Coronavirus disease 2019 (COVID-19) infection has been linked to a broad range of organ involvement including lungs, heart and blood vessels, and gastrointestinal tract.1 The most common presentation has been of viral pneumonia, characterized by progressive hypoxia, often in the absence of appropriate shortness of breath. On the other hand, dyspnea and low oxygen saturation are markers of poor prognosis.2 Thresholds for oxygen supplementation vary widely. Given the overlap between pathophysiologic effects of hypoxia and pathophysiology of COVID-19, we propose that progressive hypoxia may act as an amplifier of the COVID-19 disease process, which may conceivably be interrupted by aggressive, early, and effective oxygen supplementation.
Pathophysiology, Presentation, and Prognostic Biomarkers of COVID-19 Pneumonia
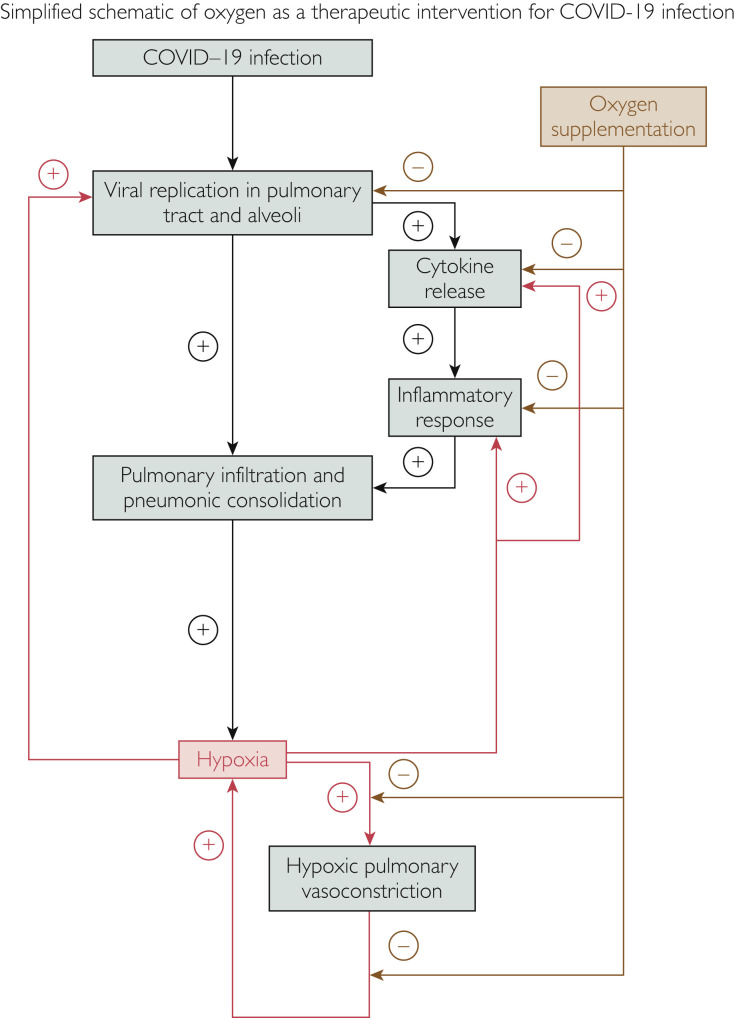
After initially infecting cells lining the nose, viral replication results in migration of infection into the pulmonary tract and alveoli, including the angiotensin-converting enzyme 2 receptor-rich alveolar type II cells which produce surfactant. In some patients, potentiated immune responses to viral infiltration elicit profuse release of pro-inflammatory cytokines, a “cytokine storm.”1 Alveolar inflammation and reduced surfactant production accelerate pneumonic consolidation and consequent hypoxia. Along with viral proliferation and inflammation, pulmonary vasoconstriction has been proposed as another potential mechanism contributing to hypoxia in COVID-19 pneumonia.1 Recent reports of COVID-19–related intravascular thrombosis also suggest that pulmonary emboli may be implicated.
