Abstract
BACKGROUND
Mesenchymal stem cells (MSCs) are an attractive tool to treat graft-versus-host disease because of their unique immunoregulatory properties. Although human bone marrow-derived MSCs (BM-MSCs) were the most widely used MSCs in cell therapy until recently, MSCs derived from human umbilical cords (UC-MSCs) have gained popularity as cell therapy material for their ethical and noninvasive collection.
AIM
To investigate the difference in mechanisms of the immunosuppressive effects of UC-MSCs and BM-MSCs.
METHODS
To analyze soluble factors expressed by MSCs, such as indolamine 2,3-dioxygenase, cyclooxygenase-2, prostaglandin E2 and interleukin (IL)-6, inflammatory environments in vitro were reconstituted with combinations of interferon-gamma (IFN-γ), tumor necrosis factor alpha and IL-1β or with IFN-γ alone. Activated T cells were cocultured with MSCs treated with indomethacin and/or anti-IL-10. To assess the ability of MSCs to inhibit T helper 17 cells and induce regulatory T cells, induced T helper 17 cells were cocultured with MSCs treated with indomethacin or anti-IL-10. Xenogeneic graft-versus-host disease was induced in NOG mice (NOD/Shi-scid/IL-2Rγnull) and UC-MSCs or BM-MSCs were treated as cell therapies.
RESULTS
Our data demonstrated that BM-MSCs and UC-MSCs shared similar phenotypic characteristics and immunomodulation abilities. BM-MSCs expressed more indolamine 2,3-dioxygenase after cytokine stimulation with different combinations of IFN-γ, tumor necrosis factor alpha-α and IL-1β or IFN-γ alone. UC-MSCs expressed more prostaglandin E2, IL-6, programmed death-ligand 1 and 2 in the in vitro inflammatory environment. Cyclooxygenase-2 and IL-10 were key factors in the immunomodulatory mechanisms of both MSCs. In addition, UC-MSCs inhibited more T helper 17 cells and induced more regulatory T cells than BM-MSCs. UC-MSCs and BM-MSCs exhibited similar effects on attenuating graft-versus-host disease.
CONCLUSION
UC-MSCs and BM-MSCs exert similar immunosuppressive effects with different mechanisms involved. These findings suggest that UC-MSCs have distinct immunoregulatory functions and may substitute BM-MBSCs in the field of cell therapy.
Keywords: Mesenchymal stem cells, Graft-versus-host disease, Umbilical cord, Cell therapy, Xenogeneic mouse model, Immunomodulation
Core Tip: Mesenchymal stem cells (MSCs) are a therapeutic approach to treat graft-versus-host disease because of their unique immunomodulatory abilities. Here, we compared and analyzed the differences and similarities between umbilical cord-derived MSCs and bone marrow-derived MSCs due to the growing needs for new sources of MSCs. We suggest that umbilical cord-derived MSCs and bone marrow-derived MSCs exhibit similar immunosuppression by different mechanisms, and umbilical cord-derived MSCs have the potentials to substitute bone marrow-derived MSCs as cell therapy products.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HSCT) is one of the most curative treatments for patients with hematological disorders, hematopoietic malignancies and immune diseases. Through HSCT, healthy hematopoietic donor cells can reconstruct the hematopoietic system of recipients. Despite the benefits of HSCT, there are risks of graft rejection, tissue toxicity, organ injury and leukemia relapse; among these risks, graft-versus-host disease (GVHD) is the major cause of death after transplantation[1]. In GVHD, alloreactive donor T cells recognize histocompatibility antigens in host cells as foreign and activate to produce inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1, IL-2 and interferon-gamma (IFN-γ)[2]. Although immunosuppressive drugs such as tacrolimus, cyclosporine and steroids are used in clinical therapy to lower GVHD mortality, a new therapeutic approach for GVHD following HSCT is needed.
Mesenchymal stem cells (MSCs) are multipotent stromal cells that have the ability to differentiate into different cell types, such as osteoblasts, chondrocytes and adipocytes[3]. Recently, MSCs have been studied for cell therapy in the field of transplantation. A major characteristic of MSCs is that they express low major histocompatibility complex II, suppressing T cells independent from the major histocompatibility complex identity between the donor and recipient[4]. Their immunosuppressive effects mainly occur through the production of soluble factors such as indolamine 2,3-dioxygenase (IDO), transforming growth factor-β, human leukocyte antigen (HLA)-G and hepatocyte growth factor[5-8]. While soluble factors are the primary mechanism by which MSCs exert immunosuppressive effects, direct cell-to-cell contact is also a contributing factor[9]. Cell-to-cell contact between CD3+ T cells and MSCs can induce CD4+CD25+ regulatory T cells[10].
Traditionally, MSCs have been isolated from bone marrow, and bone marrow-derived MSCs (BM-MSCs) have been identified as a key candidate for cell therapy[11]. However, their clinical application is restricted by the invasive procedures by which they are obtained, their decrease in proliferation and differentiation capacity with age and the difficulty of using them to treat patients with hereditary diseases[12,13]. As a result, other adult and fetal tissues have been studied as alternative sources of stem cells, including adipose tissue, umbilical cord blood, umbilical cords, amniotic fluid and placental tissue[14,15]. Although the major source of MSCs in clinical trials is bone marrow, recent studies have suggested that the allogenicity of MSCs has no significant adverse impact on the engraftment of MSCs in wound healing[16]. It is better to use freshly isolated MSCs because the five major histocompatibility complex II molecules may increase during in vitro expansion[17,18]. Additionally, the proliferative capacity of umbilical cord-derived MSCs (UC-MSCs) is higher than that of BM-MSCs. Several studies have emphasized the potential of UC-MSCs as an alternative to BM-MSCs; however, detailed comparisons of UC-MSCs and BM-MSCs are lacking.
Therefore, we compared the similarities and differences between UC-MSCs and BM-MSCs. In particular, we (1) investigated the in vitro immunoregulatory properties of UC-MSCs; (2) explored the mechanisms of immunosuppressive effects; and (3) analyzed the efficacy of UC-MSC and BM-MSC therapies to treat xenogeneic GVHD induced in severely immunodeficient NOG mice.
MATERIALS AND METHODS
Mice
Eight- to ten-week-old female NOG mice (NOD/Shi-scid/IL-2Rγnull) were purchased from the Central Institute for Experimental Animals (Kanagawa, Japan). Mice were maintained under pathogen-free conditions in an animal facility with controlled daylength (12L:12D), humidity (55% ± 5%) and temperature (22 ± 1 °C). The air in the facility passed through a HEPA filter system designed to exclude bacteria and viruses. Animals were fed mouse chow and tap water ad libitum.
Isolation and culturing of human MSCs
UCs were collected aseptically from full-term cesarean-section patients with informed consent. The UCs were transferred after collection, and the process was initiated within 24 h of delivery. The UC surface was rinsed with phosphate-buffered saline (Gibco–BRL) containing antibiotics and antifungal reagents (Antibiotic–Antimycotic, 100 ×; Gibco–BRL). In the explant method, the minced fragments were aligned and attached at regular intervals in 10-cm culture dishes. After the fragments were semi-dried and firmly attached to the bottom, culture medium was gently poured into the dishes. The cells were then washed with Alpha Minimum Essential Medium (Gibco) supplemented with 10% fetal bovine serum and seeded in 10-cm tissue culture dishes with culture medium as described above. The culture medium was refreshed once a week for 3-4 wk until fibroblast-like adherent cells reached 80%-90% confluence. The first-harvested master cells were defined as passage 0[19]. BM-MSCs (passage 2–3) were purchased from Catholic MASTER Cells (Seoul, South Korea) and maintained in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin solution (Gibco).
