Visual Abstract
Key Words: autotaxin, calcific aortic valve stenosis, lipoprotein(a), low-density lipoproteins, obesity
Abbreviations and Acronyms: apo(a), apolipoprotein(a); apoB, apolipoprotein B; ALR, adiponectin-to-leptin ratio; ATX, autotaxin; ATX-apo(a), ATX carried by Lp(a); ATX-apoB, ATX carried by apoB-containing lipoproteins; BMI, body mass index; CAD, coronary artery disease; CAVS, calcific aortic valve stenosis; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); LysoPA, lysophosphatidic acid; LysoPC, lysophosphatidylcholine; OxPLs, oxidized phospholipids
Highlights
-
•
Lipoprotein(a) is an important carrier of autotaxin and lysophosphatidic acid in the bloodstream.
-
•
Autotaxin might physically interact with apolipoprotein(a).
-
•
The plasma concentration of autotaxin transported by lipoprotein(a) may be an important predictor of calcific aortic valve stenosis.
Summary
Our objectives were to determine whether autotaxin (ATX) is transported by lipoprotein(a) [Lp(a)] in human plasma and if could be used as a biomarker of calcific aortic valve stenosis (CAVS). We first found that ATX activity was higher in Lp(a) compared to low-density lipoprotein fractions in isolated fractions of 10 healthy participants. We developed a specific assay to measure ATX-Lp(a) in 88 patients with CAVS and 144 controls without CAVS. In a multivariable model corrected for CAVS risk factors, ATX-Lp(a) was associated with CAVS (p = 0.003). We concluded that ATX is preferentially transported by Lp(a) and might represent a novel biomarker for CAVS.
Calcific aortic valve stenosis (CAVS) is the most prevalent heart valve disease, affecting 2% to 3% of individuals aged 65 years or older. The main risk factors for CAVS are age, sex, obesity, hypertension, type 2 diabetes (T2D), low-density lipoprotein cholesterol (LDL-C), and smoking (1). The main driving force of CAVS is the calcification of the aortic valve, which leads to a reduction of the aortic valve effective orifice area (2,3). Currently, the only treatment known to improve outcomes in CAVS patients is the surgical removal of the aortic valve.
Over the past 20 years, clinical and molecular studies suggested a potential role for lipoprotein(a) [Lp(a)] in the aortic valve calcification process (4, 5, 6, 7, 8). Seeking to identify genetic variants associated with aortic valve calcium accumulation, Thanassoulis et al. (9) identified a variant at the LPA locus on chromosome 6, which is strongly associated with plasma Lp(a) levels, as the top candidate locus. Lp(a) was also shown to be an important predictor of valvular outcomes in patients with mild-to-moderate CAVS (10,11). Besides its important role as a carrier of oxidized phospholipids, the molecular mechanisms through which Lp(a) might cause aortic valve calcium accumulation are poorly understood.
Autotaxin (ATX), an adipose tissue-derived phospholipase D, converts lysophosphatidylcholine (LysoPC) into lysophosphatidic acid (LysoPA), a bioactive phospholipid that can act as a signaling molecule promoting inflammation, fibrosis, and cell motility (12). LysoPA can also induce an osteogenic transition in valve interstitial cells through its binding to the LPAR1 receptor (13). Bouchareb et al. (14) and Torzewski et al. (15) have previously shown that ATX can associate with Lp(a). However, important questions remain with regard to the nature of the association between ATX and Lp(a) and its potential clinical significance. For instance, it is not known if ATX binds to apolipoprotein(a) or if the activity of ATX (or LysoPA levels) is higher on Lp(a) compared to other lipoproteins with similar size and density such as LDL. Additionally, whether the circulating levels of ATX bound to Lp(a) are associated with CAVS is also unknown. The objective of this study was to gain new knowledge about the potential interaction of ATX with atherogenic lipoproteins and to determine whether a newly developed biomarker, ATX bound to Lp(a) [ATX-apo(a)], is associated with the presence of CAVS.
Methods
Study participants
A series of consecutive patients with mild to severe CAVS who did not undergo aortic valve replacement were recruited at the echocardiography laboratory of the Québec Heart and Lung Institute (QHLI). Exclusion criteria included the presence of mitral valve stenosis, mitral insufficiency (≥moderate), aortic insufficiency (≥moderate) and heart failure (ejection fraction <40%). Cases were excluded if they had CAVS of rheumatic etiology or if they had any type of cancer that required radiotherapy in the thoracic area (breast, trachea, bronchus, or lung cancer) before the diagnosis of CAVS. Women were also excluded if they were pregnant or lactating. Monthly interrupted time series were performed to recruit individuals without CAVS undergoing Doppler echocardiography (controls with normal aortic valves). Additional controls were also recruited through the echocardiography laboratory and advertisements at the QHLI. Previous and current medical history included history of smoking, documented diagnoses of hypertension (patients receiving antihypertensive medications or having known but untreated hypertension [blood pressure ≥140/90 mm Hg]), diabetes (fasting glucose ≥7 mmol/l or treatment with antidiabetic medication), and detailed information on current medication was collected. Body weight, height, and waist circumference were measured following standardized procedures. Blood pressure and heart rate were also assessed. Peak aortic jet velocity, aortic valve area, peak and mean transvalvular gradients, and aortic valve morphology were measured by Doppler echocardiography (16, 17, 18). Patients were classified as having mild, moderate, or severe CAVS based on peak aortic jet velocity, aortic valve pressure gradient, and aortic valve area as suggested by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (19). We isolated Lp(a) from 9 healthy volunteers (5 men and 4 women) without aortic stenosis and with high Lp(a) level (>125 nmol/l). The study protocols were approved by the Ethics Committee of the QHLI and all patients signed a written informed consent.