Although most COVID-19 infections are asymptomatic, common symptoms are fever and cough, with one-third of patients complaining of shortness of breath. Infiltrates on chest x-ray and ground glass opacities on computed tomography are often evident in most of those who are admitted. Approximately one-third of patients may progress to acute respiratory distress syndrome requiring intensive care. In a study of 140 patients admitted for COVID-19 pneumonia in Wuhan, we confirmed that age, sex, comorbidities, leukopenia, and inflammatory biomarkers (C-reactive protein and D-dimer) were associated with increased mortality.2 We further showed that dyspnea on admission and hypoxia despite oxygen supplementation were also powerful independent predictors of mortality — 99% of patients who were able to maintain oxygen saturation level at greater than 90% on oxygen supplementation administered on admission survived; 69% of those with oxygen saturation level at less than or equal to 90% despite oxygen supplementation died.2 Low oxygen was also related to elevation of inflammatory biomarkers and D-dimer, suggesting that inflammation and perhaps thromboemboli may be contributing to reduced blood oxygen. However, it is possible that this may be a bi-directional relationship,2 , 3 in that low oxygen saturation may be potentiating the inflammatory, pulmonary vasoconstrictor, and thrombogenic responses (Figure ). Furthermore, while maintaining higher oxygen saturation on oxygen supplementation may indicate less severe lung disease, oxygen supplementation may conceivably help mitigate progression of COVID-19 pneumonia.
Figure.
Schematic of coronavirus disease 2019 (COVID-19) pulmonary infection showing proposed amplification of pathophysiology by hypoxia and attenuation by oxygen supplementation.
Mechanistic Role of Hypoxia in Progression of COVID-19 Pneumonia
There is ample evidence that hypoxia per se may be a contributor to, rather than simply a marker for, progressive lung inflammation, thereby providing a positive feedback loop for further hypoxia. Hypoxia potentiates inflammation.4 In mountain sickness, hypoxia triggers release of pro-inflammatory cytokines causing vascular inflammation with “vascular leakage.” Similarly, tissue hypoxia of organ grafts increases inflammation and graft failure.4 Targeting hypoxia- dependent signaling pathways by mitigating hypoxia severity may thus enable attenuation of inflammation.
Hypoxia may also promote viral replication.5 , 6 Vassilaki et al5 showed that low oxygen tension enhances hepatitis C viral replication independent of hypoxia-inducible factor-1 alpha and likely involving upregulation of oncogenes associated with glycolysis. On the other hand, hypoxia-inducible factor-1 alpha and a hypoxic microenvironment are critical for infection by oncogenic gamma herpes viruses, Kaposi sarcoma herpes virus, and Epstein Barr virus, and may play a key role in promoting viral replication and reactivation of murine gamma herpes virus.6
Hypoxia thus promotes both viral replication and inflammation. With worsening hypoxia and increased inflammation and edema, tissue hypoxia at the alveolar level is exacerbated, resulting in acceleration of this pathologic cycle (Figure), perhaps explaining why those with substrates predisposing to tissue hypoxia, such as vascular disease, diabetes, and obesity, have high COVID-19 mortality.
Hypoxia may also be implicated in pulmonary vasoconstriction, which has been proposed as a mediator of severely reduced blood oxygen in COVID-19 pneumonia.1 This vasoconstriction may be induced, at least in part, by pulmonary hypoxic vasoconstriction, also known as the von Euler-Liljestrand mechanism, whereby pulmonary blood vessels constrict in response to hypoxia, in contrast to vasodilation observed in systemic blood vessels.7
In experimental conditions, hypoxia also potentiates coagulation in part by downgrading expression of protein S.8 Hypoxia may thus also be implicated in the recently recognized COVID-19–associated predisposition to intravascular clot formation.
Although hypoxia is primarily a consequence of viral-induced pulmonary infiltration and pneumonic consolidation, the above considerations provide strong support for the construct that hypoxia in the setting of COVID-19 infection may actually potentiate viral proliferation, lung inflammation, cytokine release, pulmonary vasoconstriction, and intravascular thrombosis, thus creating an amplification circuit for worsening inflammation, accelerating hypoxia, and eventually death (Figure).
