form a network of mechanical signaling pathways that regulate cell mechanics, gene expression, and cellular behaviors relevant for immune cell functions. There are still knowledge gaps, especially regarding the identification of new mechanosensors and how mechano-signaling pathways are integrated across time and length scales. Moreover, besides the mechanosensitive interactions of integrin-ligand and TCR-pMHC, other conventional receptor-ligand binding events may also be affected by physical forces but have not been studied in the context of mechanobiology.
Single-cell technologies enable future investigations on mechanotransduction mechanisms with higher spatial and temporal resolution. For instance, single-cell force measurement techniques are developing to provide better spatiotemporal resolution and more accurate force sensing on cellular and even molecular level, such as the threshold of force required for TCR to trigger activation signaling. Also, single-cell sequencing deciphers the heterogeneous responses within a population of cells, and the gene expression profile can better define the phenotypes of immune cells. Florescence resonance energy transfer (FRET) imaging, intravital two-photon imaging and other live cell imaging techniques are useful tools to monitor cell signaling events.
Understanding these foundational knowledge of immune cell mechanobiology would further guide the design of transformative biomaterials, drug delivery platforms, and synthetic biology strategies to modulate and control immune responses. Novel biomaterials with the appropriate biophysical properties, including elasticity, viscoelasticity and tunable stiffness and porosity, are needed for more precise regulation of macrophage polarization and T cell activation. By utilizing different delivery methods, the modulation of the immune cells can be local, systematic, or at certain targeted site. While material properties are usually measured as a bulk property, decoupling bulk mechanical cues like stiffness into single factors — such as chemical composition, scaffold pore size, the degree of crosslinking — will also be important to understand underlying mechanisms and be able to design the appropriate biomaterials faster for future applications. Future investigations will also be required to fully understand how multiple cues are simultaneously processed and integrated by immune cells to regulate immune responses.
Finally, biomaterial-based vaccines to selectively stimulate desirable immune cells and suppress undesirable immune responses have much value in this season of COVID-19 pandemic outbreak. Effective antigen presentation and micro-topology on biomaterials can further facilitate the proper activation of desirable immune responses. Moreover, implantable or injectable scaffolds with optimal porosity, matrix stiffness and antigen presentation may not only generate successful immune responses in short term, but also boost the generation of memory immune cells such as memory T cells and memory B cells and promote their longevity. All in all, biomaterial innovation based on mechanotransduction of immune cells can brighten the future for vaccine development and possibly establish material platforms that are robust for generating immunity towards various diseases.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work is supported in part by grants from National Institute of Health (HL121450 and R56DE029157) and UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research.
References
- 1.Iskratsch T., Wolfenson H., Sheetz M.P. Appreciating force and shape — the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15:825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu C., Chen W., Lou J., Rittase W., Li K. Mechanosensing through immunoreceptors. Nat Immunol. 2019;20:1269–1278. doi: 10.1038/s41590-019-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huse M. Mechanical forces in the immune system. Nat Rev Immunol. 2017;17:679–690. doi: 10.1038/nri.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dustin M.L. Integrins and their role in immune cell adhesion. Cell. 2019;177:499–501. doi: 10.1016/j.cell.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 5••.Solis A.G., Bielecki P., Steach H.R., Sharma L., Harman C.C.D., Yun S., de Zoete M.R., Warnock J.N., To S.D.F., York A.G., et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573:69–74. doi: 10.1038/s41586-019-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a pioneering work to link mechanosensitive channels to innate immunity. This work demonstrates that rhythmic pressure is sensed by immune cells via the mechano-sensitive membrane channel PIEZO1 to coordinate immune defense in the lungs.