Characterization of MSCs
Fluorescence-activated cell sorting flow cytometry analysis was performed using CD29 (TS2/16; eBioscience, San Diego, CA, United States), CD44 (IM7; eBioscience), CD105 (SN6; eBioscience), CD90 (5E10, eBioscience), CD73 (AD2, eBioscience), CD45 (2D1; eBioscience), CD31 (WM-59; eBioscience), CD34 (4H11; eBioscience), HLA-ABC (W6/32; eBioscience), HLA-DR (L243; eBioscience), C-C chemokine receptor type 1 (CCR1; 5F10B29; BioLegend, San Diego, CA, United States), CCR2 (K036C2; BioLegend), CCR7 (3D12; eBioscience), C-X-C chemokine receptor type 4 (CXCR4; 12G5; eBioscience) and CXCR5 (MU5UBEE; eBioscience) antibodies to confirm that the MSC phenotype was maintained after expansion in the culture. The samples were incubated with antibodies against each surface marker for 30 min, and this treatment was followed by fluorescence-activated cell sorting. Flow cytometry analysis was performed on a LSRFortessa (BD Pharmingen, San Diego, CA, United States).
Differentiation assay
One function of MSCs is to differentiate into osteoblasts, chondrocytes or adipocytes[20]. For adipogenic differentiation assays, MSCs were seeded into 24-well plates at a density of 1 × 104 per well and induced to differentiate into adipocytes using the Human MSC Functional Identification Kit (R and D Systems, Minneapolis, MN, United States) following the manufacturer’s protocol. The medium was changed three times a week for 14-21 d. Adipocytes were detected using goat anti-mouse FABP4 polyclonal antibody.
For osteogenic and chondrogenic differentiation assays, MSCs were seeded into 12-well plates at the manufacturer-recommended density and induced to differentiate into osteoblasts or chondrocytes using StemPro Chondrogenesis/Osteogenesis Differentiation Kits (Gibco, United States) following the manufacturer’s protocols. The medium was changed three times a week for 21 d. Osteoblasts and chondrocytes were stained using Alizarin Red S and Alcian Blue, respectively.
Real-time reverse-transcription polymerase chain reaction
Total RNA was extracted using TRIzol LS reagent (Invitrogen). Isolated total RNA (2 µg) was reverse transcribed into complementary DNA at 50 °C for 2 min, followed by 60 °C for 30 min. Quantitative polymerase chain reaction was performed using the FastStart DNA Master SYBR Green I kit and a LightCycler 480 Detection system (Bio-Rad, CA, United States), as specified by the manufacturer. The crossing point was defined as the maximum of the second derivative from the fluorescence curve. Negative controls were included and contained all elements of the reaction mixture, except for template DNA. For quantification, we report relative mRNA expression levels of specific genes obtained using the 2-ΔCt method. For normalization, GAPDH, a housekeeping gene, was used. The following gene-specific primers were used: Klf4 (forward: 5'-CGAACCCACACAGGTGAGAA-3'; reverse: 5'-GAGCGGGCGAATTTCCAT-3'), OCT4 (forward: 5'-GGAGGAAGCTGACAACAATGAAA-3'; reverse: 5'-GGCCTGCACGAGGGTTT-3'), Nanog (forward: 5'-ACAACTGGCCGAAGAATAGCA-3'; reverse: 5'-GGTTCCCAGTCGGGTTCAC-3'), and GAPDH (forward: 5'-TGCCAAATATGATGACATCA-3'; reverse: 5'-GGAGTGGGTGTCGCTGTTG-3').
Luminex multiplex cytokine assay
Different cytokines (IDO, IL-6) in human cell culture supernatants were assessed with a Luminex MAGPIX instrument (Luminex, Austin, TX, United States) using the ProcartaPlex Human 4-plex immunoassay kit (Affymetrix, Santa Clara, CA, United States) according to the manufacturer’s instructions as described below. Briefly, samples were mixed with antibody-linked polystyrene beads in 96-well filter-bottom plates and incubated at room temperature for 2 h on an orbital shaker at 500-600 rpm. The plate was inserted into a magnetic plate washer and washed twice, then incubated with biotinylated detection antibody for 30 min at room temperature. Samples were washed twice as above and resuspended in streptavidin-PE. Two additional washes were performed as before with 30 min incubation at room temperature. After wash step, Reading Buffer was added to the sample. Each sample was measured in duplicate. Plates were read using a MAGPIX instrument with xPONENT 4.2 software (Luminex). Cytokine concentrations were calculated using ProcartaPlex Analyst 1.0 software (Affymetrix).
Western blot analysis
MSCs protein extractions were prepared from 1 × 105 cells by homogenization in lysis buffer with a protease/phosphatase inhibitor cocktail (Cell Signaling, Danvers, MA, United States) and centrifuged for 15 min at 14000 rpm. The protein concentration in the supernatant was measured following the Bradford method (Bio-Rad). Protein samples were separated using sodium dodecyl sulfate gel electrophoresis and transferred onto a nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Membranes were stained with primary antibodies specific to IDO, COX2, β-actin (Cell Signaling) or iNOS (R and D Systems). Then, horseradish peroxidase-conjugated secondary antibodies were added. Membranes were washed with Tris-buffered saline and Tween 20 solution, and the hybridized protein bands were detected using an enhanced chemiluminescence detection kit and Hyperfilm enhanced chemiluminescence reagents (Amersham Pharmacia Biotech).
Induction and treatment of xenogenic GVHD
Human peripheral blood was obtained from healthy volunteers with their consent, and peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Hypaque (GE Healthcare +UK Ltd., United Kingdom) density centrifugation and washed in phosphate-buffered saline. Cells were resuspended in phosphate-buffered saline and injected through the tail vein into irradiated mice. The mice received a single dose of 200 cGy gamma irradiation from a linear accelerator before injection of human PBMCs on the same day. These animals were divided into three groups (n = 12 mice/group): GVHD, UC-MSC and BM-MSC. All animals were randomly allocated and coded. All animals were monitored for clinical symptoms of GVHD (weight loss, hunched posture, fur loss, reduced mobility, skin integrity) and mean serial weight measurements. The mice that showed severe inflammation in unintended body parts causing blindness and bowel obstruction, 20% weight loss compared to mice of same age, anorexia, quadriplegia and gait impairment were administered euthanasia by CO2 exposure.
In vitro growth assay
MSCs at each passage were seeded at a density of 1 × 105 in 100 mm culture dishes. Culture dishes were incubated in 37 °C with 5% CO2 until reaching 90% confluence and then counted.
In vitro proliferation assay
Human PBMCs (1 × 105/well) from healthy adult donors were plated in 96-well flat-bottomed plates (200 μL/well). Cells were then stimulated with 5 μg/mL phytohemagglutinin (Sigma-Aldrich) and/or MSCs (5 × 103, 1 × 104, 5 × 104, 1 × 105) for 72 h, followed by the incorporation of 1 μCi/mL [3H]-thymidine (GE Healthcare, Piscataway, NJ, United States) for the last 18 h of the indicated culture period. Radioactivity was measured using a Micro Beta instrument (Pharmacia Biotech, Piscataway, NJ, United States).
In other experiments, neutralizing antibodies for human IL-10 or transforming growth factor-β mAb (10 μg/mL; R and D Systems) and chemical antagonists for COX2 (indomethacin, 20 μM; Sigma-Aldrich), iNOS [N-nitro-L-arginine methyl ester (L-NAME), 1 mM; Sigma-Aldrich], heme oxygenase 1 inducer (hemin, 50 ng/mL; Sigma-Aldrich), selective A2B adenosine receptor (alloxazine, 10 μM; Sigma-Aldrich), and CD73 (α,β-methylene ADP [APCP], 100 µM; Sigma-Aldrich) were added into the coculture. All experiments were performed in quadruple and were repeated at least twice.
