Abstract
Objectives
This study aimed to evaluate the association of chronic diseases and indigenous ethnicity on the poor prognosis of outpatients with coronavirus disease 2019 (COVID-19) and hospitalised patients in Mexico.
Study design
The study design is an observational study of consecutive COVID-19 cases that were treated in Mexican healthcare units and hospitals between February 27 and April 27, 2020.
Methods
Epidemiological, clinical and sociodemographic data were analysed from outpatients and hospitalised patients. Cox regression models were used to analyse the risk of mortality after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Results
In total, 15,529 patients with COVID-19 were characterised; 62.6% of patients were aged older than 40 years, 57.8% were men and 1.4% were of indigenous ethnicity. A high proportion had a history of diabetes (18.4%), hypertension (21.9%) and obesity (20.9%). Among hospitalised patients, 11.2% received health care in the intensive care unit. Advanced age, male sex, indigenous ethnicity and having a history of chronic diseases, such as hypertension, diabetes and obesity, were significantly associated with a high risk of death after SARS-CoV-2 infection. Diabetes and obesity were the comorbidities most highly associated with death through the models used in this study. Moreover, living in Mexico City and Mexico State (where there is easy access to medical services) and walking (rather than driving or getting public transport) were negatively associated with mortality after SARS-CoV-2 infection.
Conclusions
Diabetes, hypertension and obesity combined with older age, male sex and indigenous ethnicity increase the risk of death after SARS-CoV-2 infection in the Mexican population. It is recommended that the incidence of COVID-19 is monitored in indigenous communities, and access to health services is increased nationwide.
Keywords: SARS-CoV-2, COVID-19, Risk factors, Chronic diseases, Vulnerable populations
Highlights
-
•
Chronic diseases and indigenous ethnicity were positively associated with COVID-19 mortality.
-
•
Living in a city with more access to medical services was negatively associated with mortality.
-
•
Walking was negatively associated with mortality.
-
•
Health services coverage should be strengthened in indigenous communities.
Introduction
The novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified in December 2019 in Wuhan, China, and results in coronavirus disease 2019 (COVID-19).1 The disease is highly contagious, and each infected person could infect at least 3 other people on average.2 On March 11, 2020, the World Health Organization declared COVID-19 to be a global pandemic.3 At the time of writing, there have been more than 5.3 million cases of COVID-19 and 342,105 deaths, worldwide.4 In Mexico, the first confirmed case of COVID-19 was registered on February 28, 2020; as of May 24, 2020, there have been 65,856 COVID-19 cases reported5 and a total of 7179 COVID-19–related deaths.5 As a result of the rapidly increasing death rates from COVID-19, the key public health priority was to determine the factors that modify the prognosis of an individual after SARS-CoV-2 infection.
Diverse risk factors associated with death from COVID-19 have been reported from national records and hospital case series,6 , 7 , 8 including advanced age and male gender.9 Studies from hospitalised patients have shown that comorbidities such as diabetes mellitus, hypertension, cardiovascular disease and obesity also increase the mortality risk among patients with COVID-19.10 , 11 , 12 In addition, patients with chronic kidney disease (CKD) have a high risk for in-hospital death.13 However, few studies have analysed the association between social determinants and COVID-19 death rates. A recent study from the UK analysed ethnicity and risk of COVID-19 death and found that black individuals were at a higher risk of death than white individuals (adjusted hazard ratio [aHR]: 1.71; 95% confidence interval [CI]: 1.44–2.02); similar findings were found for the Asian population (aHR: 1.62; 95% CI: 1.43–1.82).14 , 15 , 16 Given that COVID-19 is highly contagious and there are limited data about the impact on vulnerable populations, it is important to analyse the effect of health inequalities and indigenous status on the risk of death from COVID-19 in middle-income countries.17
In low- and middle-income countries, such as Mexico, there is a high prevalence of risk factors for COVID-19 death. For example, in 2016, 69.4% of the Mexican population was overweight or obese,18 10.4% of adults aged ≥20 years had diabetes19 and 25.5% had hypertension.20 This chronic disease profile, in addition to other factors, such as the uneven distribution of healthcare resources, high mobility, poverty and ethnicity differences, could increase the risk of death from COVID-19 and it should be investigated. This study aimed to evaluate the association of the chronic disease profile and indigenous ethnicity on the poor prognosis of outpatients with COVID-19 and hospitalised patients in Mexico.
