Abstract
Mesenchymal stromal/stem cells (MSCs) are adult stem cells of stromal origin that possess self-renewal capacity and the ability to differentiate into multiple mesodermal cell lineages. They play a critical role in tissue homeostasis and wound healing, as well as in regulating the inflammatory microenvironment through interactions with immune cells. Hence, MSCs have garnered great attention as promising candidates for tissue regeneration and cell therapy. Because the inflammatory niche plays a key role in triggering the reparative and immunomodulatory functions of MSCs, priming of MSCs with bioactive molecules has been proposed as a way to foster the therapeutic potential of these cells. In this paper, we review how soluble mediators of the inflammatory niche (cytokines and alarmins) influence the regenerative and immunomodulatory capacity of MSCs, highlighting the major advantages and concerns regarding the therapeutic potential of these inflammatory primed MSCs. The data summarized in this review may provide a significant starting point for future research on priming MSCs and establishing standardized methods for the application of preconditioned MSCs in cell therapy.
Keywords: Mesenchymal stem cells, Pro-inflammatory cytokines, Alarmins, Priming, Boosting the therapeutic potential
Core Tip: The inflammatory niche plays a key role in triggering the reparative and immunomodulatory functions of mesenchymal stromal/stem cells (MSCs). This paper summarizes the data on how soluble factors in the inflammatory microenvironment, including pro-inflammatory cytokines secreted by immune cells and alarmins released by damaged cells, affect MSCs’ ability to regenerate tissue and modulate the inflammatory response. We also analyze data from in vitro and in vivo studies, which highlight the influence of these factors on the therapeutic potential of MSCs, thus providing an important background for the development of preconditioning strategies that might improve the outcomes of MSC-based cell therapies.
INTRODUCTION
Inflammation is a localized immunologic response of the tissue elicited by harmful stimuli, including pathogens, irritants, or physical injury. This complex and protective response plays a fundamental role in the regulation of tissue repair, serving to eliminate harmful stimuli and begin the healing process[1]. In fact, inflammation is considered an important initial phase, followed by cell proliferation and extracellular matrix remodeling. These phases overlap over time and each of them represents a sequence of dynamic cellular and biochemical events, contributing to tissue regeneration through the collaboration of many cell types and their soluble products[2]. Immune cells, together with blood vessels, various stromal cells, extracellular matrix components, and a plethora of secreted soluble mediators, comprise an inflammatory microenvironment capable of inducing different responses of cells within injured tissue[3].
Soluble mediators released from injured/necrotic cells or damaged microvasculature lead to enhanced endothelium permeability and infiltration of neutrophils and macrophages. Among these mediators are endogenous danger signals, known as alarmins, which are rapidly released by dying necrotic cells upon tissue damage and play an important role in promoting and enhancing the immune response[4-6]. To date, the best-characterized alarmins are the interleukin (IL)-1 family of cytokines (IL-1α and IL-33), high-mobility group protein B1 (HMGB1), S100 proteins, and heat shock proteins (Hsps)[4,7]. In addition, during the inflammatory process, the phagocytosis of necrotic cells by resident/recruited neutrophils and macrophages induces the release of various inflammatory factors, such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-1β, IL-17, and chemokines[8].
Aside from numerous soluble mediators, tissue injury mediated by immunity or infection involves an even greater number of various immune cells, including B cells, CD4+ and CD8+ T cells, and natural killer cells. While all immune cells play key roles in wound healing through the eradication of damaged tissue and invading pathogens, their excessive activation can actually aggravate the injury. Therefore, a compre-hensive understanding of inflammatory niche elements might contribute to the development of novel therapeutic strategies for the treatment of inflammatory-associated diseases, as well as conditions of failed tissue regeneration.
One of the cellular compartments participating in the inflammatory niche represents mesenchymal stromal/stem cells (MSCs). MSCs are stem cells of stromal origin that possess self-renewal capacity and the ability to differentiate into three mesodermal cell lineages, including osteocytes, chondrocytes, and adipocytes[9]. Considering their critical role in tissue homeostasis and wound healing, MSCs have garnered great attention as promising candidates for tissue regeneration. Although first isolated from the bone marrow (BM)[10], MSCs may be obtained from various fetal and adult tissues, such as the umbilical cord (UC), peripheral blood, adipose tissue (AT), and skin and dental tissues[11,12]. According to the minimum criteria proposed by the International Society for Cellular Therapy, MSCs originating from different tissues are evidenced by the property of plastic adherence in vitro and expression of various non-specific surface molecules, such as cluster of differentiation (CD)105, CD90, CD73, and CD29, in parallel with trilineage differentiation potential[13]. However, the term MSC has recently been considered inappropriate, as it has become clear that MSCs from different tissues are not the same, especially with respect to their differentiation capacities[14,15], whereas their multipotent differentiation potential has not been confirmed in in vivo conditions. Therefore, Caplan[17] recently proposed this term to stand for medicinal signaling cells[16], indicating the correlation of the therapeutic benefits of MSCs with the secretion of various bioactive molecules.
Many studies have demonstrated that MSCs contribute to tissue repair by accumulating at sites of tissue damage and inflammation, where together with resident MSCs, they exert reparative effects in two ways. One way is to replace damaged cells through differentiation, and another is related to the ability of MSCs to strongly influence the microenvironment by releasing bioactive factors and interacting with multiple cell types[18,19]. Indeed, poorly immunogenic MSCs that weakly express major histocompatibility complex (MHC) class I and lack MHC class II play a critical role in regulating the inflammatory microenvironment through interactions with immune cells such as T cells, B cells, natural killer cells, and dendritic cells[20-22]. As a result of these interactions, MSCs suppress lymphocyte proliferation and maturation of monocytes into dendritic cells, while stimulating the generation of regulatory T cells (Tregs) and M2 macrophages[23,24].
The major role in the crosstalk between MSCs and immune cells has been ascribed to soluble factors, which upon release by activated immune cells, significantly affect MSCs paracrine activity, conversely influencing immune cells. In particular, the immunosuppressive activity of MSCs has been related to the production of indoleamine-2,3-dioxygenase (IDO), nitric oxide (NO), prostaglandin-E2 (PGE2), IL-10, transforming growth factor (TGF)-β, and TNFα-stimulated gene-6[25].
Inflammatory priming of MSCs
It is believed that the inflammatory niche plays a key role in triggering the reparative function of MSCs. Namely, studies have demonstrated that the immunosuppressive potential of MSCs is not inherently expressed but requires priming by inflammatory factors, including IFN-γ, TNF-α, or IL-1β[18,26]. Moreover, it has been found that MSCs can polarize into MSC type 1 with a pro-inflammatory profile or MSC type 2 with an immunosuppressive phenotype, depending on the inflammatory condition[23,27]. On the other hand, the inflammatory microenvironment influences the differentiation potential of resident and recruited MSCs, significantly impairing their regenerative capacity. In addition, several studies have shown that MSCs of different tissue origin may exert differential sensitivity to inflammatory conditions[28,29]. These data point to the critical importance of interactions between MSCs and inflammatory factors for the outcome of wound healing.
Indeed, the regenerative potential of transplanted MSCs is affected by inflammatory conditions[30], indicating the strong influence of the recipient’s inflammatory status on the efficacy of MSC-based therapies. Interestingly, to reduce the heterogeneity of MSCs and generate more homogenous therapeutic products, MSC priming with cytokines has been proposed[31]. The application of bioactive molecules in this context has been considered a supplemental molecular signal used to foster the therapeutic potential of MSCs and contribute to establishing a favorable microenvironment for tissue repair.
The complex cytokine network has been considered a critical part of the inflammatory microenvironment, where the pleiotropic properties of pro-inflammatory cytokines play a decisive role in the healing process and tissue regeneration. TNF-α, IFN-γ, IL-1, IL-17, and IL-6 are the most common inflammatory cytokines in this complex network[32]. Moreover, another significant constituent of the inflammatory niche considers alarmins, such as IL-1α and IL-33, HMGB1, S100 proteins, and Hsps, which can promote the immune response, thereby supporting host defense and tissue repair[7]. Here, we review how these soluble mediators of the inflammatory niche influence the regenerative and immunomodulatory potential of MSCs, highlighting the major advantages and concerns regarding the therapeutic potential of inflammatory primed MSCs.