- 6.Liu C.S.C., Raychaudhuri D., Paul B., Chakrabarty Y., Ghosh A.R., Rahaman O., Talukdar A., Ganguly D. Cutting edge: Piezo1 mechanosensors optimize human T cell activation. J Immunol. 2018;200:1255–1260. doi: 10.4049/jimmunol.1701118. [DOI] [PubMed] [Google Scholar]
- 7.Panciera T., Azzolin L., Cordenonsi M., Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain N., Moeller J., Vogel V. Mechanobiology of macrophages: how physical factors coregulate macrophage plasticity and phagocytosis. Annu Rev Biomed Eng. 2019;21:267–297. doi: 10.1146/annurev-bioeng-062117-121224. [DOI] [PubMed] [Google Scholar]
- 9.McWhorter F.Y., Davis C.T., Liu W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72:1303–1316. doi: 10.1007/s00018-014-1796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meli V.S., Veerasubramanian P.K., Atcha H., Reitz Z., Downing T.L., Liu W.F. Biophysical regulation of macrophages in health and disease. J Leukoc Biol. 2019;106:283–299. doi: 10.1002/JLB.MR0318-126R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruber E.J., Leifer C.A. Molecular regulation of TLR signaling in health and disease: mechano-regulation of macrophages and TLR signaling. Innate Immun. 2020;26:15–25. doi: 10.1177/1753425919838322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada K.M., Sixt M. Mechanisms of 3D cell migration. Nat Rev Mol Cell Biol. 2019;20:738–752. doi: 10.1038/s41580-019-0172-9. [DOI] [PubMed] [Google Scholar]
- 14.Emrick M.A., Sadilek M., Konoki K., Catterall W.A. Beta-adrenergic-regulated phosphorylation of the skeletal muscle Ca(V)1.1 channel in the fight-or-flight response. Proc Natl Acad Sci U S A. 2010;107:18712–18717. doi: 10.1073/pnas.1012384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T.-H., Ly C., Christodoulides A., Nowell C.J., Gunning P.W., Sloan E.K., Rowat A.C. Stress hormone signaling through β-adrenergic receptors regulates macrophage mechanotype and function. FASEB J. 2018;33:3997–4006. doi: 10.1096/fj.201801429RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Man S.M., Ekpenyong A., Tourlomousis P., Achouri S., Cammarota E., Hughes K., Rizzo A., Ng G., Wright J.A., Cicuta P., et al. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc Natl Acad Sci U S A. 2014;111:17588–17593. doi: 10.1073/pnas.1419925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunt S.J., Fyles A., Hill R.P., Milosevic M. Interstitial fluid pressure in tumors: therapeutic barrier and biomarker of angiogenesis. Future Oncol. 2008;4:793–802. doi: 10.2217/14796694.4.6.793. [DOI] [PubMed] [Google Scholar]
- 18.Li R., Serrano J.C., Xing H., Lee T.A., Azizgolshani H., Zaman M., Kamm R.D. Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol Biol Cell. 2018;29:1927–1940. doi: 10.1091/mbc.E18-03-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma V.P., Beaty B.T., Patsialou A., Liu H., Clarke M., Cox D., Condeelis J.S., Eddy R.J. Reconstitution of in vivo macrophage-tumor cell pairing and streaming motility on one-dimensional micro-patterned substrates. Intravital. 2012;1:77–85. doi: 10.4161/intv.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Jain N., Vogel V. Spatial confinement downsizes the inflammatory response of macrophages. Nat Mater. 2018;17:1134–1144. doi: 10.1038/s41563-018-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the effect of confinement on macrophage inflammatory genes expression. When macrophage adherence is limited to a confined space, the inflammatory responses are suppressed, and such observations provide new insights into how physical cues regulate immune cell behavior.
- 21•.McWhorter F.Y., Wang T., Nguyen P., Chung T., Liu W.F. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first article to show macrophage phenotype can be modulated by cell shape. Elongation of macrophages in micropatterned substrates promotes prohealing macrophages.
