Abstract
Several studies have investigated associations between overweight/obesity and risk of developing rheumatoid arthritis, however, the evidence is not entirely consistent, and previous meta-analyses mainly included case–control studies, which can be affected by various biases. We therefore conducted a systematic review and meta-analysis of cohort studies on adiposity and risk of rheumatoid arthritis. Relevant studies were identified by searching PubMed and Embase databases. Random effects models were used to estimate summary relative risks (RRs) and 95% confidence intervals (CIs) for rheumatoid arthritis in relation to different measures of adiposity. Thirteen cohort studies (10 publications) were included. The summary RR per 5 kg/m2 increase in body mass index (BMI) was 1.11 (95% CI 1.05–1.18, I2 = 50%), but the association was restricted to women (1.15, 95% CI 1.08–1.21, I2 = 17%) and not observed in men (0.89, 95% CI 0.73–1.09, I2 = 58%). The summary RR per 5 kg/m2 increment in BMI at age 18 years was 1.17 (95% CI 1.01–1.36, I2 = 26%, n = 3), and per 10 cm increase in waist circumference was 1.13 (95% CI 1.02–1.25, I2 = 44%, n = 2). Higher BMI in middle age, BMI at age 18 years, and waist circumference were associated with increased rheumatoid arthritis risk, suggesting adiposity could be targeted for primary prevention.
Subject terms: Rheumatology, Risk factors
Introduction
Rheumatoid arthritis is a systemic autoimmune disease characterized by an inflamed synovium which can cause pain, swelling, stiffness and deformity of multiple joints1,2. Globally an estimated 23.7 million people live with rheumatoid arthritis3. There is wide geographic variation in the prevalence rates of rheumatoid arthritis between countries, with rates ranging from 0.2 to 1.1% between regions, and higher rates in high-income countries compared to low- and middle-income countries4,5. For example, prevalence rates of 0–3 cases per 1,000 persons have been reported in some areas in Africa, while rates in Northern and Western Europe are around 4–11 cases per 1,000 inhabitants, and in North America between 9 and 11 cases per 1,000 inhabitants4. These geographical differences in the occurrence of rheumatoid arthritis may be attributed to various factors, including genetic factors, socioeconomic factors, access to health services, and lifestyle factors6.
Established or suspected risk factors for rheumatoid arthritis include age7, genetic predisposition (human leukocyte antigen (HLA)-DR4)8, smoking9,10, diet10–12, physical activity13, and obesity14–24. In addition, the global prevalence of rheumatoid arthritis has been reported to be approximately two times higher in women than in men25. These sex differences suggest that hormone-related factors or other sex-specific exposures may contribute to the development of rheumatoid arthritis26. Since body fat distribution differs for men and women, it is also possible that the differences in adiposity between men and women might, at least in part, account for the sex difference in rates of rheumatoid arthritis27.
Several epidemiological studies have suggested an increased risk of rheumatoid arthritis associated with overweight and obesity14–19, but other studies found no clear association20,21. Although several meta-analyses have found positive associations between body mass index (BMI) and risk of rheumatoid arthritis22–24, they were mainly based on case–control studies which could be affected by recall and selection biases and reverse causation7. In addition, these meta-analyses only examined BMI and did not consider other measures of adiposity which might be more pertinent for rheumatoid arthritis. A few studies have suggested positive associations for BMI in early adulthood with rheumatoid arthritis15, and other studies suggested that abdominal fatness may also be important18,19, however, these results have not previously been summarised in a meta-analysis. Clarifying whether abdominal adiposity is more relevant for rheumatoid arthritis than general adiposity could contribute towards a better understanding of the underlying mechanisms and more targeted interventions for prevention and treatment. Therefore, to provide a more comprehensive assessment, we conducted an updated systematic review and meta-analysis of cohort studies to clarify the strength and shape of the association between different measures of adiposity and the risk of developing rheumatoid arthritis.
Methods
Search strategy
An electronic literature search was conducted in Embase and PubMed databases to identify all eligible studies on the association between adiposity and the risk of rheumatoid arthritis that were published up to May 10th, 2019. The following search strategy was used in PubMed and a similar search was adapted for the Embase search: (“body mass index” or body mass index[MeSH] or BMI or overweight or overweight[MeSH] or obesity or obesity[MeSH] or anthropometry or anthropometry[MeSH] or fatness or “body fatness” or “abdominal fatness” or “abdominal obesity” or abdominal obesity[MeSH] or “waist circumference” or "hip circumference" or “waist-to-hip ratio” or waist-to-hip ratio[MeSH] or adiposity or adiposity[MeSH] or "weight gain" or weight gain[MeSH] or "weight change" or weight change[MeSH] or “weight loss” or weight loss[MeSH]) AND (“rheumatoid arthritis” or rheumatoid arthritis[MeSH]) AND ("case–control" or cohort or prospective or longitudinal or retrospective or "follow-up" or "cross-sectional" or "hazard ratio" or "hazard ratios" or "relative risk" or "relative risks" or "incidence rate ratio" or "incidence rate ratios" or "odds ratio" or "odds ratios" or incidence).
Study selection
Records identified from the search strategy were screened based on the title and abstract in the first step, and then further assessed for eligibility based on the full text in the second step. Prospective and retrospective cohort studies, case-cohort studies, and nested case–control studies within cohort studies, which reported adjusted relative risk (RR) estimates (RRs, hazard ratios (HRs), incidence rate ratios, odds ratios (ORs)) and 95% confidence intervals (CIs) for rheumatoid arthritis for at least three or more categories of any measure of adiposity (e.g. BMI, waist circumference) or a risk estimate on a continuous scale were eligible for inclusion in this meta-analysis. Grey literature such as conference abstracts were excluded because they are unlikely to contain all the information required for dose–response analyses and for evaluation of study quality. Reviews, letters, comments, editorials, meta-analyses, meta-synthesis, prediction models, protocols, as well as epidemiological studies in patient populations were excluded. If there were duplicate publications from the same study population, the publication with the largest number of cases or with the most detailed information needed for dose–response meta-analyses was included. The initial screening of all references based on abstracts and titles was done by one author (TO) and a second author (DA) independently did the second part of the screening in duplicate.
Data extraction
The following data were extracted from each publication: name of the first author, publication year, country, study name or description, study period, follow-up duration, number of participants or controls, number of cases, measurement of anthropometry (e.g. measured, self-reported), exposure (e.g. BMI, waist circumference), comparison (the contrast or metric of the exposure), RR, OR, HR and 95% CI, and adjustment for covariates. If several multivariable models were reported in the paper, the effect estimate most fully adjusted for potential confounders was used for the meta-analysis.
Study quality assessment
Study quality of the included studies was assessed using the Newcastle–Ottawa scale28. Each study was appraised in terms of methodological quality categories: selection, comparability and outcome, with a total allowable score of up to 9 stars.