MSCs priming with pro-inflammatory cytokines
IFN-γ priming: One of the most studied inflammatory priming mediators is IFN-γ, which is a key player in cellular immunity regulation, heightening immune responses in infection and cancer. However, very little is known about the effects of IFN-γ on the regenerative potential of MSCs. Namely, Croitoru-Lamoury et al[33] demonstrated that IFN-γ exerts significant antiproliferative effects on mouse and human BM-MSCs (Figure 1) through IDO induction and production of downstream tryptophan metabolites, such as kynurenine, which can potentiate the suppressive effects on cell proliferation in an autocrine manner. This was the first study that linked IFN-γ-induced IDO with the control of MSC differentiation potential, as evidenced by the inhibition of both osteogenic and adipogenic marker expression in IFN-γ-primed BM-MSCs upon induction of differentiation.
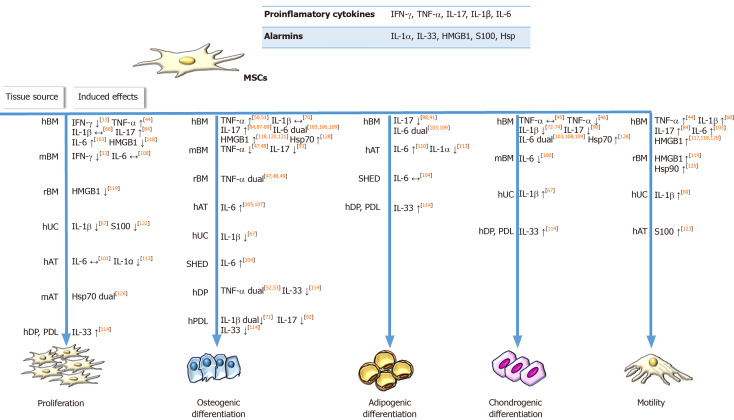
Figure 1.
Influence of inflammatory priming on regenerative features of mesenchymal stromal/stem cells under in vitro conditions. Soluble factors in the inflammatory microenvironment, including pro-inflammatory cytokines secreted by immune cells and alarmins released by damaged cells, influence mesenchymal stromal/stem cells’ (MSCs) ability to regenerate tissue by affecting their proliferation, migration and differentiation potential (osteogenic, chondrogenic, adipogenic). When applied in vitro, indicated inflammatory factors differently affect the regenerative properties of MSCs depending on the species or tissue origin of MSCs. Symbols: ↑ and ↓ represent stimulatory or inhibitory activity of priming factor, respectively; ↔ indicates no effects of priming factor. The term ’dual’ indicates the data where priming factor dually affected MSCs function depending on applied concentration or MSCs donor age. The numbers in square brackets indicate the references. IFN-γ: Interferon gamma; TNF-α: Tumor Necrosis Factor alpha; IL: Interleukin; HMGB1: High mobility group box 1; Hsp: Heat shock proteins; MSCs: Mesenchymal stromal/stem cells; h: Human; WJ: Warthon jelly; BM: Bone marrow; r: Rat; UC: Umbilical cord; AT: Adipose tissue; DP: Dental pulp; PDL: Periodontal ligament; SHED: Exfoliated deciduous teeth.
Numerous studies have demonstrated that the priming of MSCs with IFN-γ can enhance the immunosuppressive property of these cells by IDO stimulation[34]. In addition, exposure to IFN-γ has been shown to induce UC Wharton’s jelly (WJ)-MSCs to express other immunosuppressive factors, such as human leukocyte antigen G5, as well as C-X-C motif chemokine ligand (CXCL)9, CXCL10, and CXCL11[35]. Even more, IFN-γ-primed WJ-MSCs secrete more IL-6 and IL-10 upon co-culture with activated lymphocytes, increasing the percentage of Tregs, while decreasing the frequency of T helper (Th)17 cells (Table 1).
Table 1.
Influence of inflammatory priming on immunomodulatory features of mesenchymal stromal/stem cells under in vitro conditions
| Priming factor | MSCs source | Immunomodulatory effects of inflammatory priming | Suggested mechanism | Ref. | |
| Pro-inflammatory cytokines | IFN-γ | hWJ-MSCs | ↓ Th1 and Th17 cells proliferation | ↑ CXCL9, CXCL10, CXCL11, ICAM-1, VCAM-1, IDO1 and HLA-G5 gene expression | Wang et al[35], 2016 |
| ↑ Th2 and Tregs (CD4+, CD25+, CD127dim/− cells) percentage | ↓ IFNγ, TNFα but ↑ IL-10 and IL-6 secretion in co-culture with PB-MNCs | ||||
| hBM-MSCs | ↓ T-cell effector functions and Th1 cytokine (IFN-γ, TNF-α, and IL-2) production | ↑ B7H1 and B7DC (ligands for PD1) expression | Chinnadurai et al[36], 2014 | ||
| IFN-γ and TNF-α | hBM-MSCs | ↓ T cell proliferation | ↑ IDO activity | François et al[55], 2012 | |
| ↑ Monocyte differentiation into M2 (IL-10-secreting CD206+) immunosuppressive macrophages | |||||
| ↓ CD3/CD28-induced T-cell proliferation | ↓ Potency to trigger increased IFN-γ and IL-2 synthesis by activated T cells | Cuerquis et al[59], 2014 | |||
| TNF-α/IFN-γ and IL-β | rBM-MSCs | ↓ Proliferation of syngeneic lymphocytes | ↑ NO production | Murphy et al[80], 2019 | |
| IL-17 | hBM-MSCs | ↓ PHA-stimulated T-cell proliferation | ↑ IL-6 gene expression | Sivanathan et al[94], 2015 | |
| ↓ Expression of CD25 on CD4+ effector T cells | Treg-mediated IL-2 deprivation | ||||
| ↓ T cell effector function and Th1 cytokines (IFN-γ, TNF-α, IL-2) secretion | |||||
| ↑ Formation of Tregs (CD4+ CD25 high CD127 low FoxP3+) | Cell-contacts and ↑ PGE2 and TGF-β expression | ||||
| IFN-γ, TNF-α and IL-17 | mBM-MSC | ↓ T-cell proliferation | ↑ iNOS expression | Han et al [95], 2014 | |
| IFN-γ, TNF-α and IL-6 | hAT-MSC | ↓ Proliferation of PHA or MLR activated PB-MNCs | ↑ IDO expression | Crop et al[111], 2010 | |
| Alarmins | IL-1α | hBM-MSCs | ↓ IL-6, TNF-α and ↑ IL-10 secretion in LPS-activated mouse microglial BV2 cells | ↑ G-CSF secretion | Redondo-Castro et al[78], 2017 |
| HMGB1 | hBM-MSC | Unaffected inhibition of Con A-induced lymphocyte proliferation | / | Meng et al[118], 2008 |
Symbols: ↑ and ↓ represent stimulatory or inhibitory activity of priming factor, respectively. IFN-γ: Interferon gamma; TNF-α: Tumor Necrosis Factor alpha; IL: Interleukin; HMGB1: High mobility group box 1; MSCs: Mesenchymal stromal/stem cells; h: Human; WJ: Warthon jelly’s; BM: Bone marrow; r:rat; AT: Adipose tissue; Th: T helper; PHA: Phytohaemagglutinin; MLR: Mixed lymphocyte reaction; LPS: Lipopolysaccharide; Con A: Concanavalin A; CXCL: C-X-C motif chemokine ligand; ICAM-1: Intercellular adhesion molecule-1; VCAM-1: Vascular cell adhesion molecule 1; IDO: Indoleamine-pyrrole 2,3-dioxygenase; HLA-G5: Human leukocyte antigen-G molecules; PB-MNCs: Peripheral blood mononuclear cells; B7H1: B7 homolog 1; PD1: Programmed cell death protein 1; NO: Nitric oxide; PGE2: Prostaglandin E₂; TGF-β: Transforming growth factor beta; iNOS: Inducible NO synthase; G-CSF: Granulocyte - colony stimulating factor.
Another mechanism underlying the inhibitory effects of IFN-γ-primed BM-MSCs on T-cell effector functions is the upregulation of programmed death-ligand 1 (referred to as PD-L1)[36] (Table 1). However, in the context of potential MSCs’ therapeutic use, the findings from several studies which have demonstrated that MSCs priming with IFN-γ led to upregulation of class I and class II human leukocyte antigen (referred to as HLA) molecules should be considered, as they indicated more immunogenic MSCs profile that is linked to a higher susceptibility to host immune cells recognition[37,38]. Noteworthy, a recent study found that priming with IFN-γ did not increase HLA class II expression on senescent BM-MSCs but upregulated this molecule on early passage BM-MSCs, suggesting that IFN-γ priming effects can also be influenced by cell aging[39].