Laboratory data
Overnight fasting blood samples were collected in ethylenediaminetetraacetic acid tubes and immediately processed in the clinical biochemistry laboratory for the measurement of glucose, total cholesterol, LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglyceride levels. Plasma samples were also stored at -80 °C and used for measurement of Lp(a) and ATX levels. Plasma Lp(a) levels were measured by turbidimetric assay using the Tina-quant Lipoprotein(a) Gen.2 system (Cobas integra 400/800, Roche Mannheim, Germany). Plasma adiponectin and leptin concentrations were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R and D Systems, Minneapolis, Minnesota). ATX mass was also measured in plasma by ELISA according to the manufacturer’s instructions (Echelon Biosciences, Salt Lake City, Utah) (intra-assay coefficient of variation [CV] of <2%).
LDL and Lp(a) isolation and separation
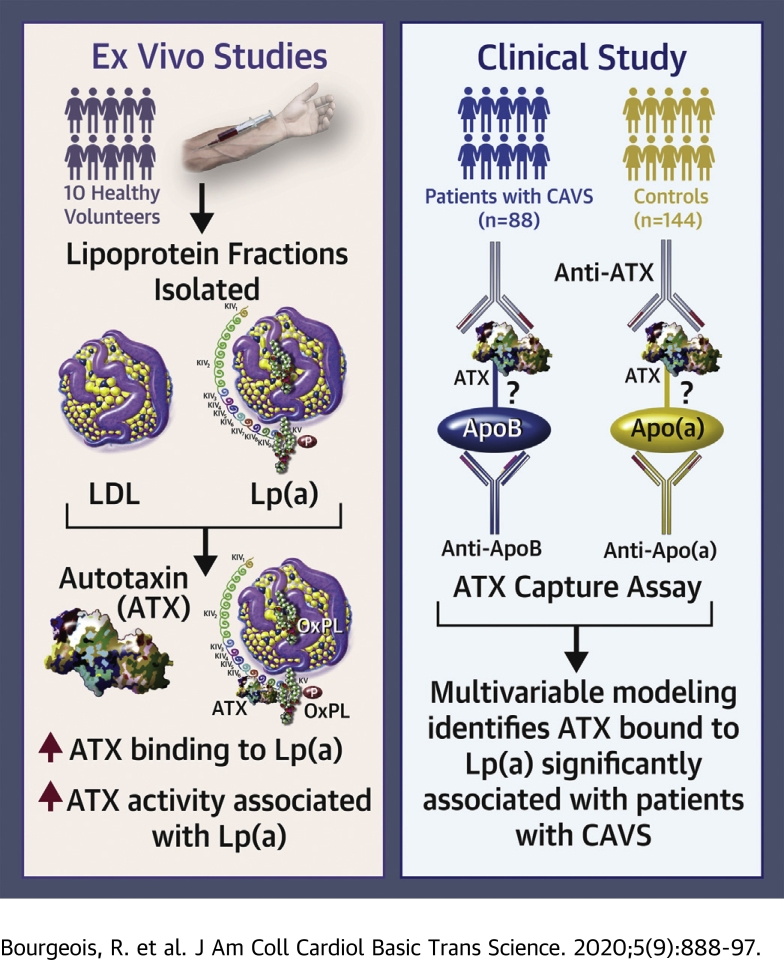
To isolate and separate Lp(a) and LDL, blood was collected into BD-Vacutainer tubes (Becton, Dickinson and Co., Franklin Lakes, New Jersey). Serum was then separated by centrifugation at 3,000 g for 15 min. Lipoproteins were separated into 3 fractions by ultracentrifugation for 9 h at 100,000 g. Lp(a)-containing fractions (assessed by Sebia Hydragel) were subjected to size exclusion chromatography with Tris-HCl buffer. Lp(a)-containing fractions were identified by western blotting. Positive Lp(a) fractions were then subjected to ion exchange chromatography and LDL and Lp(a) were separated using a NaCl gradient. After purification, samples were concentrated and equilibrated at 150 mM NaCl and purity was confirmed by Sebia Hydragel (Figure 1A). Lp(a) and LDL fractions were free of exosomes as shown by western blot using CD63 and CD9 antibodies (data not shown). Cholesterol content of lipoprotein subfractions was measured using a commercial kit (Randox, Crumlin, United Kingdom). LysoPA was measured in plasma by ELISA according to the manufacturer’s instructions (Echelon Biosciences, Salt Lake City, Utah).
Figure 1.