Methods
A secondary analysis of nationwide COVID-19 data from the Mexican Secretary of Health was carried out. This analysis is based on an observational study of consecutive COVID-19 cases that were treated in healthcare units and hospitals between February 27 and April 27, 2020. The information was extracted from the Mexican Secretary of Health, which publishes a data set on the open data platform of the Mexican government.21 COVID-19 diagnosis was made according to the Diagnosis and Treatment Guideline for COVID-19 published by the Mexican Secretary of Health.22 All cases were confirmed using real-time reverse transcription polymerase chain-reaction.19 Clinical data, such as recent exposure history, comorbidities and sociodemographic information, from outpatients and hospitalised patients were analysed. This study did not require the approval of an institutional ethics committee because it is an analysis of secondary data. The data are publicly available on the platform of the Mexican Secretary of Health under Mexico's open government data policy to facilitate the access, use, reuse and redistribution of information.
Variables
The primary endpoint was the survival time or time to death, defined as the time from the expected day of infection to death or the last follow-up date (censoring). To study mortality was a different endpoint, as there were no variables on the follow-up of patients to know whether they had recovered from COVID-19. An average incubation period of 6 days,23 an average of 14 days for recovery from the date of symptoms for outpatients24 and an average of 25 days for hospitalised patients were assumed.25 COVID-19–positive cases were analysed into two groups: outpatients and hospitalised patients. Sociodemographic variables used in this study included age as a continuous variable, sex (female vs male) and indigenous ethnicity (a person was defined as indigenous if an indigenous language was spoken by a member of the household). Mexico was divided into four geographic regions: North-western, Central, Mexico City-State of Mexico and South. Population density size was defined as <100,000 and ≥ 100,000 people. Clinical information included previous exposure to COVID-19–positive cases and pregnancy. Comorbidities were determined based on the patient's self-report on admission, were treated as categorical variables (yes vs no) and included cardiovascular disease, hypertension, diabetes, obesity, CKD, chronic obstructive pulmonary disease (COPD), asthma, disease associated to immunosuppression and additional comorbidities. Smoking status was analysed as a habit and independent variable using the Kaplan-Meier estimator and the log-rank test, and the variable was not significant.
Information on mobility trends, reported by Apple between January 13 and April 28, 2020, was used, which provides data on modes of transport (i.e. public transport, driving and walking). The data published by Apple are the percentage change from a baseline for each region. Taking into account the mean incubation period of COVID-19 infection to be 6 days and the mean duration from onset of symptoms to death to be 18 days, it was considered the average time from onset to death to be 24 days.23 , 25 Therefore, the date of symptoms was accordingly moved back 6 days based on that estimation of the probable date of infection, and the date was related with the daily reported mobility on that day.
Statistical analyses
Data for the two groups (outpatient vs hospitalised patient) were expressed as mean ± standard deviation (ȳ ± s), and they were compared using the independent group t-test or Fisher's test when appropriate. The count data were showed as a rate (%) and used an χ2 test. Survival outcomes were analysed according to the Kaplan-Meier estimator. Differences between survival curves were assessed by using the log-rank test. Cox proportional hazards models were constructed based on multivariate analysis results to evaluate the association between risk factors and survival time over the entire follow-up period, in all samples and stratified by hospitalised and non-hospitalised patients. Variables for multivariate models were chosen based on clinical relevance or a statistically significant relationship with the dependent variable. In addition, smoking status was evaluated in the Cox proportional hazards models, and it was not significant and was thus not included in the final model. Statistical analysis was performed using Stata 14.0 (StataCorp, Stata Statistical Software, 2015). A P-value of <0.05 was considered to be statistically significant.
Results
Table 1 shows the sociodemographic, clinical and epidemiological characteristics of COVID-19 cases by the type of patient care. In total, 62.6% of patients were aged older than 40 years, 57.8% were men and 1.4% were of indigenous ethnicity. Overall, 45.8% of patients with COVID-19 had come into close contact with patients who experienced symptoms of COVID-19, 1.4% of cases were pregnant and 8.9% were tobacco smokers. In the sample, the prevalence of comorbidities was high; 20.9% of them were obese, 18.4% had type 2 diabetes and 21.9% had hypertension. CKD and COPD were less prevalent at 2.3% and 2.5%, respectively. Among hospitalised cases, 11.2% received health care in the intensive care unit and 67.1% of COVID-19 cases developed pneumonia.
Table 1.