Statistical analyses
Random effects models were used to calculate summary RRs and 95% CIs per 5 kg/m2 increase in BMI and per 10 cm increase in waist circumference29. The average of the natural logarithm of the RRs was estimated and the RR from each study was weighted using random effects weights29. Given the low prevalence of rheumatoid arthritis and the moderate effect sizes observed, different effect measures (ORs, HRs) were not converted, but used directly. If studies reported results by sex or other subgroups, but not overall, the subgroup-specific RR estimates were pooled with a fixed effects model before inclusion in the overall meta-analysis. To explore the dose–response relationship between increasing levels of adiposity and the risk of developing rheumatoid arthritis, linear and nonlinear dose–response analyses were conducted. For the linear dose–response analyses, the method of Greenland and Longnecker was used30,31. Study-specific linear dose–response slopes were calculated in a logit-linear model using the estimates across at least three categories of adiposity or by using continuous estimates directly. Fractional polynomial models were used to investigate potential nonlinear associations32. The extent of heterogeneity was assessed using the I2 statistic which describes the percentage of variability across studies in a meta-analysis, and I2 values of approximately 25%, 50% and 75% indicated low, moderate and high heterogeneity, respectively33. Publication bias was assessed with funnel plots7,34, the Egger linear regression test35, and the Begg rank correlation test36. If there was evidence of publication bias as indicated by p < 0.10 for Egger’s or Begg’s test or by asymmetry in the funnel plot, a sensitivity analysis was conducted to evaluate the potential impact of publication bias on the summary estimates by excluding outlying studies in the funnel plot from the analysis. To ensure that the results were not driven by one large study or a study with an extreme result, sensitivity analyses were conducted sequentially excluding each study from the analysis and assessing its impact on the summary estimate. Subgroup analyses were conducted to evaluate whether the results differed according to the following characteristics: sex, number of cases (< 250, 250–499, ≥ 500), geographic location (Europe, North America), measurement of BMI (measured, self-reported (validated), self-reported (not validated)), duration of follow-up (< 5 years, 5–9.9 years, 10–14.9 years 15–19.9 years, ≥ 20 years) and the extent of adjustment for confounding factors including age, education, social class, smoking, alcohol intake, physical activity, diabetes, and other comorbidities. All statistical analyses were performed using Stata/IC 13.1 (StataCorp LP, Texas, US).
Results
Study selection
Figure 1 shows the flow diagram of study selection. A total of 5,269 records were identified from the Embase (3,823 records) and PubMed (1,446 records) databases by using index search terms. Based on screening the titles and abstracts, 171 potentially relevant articles were identified and evaluated for eligibility by assessing their full-texts. Ten articles including a total of 13 studies were included in the meta-analysis of adiposity and risk of rheumatoid arthritis.
Figure 1.
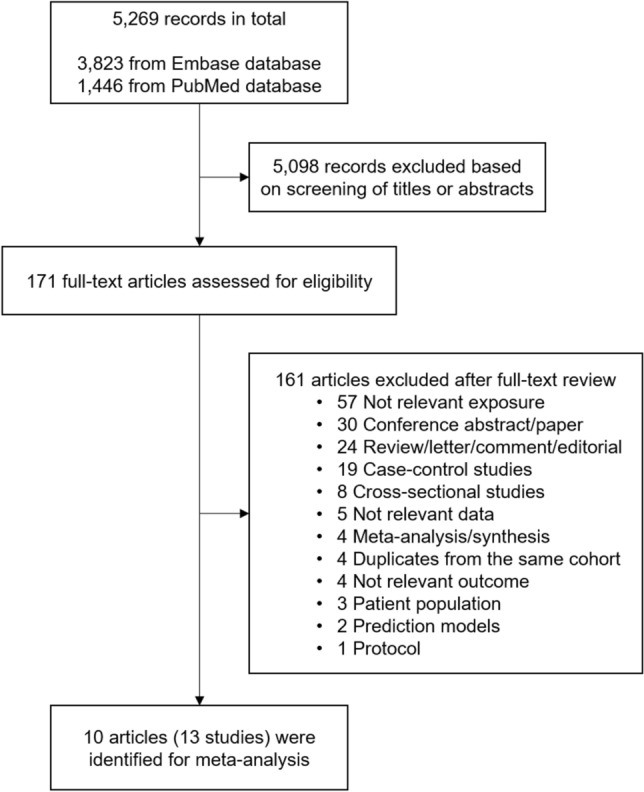
Flow diagram of study selection for the meta-analysis.
Study characteristics
The characteristics of included studies are summarised in Table 1. Among a total of 13 included studies, 8 studies were cohort studies15–18,20,37,38 and 5 studies (3 publications) were nested case–control studies within cohort studies19,21,39. Three publications included data from two studies each15,19,39, and one of these provided estimates from two different studies combined only19. While 8 studies (6 publications) included both men and women17–19,21,38,39, one study included men only37 and 4 studies (3 publications) were restricted to women only15,16,20. Eight studies (6 publications) performed anthropometric measures through a clinical examination17–19,37–39, while information on measures of adiposity was self-reported by participants in 4 studies (3 publications)15,16,20. In a nested case–control study using the UK General Practice Research Database (GPRD) conducted by Rodríguez et al.21, BMI was obtained from “physician-completed patient records”. To clarify this, we contacted the corresponding author of the study, as well as the Clinical Practice Research Datalink, who confirmed that although there is no way to be certain, it is reasonable to assume the anthropometric measurements were likely to have been performed by a physician or nurse at GP practices. Three studies (two publications, two risk estimates) reported results on waist circumference18,19, and three studies (two publications) reported on BMI in early adulthood (at age 18 years)15,20. Only one study reported on waist-to-hip ratio20, and body fat percentage18, and no studies reported results for hip circumference, thus analyses of these exposures were not possible. Most studies adjusted for potential confounders such as age, sex, smoking, and alcohol consumption. The average study quality score using the Newcastle–Ottawa scale22,28 was 8, with seven studies scoring 9 stars17–19,37,39, one study scoring 8 stars21, four studies scoring 7 stars15,16,38, and one study scoring 6 stars20 (Supplementary Table 1).
Table 1.
Characteristics of included studies for the meta-analysis.