Flow cytometry analysis
PBMCs were immunostained using various combinations of the following fluorescence-conjugated antibodies: CD4 (RPA-T4; BioLegend), CD25 (BC96; eBioscience), Foxp3 (PCH101; eBioscience), IL-17 (eBio64DEC17; eBioscience) and IFN-γ (4S.B3; eBioscience). The cells were also intracellularly stained with the following antibodies: IL-17, IFN- γ and Foxp3. Before intracellular staining, cells were stimulated in culture medium containing 25 ng/mL phorbol myristate acetate (Sigma-Aldrich), 250 ng/mL ionomycin (Sigma-Aldrich) and 1 μL/mL GolgiSTOP (BD Pharmingen) in an incubator with 5% CO2 at 37 °C for 4 h. Intracellular staining was conducted using an intracellular staining kit (eBioscience) according to the manufacturer’s protocol. Flow cytometric analysis was performed on a LSRFortessa (BD Pharmingen).
Statistical analysis
Statistical significance was determined using Student’s two-tailed t-test. In all analyses, P values less than 0.05 were considered to indicate statistical significance.
Ethics
All procedures involving animals were performed in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals and the Guidelines and Policies for Rodent Experimentation provided by the Institutional Animal Care and Use Committee of the School of Medicine of the Catholic University of Korea (Seoul, South Korea). The protocols used in this study were approved by the institutional review board of The Catholic University of Korea (CUMC-2018-0270-01).
RESULTS
Characteristics of MSCs
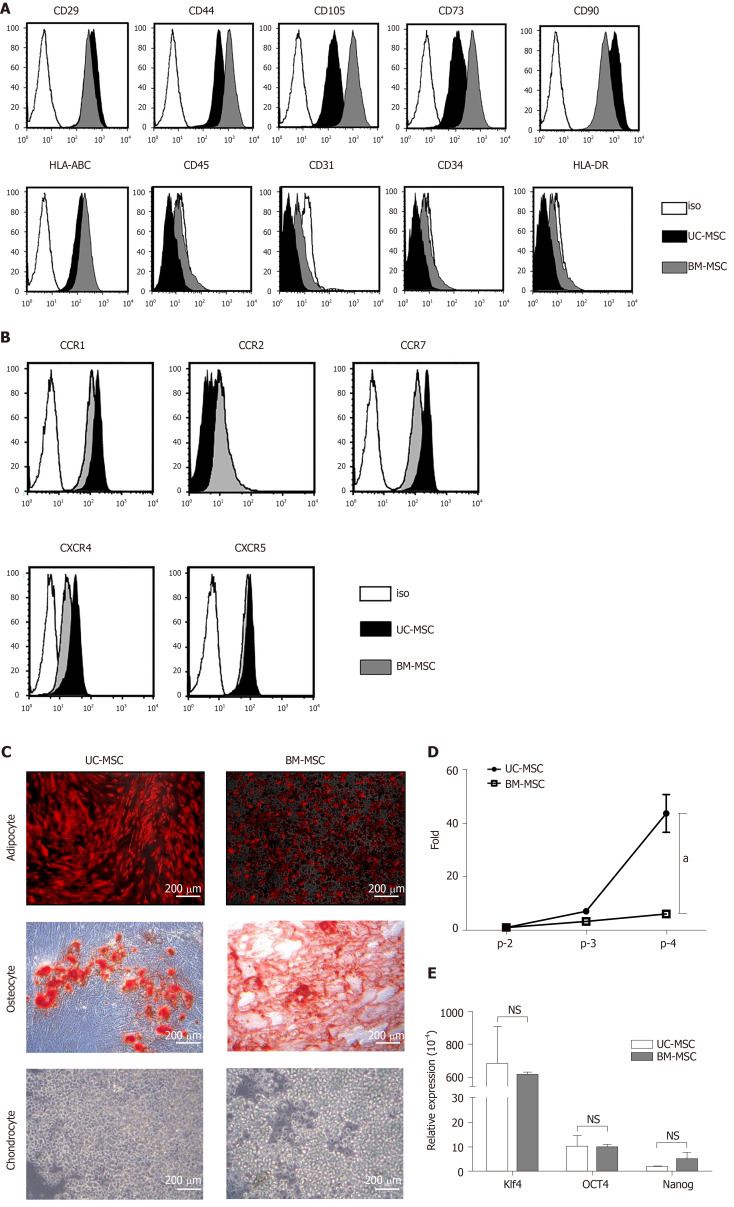
We analyzed the expression of surface proteins for immunophenotypic characterization of culture-expanded UC-MSCs and BM-MSCs. All culture-expanded MSCs showed similar spindle-shaped morphologies (data not shown) and were uniformly positive for CD29, CD44, CD105, CD73, CD90 and HLA-ABC but negative for CD45, CD31, CD34 and HLA-DR (Figure 1A).
Figure 1.
Characterization of umbilical cord-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells. A: Surface protein expression was analyzed using flow cytometry. Mesenchymal stem cells (MSCs) were assessed at the fourth passage; B: Chemokine receptor expression was analyzed at passages 3–7 by flow cytometry; C: Representative images of umbilical cord-derived MSCs and bone marrow-derived MSCs differentiation into adipocytes (bone marrow-derived MSCs: day 7; umbilical cord-derived MSCs: day 21), chondrocytes (day 21) and osteocytes (day 14). Scale bars: 200 μm. Adipocytes were detected by goat anti-mouse FABP4 polyclonal antibody under a fluorescence microscope. Osteoblasts and chondrocytes were stained with Alizarin Red S and Alcian Blue, respectively and observed under a light microscope; D: Growth rate of MSCs at different passages; and E: Pluripotency transcription factor expression levels of cultured MSCs measured by quantitative real-time polymerase chain reaction (target, GAPDH), D and E: Bone marrow-derived MSCs. Statistical analysis was performed by Student’s t-tests, aP < 0.05 vs indicated group. iso: Isotype; Klf4: Kruppel‐like factor 4; Nanog: Nanog homeobox; NS: No significance; OCT4: Octamer-binding transcription factor 4; UC-MSC: Umbilical cord-derived mesenchymal stem cells; BM-MSCs: Bone marrow-derived mesenchymal stem cells.
We used flow cytometry to examine the cell surface expression of chemokine receptors. Neither UC-MSCs nor BM-MSCs expressed CCR2. Both MSC types expressed CCR1, CCR7, CXCR4 and CXCR5 (Figure 1B).
For adipogenic induction, BM-MSCs formed lipid vacuoles after 7 d and UC-MSCs after 14 d. After they changed morphology, adipocytes were detected by goat anti-mouse FABP4 polyclonal antibody under a fluorescence microscope. For osteogenic induction, both MSCs lost their spindle shape and became irregularly shaped. After 21 d, differentiated osteocytes were stained using Alizarin Red S. Chondrocytes were induced in the micromass cultures with chondrocyte differentiation medium for 21 d. Chondrogenic-induced cells were stained with Alcian Blue. These data showed that both UC-MSCs and BM-MSCs have the potential to differentiate into adipocytes, osteocytes and chondrocytes (Figure 1C). Previous reports have suggested that donor age affects the proliferation rate of bone marrow-derived MSCs; as donors get older, the proliferation rate tends to decline[21]. We found that this trend also occurred with UC-MSCs (data not shown).
To compare the growth rates of each passage in UC-MSCs and BM-MSCs, we seeded 1 × 105 cells in 100-mm culture dishes and cultured until reaching 90% confluence. We compared the number of cells detached from the dish. The growth rate increased with passage number; however, UC-MSC growth increased more rapidly than that of BM-MSC (Figure 1D).
We then compared the expression levels of pluripotency transcription factors by quantitative real-time polymerase chain reaction. The expression levels of Klf4 and OCT4 were similar. The expression levels of Nanog in UC-MSCs and BM-MSCs were not statistically different (Figure 1E).
