Abstract
The new coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which emerged in December 2019 in Wuhan, China, has reached worldwide pandemic proportions, causing coronavirus disease 2019 (COVID-19). The clinical manifestations of COVID-19 vary from an asymptomatic disease course to clinical symptoms of acute respiratory distress syndrome and severe pneumonia. The lungs are the primary organ affected by SARS-CoV-2, with a very slow turnover for renewal. SARS-CoV-2 enters the lungs via angiotensin-converting enzyme 2 receptors and induces an immune response with the accumulation of immunocompetent cells, causing a cytokine storm, which leads to target organ injury and subsequent dysfunction. To date, there is no effective antiviral therapy for COVID-19 patients, and therapeutic strategies are based on experience treating previously recognized coronaviruses. In search of new treatment modalities of COVID-19, cell-based therapy with mesenchymal stem cells (MSCs) and/or their secretome, such as soluble bioactive factors and extracellular vesicles, is considered supportive therapy for critically ill patients. Multipotent MSCs are able to differentiate into different types of cells of mesenchymal origin, including alveolar epithelial cells, lung epithelial cells, and vascular endothelial cells, which are severely damaged in the course of COVID-19 disease. Moreover, MSCs secrete a variety of bioactive factors that can be applied for respiratory tract regeneration in COVID-19 patients thanks to their trophic, anti-inflammatory, immunomodulatory, anti-apoptotic, pro-regenerative, and proangiogenic properties.
Keywords: Mesenchymal stem cells, Stem/progenitor cells, Lung damage, Mesenchymal stem cell secretome, COVID-19 disease, COVID-19 pneumonia
Core Tip: The new severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has reached pandemic proportions, causing coronavirus disease (COVID-19), which leads to severe pneumonia. The lungs are the primary organ affected by SARS-CoV-2, with a very slow turnover for renewal. SARS-CoV-2 enters the lungs and induces immune response with cytokine storm and subsequent organ dysfunction. To date, there is no effective antiviral therapy for COVID-19. Cell-based therapy involving mesenchymal stem cells and/or their secretome is considered a supportive therapy for critically ill COVID-19 patients. Mesenchymal stem cells can regenerate severely injured respiratory tract cells through their trophic, anti-inflammatory, and immunomodulatory properties.
INTRODUCTION
The new virus, which emerged in December 2019 in Wuhan, China, was initially named coronavirus 2019-nCoV, and based on its phylogeny and taxonomy, was later renamed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It has reached worldwide pandemic proportions, causing coronavirus disease 2019 (COVID-19)[1,2]. As of 11 June 2020, the World Health Organization (WHO) Situation Report-143 states that COVID-19 has been confirmed globally in 7273958 patients and resulted in 413372 deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports).
The clinical manifestations of COVID-19 vary and include an asymptomatic disease course, acute respiratory disease, and pneumonia with different stages of severity. The asymptomatic disease course, without fever or respiratory or gastrointestinal symptoms, does not protect the patients from SARS-CoV-2 transmission, which is transmitted from person-to-person by direct contact[2,3]. Based on current observations, it is unknown whether asymptomatic patients recover without adverse organ damage, or if complications appear as the late effects of the disease in the future. Patients with clinical signs of acute respiratory disease have revealed fever and cough with no signs of pneumonia, and more than 30% of patients require oxygen therapy but not mechanical ventilation[4]. Uncommon gastrointestinal symptoms such as vomiting, nausea, and diarrhea have also been observed. The most severe symptoms of COVID-19 including fever, cough, headache, dyspnea, and sputum production have been observed in patients who developed pneumonia. Most of them (> 70%) have needed oxygen therapy, and almost 30% require mechanical ventilation[2,4]. Severely affected patients are usually older and have coexisting illnesses, including hypertension, chronic obstructive pulmonary disease, diabetes mellitus, and cardiovascular disease, which often lead to death[4-6]. Most patients with SARS-CoV-2 infection have a good clinical outcome and prognosis; however, in elderly patients (aged > 65 years) with comorbidities, severe complications can occur including acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis, coagulation dysfunction, and multiple organ failure, causing an increased risk of death[5-8].