| First author, publication year, country (ref. no) | Study name | Study period, follow-up duration | Number of participants, age, number of cases | Measurement of anthropometry | Exposure | Comparison | Relative risk, odds ratio or hazard ratio (95% CI) | Adjustment for covariates |
|---|---|---|---|---|---|---|---|---|
| Heliövaara M, 1993, Finland37 | Social Insurance Institution's Mobile Clinic Health Examination Survey | 1966/1972–1989, 18.1 years | 28,364 men ≥ 15 years 119 cases | Measured | BMI |
< 25.0 kg/m2 25.0–29.9 ≥ 30.0 |
1.0 0.8 (0.5–1.2) 0.4 (0.2–1.2) |
Age, smoking, geographical region, type of population, marital status, social class, perceived health |
| Cerhan JR, 2002, USA20 | Iowa Women’s Health Study | 1986–1997, 10.7 years |
31,336 women 55–69 years 158 cases |
Self-reported (validated) | BMI at baseline |
< 23.4 kg/m2 23.4–25.8 25.9–29.2 > 29.2 |
1.00 0.88 (0.56–1.37) 0.99 (0.64–1.53) 1.01 (0.65–1.56) |
Age |
| Waist-to-hip ratio at baseline |
< 0.773 0.773–0.825 0.826–0.886 > 0.886 |
1.00 1.01 (0.67–1.54) 0.78 (0.49–1.22) 0.86 (0.55–1.34) |
||||||
| BMI at age 18 |
< 19.6 kg/m2 19.6–21.1 21.2–22.9 > 22.9 |
1.00 1.16 (0.76–1.78) 0.87 (0.55–1.37) 0.97 (0.62–1.51) |
||||||
| BMI at age 30 |
< 21.2 kg/m2 21.2–22.6 22.7–24.6 > 24.6 |
1.00 1.09 (0.71–1.69) 0.84 (0.53–1.33) 1.10 (0.71–1.69) |
||||||
| BMI at age 50 |
< 22.7 kg/m2 22.7–24.8 24.9–27.5 > 27.5 |
1.00 0.90 (0.57–1.42) 1.15 (0.75–1.77) 0.96 (0.61–1.50) |
||||||
| Rodríguez LAG, 2009, UK21 | UK General Practice Research Database (GPRD) | 1996–1997, ~ 2 years |
Nested case–control study: 456 cases, 3,366 matched controls 20–79 years |
Physician-completed patient records (Assumed to be measured) | BMI |
< 20.0 kg/m2 20.0–24.9 25.0–30.0 > 30.0 |
0.65 (0.43–0.98) 1.00 1.18 (0.94–1.98) 0.95 (0.68–1.34) |
Age, sex, calendar year, number of referrals, and visits to a primary care physician in the previous year, smoking, alcohol intake, diabetes, cardiovascular disorders, other comorbidities, and pregnancy |
| Lu B, 2014, USA15 | Nurses’ Health Study (NHS) | 1976–2008, 25.2 years |
109,896 women 30–55 years 826 cases |
Self-reported (validated) | BMI |
18.5–24.9 kg/m2 25.0–29.9 ≥ 30.0 |
1.00 1.16 (0.99–1.35) 1.12 (0.92–1.37) |
Age, community median income, smoking, alcohol use, and physical activity |
| Cumulative average BMI |
18.5–24.9 kg/m2 25.0–29.9 ≥ 30.0 |
1.00 1.17 (1.00–1.37) 1.21 (0.97–1.50) |
||||||
| BMI at age 18 |
18.5–19.9 kg/m2 20.0–22.9 23.0–24.9 ≥ 25.0 |
1.00 0.95 (0.80–1.12) 1.18 (0.93–1.48) 1.26 (0.99–1.61) |
||||||
| Lu B, 2014, USA15 | Nurses’ Health Study II (NHSII) | 1989–2009, 17.8 years |
108,727 women 25–42 years 355 cases |
Self-reported (validated) | BMI |
18.5–24.9 kg/m2 25.0–29.9 ≥ 30.0 |
1.00 1.68 (1.30–2.17) 1.72 (1.31–2.45) |
Age, community median income, smoking, alcohol use, and physical activity |
| Cumulative average BMI |
18.5–24.9 kg/m2 25.0–29.9 ≥ 30.0 |
1.00 1.38 (1.08–1.77) 1.53 (1.16–2.01) |
||||||
| BMI at age 18 |
18.5–19.9 kg/m2 20.0–22.9 23.0–24.9 ≥ 25.0 |
1.00 1.27 (0.99–1.62) 1.10 (0.76–1.61) 1.53 (1.10–2.14) |
||||||
| Pahau H, 2014, Norway38 | Nord-Trøndelag Health Study (HUNT) | 1995/1997–2006/2008, ~ 11 years |
31,568 participants ≥ 20 years 739 cases |
Measured | BMI | Per 1 kg/m2 increase | 1.04 (1.02–1.06) | Sex, age, current smoker, former smoker, hypertension, diabetes, previous cardiovascular disease |
| Harpsøe MC, 2014, Denmark16 | Danish National Birth Cohort (DNBC) |
1996/2002–2011, 11.4 years |
75,008 pregnant women median age 30.2 years 315 cases | Self-reported (not validated) | BMI |
< 18.5 kg/m2 18.5–24.9 25.0–29.9 ≥ 30.0 |
0.82 (0.45–1.50) 1.00 1.12 (0.85–1.49) 1.53 (1.07–2.18) |
Smoking, alcohol, parity, socio-occupational status |
| Lahiri M, 2014, UK17 | European Prospective Investigation of Cancer-Norfolk |
1993/1997–2010, 14.2 years |
25,409 participants 40–79 years 138 cases | Measured | BMI |
< 25.0 kg/m2 25.0–29.9 ≥ 30.0 |
1.00 1.16 (0.78–1.74) 1.49 (0.91–2.42) |
Age, gender, smoking, alcohol, occupational class, education, diabetes mellitus, parity, breast feeding |
| Ljung L, 2016, Sweden19 | The Västerbotten Intervention Programme (VIP) and the Northern Sweden Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) project |
1986/2013-NA, NA |
Nested case–control study: 554 cases, 1,650 matched controls median age 51.9 years |
Measured | BMI, all | < 25.0 kg/m2 | 1.00 | Education and smoking, in case–control sets matched for age, sex and year of examination |
| 25.0–29.99 | 1.21 (0.96–1.52) | |||||||
| > = 30.0 | 1.45 (1.07–1.95) | |||||||
| Per 5 kg/m2 increase | 1.13 (1.00–1.28) | |||||||
| Waist circumference (men/women) |
≤ 102/ ≤ 88 cm > 102/ > 88 Per 1 cm increase |
1.00 1.40 (0.92–2.14) 1.02 (1.01–1.04) |
||||||
| Turesson C, 2016, Sweden39 | The Malmö Diet and Cancer Study (MDCS) |
1991/1996–2004, 10.5 years |
Nested case–control study: 36/135 cases (men/ women), 144/537 matched controls mean age 58.5/57.9 years |
Measured | BMI, men |
18.5–25.0 kg/m2 25.0–30.0 > 30.0 Per SD |
1.00 0.44 (0.15–1.29) 0.14 (0.01–1.44) 0.58 (0.33–1.04) |
Smoking, level of formal education and alcohol consumption, in case–control sets matched for sex and year of birth |
| BMI, women |
18.5–25.0 kg/m2 25.0–30.0 > 30.0 Per SD |
1.00 0.96 (0.57–1.61) 0.96 (0.48–1.92) 1.08 (0.86–1.36) |
||||||
| Turesson C, 2016, Sweden39 | The Malmö Preventive Medicine Program (MPMP) |
1974/1992–2004, 21 years |
Nested case–control study: 147/133 (men/ women) cases, 599/539 matched controls mean age 45.5/49.3 years |
Measured | BMI, men |
18.5–25.0 kg/m2 25.0–30.0 > 30.0 Per SD |
1.00 0.75 (0.46–1.17) 0.64 (0.20–2.02) 0.66 (0.41–1.07) |
Smoking, level of formal education and alcohol consumption, in case–control sets matched for sex and year of birth |
| BMI, women |
18.5–25.0 25.0–30.0 > 30.0 Per SD |
1.00 1.67 (0.94–2.69) 0.90 (0.36–2.26) 1.02 (0.78–1.33) |
||||||
| Linauskas A, 2019, Denmark18 | The Danish Diet, Cancer, and Health cohort |
1993/1997–2016, 20.1 years |
26,317 men and 28,720 women 50–64 years 210/456 (men/women) cases |
Measured | BMI, men |
18.5–24.99 25.0–29.99 ≥ 30.0 Per 1 kg/m2 increase |
1.00 0.87 (0.65–1.18) 1.04 (0.69–1.57) 1.01 (0.98–1.05) |
Age, smoking status and duration, socioeconomic status (education level), alcohol consumption, physical activity, and total intake of n − 3 fatty acids |
| Waist circumference, men | Per 5 cm increase | 1.03 (0.96–1.10) | ||||||
| Fat %, men | Per 5% increase | 1.03 (0.90–1.17) | ||||||
| BMI, women |
< 18.5 kg/m2 18.5–24.99 25.0–29.99 ≥ 30.0 Per 1 kg/m2 increase |
0.58 (0.19–1.83) 1.00 1.20 (0.98–1.48) 1.40 (1.08–1.83) 1.03 (1.01–1.05) |
||||||
| Waist circumference, women | Per 5 cm increase | 1.05 (1.01–1.09) | ||||||
| Fat %, women | Per 5% increase | 1.08 (1.01–1.16) |
BMI body mass index, CI confidence interval, NA not available.