Interestingly, in vivo experiments have demonstrated that IFN-γ affects the therapeutic efficacy of MSCs in a dose-dependent manner. Namely, when low concentrations of IFN-γ were used for murine BM-MSCs priming, the therapeutic effects of MSCs on experimental autoimmune encephalomyelitis in mice were completely inhibited, as demonstrated by the increased secretion of pro-inflammatory chemokine CCL2 and higher expression of MHC molecules class I and II[38]. BM-MSCs primed with higher concentrations of IFN-γ prior to use in murine models of colitis reportedly increase MSC therapeutic efficacy, as demonstrated by the significantly attenuated development and/or reduced symptoms of colitis[40]. These effects were found to be related to the increased migration of IFN-γ-primed MSCs along with their enhanced capacity to inhibit Th1 inflammatory responses, all of which contributed to decreased mucosal damage. In addition, Polchert et al[41] showed that IFN-γ-primed mouse BM-MSCs suppress graft vs host disease more efficiently than non-primed MSCs depending on the magnitude of IFN-γ exposure (Figure 2). By contrast, a recent study showed that infusion of thawed IFN-γ-primed human MSCs failed to improve retinal damage in a murine model of retinal disease[42].
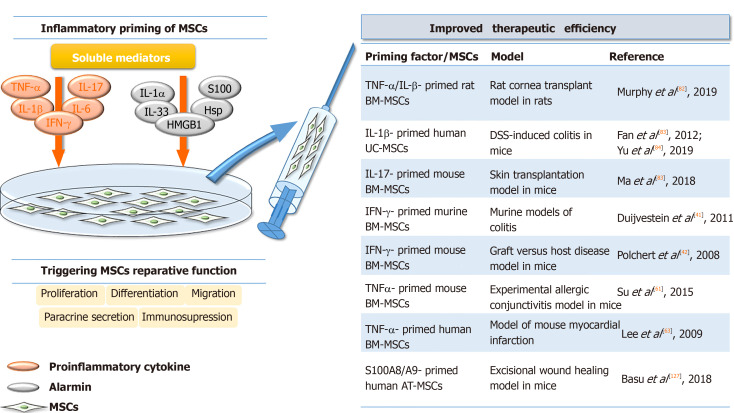
Figure 2.
Improved in vivo therapeutic potential of inflammatory primed mesenchymal stromal/stem cells. The inflammatory niche mediators, including proinflammatory cytokines and alarmins, play a key role in triggering the reparative functions of mesenchymal stromal/stem cells, influencing their paracrine secretion, proliferation, differentiation, migration and immunomodulatory potential. When applied in vivo, the improved therapeutic efficiency of inflammatory primed MSCs has been detected in various experimental models. The numbers in square brackets indicate the references. TNF-α: Tumor Necrosis Factor alpha; IL: Interleukin; IFN-γ: Interferon gamma; HMGB1: High mobility group box 1; Hsp: Heat shock proteins; BM: Bone marrow; MSCs: Mesenchymal stromal/stem cells; UC: Umbilical cord; AT: Adipose tissue; DSS: Dextran sulfate sodium.
TNF-α priming: TNF-α is a pleiotropic cytokine involved in systemic inflammation, which also affects the metabolism, growth, and differentiation potential of various cell types. Regarding the regenerative potential of TNF-α-treated MSCs, it has been determined that this cytokine promotes the proliferation of human synovial MSCs and BM-MSCs[43,44], while not affecting their clonogenic potential. Moreover, the involvement of the nuclear factor-kappa B (NF-κB) signaling pathway is implicated in TNF-α-stimulated invasion and proliferation of BM-MSCs[44] (Figure 1). Furthermore, the preconditioning of MSCs with TNF-α differentially regulates their chondrogenic differentiation depending on the tissue source and donor age (Figure 1). Namely, while TNF-α does not affect the chondrogenic capacity of human synovial MSCs and BM-MSCs[43,45], Wheiling et al[46] revealed that it inhibited the chondrogenesis of human BM-MSCs isolated from elderly donors. In addition, several studies have indicated that TNF-α alters the osteogenic differentiation of MSCs in dose-, tissue source-, and species-specific manners (Figure 1). Regarding rodent MSCs, several studies have found that TNF-α inhibits the osteogenic differentiation of MSCs[47,48], while continuous delivery of TNF-α stimulates the osteogenic differentiation of rat BM-MSCs seeded onto three-dimensional biodegradable scaffolds[49]. The enhanced osteogenic potential has also been evidenced for human BM-MSCs treated with TNF-α[50,51]. However, it dually affects dental pulp stem cells, promoting their osteogenic differentiation through the Wnt/β-catenin signaling pathway, while suppressing the osteogenesis of these cells at high concentrations[52,53].
In the context of the stronger immunomodulatory capacity of TNF-α-primed MSCs, studies have demonstrated increased secretion of the immunosuppressive molecules PGE2 and IDO, chemokine IL-8, CXCL5, CXCL6, and certain growth factors such as hepatocyte growth factor, insulin-like growth factor 1, and vascular endothelial growth factor (VEGF)[54-57] (Table 1). Moreover, it has been revealed that TNF-α priming of rat UC-MSCs suppresses the inflammatory milieu by increasing TGF-β and IL-10 expression[58]. Because the immunosuppressive effects of TNF-α are less pronounced compared to IFN-γ priming in WJ-MSCs[28], several studies have investigated the combined effects of TNF-α and IFN-γ on the immunomodulatory potential of MSCs. Indeed, when human BM-MSCs were subjected to combined pretreatment with TNF-α plus IFN-γ, more effective inhibition of CD3/CD28-induced T-cell proliferation was observed compared to non-primed MSCs[59] (Table 1). Also, combined TNF-α and IFN-γ preconditioning was shown to increase IDO activity in BM-MSCs, resulting in monocyte differentiation into M2 immunosuppressive macrophages, which further inhibited T-cell proliferation via IL-10 secretion[55].
Regarding the beneficial effects of MSC preconditioning with TNF-α, an in vivo study performed by Su et al[60] showed that the culture medium of TNFα-primed mouse BM-MSCs reduced experimental allergic conjunctivitis through multiple cyclooxygenase-2-dependent antiallergic mechanisms. In addition, the culture medium of TNF-primed human AT-MSCs has been shown to accelerate cutaneous wound closure, angiogenesis, and infiltration of immune cells in a rat excisional wound model via IL-6 and IL-8 secretion[61]. Also, in a model of mouse myocardial infarction, improved cardiac function related to decreased inflammatory responses and reduced infarct size has been documented in animals receiving TNF-α-primed human BM-MSCs[62]. By contrast, TNF priming reverses the immunosuppressive effects of mouse MSCs on T-cell proliferation, resulting in the failure of MSC treatment of murine collagen-induced arthritis[63].
IL-1β priming: IL-1β is the key mediator of inflammatory responses, which contributes to the host-defense response facilitating activation of innate immune cells[64]. Regarding the effects of IL-1β priming on MSCs proliferation and differentiation, heterogeneous results have been reported (Figure 1). Namely, while IL-1β preconditioning increases equine AT-MSCs and human synovium-derived MSCs proliferation[65,66], exposure of UC-MSCs to IL-1β results in suppressed proliferation[67]. Moreover, functional analyses of human BM-MSCs have revealed that treatment with IL-1β does not affect the proliferation of these cells, and promotes their migration and adhesion to extracellular matrix components[68]. Also, a study by Guo et al[69] demonstrated that IL-1β promoted UC-MSCs transendothelial migration through the CXCR3-CXCL9 axis, indicating the beneficial effects on MSC homing to target sites.
Many studies have described the modulatory effects of IL-1β stimulation on the osteogenic differentiation of MSCs, with conflicting results depending on the MSC tissue origin as well as IL-1β concentration (Figure 1). Sonomoto et al[70] demonstrated the ability of IL-1β to induce the osteogenic differentiation of human BM-MSCs via the Wnt pathway. Increased osteogenesis has also been found for equine AT-MSCs and human UC-MSCs treated with IL-1β[65,67]. On the other hand, depending on the concentration, IL-1β exerts dual effects on the osteogenesis of periodontal ligament-MSCs, since low doses of IL-1β promote osteogenesis by activating the bone morphogenetic protein (referred to as BMP)/Smad signaling pathway, while higher doses of the cytokine impede osteogenesis[71]. IL-1β treatment also inhibits the chondrogenesis of MSCs from the femoral intramedullary canal in a dose-dependent manner via NF-κB activation[46]. In accordance with these findings, decreased chondrogenic differentiation has been reported for BM-MSCs and synovial fluid-MSCs treated with IL-1β[72-75]. However, Hingert et al[76] showed that pretreatment of BM-MSCs with IL-1β followed by BMP-3 stimulation in a three-dimensional in vitro hydrogel model resulted in high proteoglycan accumulation and SRY-box transcription factor 9 expression, suggesting that IL-1β may be the causative factor.