Characteristics of COVID patients by the type of healthcare received.
| Variable | Outpatient [n (%)] n = 9487 | Hospitalised [n (%)] n = 6042 | P-value | Total [n (%)] n = 15,529 |
|---|---|---|---|---|
| Sex | ||||
| Female | 4437 (46.8%) | 2115 (35.0%) | <0.001 | 6552 (42.2%) |
| Male | 5050 (53.2%) | 3927 (65.0%) | 8977 (57.8%) | |
| Age group (years) | ||||
| ≤15 | 168 (1.8%) | 39 (0.65%) | <0.001 | 207 (1.3%) |
| 16 to 30 | 1853 (19.5%) | 342 (5.6%) | 2194 (14.1%) | |
| 31 to 40 | 2600 (27.4%) | 808 (13.4%) | 3408 (21.9%) | |
| 41 to 50 | 2324 (24.5%) | 1393 (23.1%) | 3717 (23.9%) | |
| 51 to 60 | 1555 (16.4%) | 1558 (25.8%) | 3113 (20.1%) | |
| 61 to 70 | 665 (7.0%) | 1111 (18.4%) | 1776 (11.4%) | |
| >70 | 322 (3.4%) | 792 (13.1%) | 1114 (7.2%) | |
| Indigenous ethnicity | ||||
| No | 9214 (99.0%) | 5787 (98.0%) | <0.001 | 15,001 (98.6%) |
| Yes | 90 (1.0%) | 119 (2.0%) | 209 (1.4%) | |
| Previous exposure to COVID-19–positive case | ||||
| No | 2561 (43.3%) | 2444 (73.5%) | <0.001 | 5005 (54.2%) |
| Yes | 3349 (56.7%) | 881 (26.5%) | 4230 (45.8%) | |
| Pregnancy | ||||
| No | 4330 (98.6%) | 2072 (98.6%) | 0.92 | 6402 (98.6%) |
| Yes | 62 (1.4%) | 29 (1.4%) | 91 (1.40%) | |
| Smoking | ||||
| No | 8650 (92.0%) | 5370 (89.7%) | <0.001 | 14,020 (91.1%) |
| Yes | 756 (8.0%) | 618 (10.3%) | 1374 (8.9%) | |
| Obesity | ||||
| No | 7748 (82.3%) | 4439 (74.1%) | <0.001 | 12,187 (79.1%) |
| Yes | 1662 (17.7%) | 1553 (25.9%) | 3215 (20.9%) | |
| Diabetes | ||||
| No | 8389 (89.2%) | 4176 (69.7%) | <0.001 | 12,565 (81.6%) |
| Yes | 1017 (10.8%) | 1814 (30.3%) | 2831 (18.4%) | |
| Hypertension | ||||
| No | 7987 (84.9%) | 4040 (67.5%) | <0.001 | 12,027 (78.1%) |
| Yes | 1420 (15.1%) | 1950 (32.5%) | 3370 (21.9%) | |
| Cardiovascular disease | ||||
| No | 9235 (98.2%) | 5701 (95.3%) | <0.001 | 14,936 (97.1%) |
| Yes | 171 (1.8%) | 282 (4.7%) | 453 (2.9%) | |
| Chronic kidney disease | ||||
| No | 9311 (99.0%) | 5720 (95.6%) | <0.001 | 15,031 (97.7%) |
| Yes | 93 (1.0%) | 266 (4.4%) | 359 (2.3%) | |
| Chronic obstructive pulmonary disease | ||||
| No | 9295 (98.8%) | 5714 (95.4%) | <0.001 | 15,009 (97.5%) |
| Yes | 113 (1.2%) | 276 (4.6%) | 389 (2.5%) | |
| Asthma | ||||
| No | 9040 (96.1%) | 5810 (97.1%) | <0.05 | 14,850 (95.5%) |
| Yes | 366 (3.9%) | 176 (2.9%) | 542 (3.5%) | |
| Disease associated to immunosuppression | ||||
| No | 9293 (98.8%) | 5804 (96.9%) | <0.001 | 15,097 (98.1%) |
| Yes | 113 (1.2%) | 183 (3.1%) | 296 (1.9%) | |
| Additional comorbidity | ||||
| No | 9026 (96.1%) | 5697 (95.3%) | <0.001 | 14,723 (95.8%) |
| Yes | 368 (3.9%) | 282 (4.7%) | 650 (4.2%) | |
| IUC | ||||
| No | NA | 5364 (88.8%) | NA | 5364 (88.8%) |
| Yes | NA | 676 (11.2%) | 676 (11.2%) | |
| Pneumonia | ||||
| No | 8952 (94.4%) | 1989 (32.9%) | <0.001 | 10,941 (70.5%) |
| Yes | 535 (5.6%) | 4053 (67.1%) | 4588 (29.5%) | |
COVID-19, coronavirus disease 2019; IUC, intensive unit care.