Body mass index and risk of rheumatoid arthritis
Thirteen studies (ten publications, twelve risk estimates)15–21,37–39, involving a total of 473,641 participants and 4,777 cases were included in the linear dose–response meta-analysis of BMI and rheumatoid arthritis, and the summary RR per 5 kg/m2 increase in BMI was 1.11 (95% CI 1.05–1.18, I2 = 50%; Fig. 2A). The funnel plot (Supplementary Fig. 1) and Begg's test (P = 0.09) showed some suggestion of publication bias, but not Egger's test (P = 0.14). When excluding one outlying study (Heliövaara et al.37), the summary RR per 5 kg/m2 increase in BMI was 1.14 (95% CI 1.08–1.19), the heterogeneity was reduced (I2 = 26%, P = 0.20; Supplementary Fig. 2), and the P values for Begg’s test and Egger’s test were 0.21 and 0.37, respectively. In sensitivity analyses excluding one study at a time from the meta-analysis, the summary RR per 5 kg/m2 increase in BMI ranged from 1.10 (95% CI 1.04–1.16, I2 = 38%) when the NHSII study by Lu et al.15 was excluded, to 1.14 (95% CI 1.08–1.19, I2 = 26%) when the study by Heliövaara et al.37 was excluded (Supplementary Fig. 3). Twelve studies (nine publications, 442,073 participants and 4,038 cases) were included in the nonlinear dose–response analysis, which revealed evidence of a positive dose–response relationship and no indication of a nonlinear association (Pnonlinearity = 0.56) (Fig. 2B).
Figure 2.
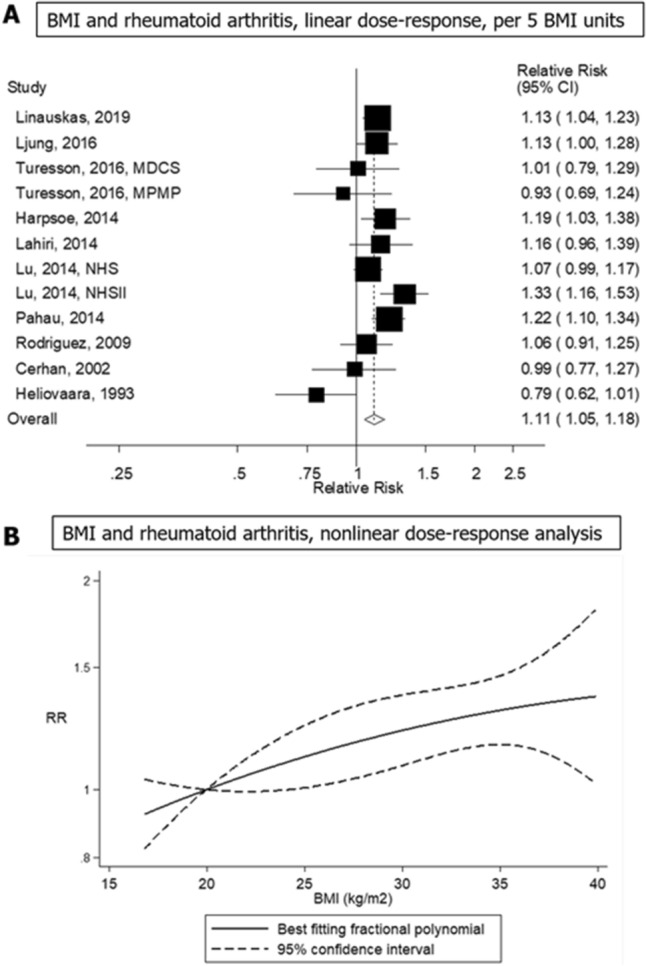
Linear and non-linear dose–response meta-analysis of body mass index and risk of rheumatoid arthritis, per 5 kg/m2.
Body mass index in early adulthood and risk of rheumatoid arthritis
Three cohort studies (two publications, 249,959 participants and 1,263 cases)15,20 were included in the analysis of BMI in early adulthood (at age 18 years) and risk of developing rheumatoid arthritis. The summary RR per 5 kg/m2 increase in BMI in early adulthood (at age 18 years) was 1.17 (95% CI 1.01–1.36, I2 = 26%; Fig. 3A). There was no evidence of publication bias by inspection of the funnel plot (Supplementary Fig. 4), or with Egger's test (P = 0.99) or Begg’s test (P = 0.57), although the number of studies was limited. There was no evidence of a nonlinear association between BMI in early adulthood (at age 18 years) and the risk of rheumatoid arthritis (Pnonlinearity = 0.99) (Fig. 3B).
Figure 3.