The basis of the pathogenesis of SARS-CoV-2 virus is binding of the S protein, expressed on the surface of the coronavirus, to angiotensin-converting enzyme 2 (ACE2) receptors[9-11], which are widely distributed on the surface of human cells, especially alveolar type II cells, and on the capillary endothelium of the lung[12]. Moreover, cellular transmembrane protease, serine 2 (TMPRSS2), which is abundantly expressed on alveolar cells, is essential for SARS-CoV-2 entry into target cells and spreading[13,14]. In addition, ACE2 receptors are present on the cells in different tissues and organs including the heart, liver, kidney, and gut. The SARS-CoV-2 virus uses the ACE2 receptor for entry and initiates fusion with the host cells, infecting them and inducing the immune response from the host’s innate immune system[9]. The virus-induced immune response leads to the accumulation of immunocompetent cells, which produce a large number of proinflammatory cytokines, leading to target organ damage, and consequently, fatal organ dysfunction. One of the first studies with COVID-19 patients reported that severely affected patients with pneumonia, in the acute phase of the disease, had high levels of proinflammatory cytokines and chemokines including interleukin-1β (IL-1β), IL-1 receptor antagonist (IL-1Ra), IL-7, IL-8, IL-9, IL-10, basic fibroblast growth factor (bFGF), granulocyte-colony stimulating factor, granulocyte-macrophage colony-stimulating factor, interferon-γ (IFN-γ), IFN-γ-induced protein 10 kDa, macrophage inflammatory protein-1 (MIP-1α), MIP-1β, and tumor necrosis factor-α (TNF-α)[6]. Activity of the innate immune response is necessary to contain and eliminate the virus infection; however, an out-of-control immune response leads to immunopathological changes.
Current therapies for COVID-19
To date, there are no effective therapies against COVID-19. To overcome this problem, global medical, scientific, pharma and funding groups have rapidly initiated more than 500 COVID-19 clinical trials based on currently available anti-viral drugs in various combinations[15]. Current antiviral therapies are based on experience in treatment strategies against the previously recognized SARS-CoV and MERS-CoV[16]. Remdesivir and chloroquine or hydroxychloroquine are commonly used to treat pneumonia in COVID-19 patients[17-20]. Other currently available potent antiviral agents and their combinations repurposed for COVID-19 treatment are also widely used[5,21,22]. New therapies include passive antibody transfer from the sera of convalescent patients[23,24] and blocking the ACE2 receptor by the serine protease TMPRSS2 inhibitor (approved for clinical use)[13].
The ACE2 receptor is not expressed in the bone marrow, lymph nodes, spleen, or on immune cells such as T and B lymphocytes and macrophages[12]. This biological feature of these cells suggests that immunotherapy can be used to treat severely infected patients. In search of new treatment modalities for COVID-19, early studies on cell-based therapy with mesenchymal stem cells (MSCs) have been employed as supportive treatment for critically ill patients[25]. Currently, a total of 28 trials exploring the potential of MSCs and their derivates for the treatment of critically ill COVID-19 patients have been approved and registered at the WHO International Clinical Trials Registry Platform www.clinicaltrials.gov[15], and more than 20 have been registered in the Chinese clinical trial registry (www.chictr.org.cn)[26].
LUNG STEM/PROGENITOR CELLS AND TISSUE HOMEOSTASIS
Most human tissues and organs, including the pulmonary tract, contain stem/ progenitor cells responsible for the maintenance of tissue homeostasis[27]. The lung is a conditionally renewing organ, and in normal conditions, the turnover of airway epithelial cells is less than 1% per day, in contrast to other adult organs such as the skin, intestines, or bone marrow. However, severe damage increases the self-renewing capability of different types of endogenous epithelial stem/progenitor cells that reside in the lung and are important for regulation of the regeneration of damaged tissue[28]. The lung contains various types of epithelial cells that reside in several regions along the pulmonary airways.