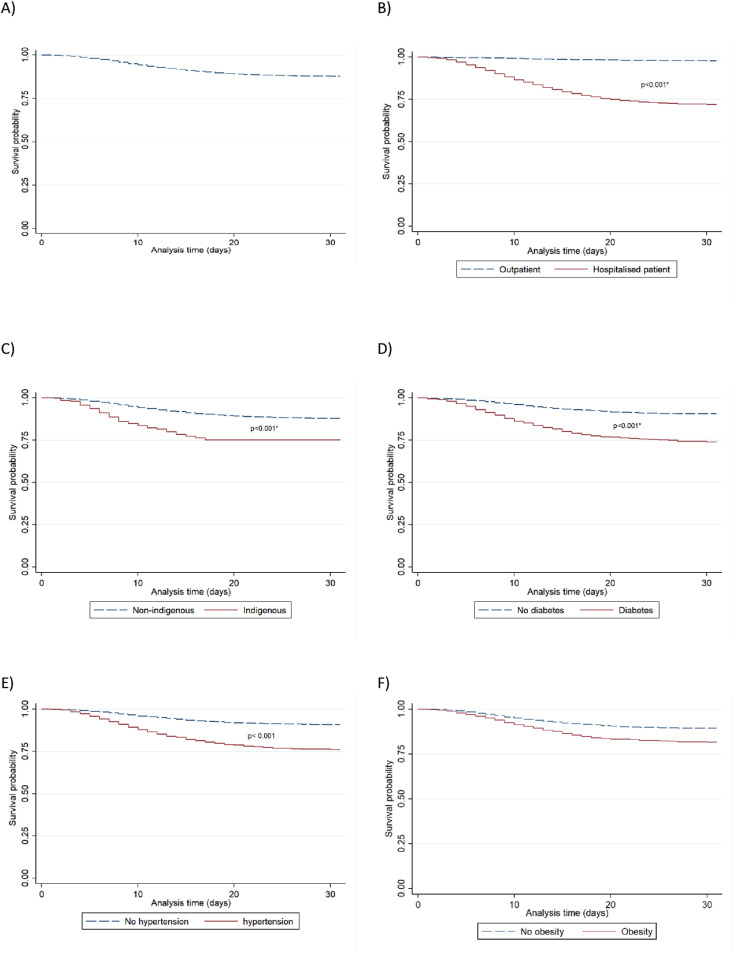
Fig. 1 shows the Kaplan-Meier survival curves for mortality among adults with SARS-CoV-2 infection enrolled in medical institutions during the study period. Overall, the median survival was 33 days (range 32–35), 26 days (range 22–28) for outpatients and 33 days (range 32–42) for hospitalised patients. Regarding survival, 1434 (9.2%) patients died of COVID-19. The overall Kaplan Meier curve (Fig. 1a) shows an increased risk of mortality after 5 days of follow-up. Kaplan-Meier survival curves stratified by the type of patient care (Fig. 1b) displayed that the overall mortality rate was higher for hospitalised patients than for outpatients. The indigenous population showed a lower survival probability than non-indigenous (Fig. 1c). Individuals with diabetes, hypertension or obesity had a decreased overall survival after COVID-19 infection compared with people without these comorbidities (Fig. 1d, e, 1f). In addition, men showed a higher mortality than women (10.92% vs 6.93%, respectively, P < 0.001). Similarly, patients with COVID-19 and cardiovascular disease, CKD or COPD exhibited higher death rates than those without these conditions (Table S1in the supplementary information).
Fig. 1.
Survival probability from symptom onset to death. (a) overall, (b) outpatient vs hospitalised patients, (c) indigenous vs non-indigenous, (d) diabetes vs no diabetes, (e) hypertension vs no hypertension, (f) obesity vs no obesity.