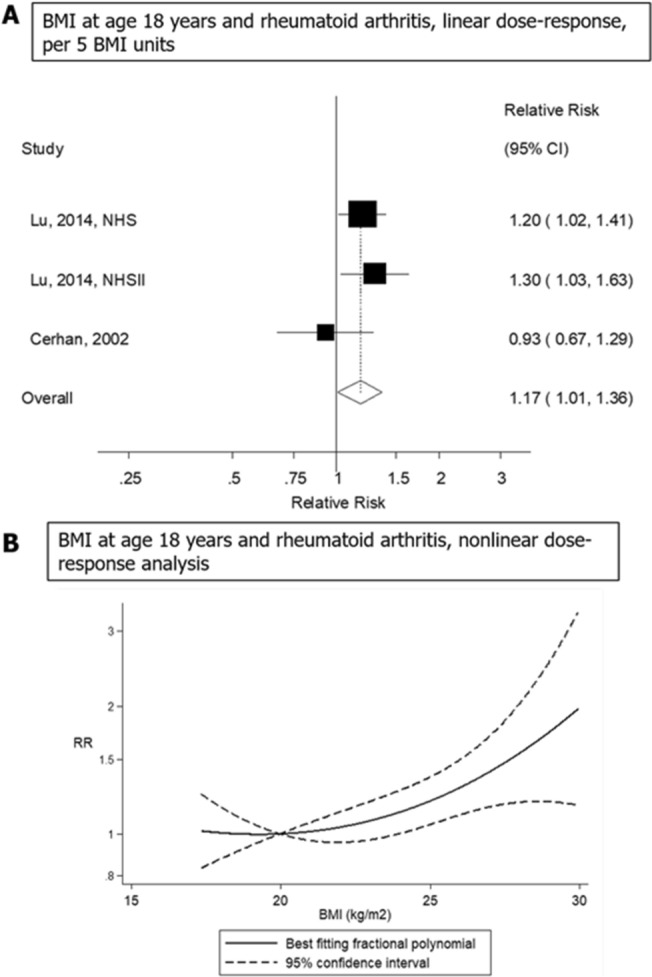
Linear and non-linear dose–response meta-analysis of body mass index in early adulthood (at age 18 years) and risk of rheumatoid arthritis, per 5 kg/m2.
Waist circumference and risk of rheumatoid arthritis
Three prospective studies (two publications, two risk estimates, 55,584 participants and 804 cases)18,19 were included in the meta-analysis of waist circumference and risk of rheumatoid arthritis. The summary RR per 10 cm increase in waist circumference was 1.13 (95% CI 1.02–1.25, I2 = 44%; Fig. 4). Nonlinear dose–response analyses were not possible because both publications reported results on a continuous scale.
Figure 4.
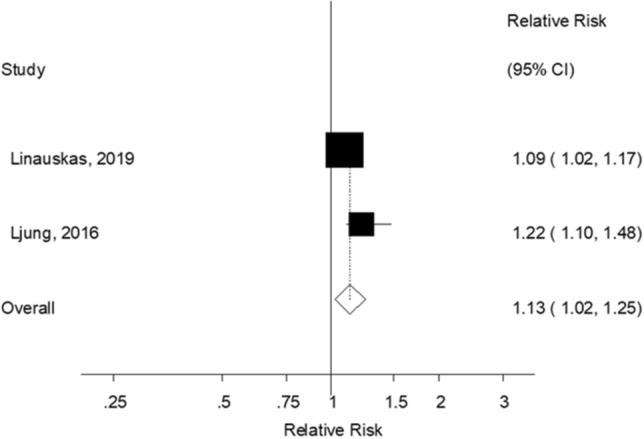
Linear dose–response meta-analysis of waist circumference and risk of rheumatoid arthritis, per 10 cm.
Subgroup analyses
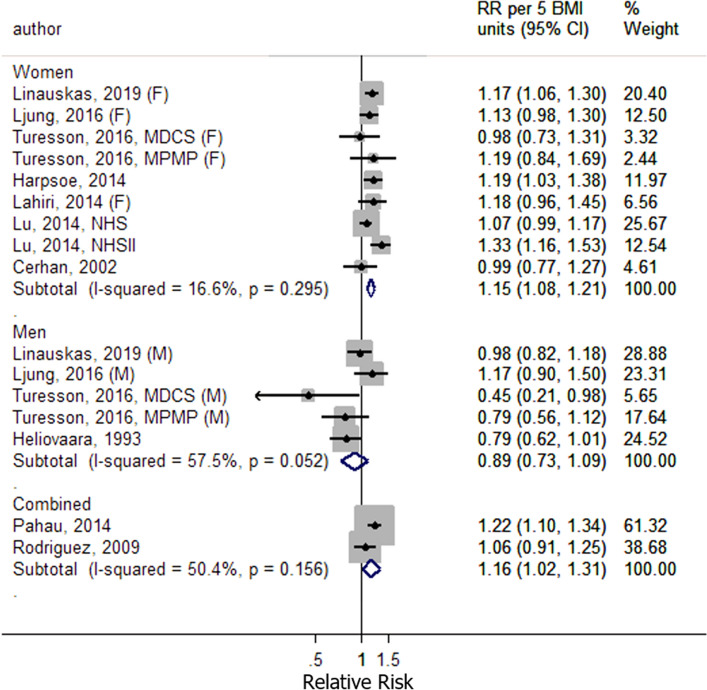
To investigate potential heterogeneity in the results, subgroup analyses were conducted stratified by sex, geographic location, number of cases, BMI measurement, duration of follow-up and adjustment for confounding factors. There was evidence of heterogeneity by sex (P = 0.02), with a positive association observed in women 1.15 (95% CI 1.08–1.21, I2 = 17%, Fig. 5), but not in men 0.89 (95% CI 0.73–1.09, I2 = 58%; Fig. 5) (Table 2). The remaining subgroup analyses showed little evidence of heterogeneity between subgroups (Table 2).
Figure 5.
Sex-specific subgroup analyses for the linear dose–response meta-analysis of body mass index and risk of rheumatoid arthritis.
Table 2.
Subgroup analyses for the linear dose–response meta-analysis of body mass index and risk of rheumatoid arthritis.