Autotaxin Activity and Lysophosphatidic Acid Content in Lipoprotein(a) Versus Low-Density Lipoproteins
Purity confirmation by Sebia Hydragel (A), measurement of autotaxin (ATX) activity (B), and lysophoshphatidic acid (LysoPA) content (C) in lipoprotein (a) [Lp(a)] and low-density lipoprotein (LDL) of healthy participants. ATX activity (relative light absorbent unit) and LysoPA content (μM) were normalized by cholesterol content (μmol/l). Asterisk (∗) indicates values significantly different from LDL (p = 0.016 for ATX activity [B] and p = 0.031 for LysoPA content [C]). VLDL = very low density lipoprotein.
ATX activity
Autotaxin activity in isolated fractions of LDL and Lp(a) was determined by assessing the cleavage of the ATX-specific fluorogenic substrate FS-3 (Echelon Biosciences, L-2000). Assays were conducted in a final volume of 210 μl comprising 180 μl assay buffer (50 mM Tris-HCl pH 8.0 containing 1 mg/ml bovine serum albumin [BSA], 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1mM CaCl2), 5 μl of FS-3 (final concentration 5 μM) and 25 μl of each sample. The reaction was allowed to proceed at 37 °C for 17 h. Fluorescence was measured with a FilterMax F3 microplate reader (Molecular Devices, Wokingham, United Kingdom). ATX activity was quantified by measuring the fluorescence increase at 528 nm with excitation at 485 nm.
Capture assay
The detection of ATX on lipoproteins containing apo(a) [Lp(a)] or apoB-100 was measured with an antibody capture assay. Microtiter 96-well medium binding plates (Greiner Bio-One, Kremsmunster, Austria) were coated with anti-apoB-100 (ab20737, Abcam, Cambridge, United Kingdom) or anti-apo(a) (MABS1284, Millipore, Burlington, Massachusetts) antibodies overnight at 4°C (1 μg/ml, 50 μl/well). Excess material was removed. Plates were then blocked for 2 h with PBST -5% BSA. After washing, plasma was added at 1:5 dilution (phosphate-buffered saline solution with Tween [PBST] -1% BSA; 50 μl/well) for 2 h at 37°C. After washing, rabbit anti-human ATX antibody (PA5-12478, ThermoFisher, Waltham, Massachusetts) (4 μg/ml; 50 μl/well), was added and incubated for 2 h. Horseradish peroxidase–labeled goat anti-rabbit antibody (AP132P, Millipore) (0.03 μg/ml; 50 μl/well) was added overnight at 4°C. After a final washing step, 100 μl of o-phenylenediamine dihydrochloride (OPD) (34005, ThermoFisher) was added for 30 min, followed by 100 μl of sulfuric acid (2.5 M) to stop the reaction. Absorbance was read on a spectrophotometer at 492 nm according to the manufacturer’s instructions. Results were expressed in relative light absorbance units after subtraction of the background (PBST-1% BSA).
Binding assay
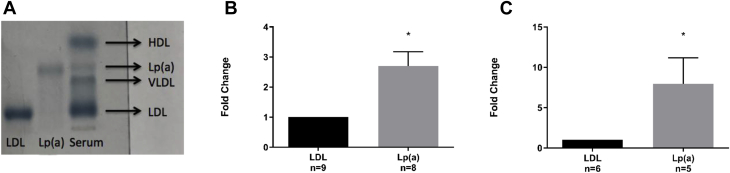
High-binding capacity microtiter 96-well plates (polystyrene, Greiner Bio-One) were coated overnight at 4°C with recombinant ATX (5 μg/ml) in carbonate buffer. Excess material was removed, and plates were blocked with PBST-1% BSA (150 μl/well) for 1 h. After washing, increasing concentrations of PBST-diluted apo(a) (0.05 μM to 1 μM; 100 μl/well) were added and incubated for 2 h at room temperature. After washing, apo(a) antibody (1 μg/ml, 100 μl/well) was added for 1 h at room temperature, followed by the secondary horseradish peroxidase–conjugated antibody for 45 min (1/10,000 dilution; 100 μl/well). Following PBST-1% BSA washes, absorbance was read at 405 nm. Purified apo(a) was obtained following the previously described method (20). Plasmid encoding a 17-kringle-containing form of recombinant apo(a) was constructed and transfected into human embryonic kidney (HEK 293) cells as previously described (20,21). Recombinant-apo(a) was purified from the conditioned medium of stably-expressing cell lines by lysine–Sepharose (GE Healthcare, Chicago, Illinois) affinity chromatography as previously reported (20).
Statistical analysis
Continuous data are expressed as mean ± SD and were compared between 2 groups using Student's t-test; and categorical data are presented as counts with percentage and compared using the chi square test. Normality of continuous variables was assessed by the Shapiro-Wilk test and log-transformed variables were entered in the model if they were not normally distributed. Univariable and multivariable logistic regression models were performed to examine the association between ATX-apo(a) and the presence of CAVS. The variables selected for the models were influenced by CAVS case-control status (p < 0.05). Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). Logistic regression was used to determine the respective contribution of ATX-apo(a) and body mass index (BMI) based on the cutpoints that were identified by sensitivity and specificity analyses (area under the receiver-operating characteristic curve for CAVS presence). Spearman's correlation coefficient (rs) was used to compare 2 continuous variables. Statistical analyses were performed using JMP software (V.14). A p value ≤0.05 was considered statistically significant.