Oxygen Supplementation as an Early-Stage Intervention in COVID-19 Infection
The effects of hypoxia on the pathophysiologic mechanisms of COVID-19 pneumonia provide a strong rationale for oxygen supplementation as a therapeutic intervention. It is important that oxygen supplementation be delivered early so as to interrupt viral replication, inflammation, pulmonary vasoconstriction, and clot formation (Figure). Although oxygen delivery could conceivably have side effects, given the high mortality of COVID-19 pneumonia and lack of effective therapies, oxygen would constitute a relatively benign and economical intervention. Nevertheless, in the absence of more compelling data, administration of oxygen should be limited to those patients in whom hypoxia is evident. Until evidence that oxygen is beneficial in the absence of hypoxia, indiscriminate treatment may divert resources from where they are needed, such as where oxygen supplies are limited. In a recent meta-analysis, Ferreyro et al9 reported that in patients with acute hypoxemic respiratory failure, more effective methods for oxygen administration, including helmet and face mask noninvasive ventilation, were associated with lower risk of endotracheal intubation and death, as compared with standard oxygen therapy. In an accompanying editorial, Patel et al10 discuss the applicability of these data to oxygen supplementation in the context of the COVID-19 pandemic. However, given that COVID-19 is highly contagious, strict precautions need to be taken with regard to aerosol generation of highly infectious viral particles during noninvasive ventilation, both in the home and hospital environments. Clinical judgement should be followed in considering other risks of oxygen therapy in any particular patient.
For patients who may not necessarily require admission, the ability to administer oxygen continuously in the home environment or a non–hospital care environment is well established in most health care systems. Considering the widespread infections in parts of South America and Africa, where intensive care resources are few, the opportunity to treat large numbers of patients in non–hospital care facilities with oxygen is attractive. Early, aggressive, and effective administration of noninvasive oxygen supplementation in non–intensive care unit (ICU) settings may conceivably mitigate the need for more invasive ICU-dependent ventilatory interventions. Nevertheless, access to care in less-developed countries may be problematic, requiring other strategies for delivering oxygen therapy. If aggressive oxygen supplementation is beneficial in more comprehensive health care settings, hyperbaric oxygen as a further step may possibly alleviate advanced cases of COVID-19 pneumonia.
Conclusion
The hallmark of COVID-19 morbidity is pneumonia, mediated by viral binding to angiotensin-converting enzyme 2 receptors in the respiratory tract and alveoli. Patients may present with severe hypoxia even in the absence of respiratory muscle fatigue. Hypoxia progression may also be insidious in that patients may not be short of breath despite relatively low blood oxygen levels. Consequences of progressive hypoxia may include potentiation of viral proliferation, cytokine release, inflammation, intravascular coagulation, and pulmonary hypoxic vasoconstriction, which are also pathophysiologic characteristics of COVID-19 disease progression. Attenuation of hypoxia may conceivably mitigate one or more of these pathophysiologic processes. We thus propose that early administration of oxygen in patients with evidence of oxygen desaturation may be a relatively safe, economical, accessible, and effective intervention for patients with COVID-19 pneumonia.
Footnotes
Potential Competing Interests: The authors have no reported no potential competing interests.
References
- 1.Wadman M., Couzin-Frankel J., Kaiser J., Matacic C. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. 2020. Science. https://www.sciencemag.org/news/2020/04/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes [DOI] [PubMed]
- 2.Xie J., Covassin N., Fan Z., et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashani K.B. Hypoxia in COVID-19: sign of severity or cause for poor outcomes. Mayo Clin Proc. 2020;95(6):1094–1096. doi: 10.1016/j.mayocp.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vassilaki N., Kalliampakou K.I., Kotta-Loizou I., et al. Low oxygen tension enhances hepatitis C virus replication. J Virol. 2013;87(5):2935–2948. doi: 10.1128/JVI.02534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Rodríguez D.M., Kirillov V., Krug L.T., Mesri E.A., Andreansky S. A role of hypoxia- inducible factor 1 alpha in Murine Gammaherpesvirus 68 (MHV68) lytic replication and reactivation from latency. PLoS Pathog. 2019;15(12):e1008192. doi: 10.1371/journal.ppat.1008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer N., Dietrich A., Schermuly R.T., et al. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Resp J. 2008;32(6):1639–1651. doi: 10.1183/09031936.00013908. [DOI] [PubMed] [Google Scholar]
- 8.Pilli V.S., Datta A., Afreen S., Catalano D., Szabo G., Majumder R. Hypoxia downregulates protein S expression. Blood. 2018;132(4):452–455. doi: 10.1182/blood-2018-04-841585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreyro B.L., Angriman F., Munshi L., et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure A systematic review and meta-analysis. JAMA. 2020;324(1):57–67. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel B.K., Kress J.P., Hall J.B. Alternatives to invasive ventilation in the COVID-19 pandemic. JAMA. 2020;324(1):43–44. doi: 10.1001/jama.2020.9611. [DOI] [PubMed] [Google Scholar]