Table 2 shows the associated factors with the survival probability after SARS-CoV-2 infection to the overall sample population during the follow-up period and stratified by the type of patient care (i.e. outpatient vs hospitalised patient). The Cox regression models showed that advanced age significantly increased the likelihood of death (overall sample model aHR = 1.04, P < 0.001; outpatient model aHR = 1.03, P < 0.001; hospitalised patient model aHR = 1.05, P < 0.001). Male sex showed increased death rates (overall sample model aHR = 1.46, P < 0.001; outpatient model aHR = 1.34, P < 0.001; hospitalised patient model aHR = 1.35, P = 0.114). Indigenous ethnicity was also associated with an increased likelihood of death (overall sample model aHR = 1.47, P = 0.020; outpatient model aHR = 1.48, P = 0.026; hospitalised patient model aHR = 2.47, P = 0.085). Hypertension, diabetes and obesity were significantly associated with a high risk of death after SARS-CoV-2 infection, and the highest risk of death was obtained by the combination of diabetes plus obesity (overall sample model aHR = 2.28, P < 0.001; outpatient model aHR = 1.89, P < 0.001; hospitalised patient model aHR = 2.84, P = 0.023). Pneumonia, COPD, immunosuppression and CKD were positively associated with mortality. Finally, living in Mexico City or in the State of Mexico (overall sample model aHR = 0.50, P < 0.001; outpatient model aHR = 0.50, P < 0.001; hospitalised patient model aHR = 0.48, P = 0.005) and walking as a mode of transport (overall sample model aHR = 0.95, P = 0.036; outpatient model aHR = 0.94, P = 0.017; hospitalised patient model aHR = 0.99, P = 0.867) were negatively associated with mortality.
Table 2.
Multivariate Cox proportional hazards models exploring factors associated with mortality in patients with COVID-19.
| Variable | Overall sample model |
Stratified model |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outpatient |
Hospitalised |
|||||||||||
| aHR | 95% CI | P-value | aHR | 95% CI | P-value | aHR | 95% CI | P-value | ||||
| Age (years)a | 1.04 | 1.03 | 1.04 | 0.000∗ | 1.03 | 1.02 | 1.03 | 0.000∗ | 1.05 | 1.04 | 1.07 | 0.000∗ |
| Sex, male | 1.46 | 1.30 | 1.64 | 0.000∗ | 1.34 | 1.19 | 1.52 | 0.000∗ | 1.35 | 0.93 | 1.95 | 0.114 |
| Indigenous ethnicity, yes | 1.47 | 1.06 | 2.04 | 0.020∗ | 1.48 | 1.05 | 2.08 | 0.026∗ | 2.47 | 0.88 | 6.91 | 0.085 |
| Pneumonia, yes | 4.69 | 4.12 | 5.33 | 0.000∗ | 1.55 | 1.35 | 1.77 | 0.000∗ | 11.02 | 7.57 | 16.05 | 0.000∗ |
| Chronic obstructive pulmonary disease, yes | 1.28 | 1.04 | 1.59 | 0.022∗ | 1.31 | 1.05 | 1.64 | 0.016∗ | 1.07 | 0.47 | 2.43 | 0.874 |
| Diseases associated with immunosuppression, yes | 1.73 | 1.34 | 2.24 | 0.000∗ | 1.69 | 1.30 | 2.20 | 0.000∗ | 0.51 | 0.11 | 2.29 | 0.382 |
| Additional comorbidity, yes | 1.22 | 0.98 | 1.53 | 0.074 | 1.27 | 1.02 | 1.60 | 0.036∗ | 0.82 | 0.28 | 2.36 | 0.711 |
| Cardiovascular disease, yes | 0.85 | 0.67 | 1.06 | 0.151 | 0.86 | 0.68 | 1.09 | 0.222 | 0.91 | 0.40 | 2.06 | 0.824 |
| Chronic diseases interaction | ||||||||||||
| Hypertension, yes | 1.27 | 1.06 | 1.52 | 0.010∗ | 1.16 | 0.96 | 1.40 | 0.125 | 1.11 | 0.59 | 2.09 | 0.737 |
| Diabetes, yes | 1.43 | 1.18 | 1.74 | 0.000∗ | 1.25 | 1.02 | 1.53 | 0.029∗ | 2.09 | 1.09 | 4.00 | 0.026∗ |
| Obesity, yes | 1.91 | 1.59 | 2.29 | 0.000∗ | 1.55 | 1.28 | 1.87 | 0.000∗ | 2.06 | 1.11 | 3.82 | 0.022∗ |