Results
Comparison of ATX activity between LDL and Lp(a)
We first undertook to establish a reliable and reproducible method for LDL and Lp(a) fraction separation validated using the Sebia Hydragel results (Figure 1A). To investigate the preferential association of ATX with Lp(a), we measured ATX activity in isolated fractions of LDL and Lp(a) from healthy participants. ATX activity was significantly higher in Lp(a) compared to the LDL fraction (Figure 1B). In addition, LysoPA content was also significantly higher in the Lp(a) fraction compared to the LDL fraction (Figure 1C).
Involvement of Apo(a) moiety in ATX binding
Although a physical association between Lp(a) and ATX has been previously reported, it is presently unknown whether the interaction occurs within the LDL component or with apo(a). By using recombinant ATX coated in 96 well plates we assessed using a binding assay the interaction with purified apo(a). In this assay, we observed a dose-dependent interaction with a plateau at a concentration of ∼1,000 nM of apo(a) (Figure 2).
Figure 2.
Binding Curve of Apo(a) to ATX
The binding curve is fitted by a nonlinear model in Prism. Apo(a) = apolipoprotein(a); ATX = autotaxin.
Measurement of ATX bound to Apo(a) in plasma
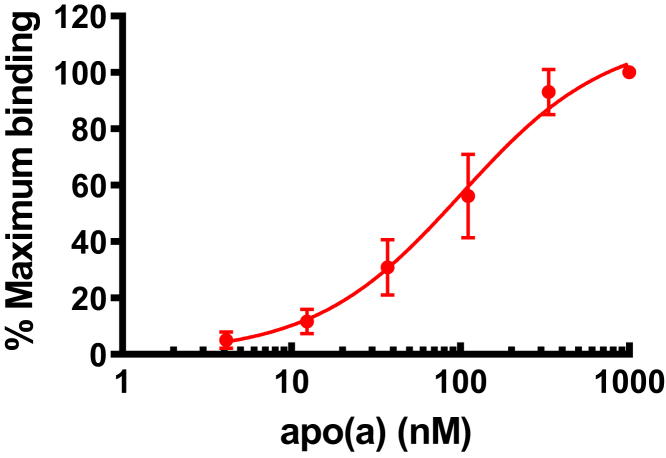
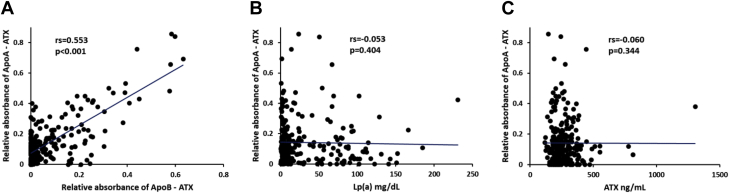
Having established that ATX activity is higher in isolated Lp(a) fractions, we sought to establish a quantitative assay to measure ATX-apo(a) in the plasma. We developed a capture assay to measure ATX bound to apo(a) and apoB (see Methods). In 232 subjects, including control and CAVS cases (Table 1), we found that the distribution of both ATX-apo(a) and ATX-apoB were skewed to the left (Figure 3). ATX-apo(a) correlated significantly (rs = 0.553; p < 0.0001) (Spearman correlation) with ATX-apoB, but not with Lp(a) or total ATX level measured by ELISA (Figure 4).
Table 1.
Clinical Characteristics of Study Participants
| Total N = 232 |
Without CAVS N = 144 |
With CAVS N = 88 |
p Value | |
|---|---|---|---|---|
| Clinical | ||||
| Age, yrs | 64.8 ± 8.9 | 62.7 ± 7.5 | 68.3 ± 9.8 | <0.001 |
| Male | 133 (57.3) | 74 (51.4) | 59 (67.1) | 0.019 |
| BMI, kg/m2 | 28.8 ± 5.5 | 27.6 ± 5.0 | 30.8 ± 5.8 | <0.001 |
| Waist circumference, cm | 101.1 ± 14.8 | 97.2 ± 13.2 | 107.5 ± 15.2 | <0.001 |
| Hypertension | 116 (50.0) | 49 (34.0) | 67 (76.1) | <0.001 |
| Diabetes | 47 (20.3) | 20 (13.9) | 27 (30.7) | 0.002 |
| Obesity (BMI ≥30 kg/m2) | 86 (37.1) | 40 (27.8) | 46 (52.3) | <0.001 |
| Medication | ||||
| Statins | 121 (52.2) | 53 (36.8) | 68 (77.3) | <0.001 |
| Calcium-channel inhibitors | 46 (19.8) | 12 (8.3) | 34 (38.6) | <0.001 |
| ACE inhibitors | 44 (19.0) | 20 (13.9) | 24 (27.3) | 0.013 |
| ARA | 51 (22.0) | 19 (13.2) | 32 (36.4) | <0.001 |
| Bicuspid AV | 15 (6.5) | 1 (0.7) | 14 (15.9) | <0.001 |
| CAVS severity | <0.001 | |||
| mild | 26 (11.2) | 0 (0) | 26 (11.2) | |
| moderate | 42 (18.1) | 0 (0) | 42 (18.1) | |
| severe | 20 (8.6) | 0 (0) | 20 (8.6) | |
| Laboratory Data | ||||
| LDL cholesterol, mg/dl | 93.3 ± 39.3 | 101.8 ± 39.3 | 79.3 ± 35.3 | <0.001 |
| HDL cholesterol, mg/dl | 53.0 ± 17.5 | 53.7 ± 17.7 | 51.8 ± 17.1 | 0.423 |
| Triglycerides, mg/dl | 156.9 ± 80.0 | 160.4 ± 77.3 | 151.1 ± 84.2 | 0.390 |
| Lp(a), nmol/l | 73.3 ± 101.4 | 63.3 ± 87.9 | 89.6 ± 119.0 | 0.055 |
| ATX, ng/ml | 266.9 ± 115.4 | 264.2 ± 79.7 | 271.4 ± 157.7 | 0.645 |
| ATX-apo(a) | 0.14 ± 0.14 | 0.12 ± 0.12 | 0.17 ± 0.17 | 0.034 |
| ATX-apoB | 0.07 ± 0.12 | 0.07 ± 0.10 | 0.08 ± 0.14 | 0.257 |
Values are mean ± SD or n (%)
ACE = angiotensin-converting enzyme; apo(a) = apolipoprotein(a); apoB = apolipoprotein B-100; ARA = angiotensin II receptor antagonists; ATX = autotaxin; BMI = body mass index; CAVS = calcific aortic valve stenosis; HDL = high-density lipoprotein; LDL = low-density lipoprotein; Lp(a) = lipoprotein(a).