Endogenous epithelial stem and progenitor cells in the adult lung are organized specifically according to their regional decomposition and functional activity along the proximal-distal axis of the pulmonary tract. The proximal part of the respiratory tract encompasses the cartilaginous trachea, lined by columnar pseudostratified epithelial cells, and different types of stem/progenitor cells with distinct roles in lung regeneration, including basal, secretory, ciliated, and neuroendocrine cells. The regenerative processes in the pulmonary tract involve local stem/progenitor cells, which are characterized by high proliferative activity during the perinatal period and a slow turnover during adulthood. In response to injury, a population of basal cells, which represent the stem/progenitor cells of the bronchiolar epithelium, migrate from the bronchiolar niche into the damaged alveolar epithelium and proliferate in order to repair the lung alveolar cells[28,29]. The distal part of the airways is lined by a columnar epithelium, and includes secretory club cells (also known as Clara cells) and populations of ciliated cells, goblet cells, and pulmonary neuroendocrine cells[28,29]. To maintain epithelial homeostasis, club cells are capable of self-renewal and can generate ciliated cells, whereas ciliated cells do not have self-regeneration capacity[29]. Another population of stem and progenitor cells residing in the distal airway, involved in epithelial homeostasis and regeneration, is a rare population of cells called the bronchioalveolar stem/progenitor cells with self-renewal potential. Their number increases following bronchiolar damage, and these cells are able to differentiate into bronchiolar and alveolar colonies, thus contributing to tissue repair[28]. The terminal part of the airway tree is composed of alveoli containing specific alveolar progenitor cells, which differentiate into surfactant-producing alveolar epithelial cells type II and squamous gas-exchanging alveolar epithelial cells type I, responsible for the maintenance and restoration of the gas exchange units of the distal part of the pulmonary tract[28,29].
Mesenchymal stromal/stem cells residing in the lung constitute a key component supporting epithelial progenitor niches along the proximal-distal axis of the airway tree. The lung mesenchymal stromal/stem cells secrete a variety of bioactive factors, including FGF10, a critical trophic factor necessary for coordinating differentiation in the developing lung and supporting epithelial regeneration in steady-state conditions and after injury[29]. Moreover, lung mesenchymal stromal/stem cells modulate the local microenvironment via a paracrine-mediated anti-inflammatory effect and support the proliferation and differentiation of lung epithelial progenitor cells.
The different populations of endogenous stem and progenitor cells residing in distinct niches of the pulmonary tract contribute to region-specific epithelial cell repair, and the balance between the immune regulation and promotion of tissue regeneration ensures homeostasis of the lung[27].
BIOLOGICAL PROPERTIES OF MSCS IN TISSUE REGENERATION
MSCs are multipotent cells, which are able to differentiate into different types of cells of mesenchymal origin including alveolar epithelial cells, lung epithelial cells, and vascular endothelial cells[30,31]. MSCs are extensively studied for their clinical application in regenerative medicine due to their trophic, anti-inflammatory, and immunomodulatory properties[32,33]. The capability of MSCs to restore tissues is also accomplished through their ability to secrete a variety of bioactive proteins, including growth factors and chemokines, to induce the proliferation of tissue-resident progenitor cells and angiogenesis[33]. In response to inflammatory cytokines, such as IL-1, IL-2, IL-12, TNF-α, and IFN-γ secreted by immunocompetent cells, MSCs secrete a variety of growth factors and anti-inflammatory proteins including prostaglandin 2 (PGE 2), transforming growth factor-1β (TGF-β1), stromal-derived factor-1 (SDF-1), IL-4, IL-6, IL-10, and IL-1Ra[31]. Soluble factors secreted by the MSCs prevent the proliferation and function of many immunocompetent cells including T lymphocytes, B lymphocytes, natural killer cells, monocytes, macrophages, and dendritic cells. The immunomodulatory activity of MSCs involves decreasing the level of IFN-γ and increasing the level of IL-4 and IL-10, thus promoting a shift from T helper type 1 (Th1) to Th2 lymphocytes and a shift in macrophage balance from the M1 (proinflammatory) to M2 (anti-inflammatory) phenotype[31,34,35].
The trophic properties of MSCs are associated with the secretion of growth factors and chemokines, such as TGF-α, TGF-β, hepatocyte growth factor (HGF), epithelial growth factor (EGF), insulin-like growth factor 1, bFGF, vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), and other bioactive factors involved in cell proliferation and angiogenesis, as confirmed by many studies[31,36] including research conducted by the author of this article[33,37,38].