Several studies have indicated changes in the secretory profile of MSCs primed with IL-1β, as well as the significance of IL-1β priming in combination with other factors. Regarding gingival-MSCs, IL-1β preconditioning induces the expression of TGF-β and matrix metalloproteinase agonists[77], while in human BM-MSCs, IL-1β increases granulocyte colony-stimulating factor (referred to as G-CSF)[78], IL-6, VEGF, CXCL1, and CCL2 chemokines[79]. The immunosuppressive activities of rat BM-MSCs were shown to be significantly promoted after preconditioning with TNF-α or IFN-γ in combination with IL-β, as the decreased proliferation of syngeneic lymphocytes in vitro was demonstrated[80] (Table 1). These effects were confirmed by in vivo experiments in a rat cornea transplant model, where after transplantation of syngeneic MSCs primed with TNF-α/IL-β enhanced graft survival (up to 70%) was observed compared to unprimed MSCs (up to 50%). In addition, the increased number of Tregs and reduced expression of pro-inflammatory cytokines in the draining lymph node of these animals were found, whereas there was an increased number of regulatory monocyte/macrophage cells and Tregs in the lungs and spleen[80].
Administration of IL-β-primed human UC-MSCs in mice with dextran sulfate sodium-induced colitis also increases the number of Tregs and Th2 cells, while reducing Th1 and Th17 cells in the spleen and mesenteric lymph nodes[81]. A recent study by Yu et al[82] emphasized the role of PGE2 and IDO induction in the observed immunosuppressive effects of umbilical cord blood-MSCs primed with IL-1β and IFN-γ in the same disease model. Moreover, another recent study showed that the culture medium of IL-1β-primed AT-MSCs increased the phagocytic capacity of neutrophils, which may contribute to inflammation resolution, removal of tissue debris, and support of tissue repair in joint pathology[83]. Overall, the results indicating the immunosuppressive phenotype of IL-1β-primed MSCs strongly suggest that this cytokine might promote the therapeutic efficacy of MSCs in disorders related to an exaggerated immune response (Figure 2).
IL-17 priming: IL-17 is another pro-inflammatory cytokine that plays a pivotal role in linking the immune and hematopoietic systems, while also contributing to the pathogenesis of numerous autoimmune and inflammatory diseases. However, the effects that this cytokine exerts on MSCs are still not fully understood. To date, it has been shown that IL-17 stimulates the proliferation of mouse and human BM-MSCs, as well as the migration of human BM-MSCs and trans-endothelial migration of peripheral blood MSCs[84-86] (Figure 1). Regarding the differentiation potential, published results have shown that IL-17 priming enhances osteogenic[84,87-89], but inhibits chondrogenic[90] and adipogenic[88,91] differentiation in human BM-MSCs. Moreover, IL-17 can decrease the osteogenic differentiation of periodontal ligament-MSCs through extracellular signal-regulated protein kinases 1 and 2 (referred to as ERK1/2), and c-Jun N-terminal kinase mitogen-activated protein kinases[92]. Research related to the effects of IL-17 on the differentiation potential of mouse BM-MSCs has led to conflicting results, as one study found that IL-17 did not affect the differentiation potential of MSCs towards osteoblasts[85], whereas another showed suppressed osteogenic differentiation of these cells mediated by IκB kinase and NF-κ B[93].
Regarding the immunomodulatory activity of MSCs, IL-17 priming enhances the immunosuppressive features of MSCs. While IL-17 has no impact on MSC markers and the low immunogenic phenotype of human BM-MSCs, IL-17-primed MSCs suppress T-cell proliferation and inhibit CD25 expression and expression of Th1 cytokines, including IFN-γ, TNF-α, and IL-2[94]. Moreover, a study showed that mouse MSCs pretreated with IFN-γ and TNF-α in combination with IL-17 significantly reduced T-cell proliferation via the inducible nitric oxide synthase (referred to as iNOS) pathway[95] (Table 1). The same study confirmed the immunosuppressive activity of BM-MSCs primed with IL-17 in vivo in a mouse model of concanavalin A-induced liver injury. However, another study showed that IL-17 significantly reduced the suppressive capacity of olfactory ecto-MSCs on CD4+ T cells, mainly through the downregulation of suppressive factors (PD-L1, iNOS, IL-10, and TGF-β)[96]. The positive effect of IL-17 on the immunomodulatory features of MSCs has been confirmed in in vivo studies with different animal models. Namely, in a study in which mouse BM-MSCs were treated with IL-17 prior to their use in ischemia-reperfusion acute kidney injury, a significant decrease in IL-6, TNF-α, and IFN-γ levels and higher spleen and kidney Treg levels were shown compared to mice that received non-primed MSCs[97]. In another work, IL-17-primed mouse BM-MSCs used in a skin transplantation model were found to increase the Treg subpopulation as well as IL-10 and TGF-β levels, significantly prolonging graft survival[98].
IL-6 priming: Pleiotropic effects on immune regulation, hematopoiesis, and tissue regeneration are exerted by another inflammatory cytokine, IL-6[99]. Preconditioning MSCs with IL-6 has been shown to influence their behaviors in different manners depending on the tissue origin of the MSCs (Figure 1). Namely, a few studies have reported conflicting data showing that IL-6 has no effect on the proliferation of human AT-MSCs and mouse BM-MSCs[100,101], whereas in other studies, IL-6 increased the proliferation of human placenta-derived MSCs and BM-MSCs[102,103]. Moreover, the stimulating effect of IL-6 on BM-MSCs growth and in vitro wound healing is mediated by ERK1/2 activation[103]. It has also been reported that IL-6 differentially influences stem cell differentiation (Figure 1). Priming with IL-6 under osteogenic induction conditions has been shown to enhance mineralization and alkaline phosphatase expression in human BM-MSCs, AT-MSCs, and stem cells from human exfoliated deciduous teeth (called SHEDs)[104-107]. However, studies using lower concentrations of IL-6 have shown no effect on osteogenic differentiation potency of human BM-MSCs[103]. Although IL-6 inhibits the chondrogenic differentiation of human BM-MSCs when added during differentiation induction[103], concomitant supplementation with IL-6 and soluble IL-6 receptor contributes to the enhanced chondrogenesis of this type of MSC[108]. Treatment with IL-6 during or prior to adipogenic differentiation induction reduces the adipogenesis capacity of human BM-MSCs[103], while other studies have reported no or positive effects of IL-6 on the adipogenic ability of human BM-MSCs, AT-MSCs, and SHEDs[104,109,110].
Even less is known about the immunomodulatory potential of IL-6-preconditioned MSCs. In this context, few studies have analyzed IL-6 effects in combination with other pro-inflammatory cytokines. Namely, altered immunological status has been reported for AT-MSCs primed with a combination of IFN-γ, TNF-α, and IL-6 (Table 1), as shown by the upregulated expression of HLA class I and class II, as well as CD40[111], indicating a potentially more immunogenic phenotype. In addition, although the same priming conditions have no effect on AT-MSCs differentiation capacity, they enhance their immunosuppressive activity mainly through increased IDO expression. Another study demonstrated that human AT-MSCs and BM-MSCs primed with another combination of pro-inflammatory cytokines, IL-1, IL-6, and IL-23, exerted increased differentiation potential towards osteogenic and adipogenic lineages, while their morphology, immunophenotype (except upregulated CD45), and costimulatory molecule expression were similar to non-primed cells. Also, primed MSCs showed increased TGF-β and decreased IL-4 production, while their suppressive effect on T-cell proliferation was comparable to controls[112] Although these findings suggest that priming with IL-6 might promote the therapeutic efficacy of MSCs in the treatment of various inflammatory and autoimmune disorders, no study investigating the in vivo therapeutic potential of MSCs preconditioned with IL-6 (alone or combined with other cytokines) has been performed to date.
MSCs priming with alarmins
Alarmins are constitutively expressed inside cells and exert various functions under physiological conditions. Upon tissue damage induced by pathogens or physical/chemical injuries, dying necrotic cells passively and rapidly release alarmins outside of cells to promote the immune response and support tissue repair[4,5,6]. In addition to these activities, alarmins are involved in many other processes, such as cellular homeostasis, wound healing, and tumor development[6,7]. The best-characterized alarmins, such as IL-1α, IL-33, HMGB1, S100 proteins, and Hsps, will be discussed here in the context of their effects on MSC biology and potential role in tissue repair.