| Diabetes + hypertension, yes | 1.65 | 1.38 | 1.97 | 0.000∗ | 1.40 | 1.16 | 1.68 | 0.000∗ | 2.05 | 1.07 | 3.90 | 0.030∗ |
| Obesity + hypertension, yes | 1.66 | 1.30 | 2.14 | 0.000∗ | 1.44 | 1.11 | 1.86 | 0.006∗ | 1.08 | 0.41 | 2.85 | 0.884 |
| Diabetes + obesity, yes | 2.28 | 1.74 | 2.99 | 0.000∗ | 1.89 | 1.42 | 2.51 | 0.000∗ | 2.84 | 1.15 | 7.00 | 0.023∗ |
| Diabetes + obesity + hypertension, yes | 2.16 | 1.74 | 2.68 | 0.000∗ | 1.62 | 1.29 | 2.05 | 0.000∗ | 5.94 | 3.23 | 10.91 | 0.000∗ |
| Chronic kidney disease, yes | 1.60 | 1.29 | 2.00 | 0.000∗ | 1.56 | 1.24 | 1.96 | 0.000∗ | 1.97 | 0.76 | 5.09 | 0.162 |
| Intensive care unit, yes | – | – | – | – | – | – | – | – | 1.81 | 1.57 | 2.09 | 0.000∗ |
| Region | ||||||||||||
| North-west | 0.84 | 0.68 | 1.03 | 0.097 | 0.93 | 0.74 | 1.16 | 0.527 | 0.71 | 0.38 | 1.36 | 0.303 |
| Central | 1.05 | 0.87 | 1.26 | 0.641 | 1.04 | 0.86 | 1.27 | 0.672 | 0.53 | 0.25 | 1.12 | 0.097 |
| Mexico City-State of Mexico | 0.50 | 0.44 | 0.57 | 0.000∗ | 0.50 | 0.44 | 0.58 | 0.000∗ | 0.48 | 0.29 | 0.80 | 0.005∗ |
| South | 0.96 | 0.82 | 1.13 | 0.614 | 0.91 | 0.76 | 1.07 | 0.256 | 0.85 | 0.51 | 1.41 | 0.522 |
| Density (>100,000 habitants) | 1.07 | 0.91 | 1.26 | 0.392 | 1.07 | 0.90 | 1.27 | 0.443 | 0.64 | 0.39 | 1.04 | 0.069 |
| Mode of transport | ||||||||||||
| Driving | 1.03 | 1.00 | 1.07 | 0.079 | 1.05 | 1.01 | 1.09 | 0.023∗ | 0.98 | 0.87 | 1.10 | 0.688 |
| Public transport | 1.02 | 1.00 | 1.04 | 0.110 | 1.02 | 1.00 | 1.04 | 0.125 | 1.03 | 0.96 | 1.10 | 0.406 |
| Walking | 0.95 | 0.91 | 1.00 | 0.036∗ | 0.94 | 0.90 | 0.99 | 0.017∗ | 0.99 | 0.86 | 1.14 | 0.867 |
∗ statistically significative, P-value<0.05. aHR = adjusted hazard ratio; CI, confidence interval; COVID-19, coronavirus disease 2019.
Age is continuous variable.
Discussion
Firstly, our findings suggest that the presence of several different factors can influence the course of SARS-CoV-2 infection. Advanced age, male sex, hypertension, diabetes and obesity were significantly associated with a high risk of death after SARS-CoV-2 infection. The combination of diabetes plus obesity comorbidities resulted in the highest risk of death through the statistical models used in this study. Living in Mexico City or the State of Mexico and walking as the mode of transport were negatively associated with mortality through the models. Secondly, the results from this study found a strong association between risk of death and indigenous ethnicity, which may suggest an increased risk of death in vulnerable groups.
The results of this study indicate that for patients who have not recovered in the average estimated time, the risk of dying is high, particularly among hospitalised patients, indigenous individuals and patients with diabetes, hypertension and obesity. During the 32 days of follow-up, there were 1434 (9.23%) deaths from COVID-19. Similar studies have found different rates of death. For example, a study from Wuhan, China, found that 16.5% of patients died during the 32 days of the follow-up.26 The Cheng et al study found a death rate of 16.1%.13 One study from two hospitals found the rate of death to be 29%.7 Additionally, a nationwide study in China found the mortality rate to be 3.1%.7 , 11 , 13 , 26 Differences in these results compared with the present study could be attributed to the sample characteristics, including prevalence of risk factors in the study population and access to health treatment.