The advantage of MSCs as a therapeutic option is the low or moderate expression of human leukocyte antigen (HLA) class I antigens and the lack of expression of HLA class II antigens, which makes MSCs “undetectable” by recipient immunocompetent cells in the allogeneic condition. However, a proinflammatory environment and IFN-γ production may increase the expression of their HLA class II antigens[31]. The immunomodulatory activity of MSCs related to dendritic cells is associated with their capacity to produce anti-inflammatory factors (PGE 2 and TGF-β), which inhibit the activation and maturation of dendritic cells, impairing their function[31].
MSCS AS SUPPORTIVE THERAPY IN COVID-19 PATIENTS
COVID-19 triggers a strong immune response with cytokine storm, especially in the lower airway, leading to lung damage[5,6]. MSCs are the ideal candidate for respiratory tract regeneration because they not only contribute to structural tissue repair but also have immunomodulatory, anti-inflammatory, proangiogenic, and anti-fibrotic properties[39,40]. This biological activity of MSCs may also affect tissue repair through modulation of the local microenvironment. The immunomodulatory properties of MSCs can diminish the inflammatory response and ameliorate the cytokine storm, as documented in clinical trials conducted among patients with steroid-resistant graft-versus-host disease[41] and among patients with an autoimmune disease[42]. Cell-based therapies with allogeneic MSCs of bone marrow or adipose tissue origin have also been applied to patients with an acute lung injury and ARDS[43-45]. In these studies, the administration of MSCs was safe and feasible; however, the clinical effect suggests that this strategy needs further optimization. ARDS is characterized by substantial damage to the capillary endothelium and alveolar epithelium, which leads to an increase in alveolar-capillary permeability, causing pulmonary edema and the formation and accumulation of inflammatory cells in the interstitial and alveolar space[46]; extensive regeneration of the tissues is required to restore pulmonary function.
A clinical study that used MSCs to treat patients infected with influenza A (H7N9), who displayed symptoms similar to COVID-19 patients including cough, fever, shortness of breath, and dyspnea accompanied by ARDS and subsequent pneumonia, as well as corresponding multi-organ dysfunction, suggested that MSCs can be used as supportive therapy to treat SARS-CoV-2-infected patients[47].
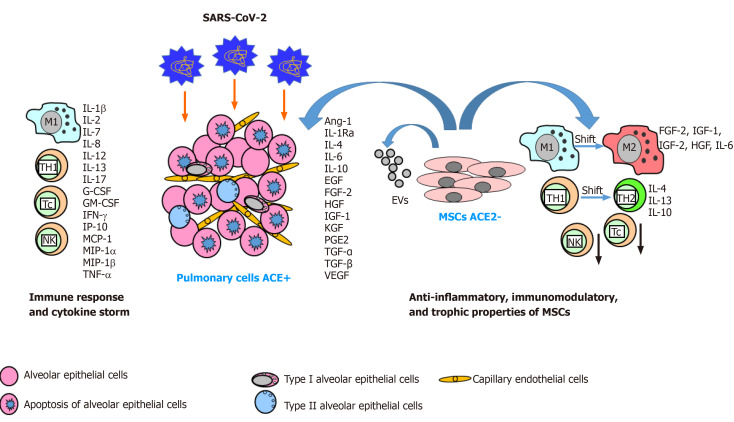
In the case of patients with COVID-19, MSCs may attenuate the cytokine storm by means of paracrine secretion of a variety of anti-inflammatory cytokines including TGF-β1, SDF-1, IL-4, IL-6, IL-10, and IL-1Ra, which decrease the overactivation of immunocompetent cells, thus regulating the inflammatory response (Figure 1). A decreased immune response modifies the microenvironment of the damaged tissue and promotes tissue repair and regeneration. It is well known that MSCs transplanted intravenously are trapped by organs with a large capillary bed including the liver, spleen, and lung. MSCs accumulating in the lung may improve the pulmonary microenvironment, protect alveolar epithelial cells, prevent dysfunction of capillary endothelial cells, and prevent pulmonary fibrosis, thus helping to recover lung function[25,48]. Moreover, systemic delivery of MSCs may ameliorate multi-organ dysfunction associated with SARS-CoV-2 infection including cardiovascular, renal, or hepatic damage[25]. The therapeutic potential of MSCs for the treatment of patients in critical condition caused by COVID-19 pneumonia was proved in a pilot study on intravenous MSC transplantation[25]. The delivery of MSCs significantly improved the functional outcome and pulmonary function of the patients within 2 d following transplantation. This effect was associated with the immunomodulatory properties of MSCs, which caused a shift in the immune response from Th1 towards Th2, resulting in a decreased level of the proinflammatory cytokine TNF-α and an increased level of the anti-inflammatory cytokine IL-10. The MSC therapy also resulted in a high production of the proangiogenic VEGF, which can help restore the function of capillary endothelial cells. A very important biological characteristic of MSCs, assessed by 10x scRNA-seq analysis, showed that MSCs transplanted to patients did not express the ACE2 receptor or TMPRSS2, thus providing resistance to COVID-19 infection[25].