Figure 3.
Distribution of ATX-Apo(a) and ATX-ApoB of Study Participants
Distribution of ATX-apo(a) (A) and ATX-apoB (B) in patients with (cases) and without (controls) calcific aortic valve stenosis. apoB = apolipoprotein B; other abbreviations as in Figures 1 and 2.
Figure 4.
Spearman Correlation Between Plasma Levels of Biomarkers
Spearman correlation between plasma levels of ATX-apo(a) and ATX-apoB (A), ATX-apo(a) and Lp(a) (B), ATX-apo(a) and ATX (C) Abbreviations as in Figures 1, 2, and 3.
Association between ATX and CAVS
We next evaluated whether the ATX capture assay on lipoproteins is a predictor of CAVS. Clinical and laboratory characteristics of patients with and without CAVS are presented in Table 1. Significant differences in some of the clinical risk factors (age, sex, BMI, and hypertension) were noted with patients in the CAVS group, having a higher proportion of males, patients with hypertension, T2D, and obesity. The proportion of patients treated with statins, calcium channel inhibitors, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) was also higher in the CAVS group. Also, patients with CAVS had higher levels of ATX-apo(a). Levels of circulating ATX and ATX-apoB were similar between the 2 groups.
For ATX-apo(a), we selected a cutpoint to simultaneously maximize the sensitivity and specificity (area under the receiver-operating characteristic curve) of the model. This was not done for ATX-apoB because we found no difference between CAVS case and controls. In a multivariable model (model 1), corrected for age, sex, BMI, T2D, hypertension, pharmacotherapy (calcium channel blockers and statins), HDL-C, and corrected LDL-C, we found that circulating ATX-apo(a) was associated with CAVS (Table 2). In a second model (model 2) where log [Lp(a)] was added into model 1, ATX-apo(a) remained associated with the risk of CAVS. We ran the same analyses replacing BMI with the adiponectin-to-leptin ratio (a marker of adipose tissue dysfunction) and found comparable results (data not shown).
Table 2.
Univariable and Multivariable Logistic Regression Analyses for CAVS Presence
| Univariable Analysis |
Model 1∗ |
Model 2† |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age, yrs | 1.08 (1.05-1.12) | <0.001 | 1.05 (1.01-1.10) | 0.017 | 1.05 (1.01-1.10) | 0.018 |
| Male | 1.92 (1.11-3.35) | 0.019 | 1.53 (0.73-3.20) | 0.254 | 1.53 (0.73-3.21) | 0.256 |
| BMI, kg/m2 | 1.12 (1.06-1.18) | <0.001 | 1.10 (1.02-1.18) | 0.012 | 1.10 (1.02-1.18) | 0.013 |
| Diabetes, yes | 2.74 (1.43-5.28) | 0.002 | 0.60 (0.23-1.57) | 0.294 | 0.60 (0.23-1.57) | 0.295 |
| Hypertension, yes | 6.19 (3.40-11.26) | <0.001 | 1.41 (0.43-4.70) | 0.574 | 1.41 (0.42-4.70) | 0.575 |
| CA channel inhibitors, yes | 6.93 (3.34-14.38) | <0.001 | 3.52 (1.35-9.19) | 0.008 | 3.52 (1.34-9.25) | 0.009 |
| Statins, yes | 5.84 (3.20-10.67) | <0.001 | 2.35 (0.94-5.89) | 0.066 | 2.35 (0.92-5.98) | 0.072 |
| ACE inhibitors, yes | 2.33 (1.19-4.52) | 0.013 | 1.62 (0.48-5.49) | 0.437 | 1.62 (0.48-5.49) | 0.437 |
| ARA, yes | 3.76 (1.96-7.20) | <0.001 | 2.34 (0.69-7.95) | 0.169 | 2.34 (0.69-7.96) | 0.170 |
| Corrected LDL-C, mg/dl | 0.96 (0.95-0.98) | <0.001 | 1.00 (0.97-1.02) | 0.715 | 1.00 (0.96-1.03) | 0.775 |
| HDL-C, mg/dl | 0.99 (0.98-1.01) | 0.419 | 1.02 (0.99-1.04) | 0.196 | 1.02 (0.99-1.04) | 0.196 |
| Log(Triglycerides, mg/dl) | 0.69 (0.39-1.20) | 0.187 | 0.61 (0.27-1.38) | 0.232 | 0.61 (0.27-1.38) | 0.232 |
| Log(Lp(a), nmol/l) | 1.10 (0.93-1.31) | 0.272 | 1.00 (0.74-1.34) | 0.982 | — | — |
| ATX- apo(a)>0.096 | 2.10 (1.21-3.64) | 0.007 | 2.80 (1.39-5.66) | 0.003 | 2.80 (1.39-5.66) | 0.003 |
CA = calcium; CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; OR = odds ratio; other abbreviations as in Table 1.