Immunomodulatory effects of MSCs with IFN-γ stimulation
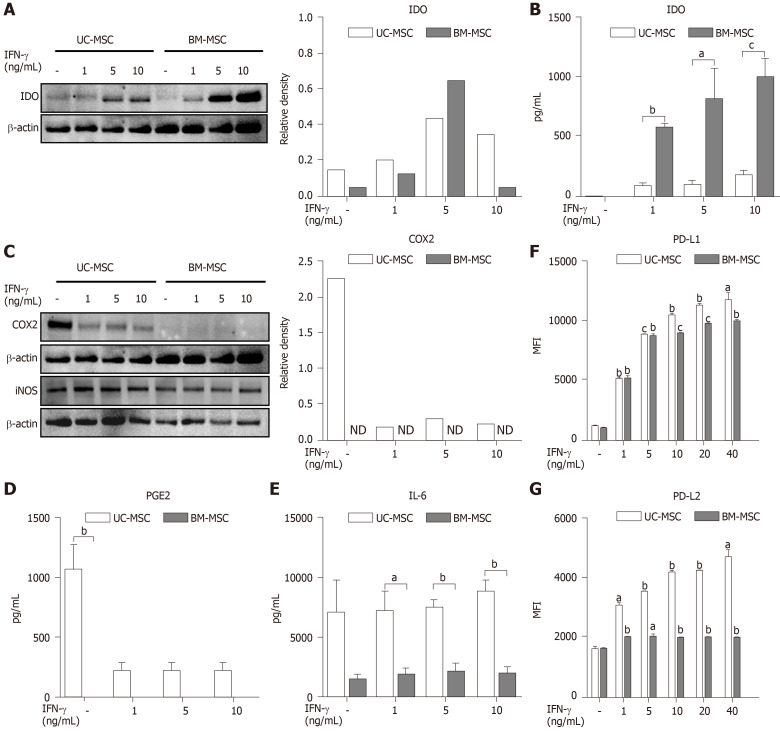
The immunosuppressive effects of MSCs are enhanced when stimulated by pro-inflammatory cytokine IFN-γ[5]. Therefore, we created inflammatory conditions using different concentrations of IFN-γ and examined the expression levels of soluble factors representing the immunosuppressive capacity of MSCs. Stimulated MSCs were detached and lysed to analyze IDO by western blot. The IDO levels of both UC-MSCs and BM-MSCs increased in a dose-dependent manner with IFN-γ. However, BM-MSCs expressed more IDO than UC-MSCs (Figure 2A). We collected cultured medium to assess the IDO concentrations. The concentration of IFN-γ was positively correlated with IDO levels produced by both UC-MSCs and BM-MSCs; however, IDO in BM-MSC culture supernatant was significantly higher than that of UC-MSCs (Figure 2B).
Figure 2.
Immunomodulatory effects of interferon-gamma-stimulated mesenchymal stem cells. Umbilical cord-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells were stimulated with 0, 1, 5 or 10 ng/mL of interferon-gamma in culture for 72 h. A and C: Expression of indoleamine 2,3-dioxygenase (A) and cyclooxygenase-2 (C) of interferon-gamma-stimulated mesenchymal stem cells measured by western blotting; B, D and E: Expression of indoleamine 2,3-dioxygenase (B), prostaglandin E2 (D), and interleukin-6 (E) in cultured medium measured by ELISA; F and G: Expression of PD-L1 (F) and PD-L2 (G) after interferon-gamma stimulation analyzed by flow cytometry. Statistical analysis was performed by Student’s t-tests, aP < 0.05; bP < 0.01; cP < 0.0001 vs indicated group. B, D and E: aP < 0.05; bP < 0.01; cP < 0.0001 vs first bar of each group (F and G). BM-MSC: Bone marrow-derived mesenchymal stem cells; IDO: Indoleamine 2,3-dioxygenase; IFN-γ: Interferon-gamma; IL-6: Interleukin 6; iNOS: Inducible nitric oxide synthase; MFI: Mean fluorescence intensity; ND: Not detected; PGE2: Prostaglandin E2; UC-MSC: Umbilical cord-derived mesenchymal stem cells.
Next, UC-MSCs and BM-MSCs were assessed for their expression of inflammatory mediators. When stimulated with IFN-γ, COX2 in UC-MSCs decreased in a dose-dependent manner, although BM-MSCs did not express COX2 with or without IFN-γ (Figure 2C). Also, IFN-γ stimulation did not affect expression of iNOS in both UC-MSCs and BM-MSCs (Figure 2C). According to recent studies, prostaglandin E2 (PGE2) is considered a major immunomodulatory factor of MSCs[22]. Therefore, we measured the concentrations of PGE2 and IL-6, which is increased by PGE2 in cultured medium[23]. PGE2 was notably secreted in UC-MSCs (Figure 2D). Interestingly, IL-6 levels were significantly high in UC-MSCs, and IFN-γ stimulation did not affect IL-6 levels (Figure 2E).
PD-L1 is known to maintain cancer cells as an immunosuppressive factor, which is necessary for cancer to evade the immune system[24]. In contrast, PD-L1 may act as an immunomodulatory factor for MSCs, which are expected to suppress the immune system. PD-L1 is also needed for cell-to-cell contact between MSCs and T cells. We analyzed the expression of PD-L1 and PD-L2 in UC-MSCs and BM-MSCs with or without IFN-γ stimulation. Overall, UC-MSCs expressed higher levels of PD-L1 (Figure 2F) and PD-L2 (Figure 2G). We also found that PD-L2 expression increased upon IFN-γ treatment in UC-MSCs but was consistent in BM-MSCs (Figure 2G).
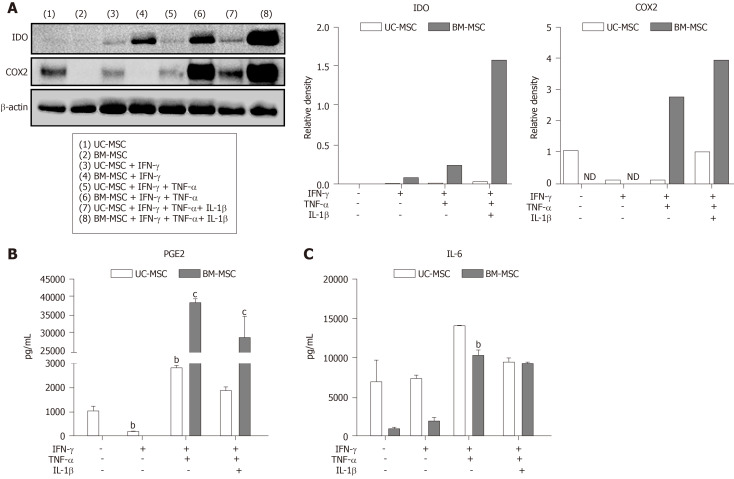
Immunomodulatory effects of MSCs with combinations of diverse cytokines
To enhance their immunomodulatory capacity, we stimulated MSCs with multiple combinations of IFN-γ, TNF-α and IL-1β. We found that UC-MSCs and BM-MSCs responded differently to the combinations. Western blot revealed that combinations of IFN-γ, TNF-α and IL-1β elevated the expression level of both IDOs. IFN-γ treatment alone did not increase IDO and COX2, but stimulation with TNF-α and IL-1β did (Figure 3A). Conversely, the protein levels in UC-MSCs were not significantly affected by stimulation (Figure 3A). Next, we analyzed PGE2 and IL-6 in cultured medium with combination stimulations. Interestingly, BM-MSCs, which did not secrete detectable amounts of PGE2 with IFN-γ, secreted high levels of PGE2 and IL-6 with combinations of IFN-γ and TNF-α. Moreover, combinations of cytokines enhanced the secreted levels of PGE2 and IL-6 (Figure 3B and 3C).
Figure 3.