Figure 1.
Immune response to severe acute respiratory syndrome coronavirus-2 infection and immunoregulatory activity of mesenchymal stem cells for treatment in patients with coronavirus disease 2019 pneumonia. Upon entry into the alveolar epithelium, SARS-CoV-2 triggers a strong immune response with cytokine storm. Cytokines and trophic factors released by MSCs (ACE2 negative) and their derivate EVs modulate the inflammatory microenvironment within the damaged pulmonary cells and modulate immune response, promoting a shift from T helper type 1 to Th2 lymphocytes, and a shift in macrophage balance from the M1 (proinflammatory) to M2 (anti-inflammatory) phenotype, and decreasing the activity of cytotoxic T lymphocytes (Tc) and natural killer lymphocytes. ACE2: Angiotensin-converting enzyme 2; MSCs: Mesenchymal stem cells; Ang-1: Angiopoietin-1; SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2; COVID-19: Coronavirus disease 2019; bFGF: Basic fibroblast growth factor; EGF: Epithelial growth factor; EVs: Extracellular vesicles; G-CSF: Granulocyte-colony stimulating factor; GM-CSF: Granulocyte-macrophage colony-stimulating factor; HGF: Hepatocyte growth factor; IFN-γ: Interferon-γ; IGF-1: Insulin-like growth factor-1; IL-: Interleukin-; KGF: Keratinocyte growth factor; MIP-1α: Macrophage inflammatory protein-1α; MIP-1β: Macrophage inflammatory protein-1β; SDF-1: Stromal-derived factor-1; TGF-α: Transforming growth factor-α; TGF-β: Transforming growth factor-β; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor; MCP-1: Monocyte chemoattractant protein-1; PGE2: Prostaglandin 2.
The progression of COVID-19 leads to the development of pulmonary diseases, such as idiopathic pulmonary fibrosis or ARDS, commonly associated with damage to alveolar epithelial cells, which in turn is related to a severe hypoxia of the alveolar cells, leading to a massive apoptosis, therefore, contributing to the pathophysiology of lung fibrosis[49]. Experimental studies have indicated that not only MSCs but also their derivates, such as a conditioned medium containing a variety of bioactive factors or extracellular vesicles (EVs) (microvesicles and exosomes) carrying various cytoplasmic components, including lipids, DNA fragments, and RNA (including mRNA and microRNA), contribute to the recovery of alveolar epithelial cells and endothelial cells and modify the function of inflammatory infiltrates in paracrine and endocrine manners[50,51].
An experimental study on the lung alveolar cells of the rat showed that conditioned medium of human MSC culture had a paracrine anti-apoptotic effects on the hypoxia-induced apoptosis of the alveolar cells. The anti-apoptotic properties of MSCs involve the secretion of keratinocyte growth factor and HGF, which downregulate the proapoptotic signal caused by the hypoxia-inducible factor-1 alpha and reactive oxygen species[49]. A similar study in a rat model using conditioned medium from a culture of MSCs of bone marrow or adipose tissue origin confirmed that MSC-derived bioactive factors protected alveolar epithelial cells from damage in hypoxic conditions by decreasing the secretion of proinflammatory cytokines, augmenting the production of IL-10, and delaying cell apoptosis[52].