Model 1: Age, sex, BMI, diabetes, hypertension, calcium channel inhibitor, statins, ACE inhibitors, ARA, corrected LDL-C, HDL, triglycerides, ATX- apo(a).
Model 2: Model 1 + Lp(a).
Contributions of ATX-Apo(a) and BMI to CAVS
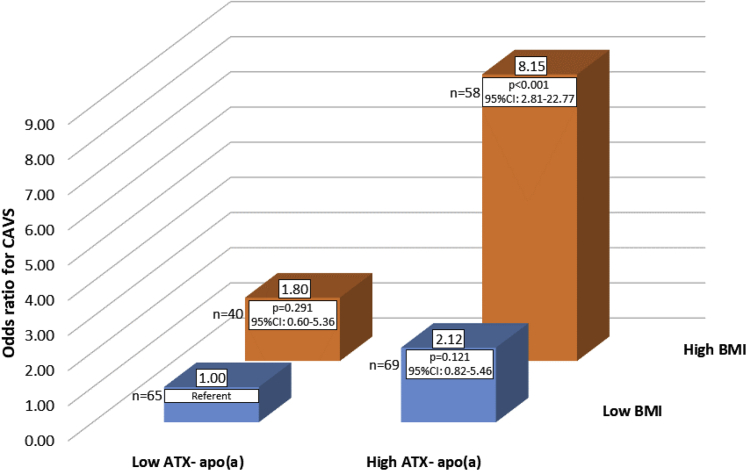
Given that circulating ATX is primarily secreted by adipose tissue, we examined the possible joint contributions of ATX-apo(a) and obesity on the CAVS risk. We then performed exploratory analyses with groups based on cutpoints ATX-apo(a) and BMI that showed the highest sensitivity and specificity according to the area under the receiver-operating characteristic curve. After corrections (same as model 2) we found that, compared to patients with lower ATX-apo(a) and BMI (referent), patients with higher ATX-apo(a) and BMI had a substantially increased risk of CAVS presence (OR: 8.15; 95% CI: 2.81 to 22.77; p < 0.001) (Figure 5). Finally, we sought to determine whether patients with T2D had higher or lower levels of ATX-apo(a) or ATX-apoB compared to patients without T2D and found no differences. We also separated our study population according to BMI and according to the adiponectin-to-leptin ratio (equal or above versus lower than top tertile) and again, we found no differences (data not shown).
Figure 5.
OR for Aortic Stenosis According to BMI and ATX-Apo(a)
Odds ratio (OR) for aortic stenosis (calcific aortic valve stenosis [CAVS]) in patients according to ATX-apo(a) and body mass index (BMI). OR calculated after corrections (same as Model 2). Apo(a) = apolipoprotein(a).
Discussion
Over the past 5 years, a series of genetic association, observational, and mechanistic studies have provided evidence that Lp(a) may represent a causal risk factor for CAVS. The mechanisms through which Lp(a) causes CAVS remain elusive. In this study, we aimed to further document the relationship between Lp(a) and CAVS risk by studying the interaction between Lp(a) and ATX. We first showed that ATX activity and LysoPA content were significantly elevated in the Lp(a) compared to LDL fraction. Additional in vitro experiments revealed that ATX binds to the apo(a) moiety of the Lp(a) particles at very low (nM) concentrations. We then conducted a clinical investigation with a case-control design showing that circulating ATX bound to Lp(a) was associated with CAVS. The combination of an elevated ATX- apo(a) and an elevated BMI was associated with CAVS. Although additional studies will be needed to confirm these findings, this analysis suggests that ATX-apo(a) may represent a potential link between obesity, Lp(a), and CAVS and a new biomarker of CAVS risk.
ATX was first identified as a motility factor in tumorigenesis (22,23). It is a secreted glycoprotein that belongs to the ectonucleotide pyrophosphatase/ phosphodiesterase (NPP) family. ATX converts LysoPC into LysoPA, which promotes cell motility, inflammation, fibrosis, and calcification (24,25). Several tissues or organs may contribute to circulating levels of ATX or LysoPA. Adipose tissue-specific deletion of ATX resulted in a 40% reduction of plasma LysoPA levels (26, 27, 28). Rancoule et al. (29) have also reported evidence that ATX expression is considerably higher in the visceral adipose tissue of obese compared to non-obese individuals. We have also reported that bariatric surgery is associated with reduction in ATX plasma levels in patients with severe obesity (30). In addition, 2 large prospective studies (24) have reported that excess body weight and abdominal obesity were associated with CAVS incidence.