- 22.Handorf A., Zhou Y., Halanski M., Li W.-J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis. 2015;11:1–15. doi: 10.1080/15476278.2015.1019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sridharan R., Cavanagh B., Cameron A.R., Kelly D.J., O’Brien F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47–59. doi: 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto T., Takagi Y., Kawamoto E., Park E.J., Usuda H., Wada K., Shimaoka M. Reduced substrate stiffness promotes M2-like macrophage activation and enhances peroxisome proliferator-activated receptor gamma expression. Exp Cell Res. 2018;367:264–273. doi: 10.1016/j.yexcr.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Ji Y., Li J., Wei Y., Gao W., Fu X., Wang Y. Substrate stiffness affects the immunosuppressive and trophic function of hMSCs via modulating cytoskeletal polymerization and tension. Biomater Sci. 2019;7:5292–5300. doi: 10.1039/c9bm01202h. [DOI] [PubMed] [Google Scholar]
- 26.Kumar B.V., Connors T.J., Farber D.L. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rushdi M., Li K., Yuan Z., Travaglino S., Grakoui A., Zhu C. Mechanotransduction in T cell development, differentiation and function. Cells. 2020;9 doi: 10.3390/cells9020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossy J., Laufer J.M., Legler D.F. Role of mechanotransduction and tension in T cell function. Front Immunol. 2018;9:2638. doi: 10.3389/fimmu.2018.02638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B., Chen W., Evavold B.D., Zhu C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell. 2014;157:357–368. doi: 10.1016/j.cell.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Hong J., Ge C., Jothikumar P., Yuan Z., Liu B., Bai K., Li K., Rittase W., Shinzawa M., Zhang Y., et al. A TCR mechanotransduction signaling loop induces negative selection in the thymus. Nat Immunol. 2018;19:1379–1390. doi: 10.1038/s41590-018-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is the first study to investigate the type of bonds during selection of T lymphocytes in thymus. It shows that negative selection exerts cooperative TCR–pMHC–CD8 trimolecular catch bonds whereas positive selection formed short-lived slip bonds.
- 31•.Kumari S., Mak M., Poh Y.-C., Tohme M., Watson N., Melo M., Janssen E., Dustin M., Geha R., Irvine D.J. Cytoskeletal tension actively sustains the migratory T-cell synaptic contact. EMBO J. 2020;39 doi: 10.15252/embj.2019102783. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study explains the force generation mechanism through cytoskeleton tension during T cell synaptic contact. WASP acts as a central regulator of synapse symmetry by nucleation of actin foci and is degraded after activation.
- 32.Feng Y., Reinherz E.L., Lang M.J. alphabeta T cell receptor mechanosensing forces out serial engagement. Trends Immunol. 2018;39:596–609. doi: 10.1016/j.it.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayya V., Judokusumo E., Abu Shah E., Peel C.G., Neiswanger W., Depoil D., Blair D.A., Wiggins C.H., Kam L.C., Dustin M.L. Durable interactions of T cells with T cell receptor stimuli in the absence of a stable immunological synapse. Cell Rep. 2018;22:340–349. doi: 10.1016/j.celrep.2017.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibener L.V., Fernandes R.A., Kolawole E.M., Carbone C.B., Liu F., McAffee D., Birnbaum M.E., Yang X., Su L.F., Yu W., et al. Isolation of a structural mechanism for uncoupling T cell receptor signaling from peptide-MHC binding. Cell. 2018;174:672–687.e27. doi: 10.1016/j.cell.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu P., Zhang T., Liu B., Fei P., Cui L., Qin R., Zhu H., Yao D., Martinez R.J., Hu W., et al. Mechano-regulation of peptide-MHC Class I conformations determines TCR antigen recognition. Mol Cell. 2019;73:1015–1027.e7. doi: 10.1016/j.molcel.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Lin J.J.Y., Low-Nam S.T., Alfieri K.N., McAffee D.B., Fay N.C., Groves J.T. Mapping the stochastic sequence of individual ligand-receptor binding events to cellular activation: T cells act on the rare events. Sci Signal. 2019;12 doi: 10.1126/scisignal.aat8715. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study emphasizes the importance of stochastic sequence and binding kinetics during T cell activation. Dwell time of pMHC-TCR binding that triggers T cell activation is an order of magnitude longer than the average agonist pMHC-TCR dwell time.