A high risk of death was found to be associated with two variables: the first was indigenous ethnicity, which increased the likelihood of death after SARS-CoV-2 infection by approximately 47%. Previous studies have described the effect of ethnicity on the risk of COVID-19 death; for example, one study from the US documented a high death rate in Afro-American populations, as is the case of Louisiana, where 70% of COVID-19 deaths have been in the Afro-American population. Increased death rates have also been attributed to limited access to health care, high prevalence of chronic conditions and reduced social distancing in the low-wage essential services that cannot be performed remotely.27 In Mexico, indigenous people could be living in such situations, with limited health care as a result of the low distribution of human resources and healthcare staff in rural areas.28 Other factors could be the high prevalence of chronic conditions among indigenous people; the prevalence of metabolic syndrome in this population has been recorded as 50.3% and the prevalence of high blood pressure was 42.7%.29 The reduced risk of COVID-19 deaths (50%, P < 0.001) seen in Mexico City and the State of Mexico could be attributed to the easy access to medical services and resulting treatment of the disease in this area; previous evidence from a Chinese study suggests that mortality is correlated to healthcare resource availability.30
Chronic diseases are common factors associated with the risk of death from COVID-19. A strong positive association was found between metabolic conditions and death. Previous studies have explained the effect of comorbidities on patients with COVID-19. Patients with diabetes, often associated with obesity and hypertension, may be more susceptible to an inflammatory reaction, eventually leading to rapid progression and adverse prognosis of COVID-19.31 In obese patients, there is an increased risk of inflammation due to adipose tissue potential. Hyperexpression of ACE2 is also found in obese patients, and thromboembolism events are frequent.32 SARS-CoV-2 infection induces an alteration of insulin secretion and may increase resistance to insulin action, which could lead to acute decompensation in patients with diabetes.33 A high level of troponin and natriuretic peptides have been found in critically ill patients, where the main proposed mechanism is inflammation of the vascular system that can result in diffuse microangiopathy with thrombosis. Inflammation of the myocardium can induce myocarditis, heart failure, cardiac arrhythmias, acute coronary syndrome, rapid deterioration and sudden death.34
Currently, social distancing is the only effective tool recommended to slow the spread of COVID-19.35 In some countries, such as Italy, Spain and China, lockdown measures have been extreme; however, in Mexico, the measures were more moderate considering the vulnerability of the population. In this study, it was identified that the mobility reduction is very important in controlling and reducing the risk of death from COVID-19. There are significant associations between mobility trends and COVID-19 death risk: driving and public transport were positively associated with the risk of death. Walking was negatively associated with the risk of death and showed that it is the most sustainable option and allows guarantees social distancing.
Limitations
This study has several limitations. It is a case series study based on the Mexican registry data; thus, the proportion of severe and critical patients and fatality rate might be different to the whole infected population. The follow-up was a relatively short duration, and some patients remained in hospital at the time of writing. Further studies that explore the associations in a sufficiently long time frame are warranted. As with other observational studies, our findings do not provide direct inference about the causation or reverse causation of comorbidities and the poor clinical outcomes. Finally, in this work, no information on the date of recovery is available, although we were able to find information on the time of recovery in the existing literature.
Conclusions
It was found that chronic conditions experienced in Mexican population, such as diabetes, hypertension and obesity, combined with older age, male sex and indigenous ethnicity increase the risk of death after SARS-CoV-2 infection. However, living in Mexico City or in the State of Mexico (an area with a high access to medical services) and walking as a mode of transport were negatively associated with mortality under the adjusted model. It is recommended that the incidence of COVID-19 is monitored in indigenous communities and the impact of SARS-CoV-2 infection in other vulnerable groups is assessed.
Author statements
Ethical approval
This study did not require the approval of an institutional ethics committee because it is an analysis of secondary data. The data are publicly available on the platform of the Mexican Secretary of Health under Mexico's open government data (OGD) policy to facilitate the access, use, reuse and redistribution of information.
Funding
None.
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2020.09.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. 2020:1–7. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alimohamadi Y., Taghdir M., Sepandi M. The estimate of the basic reproduction number for novel coronavirus disease (COVID-19): a systematic review and meta-analysis. Korean J Prev Med. 2020;53:151–157. doi: 10.3961/jpmph.20.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control (ECDC) European Centre for Disease Prevention and Control (ECDC); 2020. COVID-19 situation update worldwide, as of 21 May 2020.https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [Internet] Available from: [Google Scholar]
- 5.Secretaría de Salud . 2020. Covid-19 México. Subsecretaría de Prevención y promoción la Salud.https://coronavirus.gob.mx/ Available from: [Google Scholar]
- 6.Chen T., Wu D., Chen H., Yan W., Yang W., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;S0163–4453(20):30234–30236. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim N.Y., Ha E., Moon J.S., Lee Y.-H.H., Choi E.Y. Acute hyperglycemic crises with coronavirus disease-19: case reports. Diabetes Metab J. 2020;44(2):349–353. doi: 10.4093/dmj.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W., Liang W., Zhao Y., Liang H.-R., Chen Z-Sh, Yi-Min Li. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cure E., Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):349–350. doi: 10.1016/j.dsx.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson E, Walker AJ, Bhaskaran KJ, et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv [Internet]. Available from: http://medrxiv.org/content/early/2020/05/07/2020.05.06.20092999.abstract. [Accessed May 21, 2020].