Immunomodulatory effects of mesenchymal stem cells treated with combinations of cytokines. Umbilical cord-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells were stimulated with different combinations of interferon-gamma (5 ng/mL), tumor necrosis factor alpha (5 ng/mL), and/or IL-1β (5 ng/mL) for 72 h. A: Expression levels of indoleamine 2,3-dioxygenase and cyclooxygenase-2 of cytokine-stimulated mesenchymal stem cells measured by western blotting; B and C: Expression levels of prostaglandin E2 (B) and interleukin-6 (C) in culture medium measured by ELISA. Statistical analysis was performed by Student’s t-tests, aP < 0.05; bP < 0.01; cP < 0.0001 vs first bar of each group. (B and C) BM-MSC: Bone marrow-derived mesenchymal stem cells; IDO: Indoleamine 2,3-dioxygenase; IFN-γ: Interferon-gamma; IL-1β: Interleukin 1 beta; IL-6: Interleukin 6; ND: Not detected; PGE2: Prostaglandin E2; TNF-α: Tumor necrosis factor alpha; UC-MSC: Umbilical cord-derived mesenchymal stem cells.
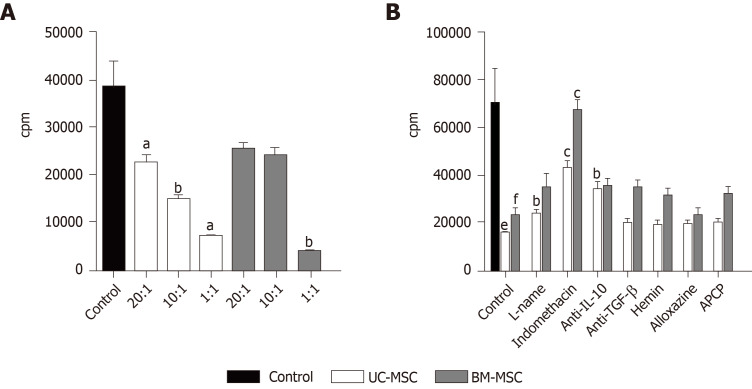
T cell inhibition
To test the immunosuppressive effects of the MSCs in vitro, we cultured phytohemagglutinin stimulated-human PBMCs in the presence of either UC-MSCs or BM-MSCs. When activated T cells were cocultured with MSCs, there was a decrease in T cell proliferation. Also, the immunosuppressive effects of UC-MSCs increased as the MSC-to-T cell ratio increased (Figure 4A). In the case of BM-MSCs, the immunosuppressive effects also tended to increase as MSC-to-T cell ratio increased; however, suppression effects of 20:1 and 10:1 were not significantly different (Figure 4A). Next, we inhibited various soluble factors with the respective inhibitors and assessed the effects on immunosuppressive activity. In both MSCs, the proliferation of T cells was recovered when cells were treated with indomethacin, which inhibits the COX2 pathway. This indicated that COX2 plays a critical role in the immunomodulation of MSCs. Another factor that restored T cell proliferation was anti-IL-10 (inhibitor of IL-10) in UC-MSCs and L-NAME (inhibitor of iNOS) in BM-MSCs. These findings suggested that UC-MSCs and BM-MSCs may regulate T cells through different pathways (Figure 4B).
Figure 4.
T cell inhibition by mesenchymal stem cells. T cell proliferation was inhibited by either umbilical cord-derived mesenchymal stem cells (MSCs) or bone marrow-derived MSCs in coculture. A: Human peripheral blood mononuclear cells (1 × 105/well) from heathy adult donors were stimulated with phytohemagglutinin (5 μg/mL) with or without MSCs (5 × 103, 1 × 104, 5 × 104, 1 × 105) for 72 h. In the final 18 h, 3H-thymidine was added to the cultures, aP < 0.05; bP < 0.01; cP <0.0001 vs control of each group, eP < 0.01; fP < 0.0001 vs control; and B: Human peripheral blood mononuclear cells were treated with L-NAME (iNOS inhibitor), indomethacin (cyclooxygenase-2 inhibitor), anti-IL-10, anti-transforming growth factor-β, hemin (heme oxygenase inducer), alloxazine (selective A2B adenosine receptor antagonist), or adenosine 5'-(α,β-methylene)diphosphate (CD73 inhibitor) with or without MSCs for 72 h. In the final 18 h, 3H-thymidine was added to the cultures. Statistical analysis was performed by Student’s t-tests aP < 0.05; bP < 0.01; cP < 0.0001 vs control. Anti-IL-10: Anti-interleukin 10 antibody; Anti-TGF-β: Anti-transforming growth factor β antibody; APCP: Adenosine 5'-(α,β-methylene)diphosphate; BM-MSC: Bone marrow-derived mesenchymal stem cells; cpm: Count per minute; L-NAME: N-nitro-L-arginine methyl ester; UC-MSC: Umbilical cord-derived mesenchymal stem cells.
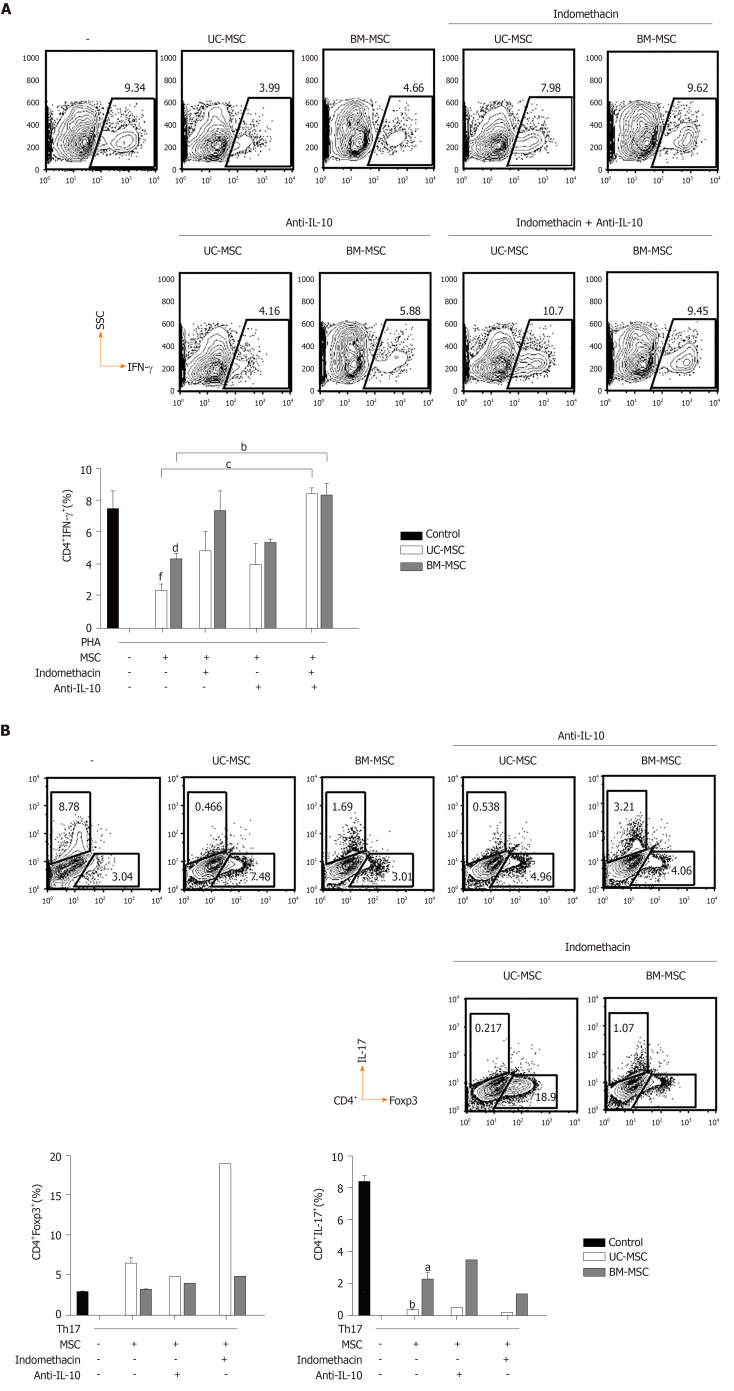
Because IL-10 and PGE2 play important roles in the immunomodulatory effects of MSCs, we evaluated the effects of inhibiting PGE2 and/or IL-10 on the immunomodulatory functions of MSCs. Because PGE2 is produced via the COX2 pathway, we inhibited COX2 or/and IL-10 with indomethacin and anti-IL-10, respectively. In GVHD, the balance between T cell subpopulations, especially T helper cell 17 (Th17), Th1 and regulatory T cell (Treg) populations, is very important. Therefore, we investigated how the inhibition of these two factors affected MSCs to modulate Th17, Th1 and Treg populations. First, we cocultured phytohemagglutinin-activated T cells with MSCs and treated them with indomethacin and/or anti-IL-10 to assess the effects on IFN-γ-producing CD4+ T cells. Next, we induced Th17 and cocultured them with MSCs in the presence of each inhibitor to identify changes in Th17 and Treg populations. In UC-MSCs, COX2 suppression increased the population of IFN-γ-producing CD4+ T cells and decreased the population of Treg cells. COX2 inhibition did not affect the population of IL-17-producing CD4+ T cells. In BM-MSCs, COX2 suppression increased the population of IFN-γ-producing and IL-17-producing CD4+ T cells. However, Treg cells were not affected. Through IL-10 inhibition, we concluded that IL-10 was more critical for UC-MSCs than BM-MSCs to suppress Th1 cells (Figure 5A and 5B).