IL-1α and IL-33 are both dual-function cytokines that are localized in the nucleus under homeostatic conditions, where they function as transcription factors. Data on their extracellular effects on MSCs are very elusive. It has been demonstrated that IL-1α stimulates the expression of trophic factor G-CSF via IL-1 receptor type 1 signaling in human BM-MSCs. In addition, the conditioned medium of IL-1α-primed BM-MSCs was shown to inhibit the secretion of inflammatory and apoptotic markers in lipopolysaccharide-activated mouse microglial BV2 cells and increase secretion of the anti-inflammatory IL-10 cytokine[78], suggesting that MSCs priming with IL-1α favors their immunosuppressive activities (Table 1). Another recent study showed that IL-1α decreased the proliferative and adipogenic differentiation capacity of AT-MSCs, whereby adipogenesis was inhibited predominantly during the early phase of differentiation via NF-κB and ERK1/2 pathways with subsequent stimulation of pro-inflammatory cytokines, such as IL-8, IL-6, CCL2, and IL-1β, during adipogenic differentiation of AT-MSCs[113] (Figure 1). Since the effects of IL-1α are conspicuous at the beginning of the differentiation process, it is important to further examine IL-1α priming in the context of MSC differentiation. It was recently demonstrated that without changing MSC marker expression[114,115], IL-33 treatment has the potential to modify the regenerative[114] and immunomodulatory characteristics of MSCs[115]. Our recent study demonstrated that IL-33 treatment reduced periodontal ligament-MSCs and dental pulp MSCs osteogenesis but supported their proliferation, clonogenicity, and stemness (Figure 1). Both MSC types primed with IL-33 maintained their differentiation capacity, while increased alkaline phosphatase activity was also observed, indicating that IL-33 may contribute to the preservation of the dental stem cell pool[114]. Research conducted by Terraza et al[115] demonstrated that IL-33 with IFN-γ stimulated the high expression of IL-6, TGF-β, and iNOS in mouse BM-MSCs. Despite the scarce data on IL-1α and IL-33 priming of MSCs, overall data indicate that preconditioning with these molecules should be additionally explored as an MSC priming strategy.
Another nuclear alarmin is HMGB1, a non-histone DNA-binding protein involved in the maintenance of the chromatin structure and gene expression regulation[116]. The knowledge on the effects of released HMGB1 on MSCs functions is still contradictory, as its stimulatory[117] as well as inhibitory[118,119] actions on MSCs proliferation have been reported. The promoted migratory capacity of MSCs primed with HMGB1 has also been demonstrated[117-119], indicating its beneficial effects for MSC functional adjustment in therapeutic use. In the presence of HMGB1, the osteogenic differentiation of MSCs is also induced[118,120,121] (Figure 1). Moreover, HMGB1 stimulates the secretion of various cytokines by MSCs including macrophage CSF, eotaxin-3, epidermal growth factor receptor, VEGF, angiopoietin-2, CCL-5, urokinase plasminogen activator receptor, and macrophage migration inhibitory factor, which may be associated with the induced osteogenic differentiation under HMGB1 influence[121]. Furthermore, in rat BM-MSCs, along with promoted MSC migration, HMGB1 stimulates VEGF-induced differentiation to endothelial cells but decreases their proliferation and platelet-derived growth factor-induced differentiation to smooth muscle cells[119]. These findings indicate that HMGB1 priming could be a significant factor in tissue engineering for MSC-guided differentiation. Regarding immunomodulatory functions, it has been reported that HMGB1 priming has no effect on BM-MSCs ability to inhibit the proliferation of concanavalin A-stimulated lymphocytes in vitro[118] (Table 1), but additional research is needed to confirm these functions.
Unlike IL-1α, IL-33 and HMGB1 alarmins, S100 proteins, and Hsps are located in the cytoplasm during homeostasis[4]. The effect of extracellular S100A6 has been investigated on MSCs derived from WJ of the UC, and the results have shown the ability of this molecule to increase cellular adhesion and reduce their proliferation capacity by interacting with integrin β1[122] (Figure 1). Regardless, pretreatment of human AT-MSCs with S100A8/A9 and their subsequent application to wounds induced in C57BL/6 mice significantly improve wound healing due to transcriptome expression profile changes related to the enhanced protective MSCs phenotype[123]. Regarding the Hsp protein family, it has been demonstrated that Hsp90 increases viability and protects rat BM-MSCs against apoptosis, simultaneously increasing the paracrine effect of MSCs[124]. Another study showed that Hsp90α promotes rat MSCs migration, possibly mediated by the increased secretion of MMPs, SDF-1/CXCR4, and vascular cell adhesion protein 1[125] (Figure 1). Interestingly, the dual effects of Hsp70 have been demonstrated depending on the age of the MSCs. Namely, in a study by Andreeva et al[126], Hsp70 increased the growth of aged but not young mouse AT-MSCs (Figure 1), suggesting the potential beneficial effects of Hsp70 priming. Moreover, the important role of Hsp70 in the osteogenesis of human MSCs is demonstrated by increased alkaline phosphatase activity and MSC mineralization[127,128].
CONCLUSION
As MSCs are crucial cellular components for tissue repair, it is essential to understand how the inflammatory microenvironment modulates the functionality of these cells. The beneficial effects of MSCs have been demonstrated, but due to the large heterogeneity detected within MSC populations, the success of their application in clinical trials has been limited. Moreover, it is believed that the inflammatory niche is indispensable for triggering MSC activity in an appropriate manner. Therefore, preconditioning methods have been applied to enhance and/or adjust MSCs functionality, including their regenerative and immunomodulatory status. To date, studies of the MSCs response to soluble factors featuring the inflammatory niche have been mostly focused on the effects provoked during their presence, pointing to the necessity of further exploring the durability of these changes. In this work, we collected data on the therapeutic potential of MSCs treated with pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, IL-17 and IL-6) and alarmins (IL-1α, IL-33, HMGB1, S100 proteins, and Hsps) that are predominantly released at the site of the damaged tissue.
The reviewed data strongly indicate that all aforementioned factors possess the ability to modify the regenerative and immunomodulatory activities of MSCs, and the effects of these factors depend on the MSC tissue and species origin, as well as on donor age and cellular aging (senescence) status. In addition, different effects have been reported depending on the priming factor concentration and their selected combinations, as well as on the disease model, indicating that all of these aspects together should be carefully considered in relation to specific application requirements. Importantly, the effects of primed MSCs have been demonstrated in various animal wound and disease models, suggesting the validity of priming approaches for MSC therapy. Indeed, priming MSCs with certain inflammatory factors, such as TNF-α, IL-β, IFN-γ or S100A8/A9, contribute to the suppression of graft vs host disease and colitis, as well as to improved corneal and skin graft survival, mediated by their dominant immunosuppressive activity (Figure 2). Together, the data summarized in this paper provide a significant starting point for future research on priming MSCs and set future directions for establishing standardized methods for the application of preconditioned MSCs in cell therapy.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest for this article.
Manuscript source: Invited manuscript
Peer-review started: April 14, 2020
First decision: April 29, 2020
Article in press: September 1, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farahzadi R S-Editor: Liu M L-Editor: A P-Editor: Xing YX
Contributor Information
Aleksandra Jauković, Laboratory for Experimental Hematology and Stem Cells, Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia. aleksandra@imi.bg.ac.rs.
Tamara Kukolj, Laboratory for Experimental Hematology and Stem Cells, Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia.
Hristina Obradović, Laboratory for Experimental Hematology and Stem Cells, Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia.
Ivana Okić-Đorđević, Laboratory for Experimental Hematology and Stem Cells, Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia.
Slavko Mojsilović, Laboratory for Experimental Hematology and Stem Cells, Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia.
Diana Bugarski, Laboratory for Experimental Hematology and Stem Cells, Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia.