- 37.Thauland T.J., Hu K.H., Bruce M.A., Butte M.J. Cytoskeletal adaptivity regulates T cell receptor signaling. Sci Signal. 2017:10. doi: 10.1126/scisignal.aah3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colin-York H., Javanmardi Y., Skamrahl M., Kumari S., Chang V.T., Khuon S., Taylor A., Chew T.-L., Betzig E., Moeendarbary E., et al. Cytoskeletal control of antigen-dependent T cell activation. Cell Rep. 2019;26:3369–3379.e5. doi: 10.1016/j.celrep.2019.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majedi F.S., Hasani-Sadrabadi M.M., Thauland T.J., Li S., Bouchard L.-S., Butte M.J. T-cell activation is modulated by the 3D mechanical microenvironment. bioRxiv. 2019 doi: 10.1101/580886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng K.P., Majedi F.S., Thauland T.J., Butte M.J. Tissue mechanics controls T-cell activation and metabolism. bioRxiv. 2019 doi: 10.1101/581322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majedi F.S., Hasani-Sadrabadi M.M., Thauland T.J., Li S., Bouchard L.-S., Butte M.J. Augmentation of T-cell activation by oscillatory forces and engineered antigen-presenting cells. Nano Lett. 2019;19:6945–6954. doi: 10.1021/acs.nanolett.9b02252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Jin W., Tamzalit F., Chaudhuri P.K., Black C.T., Huse M., Kam L.C. T cell activation and immune synapse organization respond to the microscale mechanics of structured surfaces. Proc Natl Acad Sci U S A. 2019;116:19835–19840. doi: 10.1073/pnas.1906986116. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study captures the spatial complexity of mechanical stiffness. By observing the cell interaction with elastomer micropillar arrays, they show that T cells can sense their surrounding local rigidity.
- 43.Benard E., Nunes J.A., Limozin L., Sengupta K. T cells on engineered substrates: the impact of TCR clustering is enhanced by LFA-1 engagement. Front Immunol. 2018;9:2085. doi: 10.3389/fimmu.2018.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu R., Whitlock B.M., Husson J., Le Floc’h A., Jin W., Oyler-Yaniv A., Dotiwala F., Giannone G., Hivroz C., Biais N., et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell. 2016;165:100–110. doi: 10.1016/j.cell.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamzalit F., Wang M.S., Jin W., Tello-Lafoz M., Boyko V., Heddleston J.M., Black C.T., Kam L.C., Huse M. Interfacial actin protrusions mechanically enhance killing by cytotoxic T cells. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aav5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gossett D.R., Tse H.T.K., Lee S.A., Ying Y., Lindgren A.G., Yang O.O., Rao J., Clark A.T., Di Carlo D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci U S A. 2012;109:7630–7635. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nyberg K.D., Hu K.H., Kleinman S.H., Khismatullin D.B., Butte M.J., Rowat A.C. Quantitative deformability cytometry: rapid, calibrated measurements of cell mechanical properties. Biophys J. 2017;113:1574–1584. doi: 10.1016/j.bpj.2017.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosendahl P., Plak K., Jacobi A., Kraeter M., Toepfner N., Otto O., Herold C., Winzi M., Herbig M., Ge Y., et al. Real-time fluorescence and deformability cytometry. Nat Methods. 2018;15:355–358. doi: 10.1038/nmeth.4639. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh J.Y., Keating M.T., Smith T.D., Meli V.S., Botvinick E.L., Liu W.F. Matrix crosslinking enhances macrophage adhesion, migration, and inflammatory activation. APL Bioeng. 2019;3:16103. doi: 10.1063/1.5067301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambert L.H., Goebrecht G.K.E., De Leo S.E., O’Connor R.S., Nunez-Cruz S., Li T.