MSC-derived EVs, similarly to their parent cells, exhibit proregenerative, anti-inflammatory, anti-apoptotic, anti-oxidative, prometabolomic, and immunoregulatory properties with respect to the damaged tissue microenvironment. The effectiveness of treatment of lung damage using MSC-derived EVs is currently being tested in vitro and in different preclinical experimental models[51]. The beneficial effects of MSC-derived EVs have been demonstrated in influenza-induced acute lung injury in a pig model. Studies have shown that MSC-derived EVs, delivered 12 h after virus infection, result in reduced viral replication and shedding and a decreased level of proinflammatory cytokines[53]. In addition to anti-inflammatory factors, MSC-derived EVs also contain Ang-1 mRNA, an angiogenic trophic factor that is essential in endothelial cell stabilization, and during injury, diminishes the interactions between leukocytes and vessel endothelial cells, and preserves vascular barrier integrity. The therapeutic effects of MSC-derived EVs in an experimental murine model of lipopolysaccharide-induced acute lung damage confirmed that a transfer of Ang-1 mRNA by EVs significantly contributed to the restoration of pulmonary capillary permeability[54]. Moreover, MSC-derived EVs affected the immunomodulatory properties of the murine macrophage cell line by suppressing the expression of TNF-α and inducing the secretion of IL-10, thus attenuating inflammation. Transfer of Ang-1 mRNA to the injured endothelium restores partial protein permeability across the injured human lung microvascular endothelial cells through the internalization of MSC-derived EVs into the injured cells[55]. A study in an experimental mouse model revealed that exosomes, isolated from MSCs derived from human Wharton’s jelly and bone marrow, improved lung development, decreased lung fibrosis, and improved pulmonary vascular remodeling in a neonatal hyperoxia model of bronchopulmonary dysplasia. Exosomes originating from MSCs act as a paracrine anti-inflammatory mediator by modifying the pulmonary macrophage phenotype from M1 (proinflammatory) to M2 (anti-inflammatory) and suppressing lung inflammation and the immune response in favor of proper organ development[34].
MSC-derived EVs, widely used in pre-clinical experimental models of pulmonary injury and disease, are a promising alternative to MSC-based therapy[51]. However, many scientific and clinical questions regarding EV production, purification, characterization, route of delivery (intravenous or inhalation), and bio-distribution need to be explored before clinical application. The first prospective non-randomized single-center clinical study using bone marrow-derived exosomes (ExoFloTM) was performed to address the safety and efficacy for the treatment of severely affected COVID-19 patients[56]. Twenty-four patients with severe and moderate-to-severe symptoms of ARDS enrolled in this study received a single dose of ExoFloTM administered intravenously, and no adverse effect was observed 72 h after exosome delivery. The survival rate was 83%, and the study demonstrated a profound reversal of hypoxia, downregulation of cytokine storm, and immune reconstitution. However, the biological characteristics of the delivered exosomes (ExoFloTM) were not presented. To determine the therapeutic potential of the applied therapy, future randomized controlled trials with a detailed characterization of the delivered exosomes are needed.
Extensive research on COVID-19 treatment have suggested that the MSC secretome should be employed as a supportive therapy in patients infected with SARS-CoV-2. Experimental pre-clinical studies on the biological activity of the MSC secretome, composed of both soluble bioactive factors (including cytokines, chemokines, and trophic factors) and EVs, suggest that the MSC secretome can be applied for cell-free therapy in severely affected COVID-19 patients[57]. The bioactive proteins and EVs released by MSCs activate endogenous lung stem/progenitor cells, inducing their proliferation and differentiation, inhibit apoptosis, diminish inflammatory response, restore capillary barrier function, and reduce fibrosis and can also be used to treat acute and chronic lung injury, as they act similarly to parental MSCs[50,57]. The advantages of cell-free therapy with the MSC secretome is its formulation as inhalable dosage forms or injectable dosage forms for potential clinical use[58]. Both types of formulation are stored as freeze-dried powder and can be used to treat critically ill patients with COVID-19 pneumonia. The authors of the study introduced two Chinese clinical trials to investigate the inhaled secretome for the treatment of COVID-19 pneumonia (NCT04276987) and assess its tolerance in healthy volunteers (NCT04313647)[57].