References
- 1.Hall SW, Cooke A. Autoimmunity and inflammation: murine models and translational studies. Mamm Genome. 2011;22:377–389. doi: 10.1007/s00335-011-9338-2. [DOI] [PubMed] [Google Scholar]
- 2.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 3.Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 4.Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: Feel the Stress. J Immunol. 2017;198:1395–1402. doi: 10.4049/jimmunol.1601342. [DOI] [PubMed] [Google Scholar]
- 6.Kapurniotu A, Gokce O, Bernhagen J. The Multitasking Potential of Alarmins and Atypical Chemokines. Front Med (Lausanne) 2019;6:3. doi: 10.3389/fmed.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang, Han Z, Oppenheim JJ. Alarmins and immunity. Immunol Rev. 2017;280:41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 9.Klimczak A, Kozlowska U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor Cells: Their Role in Tissue Homeostasis. Stem Cells Int. 2016;2016:4285215. doi: 10.1155/2016/4285215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JA. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology. 2015;71:181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 14.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, Cossu G, Serafini M, Sampaolesi M, Tagliafico E, Tenedini E, Saggio I, Robey PG, Riminucci M, Bianco P. No Identical "Mesenchymal Stem Cells" at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Reports. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017;6:1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 19.Fayyad-Kazan M, Fayyad-Kazan H, Lagneaux L, Najar M. The potential of mesenchymal stromal cells in immunotherapy. Immunotherapy. 2016;8:839–842. doi: 10.2217/imt-2016-0037. [DOI] [PubMed] [Google Scholar]
- 20.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 21.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espagnolle N, Balguerie A, Arnaud E, Sensebé L, Varin A. CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells. Stem Cell Reports. 2017;8:961–976. doi: 10.1016/j.stemcr.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018;2018:3057624. doi: 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noronha NC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:131. doi: 10.1186/s13287-019-1224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. doi: 10.1371/journal.pone.0009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najar M, Raicevic G, Fayyad-Kazan H, De Bruyn C, Bron D, Toungouz M, Lagneaux L. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev Rep. 2012;8:1188–1198. doi: 10.1007/s12015-012-9408-1. [DOI] [PubMed] [Google Scholar]
- 30.Sui BD, Hu CH, Liu AQ, Zheng CX, Xuan K, Jin Y. Stem cell-based bone regeneration in diseased microenvironments: Challenges and solutions. Biomaterials. 2019;196:18–30. doi: 10.1016/j.biomaterials.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 31.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, Dominici M, Fibbe WE, Gee AP, Gimble JM, Hematti P, Koh MB, LeBlanc K, Martin I, McNiece IK, Mendicino M, Oh S, Ortiz L, Phinney DG, Planat V, Shi Y, Stroncek DF, Viswanathan S, Weiss DJ, Sensebe L. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Croitoru-Lamoury J, Lamoury FM, Caristo M, Suzuki K, Walker D, Takikawa O, Taylor R, Brew BJ. Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO) PLoS One. 2011;6:e14698. doi: 10.1371/journal.pone.0014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Yang Q, Wang Z, Tong H, Ma L, Zhang Y, Shan F, Meng Y, Yuan Z. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton's jelly as sources of cell immunomodulatory therapy. Hum Vaccin Immunother. 2016;12:85–96. doi: 10.1080/21645515.2015.1030549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol. 2014;192:1491–1501. doi: 10.4049/jimmunol.1301828. [DOI] [PubMed] [Google Scholar]
- 37.Noone C, Kihm A, English K, O'Dea S, Mahon BP. IFN-γ stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. 2013;22:3003–3014. doi: 10.1089/scd.2013.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafei M, Birman E, Forner K, Galipeau J. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009;17:1799–1803. doi: 10.1038/mt.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinnadurai R, Rajan D, Ng S, McCullough K, Arafat D, Waller EK, Anderson LJ, Gibson G, Galipeau J. Immune dysfunctionality of replicative senescent mesenchymal stromal cells is corrected by IFNγ priming. Blood Adv. 2017;1:628–643. doi: 10.1182/bloodadvances.2017006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]
- 41.Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft vs host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burand AJ, Gramlich OW, Brown AJ, Ankrum JA. Function of Cryopreserved Mesenchymal Stromal Cells With and Without Interferon-γ Prelicensing is Context Dependent. Stem Cells. 2017;35:1437–1439. doi: 10.1002/stem.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shioda M, Muneta T, Tsuji K, Mizuno M, Komori K, Koga H, Sekiya I. TNFα promotes proliferation of human synovial MSCs while maintaining chondrogenic potential. PLoS One. 2017;12:e0177771. doi: 10.1371/journal.pone.0177771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Böcker W, Docheva D, Prall WC, Egea V, Pappou E, Rossmann O, Popov C, Mutschler W, Ries C, Schieker M. IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. J Mol Med (Berl) 2008;86:1183–1192. doi: 10.1007/s00109-008-0378-3. [DOI] [PubMed] [Google Scholar]
- 45.Jagielski M, Wolf J, Marzahn U, Völker A, Lemke M, Meier C, Ertel W, Godkin O, Arens S, Schulze-Tanzil G. The influence of IL-10 and TNFα on chondrogenesis of human mesenchymal stromal cells in three-dimensional cultures. Int J Mol Sci. 2014;15:15821–15844. doi: 10.3390/ijms150915821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Müller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60:801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009;17:735–742. doi: 10.1016/j.joca.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS, Nanes MS. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 49.Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-alpha on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010;31:1666–1675. doi: 10.1016/j.biomaterials.2009.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croes M, Oner FC, Kruyt MC, Blokhuis TJ, Bastian O, Dhert WJ, Alblas J. Proinflammatory Mediators Enhance the Osteogenesis of Human Mesenchymal Stem Cells after Lineage Commitment. PLoS One. 2015;10:e0132781. doi: 10.1371/journal.pone.0132781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45:367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 52.Qin Z, Fang Z, Zhao L, Chen J, Li Y, Liu G. High dose of TNF-α suppressed osteogenic differentiation of human dental pulp stem cells by activating the Wnt/β-catenin signaling. J Mol Histol. 2015;46:409–420. doi: 10.1007/s10735-015-9630-7. [DOI] [PubMed] [Google Scholar]
- 53.Feng G, Zheng K, Song D, Xu K, Huang D, Zhang Y, Cao P, Shen S, Zhang J, Feng X, Zhang D. SIRT1 was involved in TNF-α-promoted osteogenic differentiation of human DPSCs through Wnt/β-catenin signal. In Vitro Cell Dev Biol Anim. 2016;52:1001–1011. doi: 10.1007/s11626-016-0070-9. [DOI] [PubMed] [Google Scholar]
- 54.Lee MJ, Kim J, Kim MY, Bae YS, Ryu SH, Lee TG, Kim JH. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J Proteome Res. 2010;9:1754–1762. doi: 10.1021/pr900898n. [DOI] [PubMed] [Google Scholar]
- 55.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 56.Zhang A, Wang Y, Ye Z, Xie H, Zhou L, Zheng S. Mechanism of TNF-α-induced migration and hepatocyte growth factor production in human mesenchymal stem cells. J Cell Biochem. 2010;111:469–475. doi: 10.1002/jcb.22729. [DOI] [PubMed] [Google Scholar]
- 57.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291:R880–R884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 58.Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NAC, Rosdiana I, Munir D. The Role of TNF-α induced MSCs on Suppressive Inflammation by Increasing TGF-β and IL-10. Open Access Maced J Med Sci. 2018;6:1779–1783. doi: 10.3889/oamjms.2018.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuerquis J, Romieu-Mourez R, François M, Routy JP, Young YK, Zhao J, Eliopoulos N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-γ and tumor necrosis factor-α stimulation. Cytotherapy. 2014;16:191–202. doi: 10.1016/j.jcyt.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Su W, Wan Q, Huang J, Han L, Chen X, Chen G, Olsen N, Zheng SG, Liang D. Culture medium from TNF-α-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136:423–32.e8. doi: 10.1016/j.jaci.2014.12.1926. [DOI] [PubMed] [Google Scholar]