| Study groups | n | RR | (95% CI) | I2 (%) | P value for heterogeneity | |
|---|---|---|---|---|---|---|
| Within each subgroup | Between subgroups | |||||
| All studies | 12 | 1.11 | (1.05–1.18) | 50.2 | 0.002 | |
| Sex | 0.06 | |||||
| Men | 5 | 0.89 | (0.73–1.09) | 57.5 | 0.05 | |
| Women | 9 | 1.15 | (1.08–1.21) | 16.6 | 0.30 | |
| Combined | 2 | 1.16 | (1.02–1.31) | 50.4 | 0.16 | |
| Geographic location | 0.65 | |||||
| Europe | 9 | 1.10 | (1.03–1.18) | 44.7 | 0.07 | |
| North America | 3 | 1.14 | (0.96–1.34) | 73.8 | 0.02 | |
| Number of cases | 0.22 | |||||
| < 250 | 4 | 0.99 | (0.84–1.16) | 50.0 | 0.11 | |
| 250–499 | 4 | 1.15 | (1.01–1.31) | 58.2 | 0.07 | |
| ≥ 500 | 4 | 1.13 | (1.08–1.19) | 16.3 | 0.31 | |
| BMI measurement | 0.41 | |||||
| Measured | 8 | 1.09 | (1.01–1.18) | 49.5 | 0.05 | |
| Self-reported (validated) | 3 | 1.14 | (0.96–1.34) | 73.8 | 0.02 | |
| Self-reported (not validated) | 1 | 1.19 | (1.03–1.38) | – | – | |
| Duration of follow-up | 0.99 | |||||
| < 5 years | 1 | 1.06 | (0.91–1.25) | – | – | |
| 5–9.9 years | 1 | 1.13 | (1.00–1.28) | – | – | |
| 10–14.9 years | 4 | 1.14 | (1.04–1.26) | 19.2 | 0.29 | |
| 15–19.9 years | 2 | 1.04 | (0.62–1.72) | 92.6 | < 0.0001 | |
| ≥ 20 years | 4 | 1.11 | (1.05–1.17) | 4.4 | 0.37 | |
| Adjustment for confounders | ||||||
| Age | 0.55 | |||||
| Yes | 11 | 1.10 | (1.03–1.18) | 53.5 | 0.02 | |
| No | 1 | 1.19 | (1.03–1.38) | – | – | |
| Education | 0.87 | |||||
| Yes | 4 | 1.13 | (1.06–1.20) | 0.0 | 0.84 | |
| No | 8 | 1.10 | (1.00–1.20) | 67.0 | 0.003 | |
| Social class | 0.91 | |||||
| Yes | 6 | 1.09 | (0.97–1.24) | 70.9 | 0.004 | |
| No | 6 | 1.14 | (1.08–1.19) | 0.0 | 0.46 | |
| Smoking | 0.49 | |||||
| Yes | 11 | 1.12 | (1.05–1.19) | 52.5 | 0.02 | |
| No | 1 | 0.99 | (0.77–1.27) | – | – | |
| Alcohol intake | 0.33 | |||||
| Yes | 7 | 1.14 | (1.07–1.21) | 29.1 | 0.21 | |
| No | 5 | 1.03 | (0.90–1.19) | 70.4 | 0.009 | |
| Physical activity | 0.37 | |||||
| Yes | 3 | 1.16 | (1.04–1.29) | 69.4 | 0.04 | |
| No | 9 | 1.08 | (1.00–1.17) | 48.0 | 0.05 | |
| Diabetes | 0.52 | |||||
| Yes | 3 | 1.17 | (1.08–1.26) | 1.7 | 0.36 | |
| No | 9 | 1.09 | (1.01–1.18) | 57.2 | 0.02 | |
| Other comorbidities | 0.61 | |||||
| Yes | 2 | 1.16 | (1.02–1.31) | 50.4 | 0.16 | |
| No | 10 | 1.10 | (1.03–1.18) | 52.3 | 0.03 | |
n refers to number of risk estimates (one publication reported results for two studies combined so the total number of studies is 13).
Discussion
In this meta-analysis, higher BMI in middle age, BMI in early adulthood, and waist circumference were associated with an increased risk of rheumatoid arthritis. There was no evidence of nonlinearity, and positive dose–response relationships were apparent for both BMI in middle age and BMI in early adulthood. The positive association between BMI in middle age and rheumatoid arthritis was restricted to women, and no clear association was observed in men; this could be due to an interaction with hormone-related factors or other sex-specific exposures, which may contribute to the higher prevalence of rheumatoid arthritis in women26. It has also been reported that differences in body fat distribution between men and women may contribute to sex differences in the risk of rheumatoid arthritis27. Due to a lack of data from primary studies, it was not possible to evaluate the sex-specific associations for waist circumference, and further research is needed to determine whether the sex difference in association is only for BMI, or also for other measures of adiposity.
The current findings are consistent with a previous meta-analysis, which also found increased risk of rheumatoid arthritis with increasing BMI22,23. Feng et al.23, reported a summary RR for every 5 kg/m2 increase in BMI of 1.13 (95% CI 1.01–1.26, I2 = 83.0%) based on data from 5 cohort studies and 6 case–control studies. The heterogeneity between studies was much lower in the current meta-analysis than in the previous meta-analysis, and this is probably to a large extent because we only included cohort studies. This avoids recall bias and reduces the possibility of selection bias and reverse causation. The larger number of cohort studies in the present analysis contributed to more precise risk estimates, and enabled assessment of associations of other adiposity measures, such as waist circumference and BMI in early adulthood, with risk of rheumatoid arthritis. Some studies have suggested that bariatric surgery may improve symptoms in patients with rheumatoid arthritis40,41, however, a recent Swedish study found no significant association between bariatric surgery and the risk of developing rheumatoid arthritis (HR = 0.92, 95% CI 0.59–1.46), but statistical power to detect a moderate association may have been low and confidence intervals were wide42.
The finding that high BMI in early adulthood (at age 18 years) was associated with an increased risk of rheumatoid arthritis might suggest that early life risk factors may be of importance in the aetiology of rheumatoid arthritis43,44. In a review by Colebatch et al.43, the authors summarised evidence on the effect of early life environmental factors on the risk of future rheumatoid arthritis. Among the findings, it was reported that early initiation of breastfeeding may be associated with a reduced risk of rheumatoid arthritis45, while high birth weight45,46 and maternal smoking in pregnancy47 may contribute to an increased risk of rheumatoid arthritis. On the other hand, high BMI in early adulthood is correlated with high BMI in middle age48, and it is possible that longer duration of obesity may confer an even greater risk of rheumatoid arthritis.
With regard to the underlying mechanisms that could contribute to the observed associations, it is known that adipose tissue plays an important role in the inflammatory process49. This is supported by evidence that adipocytes produce not only adipocytokines but also inflammatory cytokines49. Thus, adiposity can increase systemic inflammation, leading to increased susceptibility for developing rheumatoid arthritis.
There are several strengths of this study. The current meta-analysis had 2–3 times as many cohort studies as three meta-analyses published in 2015, 2016 and 201822–24, and we identified two additional cohort studies18,38 that were not identified in the most recently published meta-analysis in 201950. The inclusion of additional studies and a large total number of cases (n = 4,777) and nearly 0.5 million participants provided sufficient statistical power to detect moderate associations and contributed to more robust results. Another major advantage of the current meta-analysis was that it only included prospective cohort studies. While the previous meta-analyses included many retrospective case–control studies that may have been affected by recall and selection biases22–24,50, the current meta-analysis is less likely to have been affected by such biases. Detailed dose–response analyses were conducted to clarify the dose–response relationship between adiposity and rheumatoid arthritis, and detailed subgroup analyses were conducted to investigate sources of heterogeneity. A novelty of this study was the analyses of additional measures of adiposity, specifically waist circumference and BMI in early adulthood, with risk of rheumatoid arthritis. BMI is considered to be a useful indicator of obesity, but this index does not reflect abdominal body fat distribution, and the accuracy of the index can vary according to age, sex and body composition51. When developing prevention interventions for rheumatoid arthritis, it may be informative to assess the association between several other measures of adiposity and the risk of developing rheumatoid arthritis.
There are several limitations of this study that should be noted. In some of the included studies anthropometric measurements were self-reported. Consequently, the results might have been influenced by misclassification of the exposure. However, nine of the thirteen included studies performed anthropometric measurements through a clinical examination17–19,21,37–39, and showed similar results to the overall summary estimate. Only four studies used self-reported data15,16,20. There was no heterogeneity in associations in subgroup analyses stratified by whether anthropometric measures were measured or self-reported.