Figure 5.
Roles of prostaglandin E2 and interleukin-10 in the immunomodulatory functions of mesenchymal stem cells. A: Human peripheral blood mononuclear cells were stimulated with phytohemagglutinin (10 μg/mL) and cocultured with mesenchymal stem cells treated with indomethacin (20 μM) and/or anti- interleukin (IL)-10 (5 μg/mL). Then, interferon-gamma-producing CD4+ T cells were analyzed by flow cytometry, aP < 0.05; bP < 0.01 vs first bar; B: Human peripheral blood mononuclear cells were treated with anti-interferon-gamma (10 μg/mL), anti-IL-4 (10 μg/mL), IL-6 (10 ng/mL), IL-23 (5 ng/mL), IL-1β (10 ng/mL), tumor necrosis factor alpha-α (5 ng/mL) and transforming growth factor-β (2 ng/mL) to induce T helper 17 cells. Induced T helper 17 cells were cocultured with mesenchymal stem cells treated with indomethacin (20 μM) and/or anti-IL-10 (5 μg/mL). Populations of CD4+Foxp3+ T cells and CD4+IL-17+ T cells were analyzed by flow cytometry. Statistical analysis was performed by Student’s t-tests, aP < 0.05; bP < 0.01; cP < 0.0001 vs indicated group, dP < 0.05; fP < 0.0001 vs phytohemagglutinin control. Anti-IL-10: Anti-interleukin 10 antibody; BM-MSC: Bone marrow-derived mesenchymal stem cells; IFN-γ: Interferon-gamma; IL-17: Interleukin 17; MSC: Mesenchymal stem cells; PHA: Phytohemagglutinin; Th17: T helper 17; UC-MSC: Umbilical cord-derived mesenchymal stem cells.
MSC therapy in a humanized GVHD mouse model
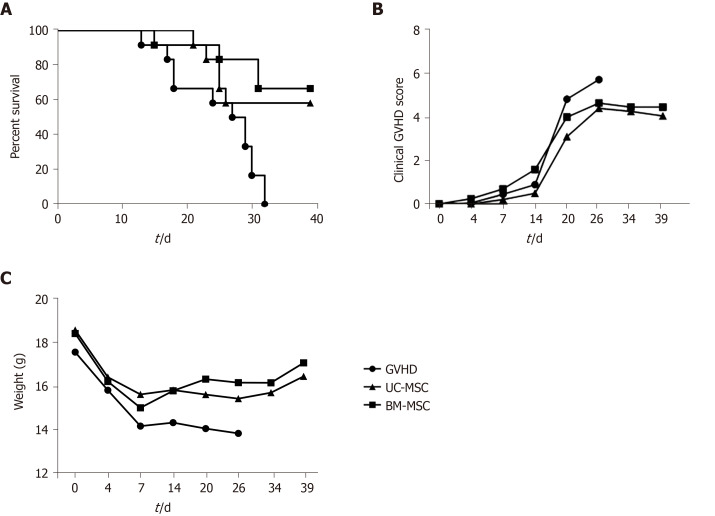
To assess the immunosuppressive activity of MSCs in vivo, we established a humanized GVHD mouse model in NOG mice that were exposed to a 200-cGy dose of radiation, and human PBMCs (2 × 107) were transferred intravenously to induce GVHD. At days 0, 7 and 14 after GVHD induction, mice were administered with UC-MSCs or BM-MSCs (1 × 105) for cell therapy. Mice were monitored at different time points after GVHD induction for survival, body weight and clinical GVHD scores. Figure 6 presents the combined results of two independent experiments under the same conditions. Most untreated GVHD-induced mice died between days 20 and 32. At 1 wk after the final death of untreated mice, survivors were treated with UC-MSCs (6/12) and BM-MSCs (8/12). MSC therapy significantly increased the survival rate (Figure 6A). Mice treated with MSCs also had less severe GVHD symptoms, such as declines in weight, activity, posture, fur texture and skin integrity (Figure 6B and 6C).
Figure 6.
Inhibitory effects of mesenchymal stem cells therapy on graft-versus-host disease severity. A-C: NOG mice were administered 200 cGy of total body irradiation before transplantation of human peripheral blood mononuclear cells (2 × 107). At days 0, 7 and 14 after transplantation, mice were administered with umbilical cord-derived mesenchymal stem cells or bone marrow-derived mesenchymal stem cells (1 × 105). All NOG mice were monitored for survival (A), clinical signs of graft-versus-host disease (B) and weight (C). BM-MSC: Bone marrow-derived mesenchymal stem cells; GVHD: Graft-versus-host disease; UC-MSC: Umbilical cord-derived mesenchymal stem cells.
DISCUSSION
The use of MSCs to treat various diseases has been gaining popularity due to their immunosuppression abilities. Studies have shown that MSCs play a critical role in injury healing[16,25], Crohn’s disease[26], systemic lupus erythematosus[27] and rheumatoid arthritis[28]. MSC therapy has been applied for many years to treat GVHD, which is a lethal disease occurring after allogeneic hematopoietic stem cell or organ transplantation. Several treatments, such as steroids with or without calcineurin inhibitors, are used to treat GVHD, but newer therapies are needed due to the poor prognosis of current methods. Since Le Blanc et al[11] first reported that adult BM-MSCs were effective in treating severe treatment-resistant grade IV acute GVHD, many clinical trials have confirmed the potential of MSC therapy for GVHD. Given these promising results, MSCs have been derived from various sources, such as adipose tissue, molar cells, amniotic fluid and umbilical cords. However, it remains unclear which source is best, or how MSCs of different sources regulate other cells[29]. Recently, UC-MSCs have gained attention for their ease of collection from a young source.
In this study, we compared UC-MSCs and BM-MSCs not only to find the difference in degree of immunoregulation but also to discover the difference in factors affecting immunoregulation. Specifically, we compared the phenotypic and functional features of human UC-MSCs and human BM-MSCs. Our findings demonstrated that UC-MSCs and BM-MSCs share similar phenotypic characteristics and immunomodulation capacities. Furthermore, we suggest that UC-MSCs and BM-MSCs may suppress activated immune systems via different pathways.