- 61.Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131:1559–1567. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 62.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noël D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 64.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brandt L, Schubert S, Scheibe P, Brehm W, Franzen J, Gross C, Burk J. Tenogenic Properties of Mesenchymal Progenitor Cells Are Compromised in an Inflammatory Environment. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumura E, Tsuji K, Komori K, Koga H, Sekiya I, Muneta T. Pretreatment with IL-1β enhances proliferation and chondrogenic potential of synovium-derived mesenchymal stem cells. Cytotherapy. 2017;19:181–193. doi: 10.1016/j.jcyt.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Yang C, Chen Y, Li F, You M, Zhong L, Li W, Zhang B, Chen Q. The biological changes of umbilical cord mesenchymal stem cells in inflammatory environment induced by different cytokines. Mol Cell Biochem. 2018;446:171–184. doi: 10.1007/s11010-018-3284-1. [DOI] [PubMed] [Google Scholar]
- 68.Carrero R, Cerrada I, Lledó E, Dopazo J, García-García F, Rubio MP, Trigueros C, Dorronsoro A, Ruiz-Sauri A, Montero JA, Sepúlveda P. IL1β induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-κB. Stem Cell Rev Rep. 2012;8:905–916. doi: 10.1007/s12015-012-9364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo YC, Chiu YH, Chen CP, Wang HS. Interleukin-1β induces CXCR3-mediated chemotaxis to promote umbilical cord mesenchymal stem cell transendothelial migration. Stem Cell Res Ther. 2018;9:281. doi: 10.1186/s13287-018-1032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sonomoto K, Yamaoka K, Oshita K, Fukuyo S, Zhang X, Nakano K, Okada Y, Tanaka Y. Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 2012;64:3355–3363. doi: 10.1002/art.34555. [DOI] [PubMed] [Google Scholar]
- 71.Mao CY, Wang YG, Zhang X, Zheng XY, Tang TT, Lu EY. Double-edged-sword effect of IL-1β on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-κB, MAPK and BMP/Smad signaling pathways. Cell Death Dis. 2016;7:e2296. doi: 10.1038/cddis.2016.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Felka T, Schäfer R, Schewe B, Benz K, Aicher WK. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009;17:1368–1376. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 73.Gao B, Gao W, Wu Z, Zhou T, Qiu X, Wang X, Lian C, Peng Y, Liang A, Qiu J, Zhu Y, Xu C, Li Y, Su P, Huang D. Melatonin rescued interleukin 1β-impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res Ther. 2018;9:162. doi: 10.1186/s13287-018-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 75.Liu W, Sun Y, He Y, Zhang H, Zheng Y, Yao Y, Zhang Z. IL-1β impedes the chondrogenic differentiation of synovial fluid mesenchymal stem cells in the human temporomandibular joint. Int J Mol Med. 2017;39:317–326. doi: 10.3892/ijmm.2016.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hingert D, Barreto Henriksson H, Brisby H. Human Mesenchymal Stem Cells Pretreated with Interleukin-1β and Stimulated with Bone Morphogenetic Growth Factor-3 Enhance Chondrogenesis. Tissue Eng Part A. 2018;24:775–785. doi: 10.1089/ten.TEA.2017.0087. [DOI] [PubMed] [Google Scholar]
- 77.Magne B, Dedier M, Nivet M, Coulomb B, Banzet S, Lataillade JJ, Trouillas M. IL-1β-Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing via Matrix Metalloproteinases and Transforming Growth Factor-β1. J Invest Dermatol. 2020;140:688–698.e21. doi: 10.1016/j.jid.2019.07.721. [DOI] [PubMed] [Google Scholar]
- 78.Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8:79. doi: 10.1186/s13287-017-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amann EM, Groß A, Rojewski MT, Kestler HA, Kalbitz M, Brenner RE, Huber-Lang M, Schrezenmeier H. Inflammatory response of mesenchymal stromal cells after in vivo exposure with selected trauma-related factors and polytrauma serum. PLoS One. 2019;14:e0216862. doi: 10.1371/journal.pone.0216862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murphy N, Treacy O, Lynch K, Morcos M, Lohan P, Howard L, Fahy G, Griffin MD, Ryan AE, Ritter T. TNF-α/IL-1β-licensed mesenchymal stromal cells promote corneal allograft survival via myeloid cell-mediated induction of Foxp3+ regulatory T cells in the lung. FASEB J. 2019;33:9404–9421. doi: 10.1096/fj.201900047R. [DOI] [PubMed] [Google Scholar]
- 81.Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu Y, Yoo SM, Park HH, Baek SY, Kim YJ, Lee S, Kim YL, Seo KW, Kang KS. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J Tissue Eng Regen Med. 2019;13:1792–1804. doi: 10.1002/term.2930. [DOI] [PubMed] [Google Scholar]
- 83.van Dalen SCM, Blom AB, Walgreen B, Slöetjes AW, Helsen MMA, Geven EJW, Ter Huurne M, Vogl T, Roth J, van de Loo FAJ, Koenders MI, Casteilla L, van der Kraan PM, van den Bosch MHJ, van Lent PLEM. IL-1β-Mediated Activation of Adipose-Derived Mesenchymal Stromal Cells Results in PMN Reallocation and Enhanced Phagocytosis: A Possible Mechanism for the Reduction of Osteoarthritis Pathology. Front Immunol. 2019;10:1075. doi: 10.3389/fimmu.2019.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, Lee Y, Kim HH. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16:1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 85.Mojsilović S, Krstić A, Ilić V, Okić Djordjević I I, Kocić J, Trivanović D, Santibañez JF, JovčićG, Bugarski D. IL-17 and FGF signaling involved in mouse mesenchymal stem cell proliferation. Cell Tissue Res. 2011;346:305–316. doi: 10.1007/s00441-011-1284-5. [DOI] [PubMed] [Google Scholar]
- 86.Krstić J, Obradović H, Jauković A, Okić Djordjević I, Trivanović D, Kukolj T, Mojsilović S, Ilić V, Santibañez JF, Bugarski D. Urokinase type plasminogen activator mediates Interleukin-17-induced peripheral blood mesenchymal stem cell motility and transendothelial migration. Biochim Biophys Acta. 2015;1853:431–444. doi: 10.1016/j.bbamcr.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 87.Croes M, Öner FC, van Neerven D, Sabir E, Kruyt MC, Blokhuis TJ, Dhert WJA, Alblas J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262–270. doi: 10.1016/j.bone.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 88.Noh M. Interleukin-17A increases leptin production in human bone marrow mesenchymal stem cells. Biochem Pharmacol. 2012;83:661–670. doi: 10.1016/j.bcp.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 89.Osta B, Lavocat F, Eljaafari A, Miossec P. Effects of Interleukin-17A on Osteogenic Differentiation of Isolated Human Mesenchymal Stem Cells. Front Immunol. 2014;5:425. doi: 10.3389/fimmu.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kondo M, Yamaoka K, Sonomoto K, Fukuyo S, Oshita K, Okada Y, Tanaka Y. IL-17 inhibits chondrogenic differentiation of human mesenchymal stem cells. PLoS One. 2013;8:e79463. doi: 10.1371/journal.pone.0079463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol. 2009;77:1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Djordjević IO, Kukolj T, Krstić J, Trivanović D, Obradović H, Santibañez JF, Mojsilović S, Ilić V, Bugarski D, Jauković A. The inhibition of periodontal ligament stem cells osteogenic differentiation by IL-17 is mediated via MAPKs. Int J Biochem Cell Biol. 2016;71:92–101. doi: 10.1016/j.biocel.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 93.Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN, Soo C, Al Hezaimi K, Zou W, Chen X, Mooney DJ, Wang CY. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc Natl Acad Sci USA. 2013;110:9469–9474. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sivanathan KN, Rojas-Canales DM, Hope CM, Krishnan R, Carroll RP, Gronthos S, Grey ST, Coates PT. Interleukin-17A-Induced Human Mesenchymal Stem Cells Are Superior Modulators of Immunological Function. Stem Cells. 2015;33:2850–2863. doi: 10.1002/stem.2075. [DOI] [PubMed] [Google Scholar]