Lp(a) is an important carrier of oxidized phospholipids (OxPLs). LysoPA, which is also generated during the oxidation of lipoproteins [LDL or Lp(a)] (31, 32, 33), is a bioactive lipid that promotes a pro-inflammatory state and drives the mineralization of the aortic valve (14). In human explanted CAVS tissues, ATX activity and LysoPA levels were increased by several-fold compared to noncalcified aortic valves (14). The pro-inflammatory effect as well as the mineralization associated with LysoPA occurs through an RHOA/NF-κB/ bone morphogenetic 2 (BMP2) pathway (13). In a mouse model of CAVS, the exogenous administration of LysoPA exacerbated the mineralization of the aortic valve (14). However, because mice do not express apo(a), the interaction between Lp(a), apo(a), and ATX needs to be documented in humans. Our data suggest protein-protein interaction between ATX and apo(a) both in vitro and within human plasma. These findings are in line with a higher activity of ATX and an elevated LysoPA content in the Lp(a) compared to the LDL fraction (14).
A recent study by Zheng et al. (34) showed the role of Lp(a) in aortic valve microcalcification and CAVS progression in a combined analysis of the SALTIRE (Study Investigating the Effect of Drugs Used to Treat Osteoporosis on the Progression of Calcific Aortic Stenosis) and Ring of Fire studies. In that analysis, similar to our results, no correlations were observed between circulating levels of Lp(a) and ATX-apo(a). That analysis also reported that participants with higher ATX-apo(a) levels have faster hemodynamic progression of CAVS. However, this finding was limited to patients with lower Lp(a) levels. Altogether, although replication of our work will be required, our results as well as those of Zheng et al. (34) suggest that ATX-apo(a) might be relevant for both the initiation and the progression of CAVS.
Study limitations
Lp(a) is a significant risk factor for coronary artery disease (CAD) and CAVS. Nsaibia et al. (35) has previously shown that circulating ATX activity was higher in patients with CAD and CAVS versus patients with only CAD. In the present work, we emphasized that ATX-Lp(a) is a predictor of CAVS. As this work is descriptive and included a small number of individuals, we believe that prospective studies are needed to further investigate the potential causal role of ATX-apo(a) on the development of CAVS. Although our data suggest that ATX-apo(a) was associated with the presence of CAVS, we could not determine whether ATX activity specifically in the Lp(a) or LDL fraction were higher in patients with CAVS compared to controls. Additionally, although our results support the notion that the circulating ATX-apo(a) complex may arise from adipose, this has yet to be experimentally demonstrated. In this regard, Otto et al. (36) identified fibrofatty (intracellular neutral lipid) degeneration of aortic valves with aging, including the presence of adipose cells interposed between the fibrosa and ventricularis layers of the leaflet. Whether these cells secrete ATX is unknown at the moment.
Conclusions
The present study showed that ATX activity and LysoPA contents are higher in Lp(a) compared to LDL fraction. ATX bound to Lp(a) is strongly associated with the presence of CAVS. Hence, the pathophysiologic role of Lp(a) in aortic valve disease could be attributable, at least in part, to the unique proteome and lipidome of the Lp(a) particle.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Lp(a) carries ATX and LysoPA in the bloodstream and may contribute to CAVS.
TRANSLATIONAL OUTLOOK: Further studies should determine whether lowering of Lp(a) and Lp(a)-related molecules such as ATX and LysoPA may prevent the development and/or progression of CAVS.
Footnotes
This work was supported by the Canadian Institutes of Health Research (FRN155226 and FRN149068). Dr. Arsenault holds a junior scholar award from the Fonds de recherche du Québec: Santé (FRQS). Ms. Després is supported by a master’s training award from the FRQS. Dr. Mathieu holds a FRQS Research Chair on the Pathobiology of Calcific Aortic Valve Disease. Dr. Koschinsky is supported by a grant from the Heart and Stroke Foundation of Canada (G-17-0018740) for this work. Dr. Arsenault is a consultant for Novartis; and holds/has held research grants from Pfizer, and Ionis Pharmaceuticals. Dr. Mathieu is a consultant for Casebia Therapeutics. Dr. Koschinsky holds/has held research grants from Pfizer; is a member of advisory boards for Sanofi and Amgen; has received speaker honoraria/consulting fees from Amgen, Regeneron, and Eli Lilly; and holds/has held research contracts with Sanofi, Ionis, Eli Lilly, and Cardiovax.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Shen M., Tastet L., Bergler-Klein J., Pibarot P., Clavel M.A. Blood, tissue and imaging biomarkers in calcific aortic valve stenosis: past, present and future. Curr Opin Cardiol. 2018;33:125–133. doi: 10.1097/HCO.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 2.Mathieu P., Boulanger M.C. Basic mechanisms of calcific aortic valve disease. Can J Cardiol. 2014;30:982–993. doi: 10.1016/j.cjca.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Lindman B.R., Clavel M.A., Mathieu P. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capoulade R., Chan K.L., Yeang C. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Arsenault B.J., Boekholdt S.J., Dube M.P. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7:304–310. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu P., Arsenault B.J., Boulanger M.C., Bosse Y., Koschinsky M.L. Pathobiology of Lp(a) in calcific aortic valve disease. Expert Rev Cardiovasc Ther. 2017;15:797–807. doi: 10.1080/14779072.2017.1367286. [DOI] [PubMed] [Google Scholar]