The current status of clinical investigations of cell-based therapy for COVID-19 patients has been reviewed very well by Khoury et al[59] in the context of cell sources, doses, dosing strategies, and targeted patient populations. Khoury et al[59] also highlighted the importance of upholding ethical standards to create a rational evidence-based platform for the potential therapeutic use of cell-based therapies in patients infected with SARS-CoV-2. A very recent editorial article, introduced by worldwide famous experts in the field of infectious diseases and MSCs therapies, discusses the rationale behind the use of MSCs in the treatment trials of patients with severe COVID-19 disease[15]. The authors emphasized that the registered trials differ in design, sources of MSCs, doses and schedules of MSCs administration, and patient selection. All of these aspects indicate the need for standardizing protocols through a worldwide consortium network on cellular therapies for COVID-19 and other infectious diseases.
CONCLUSION
In summary, MSCs and their derivates, such as the MSC secretome, may have a great curative potential for COVID-19 patients thanks to their trophic, paracrine, immunomodulatory, anti-inflammatory, anti-apoptotic, anti-oxidative, and prometabolomic activities. Moreover, the immunosuppressive properties of MSCs may decrease the alloreactivity of host immune cells in allogenic conditions. MSCs are attractive candidates for the supportive therapy of severely affected COVID-19 patients thanks to their high proliferative activity, multipotent ability to regenerate tissues via direct differentiation into the desired cells and tissues, and their immunomodulatory activity. MSCs are easily obtainable from different sources, such as bone marrow, adipose tissue, skin, or perinatal tissues, including the umbilical cord, cord blood, Warton’s jelly, and amniotic fluid, and can be expanded into clinical grade and stored for potential clinical use. The most important biological characteristic of MSCs is that they do not express the ACE2 receptor or serine protease TMPRSS2, which makes them safe for the treatment of SARS-CoV-2 infection.
Footnotes
Conflict-of-interest statement: The author declares that there is no conflict of interests regarding the publication of this paper.
Manuscript source: Invited manuscript
Peer-review started: June 12, 2020
First decision: July 30, 2020
Article in press: September 1, 2020
Specialty type: Respiratory system
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Long X S-Editor: Wang DM L-Editor: Filipodia P-Editor: Xing YX
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, Ko WC, Hsueh PR. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wölfel R, Hoelscher M. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J Virol. 2019;93 doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zumla A, Wang FS, Ippolito G, Petrosillo N, Agrati C, Azhar EI, Chang C, El-Kafrawy SA, Osman M, Zitvogel L, Galle PR, Locatelli F, Gorman E, Cordon-Cardo C, O'Kane C, McAuley D, Maeurer M. Reducing mortality and morbidity in patients with severe COVID-19 disease by advancing ongoing trials of Mesenchymal Stromal (stem) Cell (MSC) therapy - Achieving global consensus and visibility for cellular host-directed therapies. Int J Infect Dis. 2020;96:431–439. doi: 10.1016/j.ijid.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 19.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CC, Wang XJ, Wang HR. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov Today. 2019;24:726–736. doi: 10.1016/j.drudis.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, Jeong SJ, Kim JH, Ku NS, Yeom JS, Roh J, Ahn MY, Chin BS, Kim YS, Lee H, Yong D, Kim HO, Kim S, Choi JY. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci. 2020;35:e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alzoughool F, Alanagreh L. Coronavirus drugs: Using plasma from recovered patients as a treatment for COVID-19. Int J Risk Saf Med. 2020;31:47–51. doi: 10.3233/JRS-201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep. 2020;16:427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klimczak A, Kozlowska U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor Cells: Their Role in Tissue Homeostasis. Stem Cells Int. 2016;2016:4285215. doi: 10.1155/2016/4285215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertoncello I, McQualter JL. Lung stem cells: do they exist? Respirology. 2013;18:587–595. doi: 10.1111/resp.12073. [DOI] [PubMed] [Google Scholar]