- 95.Han X, Yang Q, Lin L, Xu C, Zheng C, Chen X, Han Y, Li M, Cao W, Cao K, Chen Q, Xu G, Zhang Y, Zhang J, Schneider RJ, Qian Y, Wang Y, Brewer G, Shi Y. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014;21:1758–1768. doi: 10.1038/cdd.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian J, Rui K, Tang X, Wang W, Ma J, Tian X, Wang Y, Xu H, Lu L, Wang S. IL-17 down-regulates the immunosuppressive capacity of olfactory ecto-mesenchymal stem cells in murine collagen-induced arthritis. Oncotarget. 2016;7:42953–42962. doi: 10.18632/oncotarget.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bai M, Zhang L, Fu B, Bai J, Zhang Y, Cai G, Bai X, Feng Z, Sun S, Chen X. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 2018;93:814–825. doi: 10.1016/j.kint.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 98.Ma T, Wang X, Jiao Y, Wang H, Qi Y, Gong H, Zhang L, Jiang D. Interleukin 17 (IL-17)-Induced Mesenchymal Stem Cells Prolong the Survival of Allogeneic Skin Grafts. Ann Transplant. 2018;23:615–621. doi: 10.12659/AOT.909381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 100.Wei H, Shen G, Deng X, Lou D, Sun B, Wu H, Long L, Ding T, Zhao J. The role of IL-6 in bone marrow (BM)-derived mesenchymal stem cells (MSCs) proliferation and chondrogenesis. Cell Tissue Bank. 2013;14:699–706. doi: 10.1007/s10561-012-9354-9. [DOI] [PubMed] [Google Scholar]
- 101.Song HY, Jeon ES, Jung JS, Kim JH. Oncostatin M induces proliferation of human adipose tissue-derived mesenchymal stem cells. Int J Biochem Cell Biol. 2005;37:2357–2365. doi: 10.1016/j.biocel.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 102.Li D, Wang GY, Dong BH, Zhang YC, Wang YX, Sun BC. Biological characteristics of human placental mesenchymal stem cells and their proliferative response to various cytokines. Cells Tissues Organs. 2007;186:169–179. doi: 10.1159/000105674. [DOI] [PubMed] [Google Scholar]
- 103.Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nowwarote N, Sukarawan W, Kanjana K, Pavasant P, Fournier BPJ, Osathanon T. Interleukin 6 promotes an in vitro mineral deposition by stem cells isolated from human exfoliated deciduous teeth. R Soc Open Sci. 2018;5:180864. doi: 10.1098/rsos.180864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fukuyo S, Yamaoka K, Sonomoto K, Oshita K, Okada Y, Saito K, Yoshida Y, Kanazawa T, Minami Y, Tanaka Y. IL-6-accelerated calcification by induction of ROR2 in human adipose tissue-derived mesenchymal stem cells is STAT3 dependent. Rheumatology (Oxford) 2014;53:1282–1290. doi: 10.1093/rheumatology/ket496. [DOI] [PubMed] [Google Scholar]
- 106.Xie Z, Tang S, Ye G, Wang P, Li J, Liu W, Li M, Wang S, Wu X, Cen S, Zheng G, Ma M, Wu Y, Shen H. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9:13. doi: 10.1186/s13287-017-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bastidas-Coral AP, Bakker AD, Zandieh-Doulabi B, Kleverlaan CJ, Bravenboer N, Forouzanfar T, Klein-Nulend J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem Cells Int. 2016;2016:1318256. doi: 10.1155/2016/1318256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kondo M, Yamaoka K, Sakata K, Sonomoto K, Lin L, Nakano K, Tanaka Y. Contribution of the Interleukin-6/STAT-3 Signaling Pathway to Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Arthritis Rheumatol. 2015;67:1250–1260. doi: 10.1002/art.39036. [DOI] [PubMed] [Google Scholar]
- 109.Huang RL, Sun Y, Ho CK, Liu K, Tang QQ, Xie Y, Li Q. IL-6 potentiates BMP-2-induced osteogenesis and adipogenesis via two different BMPR1A-mediated pathways. Cell Death Dis. 2018;9:144. doi: 10.1038/s41419-017-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mrácek T, Cannon B, Houstek J. IL-1 and LPS but not IL-6 inhibit differentiation and downregulate PPAR gamma in brown adipocytes. Cytokine. 2004;26:9–15. doi: 10.1016/j.cyto.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 111.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Pescatori M, Stubbs AP, van Ijcken WF, Dahlke MH, Eggenhofer E, Weimar W, Hoogduijn MJ. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin Exp Immunol. 2010;162:474–486. doi: 10.1111/j.1365-2249.2010.04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pourgholaminejad A, Aghdami N, Baharvand H, Moazzeni SM. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine. 2016;85:51–60. doi: 10.1016/j.cyto.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 113.Sun X, Zou T, Zuo C, Zhang M, Shi B, Jiang Z, Cui H, Liao X, Li X, Tang Y, Liu Y, Liu X. IL-1α inhibits proliferation and adipogenic differentiation of human adipose-derived mesenchymal stem cells through NF-κB- and ERK1/2-mediated proinflammatory cytokines. Cell Biol Int. 2018;42:794–803. doi: 10.1002/cbin.10932. [DOI] [PubMed] [Google Scholar]
- 114.Kukolj T, Trivanović D, Mojsilović S, Okić Djordjević I, Obradović H, Krstić J, Jauković A, Bugarski D. IL-33 guides osteogenesis and increases proliferation and pluripotency marker expression in dental stem cells. Cell Prolif. 2019;52:e12533. doi: 10.1111/cpr.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Terraza C, Fuentes R, Pino-Lagos K. IFN-γ and IL-33 modulate mesenchymal stem cells function targeting Th1/Th17 axis in a murine skin transplantation model. Cytokine. 2018;111:317–324. doi: 10.1016/j.cyto.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 116.Martinotti S, Patrone M, Ranzato E. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther. 2015;4:101–109. doi: 10.2147/ITT.S58064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lotfi R, Eisenbacher J, Solgi G, Fuchs K, Yildiz T, Nienhaus C, Rojewski MT, Schrezenmeier H. Human mesenchymal stem cells respond to native but not oxidized damage associated molecular pattern molecules from necrotic (tumor) material. Eur J Immunol. 2011;41:2021–2028. doi: 10.1002/eji.201041324. [DOI] [PubMed] [Google Scholar]
- 118.Meng E, Guo Z, Wang H, Jin J, Wang J, Wang H, Wu C, Wang L. High mobility group box 1 protein inhibits the proliferation of human mesenchymal stem cells and promotes their migration and differentiation along osteoblastic pathway. Stem Cells Dev. 2008;17:805–813. doi: 10.1089/scd.2007.0276. [DOI] [PubMed] [Google Scholar]
- 119.Meng X, Chen M, Su W, Tao X, Sun M, Zou X, Ying R, Wei W, Wang B. The differentiation of mesenchymal stem cells to vascular cells regulated by the HMGB1/RAGE axis: its application in cell therapy for transplant arteriosclerosis. Stem Cell Res Ther. 2018;9:85. doi: 10.1186/s13287-018-0827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin F, Zhang W, Xue D, Zhu T, Li J, Chen E, Yao X, Pan Z. Signaling pathways involved in the effects of HMGB1 on mesenchymal stem cell migration and osteoblastic differentiation. Int J Mol Med. 2016;37:789–797. doi: 10.3892/ijmm.2016.2479. [DOI] [PubMed] [Google Scholar]
- 121.Feng L, Xue D, Chen E, Zhang W, Gao X, Yu J, Feng Y, Pan Z. HMGB1 promotes the secretion of multiple cytokines and potentiates the osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Exp Ther Med. 2016;12:3941–3947. doi: 10.3892/etm.2016.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jurewicz E, Góral A, Filipek A. S100A6 is secreted from Wharton's jelly mesenchymal stem cells and interacts with integrin β1. Int J Biochem Cell Biol. 2014;55:298–303. doi: 10.1016/j.biocel.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 123.Basu A, Munir S, Mulaw MA, Singh K, Crisan D, Sindrilaru A, Treiber N, Wlaschek M, Huber-Lang M, Gebhard F, Scharffetter-Kochanek K. A Novel S100A8/A9 Induced Fingerprint of Mesenchymal Stem Cells associated with Enhanced Wound Healing. Sci Rep. 2018;8:6205. doi: 10.1038/s41598-018-24425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao F, Hu XY, Xie XJ, Xu QY, Wang YP, Liu XB, Xiang MX, Sun Y, Wang JA. Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J Zhejiang Univ Sci B. 2010;11:608–617. doi: 10.1631/jzus.B1001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao F, Hu X, Xie X, Liu X, Wang J. Heat shock protein 90 stimulates rat mesenchymal stem cell migration via PI3K/Akt and ERK1/2 pathways. Cell Biochem Biophys. 2015;71:481–489. doi: 10.1007/s12013-014-0228-6. [DOI] [PubMed] [Google Scholar]
- 126.Andreeva NV, Zatsepina OG, Garbuz DG, Evgen'ev MB, Belyavsky AV. Recombinant HSP70 and mild heat shock stimulate growth of aged mesenchymal stem cells. Cell Stress Chaperones. 2016;21:727–733. doi: 10.1007/s12192-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen E, Xue D, Zhang W, Lin F, Pan Z. Extracellular heat shock protein 70 promotes osteogenesis of human mesenchymal stem cells through activation of the ERK signaling pathway. FEBS Lett. 2015;589:4088–4096. doi: 10.1016/j.febslet.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 128.Li C, Sunderic K, Nicoll SB, Wang S. Downregulation of Heat Shock Protein 70 Impairs Osteogenic and Chondrogenic Differentiation in Human Mesenchymal Stem Cells. Sci Rep. 2018;8:553. doi: 10.1038/s41598-017-18541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]