Early studies emphasized the critical roles of chemokines and their receptors in the migration of MSCs to sites of injury. For example, interactions between stromal cell-derived factor-1 and CXCR4 and between monocyte chemoattractant protein-1 and CCR2 regulate the migration of MSCs in vitro[30,31]. In this study, our data showed that UC-MSCs and BM-MSCs share similar immunophenotypic characteristics and chemokine receptors. In addition, they possess similar abilities to differentiate into adipocytes, osteocytes and chondrocytes. Moreover, the expression levels of pluripotency transcription factors such as Klf4, OCT4 and Nanog are similar between UC-MSCs and BM-MSCs. Our results provide evidence that UC-MSCs and BM-MSCs share similar phenotypic characteristics.
MSCs have enhanced immunosuppressive effects under acute inflammatory conditions, especially in the presence of the pro-inflammatory cytokine IFN-γ[5]. As expected, exposure to IFN-γ increased the expressions of IDO in both UC-MSCs and BM-MSCs. However, BM-MSCs produced more IDO than UC-MSCs with stimulation by IFN-γ.
Previous studies have claimed that combinations of cytokines upregulate IDO and COX2 levels in MSCs. For instance, upregulated IDO activity of TNF-α- and IFN-γ-activated MSCs differentiated monocytes into IL-10-secreting M2 immunosuppressive macrophages, which suppress T cell proliferation[32]. Another study observed that the induction of IDO in MSCs was regulated by IFN-β and IFN-γ[33]. Finally, IL-1β has also been shown to induce COX2[34]. In this study, we treated MSCs with diverse combinations of cytokines and assessed factors representing the immunosuppressive activity of MSCs. BM-MSCs expressed more upregulated IDO and COX2. However, UC-MSCs secreted high levels of IL-6 compared to BM-MSCs. UC-MSCs expressed low levels of IL-6 even without stimulation and high levels with cytokine stimulation. Paradoxically, while IL-6 is mostly known as a pro-inflammatory cytokine, it is also an anti-inflammatory cytokine in terms of maintaining the immunosuppressive capacity of MSCs[35]. When MSCs are differentiated, IL-6 is downregulated, which leads to loss of immune privilege in MSCs[36]. At the same time, IL-6 is claimed as a critical factor for maintaining the stemness of MSCs. Thus, the role of IL-6 in MSCs is to enhance MSC proliferation, protect MSCs from apoptosis and inhibit differentiation[37]. Based on our findings, we speculate that high levels of IL-6 in UC-MSCs are involved in suppressive effects.
Li et al[38] suggested that overexpression of IL-6 in tumor tissue enhanced PD-L1 expression, suggesting that the high levels of IL-6 in UC-MSCs might be related to the higher levels of PD-L1 and PD-L2 in UC-MSCs than BM-MSCs. In tumor tissue, PD-L1 acts as a ligand of PD-1 expressed in T cells, inhibiting anti-tumor effects[39]. Therefore, much like PD-L1 in tumor tissue suppresses T cells via PD-1, UC-MSCs may inhibit T cell proliferation through the PD-1/PD-L1 pathway.
Although the Th1-mediated response is considered a main contributor to the induction of GVHD, Th17 cells are also capable of developing lethal GVHD[40]. Also, it is well known that imbalances between Th17 and Treg cells affect the severity of GVHD[41]. In this study, we demonstrated that UC-MSCs and BM-MSCs effectively suppressed Th1 and Th17 cells and induced Treg cells.
In conclusion, we have demonstrated that UC-MSCs and BM-MSCs share similar phenotypic characteristics and immunomodulation abilities. Moreover, cytokine stimulation enhances the immunomodulation of both MSCs, although UC-MSCs and BM-MSCs exhibit different tendencies toward expression of IDO, COX2, PGE2, IL-6, PD-L1 and PD-L2. These findings suggested that UC-MSCs and BM-MSCs may induce immunosuppression via different pathways. High expression of IDO and COX2 in BM-MSCs suggests that these two factors are involved in T cell suppression. Meanwhile, high expression of IL-6, PD-L1 and PD-L2 in UC-MSCs suggests that UC-MSCs inhibited T cell proliferation through cell-to-cell contact via the PD-1/PD-L1 pathway. Finally, we demonstrated that treating a GVHD mouse model with MSCs improved the clinical score, survival and weight loss of irradiated mice. Interestingly, the mechanisms by which UC-MSCs and BM-MSCs alleviated GVHD seemed to differ, but their ability to attenuate GVHD in a mouse model was overall similar. Taken together, these findings underscore the need for future studies to identify the mechanisms of immunosuppression of different types of MSCs and further studies in mechanisms of MSCs may allow advanced MSC therapy in clinical use.
ARTICLE HIGHLIGHTS
Research background
Mesenchymal stem cells (MSCs) are a promising therapeutic approach to treat graft-versus-host disease (GVHD) because of their immunoregulatory properties. Until recently, human bone marrow-derived MSCs (BM-MSCs) were used widely as cell therapy sources. However, the needs for new source of MSCs are growing due to the invasive collection method and decrease in donors. Among many other adult and fetal tissues, human umbilical cord has emerged as a promising source of MSCs because of their ethical and noninvasive collection.
Research motivation
Although several studies have pointed out the potential of human umbilical cord-derived MSCs (UC-MSCs), the difference in immunomodulatory effects and mechanisms of BM-MSCs and UC-MSCs should be examined in greater detail.
Research objectives
In this study, we aim to investigate the difference in mechanisms of the immunosuppressive effects of UC-MSCs and BM-MSCs.
Research methods
Western blot, quantitative real-time polymerase chain reaction and luminex multiplex cytokine assay were employed to examine the expression of soluble factors after MSCs were primed with different combinations of interferon-gamma, tumor necrosis factor alpha and interleukin (IL)-1β, or interferon-gamma alone. Human peripheral blood mononuclear cells stimulated with phytohemagglutinin were cocultured with MSCs to examine the immunosuppressive effects of the MSCs in vitro. Several inhibitors of soluble factors were used to identify which soluble factors played critical roles in the immunomodulation of MSCs. Lastly, xenogeneic GVHD was induced in NOG mice (NOD/Shi-scid/IL-2Rγnull) and UC-MSCs or BM-MSCs were used as cell therapies.
Research results
BM-MSCs and UC-MSCs shared similar phenotypic characteristics and immunosuppressive effects. COX2 and IL-10 were key factors in the immunomodulatory mechanisms of both MSCs. However, upon in vitro cytokine stimulation, BM-MSCs expressed more indolamine 2,3-dioxygenase, and UC-MSCs expressed more prostaglandin E2, IL-6, PD-L1 and PD-L2. UC-MSCs and BM-MSCs established different T cell subpopulations when cultured with stimulated T cells. UC-MSCs inhibited more T helper 17 cells and induced more regulatory T cells than BM-MSCs. In a humanized GVHD mouse model, UC-MSCs and BM-MSCs showed comparable effects in attenuating GVHD.
Research conclusions
Our data provides a deeper understanding in similarities and differences between UC-MSCs and BM-MSCs. This study demonstrated that UC-MSCs and BM-MSCs exhibited similar immunosuppression in different mechanisms. Also, this study introduced that UC-MSCs have the potential to substitute for BM-MSCs as cell therapy products.
Research perspectives
In summary, we have demonstrated that UC-MSCs and BM-MSCs exhibit different tendencies toward expression of proteins known to contribute to immunosuppression although they share similar phenotypic characteristics and immunomodulation abilities. Our data also suggest that UC-MSCs and BM-MSCs induced immunosuppression through different pathways underscoring the need for future studies to identify detailed mechanisms of MSCs derived from different sources.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Veterans Health Service Medical Center for supporting this experiment. The authors would like to acknowledge Hyeon Woo Yim (The Catholic University of Korea, South Korea) for his assistance of statistical analysis.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Catholic University of Korea Catholic Medical Center Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by Institutional Animal care and Use Committee in School of Medicine, The Catholic University of Korea; approval number: CUMC-2018-0270-01.