- 8.Stewart B.F., Siscovick D., Lind B.K. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 9.Thanassoulis G., Campbell C.Y., Owens D.S. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glader C.A., Birgander L.S., Soderberg S. Lipoprotein(a), Chlamydia pneumoniae, leptin and tissue plasminogen activator as risk markers for valvular aortic stenosis. Eur Heart J. 2003;24:198–208. doi: 10.1016/s0195-668x(02)00385-8. [DOI] [PubMed] [Google Scholar]
- 11.Kamstrup P.R., Tybjaerg-Hansen A., Nordestgaard B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Schober A., Siess W. Lysophosphatidic acid in atherosclerotic diseases. Br J Pharmacol. 2012;167:465–482. doi: 10.1111/j.1476-5381.2012.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nsaibia M.J., Boulanger M.C., Bouchareb R. OxLDL-derived lysophosphatidic acid promotes the progression of aortic valve stenosis through a LPAR1-RhoA-NF-kappaB pathway. Cardiovasc Res. 2017;113:1351–1363. doi: 10.1093/cvr/cvx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouchareb R., Mahmut A., Nsaibia M.J. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132:677–690. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 15.Torzewski M., Ravandi A., Yeang C. Lipoprotein(a) associated molecules are prominent components in plasma and valve leaflets in calcific aortic valve stenosis. J Am Coll Cardiol Basic Trans Science. 2017;2:229–240. doi: 10.1016/j.jacbts.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia D., Pibarot P., Dumesnil J.G., Sakr F., Durand L.G. Assessment of aortic valve stenosis severity: a new index based on the energy loss concept. Circulation. 2000;101:765–771. doi: 10.1161/01.cir.101.7.765. [DOI] [PubMed] [Google Scholar]
- 17.Briand M., Dumesnil J.G., Kadem L. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 18.Rosenhek R., Binder T., Porenta G. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura R.A., Otto C.M., Bonow R.O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 20.Hancock M.A., Boffa M.B., Marcovina S.M., Nesheim M.E., Koschinsky M.L. Inhibition of plasminogen activation by lipoprotein(a): critical domains in apolipoprotein(a) and mechanism of inhibition on fibrin and degraded fibrin surfaces. J Biol Chem. 2003;278:23260–23269. doi: 10.1074/jbc.M302780200. [DOI] [PubMed] [Google Scholar]
- 21.Koschinsky M.L., Tomlinson J.E., Zioncheck T.F., Schwartz K., Eaton D.L., Lawn R.M. Apolipoprotein(a): expression and characterization of a recombinant form of the protein in mammalian cells. Biochemistry. 1991;30:5044–5051. doi: 10.1021/bi00234a029. [DOI] [PubMed] [Google Scholar]
- 22.Stracke M.L., Krutzsch H.C., Unsworth E.J. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. [PubMed] [Google Scholar]
- 23.Mills G.B., Moolenaar W.H. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 24.Aoki J., Inoue A., Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 2008;1781:513–518. doi: 10.1016/j.bbalip.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Tokumura A., Majima E., Kariya Y. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 26.Dusaulcy R., Rancoule C., Gres S. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res. 2011;52:1247–1255. doi: 10.1194/jlr.M014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rancoule C., Dusaulcy R., Treguer K. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie. 2014;96:140–143. doi: 10.1016/j.biochi.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Sun S., Wang R., Song J. Blocking gp130 signaling suppresses autotaxin expression in adipocytes and improves insulin sensitivity in diet-induced obesity. J Lipid Res. 2017;58:2102–2113. doi: 10.1194/jlr.M075655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rancoule C., Dusaulcy R., Treguer K. Depot-specific regulation of autotaxin with obesity in human adipose tissue. J Physiol Biochem. 2012;68:635–644. doi: 10.1007/s13105-012-0181-z. [DOI] [PubMed] [Google Scholar]
- 30.Bourgeois R., Piche M.E., Auclair A. Acute and chronic effect of bariatric surgery on circulating autotaxin levels. Physiol Rep. 2019;7 doi: 10.14814/phy2.14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinbrecher U.P., Parthasarathy S., Leake D.S., Witztum J.L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi J., Zhang W., Gu X. Lysophosphatidylcholine is generated by spontaneous deacylation of oxidized phospholipids. Chem Res Toxicol. 2011;24:111–118. doi: 10.1021/tx100305b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siess W., Zangl K.J., Essler M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng K.H., Tsimikas S., Pawade T. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol. 2019;73:2150–2162. doi: 10.1016/j.jacc.2019.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nsaibia M.J., Mahmut A., Boulanger M.C. Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J Intern Med. 2016;280:509–517. doi: 10.1111/joim.12519. [DOI] [PubMed] [Google Scholar]
- 36.Otto C.M., Kuusisto J., Reichenbach D.D., Gown A.M., O'Brien K.D. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]