- 29.Leeman KT, Fillmore CM, Kim CF. Lung stem and progenitor cells in tissue homeostasis and disease. Curr Top Dev Biol. 2014;107:207–233. doi: 10.1016/B978-0-12-416022-4.00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan XL, Zhang Z, Ma CY, Fu QL. Mesenchymal stem cells for inflammatory airway disorders: promises and challenges. Biosci Rep. 2019;39 doi: 10.1042/BSR20182160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozlowska U, Krawczenko A, Futoma K, Jurek T, Rorat M, Patrzalek D, Klimczak A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cells. 2019;11:347–374. doi: 10.4252/wjsc.v11.i6.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, Kwong A, Mitsialis SA, Kourembanas S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.L PK, Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Kraskiewicz H, Paprocka M, Bielawska-Pohl A, Krawczenko A, Panek K, Kaczyńska J, Szyposzyńska A, Psurski M, Kuropka P, Klimczak A. Can supernatant from immortalized adipose tissue MSC replace cell therapy? An in vitro study in chronic wounds model. Stem Cell Res Ther. 2020;11:29. doi: 10.1186/s13287-020-1558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krawczenko A, Bielawska-Pohl A, Paprocka M, Kraskiewicz H, Szyposzynska A, Wojdat E, Klimczak A. Microvesicles from Human Immortalized Cell Lines of Endothelial Progenitor Cells and Mesenchymal Stem/Stromal Cells of Adipose Tissue Origin as Carriers of Bioactive Factors Facilitating Angiogenesis. Stem Cells Int. 2020;2020:1289380. doi: 10.1155/2020/1289380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behnke J, Kremer S, Shahzad T, Chao CM, Böttcher-Friebertshäuser E, Morty RE, Bellusci S, Ehrhardt H. MSC Based Therapies-New Perspectives for the Injured Lung. J Clin Med. 2020;9 doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers CJ, Harman RJ, Bunnell BA, Schreiber MA, Xiang C, Wang FS, Santidrian AF, Minev BR. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J Transl Med. 2020;18:203. doi: 10.1186/s12967-020-02380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Zhang H, Liang J, Wang H, Hua B, Feng X, Gilkeson GS, Farge D, Shi S, Sun L. A Long-Term Follow-Up Study of Allogeneic Mesenchymal Stem/Stromal Cell Transplantation in Patients with Drug-Resistant Systemic Lupus Erythematosus. Stem Cell Reports. 2018;10:933–941. doi: 10.1016/j.stemcr.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Peng D, Qiu H, Yang K, Fu Z, Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020;11:169. doi: 10.1186/s13287-020-01678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Chen L, Gao H, Lu X, Yu L, Dai X, Xiang C, Li L. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 2020;23:E71–E83. [PubMed] [Google Scholar]
- 49.Bernard O, Jeny F, Uzunhan Y, Dondi E, Terfous R, Label R, Sutton A, Larghero J, Vanneaux V, Nunes H, Boncoeur E, Planès C, Dard N. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am J Physiol Lung Cell Mol Physiol. 2018;314:L360–L371. doi: 10.1152/ajplung.00153.2017. [DOI] [PubMed] [Google Scholar]
- 50.Mohammadipoor A, Antebi B, Batchinsky AI, Cancio LC. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir Res. 2018;19:218. doi: 10.1186/s12931-018-0921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Worthington EN, Hagood JS. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21072318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shologu N, Scully M, Laffey JG, O'Toole D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19102996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang XD, Shi L, Monsel A, Li XY, Zhu HL, Zhu YG, Qu JM. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 mRNA. Stem Cells. 2017;35:1849–1859. doi: 10.1002/stem.2619. [DOI] [PubMed] [Google Scholar]
- 55.Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, Lee JW. Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem Cells Transl Med. 2018;7:615–624. doi: 10.1002/sctm.17-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bari E, Ferrarotti I, Saracino L, Perteghella S, Torre ML, Corsico AG. Mesenchymal Stromal Cell Secretome for Severe COVID-19 Infections: Premises for the Therapeutic Use. Cells. 2020;9 doi: 10.3390/cells9040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bari E, Ferrarotti I, Torre ML, Corsico AG, Perteghella S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through "pharmaceuticalization" for the best formulation. J Control Release. 2019;309:11–24. doi: 10.1016/j.jconrel.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55 doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]