Heterogeneity between studies, publication bias and confounding are potentially important limitations of any meta-analysis. Some degree of heterogeneity is expected because studies may differ with regard to the assessment of adiposity, other risk factors, the outcome, underlying risk factor profile and disease rates, as well as analytical approaches including the extent of adjustment for confounding factors. Sources of heterogeneity were investigated in subgroup analyses, however, with the exception of the difference in association for men and women, there was little evidence of heterogeneity between other subgroups including geographic location, number of cases, measurement of BMI, duration of follow-up, and adjustment for confounding factors (including education, social class, smoking, alcohol, physical activity, diabetes or other co-morbidities). Although most of the individual included studies adjusted for important potential confounders, it is important to note that residual and unmeasured confounding is possible. For example, one study suggested that consumption of sugar-sweetened beverages may increase risk of rheumatoid arthritis52 and other studies have found sugar-sweetened beverages to increase weight gain53. Given that little is known with certainty about diet and rheumatoid arthritis, and most studies have not adjusted for dietary factors, we cannot exclude the possibility that the observed association partly could be due to confounding by sugar-sweetened beverages or other dietary factors. Although there was some indication of publication bias in the analysis of BMI, this appeared to be explained by one outlying study, which when excluded did not substantially alter the results, but which also appeared to explain a large part of the heterogeneity between studies. Thus, it seems less likely that publication bias would have substantially impacted the overall conclusions. Another limitation was that the study populations were limited to North American and European populations and therefore had limited geographical coverage. Therefore, further research is needed to evaluate the association between adiposity and the risk of rheumatoid arthritis in other regions, including Africa, South America and Asia–Pacific.
According to the Global Burden of Disease 2010 study, the number of people living with rheumatoid arthritis is expected to increase considerably over the coming decades, especially in low-income and middle-income countries25. In addition, overweight and obesity have become a considerable problem in most regions of the world54, including low-income and middle-income countries such as China, Brazil and Indonesia as well as in high-income countries55.
As the number of studies on adiposity variables other than BMI was limited, further studies are needed on a range of alternative adiposity measures including waist circumference, hip circumference, waist-to-hip ratio, waist-to-height ratio, BMI in early adulthood, and weight change throughout the life course, and to clarify whether the sex-specific results for BMI also apply to these other measures of adiposity. Because excess weight to a large degree is driven by diet and lifestyle factors56, it is possible that certain lifestyle-related risk factors (e.g. consumption of sugar-sweetened soft drinks and low physical activity) may contribute to the development of rheumatoid arthritis partly through an effect on adiposity52,57–59, and further studies are needed to clarify if this is the case.
Conclusion
This study confirms a positive association between levels of adiposity and the risk of developing rheumatoid arthritis. Higher BMI in middle age, BMI in early adulthood (at age 18 years), and waist circumference were associated with a higher risk of rheumatoid arthritis in dose–response meta-analyses. The current analysis suggests that excess weight may be a risk factor for rheumatoid arthritis in women, but further cohort studies are needed to clarify sex-specific associations with adiposity measures other than BMI as the available data is limited. Since most of the existing studies are from Europe and the US, additional studies are needed from other geographic regions as well. Based on these findings, prevention strategies targeting weight reduction and maintenance of a healthy weight may contribute to reducing the burden rheumatoid arthritis places on society.
Supplementary information
Author contributions
T.O., D.A., A.K.H. contributed to the study concept and design, data analysis, interpretation, and manuscript preparation. All authors were involved in the writing and review of the manuscript and approved the final version to be submitted.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
TO is an employee of Novartis Pharma K.K. During the conduct of the study, TO was an employee of GlaxoSmithKline K.K., and a postgraduate student in the School of Public Health at Imperial College London. DA and AKH declare no potential competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Dagfinn Aune and Alicia K. Heath.
Supplementary information
is available for this paper at 10.1038/s41598-020-71676-6.
References
- 1.Torpy JM, Perazza GD, Golub RM. JAMA patient page. Rheumatoid arthritis. JAMA. 2011;305(17):1824. doi: 10.1001/jama.305.17.1824. [DOI] [PubMed] [Google Scholar]
- 2.Rheumatoid Arthritis: Athritis Foundation. https://www.arthritis.org/about-arthritis/types/rheumatoid-arthritis/.
- 3.The global burden of disease: 2004 update. World Health Organization. https://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/.
- 4.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev. 2005;4(3):130–136. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin. Arthritis Rheum. 2006;36(3):182–188. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. PharmacoEconomics. 2004;22(2 Suppl 1):1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- 7.Ward H, Toledano MB, Shaddick G, Davies B, Elliott P. Oxford handbook of epidemiology for clinicians. Oxford: Oxford University Press; 2012. [Google Scholar]
- 8.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann. Rheum. Dis. 2009;69(01):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 10.Lahiri M, Morgan C, Symmons DP, Bruce IN. Modifiable risk factors for RA: prevention, better than cure? Rheumatology. 2012;51(3):499–512. doi: 10.1093/rheumatology/ker299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skoczynska M, Swierkot J. The role of diet in rheumatoid arthritis. Reumatologia. 2018;56(4):259–267. doi: 10.5114/reum.2018.77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Sparks JA, Malspeis S, Costenbader KH, Hu FB, Karlson EW, et al. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann. Rheum. Dis. 2017;76(8):1357–1364. doi: 10.1136/annrheumdis-2016-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Tedeschi SK, Lu B, Zaccardelli A, Speyer CB, Costenbader KH, et al. Long-term physical activity and subsequent risk for rheumatoid arthritis among women: A prospective cohort study. Arthritis Rheumatol. 2019;71:1460–1471. doi: 10.1002/art.40899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity (Silver Spring, Md). 2007;15(7):1827–1840. doi: 10.1038/oby.2007.217. [DOI] [PubMed] [Google Scholar]
- 15.Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen CY, Awosogba JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann. Rheum. Dis. 2014;73(11):1914–1922. doi: 10.1136/annrheumdis-2014-205459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harpsoe MC, Basit S, Andersson M, Nielsen NM, Frisch M, Wohlfahrt J, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int. J. Epidemiol. 2014;43(3):843–855. doi: 10.1093/ije/dyu045. [DOI] [PubMed] [Google Scholar]