-D., Yuan J., Milone M.C., Kam L.C. Improving T cell expansion with a soft touch. Nano Lett. 2017;17:821–826. doi: 10.1021/acs.nanolett.6b04071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saitakis M., Dogniaux S., Goudot C., Bufi N., Asnacios S., Maurin M., Randriamampita C., Asnacios A., Hivroz C. Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. eLife. 2017;6 doi: 10.7554/eLife.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang S., Lyu C., Zhao P., Li W., Kong W., Huang C., Genin G.M., Du Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat Commun. 2019;10 doi: 10.1038/s41467-019-11397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hickey J.W., Dong Y., Chung J.W., Salathe S.F., Pruitt H.C., Li X., Chang C., Fraser A.K., Bessell C.A., Ewald A.J., et al. Engineering an artificial T-cell stimulating matrix for immunotherapy. Adv Mater. 2019;31 doi: 10.1002/adma.201807359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiTommaso T., Cole J.M., Cassereau L., Buggé J.A., Hanson J.L.S., Bridgen D.T., Stokes B.D., Loughhead S.M., Beutel B.A., Gilbert J.B., et al. Cell engineering with microfluidic squeezing preserves functionality of primary immune cells in vivo. Proc Natl Acad Sci U S A. 2018;115:E10907–E10914. doi: 10.1073/pnas.1809671115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cezar C.A., Roche E.T., Vandenburgh H.H., Duda G.N., Walsh C.J., Mooney D.J. Biologic-free mechanically induced muscle regeneration. Proc Natl Acad Sci U S A. 2016;113:1534–1539. doi: 10.1073/pnas.1517517113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Cheung A.S., Zhang D.K.Y., Koshy S.T., Mooney D.J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat Biotechnol. 2018;36:160–169. doi: 10.1038/nbt.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]; This engineering scaffold mimics antigen-presenting cells and provides ex vivo expansion for primary T cells better than current technology. It consists of a fluid lipid bilayer for membrane-bound presentation of anti-CD3 and anti-CD28 and a mesoporous silica micro-rod core for sustained release of interleukin-2.
- 57.Kim J., Li W.A., Choi Y., Lewin S.A., Verbeke C.S., Dranoff G., Mooney D.J. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsieh J.Y., Smith T.D., Meli V.S., Tran T.N., Botvinick E.L., Liu W.F. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017;47:14–24. doi: 10.1016/j.actbio.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Pan Y., Yoon S., Sun J., Huang Z., Lee C., Allen M., Wu Y., Chang Y.-J., Sadelain M., Shung K.K., et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc Natl Acad Sci U S A. 2018;115:992–997. doi: 10.1073/pnas.1714900115. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mechanosensitive CAR-T cells are constructed through engineering the mechanosensor Piezo-1 and genetic transducing modules. The engineered T cells are responsive to mechanical perturbation like ultrasound waves, thus non-invasive and remote control achieved.
- 60.Chang Z.L., Lorenzini M.H., Chen X., Tran U., Bangayan N.J., Chen Y.Y. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol. 2018;14:317–324. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver W.M., Tseng P., Kunze A., Masaeli M., Chung A.J., Dudani J.S., Kittur H., Kulkarni R.P., Di Carlo D. Advances in high-throughput single-cell microtechnologies. Curr Opin Biotechnol. 2014;25:114–123. doi: 10.1016/j.copbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedemann M., Kalbitzer L., Franz S., Moeller S., Schnabelrauch M., Simon J.-C., Pompe T., Franke K. Instructing human macrophage polarization by stiffness and glycosaminoglycan functionalization in 3D collagen networks. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201600967. [DOI] [PubMed] [Google Scholar]