- 15.Wang Z., Tang K. Combating COVID-19: health equity matters. Nat Med. 2020;26(4):458. doi: 10.1038/s41591-020-0823-6. [DOI] [PubMed] [Google Scholar]
- 16.White C., Nafilyan V. Coronavirus (COVID-19) related deaths by ethnic group, England and wales: 2 March 2020 to 10 April 2020. Off Natl Stat. 2020:1–10. (March) [Google Scholar]
- 17.CEJIL. COVID 19: The survival of indigenous peoples is at risk. Available from: https://www.cejil.org/en/covid-19-survival-indigenous-peoples-risk. [Accessed May 21, 2020].
- 18.Rivera J., Colchero A., Fuentes M. Salud Pública de México; México: 2018. La Obesidad en México. Estado de la política pública y recomendaciones para su prevención y control; p. 271p. [Google Scholar]
- 19.Interim infection prevention and control recommendations for patients with known or patients under investigation for 2019 novel coronavirus (2019-nCoV) in a healthcare setting. Centers for Disease Control and Prevention; February 21, 2020. https://www.cdc.gov/coronavirus/2019- nCoV/hcp/infection-control.html Updated. [Google Scholar]
- 20.Campos-Nonato I., Hernández-Barrera L., Pedroza-Tobías A., Medina C., Barquera S. Hypertension in Mexican adults: prevalence, diagnosis and type of treatment. Ensanut MC 2016. Salud Publica Mex. 2018;60(3):233–243. doi: 10.21149/8813. [DOI] [PubMed] [Google Scholar]
- 21.Gobierno de México. 2020. Descubre Datos Abiertos de tu gobierno. México.www.datos.gob.mx Available from: [Google Scholar]
- 22.Gobierno de México. 2020. Todo sobre el COVID-19. México.https://coronavirus.gob.mx/ Available from: [Google Scholar]
- 23.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-2019)https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf Available from: [Google Scholar]
- 24.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network – United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker Ch, Imai N. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;S0091–6749(20):30495–30504. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferdinand K.C., Nasser S.A. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75(21):2746–2748. doi: 10.1016/j.jacc.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de León-Martínez L.D., de la Sierra-de la Vega L., Palacios-Ramírez A., Rodriguez-Aguilar M., Flores-Ramírez R. Critical review of social, environmental and health risk factors in the Mexican indigenous population and their capacity to respond to the COVID-19. Sci Total Environ. 2020;733:139357. doi: 10.1016/j.scitotenv.2020.139357139357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza-Caamal E.C., Barajas-Olmos F., García-Ortiz H., Cicerón-Arellano I., Martínez-Hernández A., Córdova E.J. Metabolic syndrome in indigenous communities in Mexico: a descriptive and cross-sectional study. BMC Public Health. 2020;20(1):339. doi: 10.1186/s12889-020-8378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji Y., Ma Z., Peppelenbosch M.P., Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8(4) doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg S., Kim L., Whitaker M., O´Halloran A., Cummings Ch, Holstein R. Hospitalization rates and characteristics of patients hospitalized with. Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A.K., Gupta R., Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr. 2020;14(4):283–287. doi: 10.1016/j.dsx.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Temgoua M.N., Kuate L.M., Ngatchou W. COVID-19 pandemic: do we need systematic screening of patients with cardiovascular risk factors in Low-and Middle-Income Countries (LMICs) for preventing death? Pan African Med Journal. 2020 doi: 10.11604/pamj.2020.35.2.22947. SUPPLEMENT. doi: 10.11604/pamj.supp.2020.SUPPLEMENT_PUB_VOLUME.SUPPLEMENT_NUMBER.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galbadage T., Peterson B.M., Gunasekera R.S. Does COVID-19 spread through droplets alone? Front Public Health. 2020;8:163. doi: 10.3389/fpubh.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.