- 17.Lahiri M, Luben RN, Morgan C, Bunn DK, Marshall T, Lunt M, et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register–the EPIC-2-NOAR Study) Ann. Rheum. Dis. 2014;73(1):219–226. doi: 10.1136/annrheumdis-2012-202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linauskas A, Overvad K, Symmons D, Johansen MB, Stengaard-Pedersen K, de Thurah A. Body fat percentage, waist circumference, and obesity as risk factors for rheumatoid arthritis: a Danish Cohort Study. Arthritis Care Res. (Hoboken). 2019;71(6):777–786. doi: 10.1002/acr.23694. [DOI] [PubMed] [Google Scholar]
- 19.Ljung L, Rantapaa-Dahlqvist S. Abdominal obesity, gender and the risk of rheumatoid arthritis - a nested case-control study. Arthritis Res. Ther. 2016;18(1):277. doi: 10.1186/s13075-016-1171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerhan JR, Saag KG, Criswell LA, Merlino LA, Mikuls TR. Blood transfusion, alcohol use, and anthropometric risk factors for rheumatoid arthritis in older women. J. Rheumatol. 2002;29(2):246–254. [PubMed] [Google Scholar]
- 21.Rodriguez LA, Tolosa LB, Ruigomez A, Johansson S, Wallander MA. Rheumatoid arthritis in UK primary care: incidence and prior morbidity. Scand. J. Rheumatol. 2009;38(3):173–177. doi: 10.1080/03009740802448825. [DOI] [PubMed] [Google Scholar]
- 22.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res. Ther. 2015;17:86. doi: 10.1186/s13075-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng J, Chen Q, Yu F, Wang Z, Chen S, Jin Z, et al. Body mass index and risk of rheumatoid arthritis: a meta-analysis of observational studies. Medicine. 2016;95(8):e2859. doi: 10.1097/MD.0000000000002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Sun M. A meta-analysis of the relationship between body mass index and risk of rheumatoid arthritis. EXCLI J. 2018;17:1079–1089. doi: 10.17179/excli2018-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73(7):1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 26.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 27.Finckh A, Turesson C. The impact of obesity on the development and progression of rheumatoid arthritis. Ann. Rheum. Dis. 2014;73(11):1911–1913. doi: 10.1136/annrheumdis-2014-205741. [DOI] [PubMed] [Google Scholar]
- 28.GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses: Ottawa Hospital Research Institute. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 31.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6(1):40–57. doi: 10.1177/1536867X0600600103. [DOI] [Google Scholar]
- 32.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am. J. Epidemiol. 2004;159(11):1077–1086. doi: 10.1093/aje/kwh142. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. Ed.) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. “Publication Bias”. Introduction to Meta-Analysis. Chichester: Wiley; 2009. [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 37.Heliovaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J. Rheumatol. 1993;20(11):1830–1835. [PubMed] [Google Scholar]
- 38.Pahau H, Brown MA, Paul S, Thomas R, Videm V. Cardiovascular disease is increased prior to onset of rheumatoid arthritis but not osteoarthritis: the population-based Nord-Trondelag health study (HUNT) Arthritis Res. Ther. 2014;16(2):R85. doi: 10.1186/ar4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turesson C, Bergstrom U, Pikwer M, Nilsson JA, Jacobsson LT. A high body mass index is associated with reduced risk of rheumatoid arthritis in men, but not in women. Rheumatology. 2016;55(2):307–314. doi: 10.1093/rheumatology/kev313. [DOI] [PubMed] [Google Scholar]
- 40.Sparks JA, Halperin F, Karlson JC, Karlson EW, Bermas BL. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res. 2015;67(12):1619–1626. doi: 10.1002/acr.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lespessailles E, Hammoud E, Toumi H, Ibrahim-Nasser N. Consequences of bariatric surgery on outcomes in rheumatic diseases. Arthritis Res. Ther. 2019;21(1):83. doi: 10.1186/s13075-019-1869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maglio C, Zhang Y, Peltonen M, Andersson-Assarsson J, Svensson PA, Herder C, et al. Bariatric surgery and the incidence of rheumatoid arthritis—a Swedish Obese Subjects study. Rheumatology. 2019 doi: 10.1093/rheumatology/kez275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colebatch AN, Edwards CJ. The influence of early life factors on the risk of developing rheumatoid arthritis. Clin. Exp. Immunol. 2011;163(1):11–16. doi: 10.1111/j.1365-2249.2010.04263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards CJ, Cooper C. Early environmental factors and rheumatoid arthritis. Clin. Exp. Immunol. 2006;143(1):1–5. doi: 10.1111/j.1365-2249.2005.02940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobsson LT, Jacobsson ME, Askling J, Knowler WC. Perinatal characteristics and risk of rheumatoid arthritis. BMJ (Clin. Res. Ed.) 2003;326(7398):1068–1069. doi: 10.1136/bmj.326.7398.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandl LA, Costenbader KH, Simard JF, Karlson EW. Is birthweight associated with risk of rheumatoid arthritis? Data from a large cohort study. Ann. Rheum. Dis. 2009;68(4):514–518. doi: 10.1136/ard.2007.080937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaakkola JJ, Gissler M. Maternal smoking in pregnancy as a determinant of rheumatoid arthritis and other inflammatory polyarthropathies during the first 7 years of life. Int. J. Epidemiol. 2005;34(3):664–671. doi: 10.1093/ije/dyi006. [DOI] [PubMed] [Google Scholar]
- 48.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obesity Rev. 2016;17(2):95–107. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- 49.Tolusso B, Alivernini S, Gigante MR, Ferraccioli G, Gremese E. Biomolecular features of inflammation in obese rheumatoid arthritis patients: management considerations. Expert Rev. Clin. Immunol. 2016;12(7):751–762. doi: 10.1586/1744666X.2016.1159132. [DOI] [PubMed] [Google Scholar]
- 50.Feng X, Xu X, Shi Y, Liu X, Liu H, Hou H, et al. Body mass index and the risk of rheumatoid arthritis: an updated dose-response meta-analysis. Biomed. Res. Int. 2019;2019:3579081. doi: 10.1155/2019/3579081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gremese E, Tolusso B, Gigante MR, Ferraccioli G. Obesity as a risk and severity factor in rheumatic diseases (autoimmune chronic inflammatory diseases) Front. Immunol. 2014;5:576. doi: 10.3389/fimmu.2014.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Y, Costenbader KH, Gao X, Al-Daabil M, Sparks JA, Solomon DH, et al. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr. 2014;100(3):959–967. doi: 10.3945/ajcn.114.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2013;98(4):1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, Adams RJ, Aekplakorn W, Afsana K, Aguilar-Salinas CA, Agyemang C. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregg EW, Shaw JE. Global health effects of overweight and obesity. N. Engl. J. Med. 2017;377(1):80–81. doi: 10.1056/NEJMe1706095. [DOI] [PubMed] [Google Scholar]
- 56.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeChristopher LR, Uribarri J, Tucker KL. Intake of high-fructose corn syrup sweetened soft drinks, fruit drinks and apple juice is associated with prevalent arthritis in US adults, aged 20–30 years. Nutr. Diabetes. 2016;6:e199. doi: 10.1038/nutd.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Giuseppe D, Bottai M, Askling J, Wolk A. Physical activity and risk of rheumatoid arthritis in women: a population-based prospective study. Arthritis Res. Ther. 2015;17:40. doi: 10.1186/s13075-015-0560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Tedeschi SK, Lu B, Zaccardelli A, Speyer CB, Costenbader KH, et al. Long-term physical activity and subsequent risk for rheumatoid arthritis among women: a prospective cohort study. Arthritis Rheumatol. 2019;71(9):1460–1471. doi: 10.1002/art.40899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.