Conflict-of-interest statement: The authors have nothing to disclose.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: March 20, 2020
First decision: April 25, 2020
Article in press: July 19, 2020
Specialty type: Cell and tissue engineering
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin M S-Editor: Zhang L L-Editor: Filipodia P-Editor: Xing YX
Contributor Information
Yunejin Song, Institute for Translational Research and Molecular Imaging, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Laboratory of Immune Regulation, Convergent Research Consortium for Immunologic Disease, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Department of Biomedicine and Health Sciences, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Jung-Yeon Lim, Institute for Translational Research and Molecular Imaging, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Laboratory of Immune Regulation, Convergent Research Consortium for Immunologic Disease, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Precision Immunology Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States.
Taekyu Lim, Division of Hematology Oncology, Department of Internal Medicine, Veterans Health Service Medical Center, Seoul 05368, South Korea.
Keon-Il Im, Institute for Translational Research and Molecular Imaging, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Laboratory of Immune Regulation, Convergent Research Consortium for Immunologic Disease, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Nayoun Kim, Institute for Translational Research and Molecular Imaging, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Laboratory of Immune Regulation, Convergent Research Consortium for Immunologic Disease, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Young-Sun Nam, Institute for Translational Research and Molecular Imaging, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Laboratory of Immune Regulation, Convergent Research Consortium for Immunologic Disease, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Young-Woo Jeon, Institute for Translational Research and Molecular Imaging, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Laboratory of Immune Regulation, Convergent Research Consortium for Immunologic Disease, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Department of Hematology, Catholic Blood and Marrow Transplantation Center, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Jong Chul Shin, Department of Obstetrics and Gynecology, CHA Bundang Medical Center, CHA University, Seongnam 13496, South Korea.
Hyun Sun Ko, Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
In Yang Park, Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Seok-Goo Cho, Institute for Translational Research and Molecular Imaging, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Laboratory of Immune Regulation, Convergent Research Consortium for Immunologic Disease, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Department of Hematology, Catholic Blood and Marrow Transplantation Center, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Division of Hematology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea. chosg@catholic.ac.kr.
Data sharing statement
The dataset supporting the conclusions of this article are included within in the article.
References
- 1.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 2.Imamura M, Hashino S, Kobayashi H, Kubayashi S, Hirano S, Minagawa T, Tanaka J, Fujii Y, Kobayashi M, Kasai M. Serum cytokine levels in bone marrow transplantation: synergistic interaction of interleukin-6, interferon-gamma, and tumor necrosis factor-alpha in graft-versus-host disease. Bone Marrow Transplant. 1994;13:745–751. [PubMed] [Google Scholar]
- 3.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 5.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 6.Kögler G, Radke TF, Lefort A, Sensken S, Fischer J, Sorg RV, Wernet P. Cytokine production and hematopoiesis supporting activity of cord blood-derived unrestricted somatic stem cells. Exp Hematol. 2005;33:573–583. doi: 10.1016/j.exphem.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 8.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 9.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 10.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, Sportoletti P, Falzetti F, Tabilio A. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–318. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 12.Atala A. Recent developments in tissue engineering and regenerative medicine. Curr Opin Pediatr. 2006;18:167–171. doi: 10.1097/01.mop.0000193294.94646.be. [DOI] [PubMed] [Google Scholar]
- 13.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 15.Zheng YB, Gao ZL, Xie C, Zhu HP, Peng L, Chen JH, Chong YT. Characterization and hepatogenic differentiation of mesenchymal stem cells from human amniotic fluid and human bone marrow: a comparative study. Cell Biol Int. 2008;32:1439–1448. doi: 10.1016/j.cellbi.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Tredget EE, Liu C, Wu Y. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One. 2009;4:e7119. doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M, Dulong J, Monnier D, Gourmelon P, Gorin NC, Sensebé L Société Française de Greffe de Moelle et Thérapie Cellulaire. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549–1553. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- 18.Bocelli-Tyndall C, Zajac P, Di Maggio N, Trella E, Benvenuto F, Iezzi G, Scherberich A, Barbero A, Schaeren S, Pistoia V, Spagnoli G, Vukcevic M, Martin I, Tyndall A. Fibroblast growth factor 2 and platelet-derived growth factor, but not platelet lysate, induce proliferation-dependent, functional class II major histocompatibility complex antigen in human mesenchymal stem cells. Arthritis Rheum. 2010;62:3815–3825. doi: 10.1002/art.27736. [DOI] [PubMed] [Google Scholar]
- 19.He H, Nagamura-Inoue T, Tsunoda H, Yuzawa M, Yamamoto Y, Yorozu P, Agata H, Tojo A. Stage-specific embryonic antigen 4 in Wharton's jelly-derived mesenchymal stem cells is not a marker for proliferation and multipotency. Tissue Eng Part A. 2014;20:1314–1324. doi: 10.1089/ten.TEA.2013.0333. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 21.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Kopper O, Giladi O, Golan-Lev T, Benvenisty N. Characterization of gastrulation-stage progenitor cells and their inhibitory crosstalk in human embryoid bodies. Stem Cells. 2010;28:75–83. doi: 10.1002/stem.260. [DOI] [PubMed] [Google Scholar]
- 23.Cho JS, Han IH, Lee HR, Lee HM. Prostaglandin E2 Induces IL-6 and IL-8 Production by the EP Receptors/Akt/NF-κB Pathways in Nasal Polyp-Derived Fibroblasts. Allergy Asthma Immunol Res. 2014;6:449–457. doi: 10.4168/aair.2014.6.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214:895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22:2267–2277. doi: 10.3727/096368911X582769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim JY, Im KI, Lee ES, Kim N, Nam YS, Jeon YW, Cho SG. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep. 2016;6:26851. doi: 10.1038/srep26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf D, von Lilienfeld-Toal M, Wolf AM, Schleuning M, von Bergwelt-Baildon M, Held SA, Brossart P. Novel treatment concepts for graft-versus-host disease. Blood. 2012;119:16–25. doi: 10.1182/blood-2011-08-339465. [DOI] [PubMed] [Google Scholar]
- 30.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 31.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T, Kerin MJ. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 32.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 33.Croitoru-Lamoury J, Lamoury FM, Caristo M, Suzuki K, Walker D, Takikawa O, Taylor R, Brew BJ. Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO) PLoS One. 2011;6:e14698. doi: 10.1371/journal.pone.0014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin M, Neumann D, Hoff T, Resch K, DeWitt DL, Goppelt-Struebe M. Interleukin-1-induced cyclooxygenase 2 expression is suppressed by cyclosporin A in rat mesangial cells. Kidney Int. 1994;45:150–158. doi: 10.1038/ki.1994.18. [DOI] [PubMed] [Google Scholar]
- 35.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Li SH, Wu J, Zang WF, Dhingra S, Sun L, Weisel RD, Li RK. Interleukin-6 downregulation with mesenchymal stem cell differentiation results in loss of immunoprivilege. J Cell Mol Med. 2013;17:1136–1145. doi: 10.1111/jcmm.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Xu J, Yan X, Jin K, Li W, Zhang R. Targeting Interleukin-6 (IL-6) Sensitizes Anti-PD-L1 Treatment in a Colorectal Cancer Preclinical Model. Med Sci Monit. 2018;24:5501–5508. doi: 10.12659/MSM.907439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 40.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malard F, Bossard C, Brissot E, Chevallier P, Guillaume T, Delaunay J, Mosnier JF, Moreau P, Grégoire M, Gaugler B, Mohty M. Increased Th17/Treg ratio in chronic liver GVHD. Bone Marrow Transplant. 2014;49:539–544. doi: 10.1038/bmt.2013.215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article are included within in the article.