Visual Abstract
Key Words: diastolic, neprilysin, obesity
Abbreviations and Acronyms: ARB, angiotensin receptor blocker; ATP, adenosine triphosphate; CD, control diet; GMP, guanosine monophosphate; HFHS, high-fat, high-sucrose; HFpEF, heart failure with a preserved ejection fraction; LV, left ventricular; MHD, metabolic heart disease; MNA, methoxy-2-naphthlamine; NMR, nuclear magnetic resonance; PCr, phosphocreatine; ROS, reactive oxygen species; RPP, rate × pressure product; SAC/VAL, sacubitril/valsartan; VAL, valsartan
Highlights
-
•
MHD associated with obesity, diabetes, and/or metabolic syndrome is an important precursor of HFpEF.
-
•
Mice fed a HFHS diet develop MHD with myocardial hypertrophy, fibrosis, diastolic dysfunction, and impaired energetics.
-
•
Mice on HFHS diet were treated with matched doses of VAL or sac SAC/VAL for 16 weeks.
-
•
Only SAC/VAL prevented diastolic dysfunction and fibrosis, and to a lesser extent oxidative stress, whereas VAL and SAC/VAL had similar effects on hypertrophy and energetics.
-
•
Neprilysin inhibition exerts beneficial effects on MHD that are complimentary to VAL, suggesting that SAC/VAL has promise to prevent the development of HFpEF in patients with or at risk for MHD.
Summary
Mice with obesity and metabolic heart disease (MHD) due to a high-fat, high-sucrose diet were treated with placebo, a clinically relevant dose of sacubitril (SAC)/valsartan (VAL), or an equivalent dose of VAL for 4 months. There were striking differences between SAC/VAL and VAL with regard to: 1) diastolic dysfunction; 2) interstitial fibrosis; and to a lesser degree; 3) oxidative stress—all of which were more favorably affected by SAC/VAL. SAC/VAL and VAL similarly attenuated myocardial hypertrophy and improved myocardial energetics. In mice with obesity-related MHD, neprilysin inhibition exerts favorable effects on diastolic function.
Heart failure with a preserved ejection fraction (HFpEF) is common, accounting for about one-half of all clinical heart failure (1,2). Sacubitril/valsartan (SAC/VAL) is a new class of drugs for heart failure that combines the angiotensin receptor blocker (ARB) valsartan (VAL) with the neprilysin inhibitor sacubitril, the latter resulting in increased levels of peptide substrates of neprilysin, most notably the natriuretic peptides (3). In patients with heart failure with a reduced ejection fraction (HFrEF), SAC/VAL reduced the risk of cardiovascular death and hospitalization more than an angiotensin-converting enzyme inhibitor (4). By contrast, in patients with HFpEF, clinical benefit has been less clear.
Obesity, which is frequently associated with type 2 diabetes and metabolic syndrome, is an important phenotype leading to HFpEF (1,5). Obese individuals have preclinical metabolic heart disease (MHD) at a young age, often before their fourth decade, characterized by diastolic dysfunction, myocardial hypertrophy, interstitial fibrosis, and energetic dysfunction (6, 7, 8). We have shown that mice that consume a “Western” diet that is high in fat and sucrose (HFHS) become obese, and develop metabolic syndrome with glucose intolerance and mild hypertension (9). These mice develop a cardiomyopathy characterized by left ventricular (LV) diastolic dysfunction with preserved systolic function, hypertrophy, interstitial fibrosis, and energetic dysfunction, thereby mimicking the MHD phenotype that occurs in obese patients (6,10).
The goal of this study was to determine whether SAC/VAL exerts beneficial effects on the cardiomyopathy associated with obesity that differ from those of the angiotensin receptor antagonist VAL, and thus are attributable to neprilysin inhibition. Accordingly, mice fed a HFHS diet for 4 months were treated with concurrent administration of placebo, a clinically relevant dose of SAC/VAL, or an equivalent dose of VAL. The effects of treatment were assessed at the organ, tissue, and cellular level, and cardiac energetics were measured using 31P nuclear magnetic resonance (NMR) spectroscopy in perfused beating hearts.
Methods
Experimental animals and groups
C57BL/6J mice at 8 weeks of age were fed a control diet (CD) or HFHS diet for 4 months as we have described (9). Mice were randomized by body weight and divided into 4 groups (n = 25 each) as follows: 1) CD plus placebo (water); 2) HFHS diet plus placebo (water); 3) HFHS diet plus SAC/VAL; and 4) HFHS diet plus VAL. Mice were treated with placebo, SAC/VAL (100 mg/kg/day) or VAL (50 mg/kg/day) added to the drinking water for 16 weeks concurrent with the CD or HFHS diet. The protocol was approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine.
Neprilysin activity assay
Plasma neprilysin activity was measured as described (11). Briefly, blood plasma was separated by centrifugation from whole blood collected on heparin-coated microvettes (Sarstedt, Nümbrecht, Germany). Plasma samples in triplicate were incubated at 37°C with the substrate glutaryl-Ala-Ala-Phe-4-methoxy-2-naphthylamine (Sigma-Aldrich, St. Louis, Missouri) and aminopeptidase M (R and D Systems, Minneapolis, Minnesota) to generate the fluorescent product methoxy-2-naphthlamine (MNA) in the absence or presence of a specific neprilysin inhibitor (DL-Thiorphan, Sigma-Aldrich). Fluorescence was read on a Tecan M1000 Pro multimode spectrophotometer (ex/em 340/420 nm, Tecan, Männedorf, Switzerland), compared against a MNA standard curve, and expressed as pmol MNA/h/uL plasma.
2-dimensional, m-mode, and doppler echocardiography
LV dimensions and function were measured by 2-dimensional and Doppler echocardiography using a VisualSonics Vevo 2100 high-resolution imaging system (VisualSonics, Toronto, Ontario, Canada) equipped with a 22-55-MHz MS550D transducer as we have described (9).
Organ weights and histology
Mice were sacrificed after 16 weeks on treatment, and heart, LV (with septum), lung, and liver were weighed, and tibia length was measured. LV samples were either snap frozen for RNA analysis or fixed in 10% buffered formalin, embedded with paraffin, and sectioned. Myocyte cross-sectional area and fibrosis were measured as we have described (12).
Simultaneous measurement of LV function and high-energy phosphates by 31P NMR spectroscopy in isolated beating hearts
LV function was assessed in an isolated retrograde-perfused Langendorff heart preparation as we have described (13). LV workload was increased by increasing the concentration of CaCl2 in the perfusate from 2 to 4 mmol/l, and the pacing rate from 450 beats/min to 600 beats/min, as we have described (14,15). Workload was estimated as the rate × pressure product (RPP = heart rate × developed pressure) (14,15). High-energy phosphates were measured by 31P NMR spectroscopy (161.4 MHz, 9.4-T) using a Varian VNMRS spectrometer (Varian Medical Systems, Palo Alto, California), as we have described (15,16).
Immunohistochemistry for 4-hydroxy-2-nonenal
Immunohistochemistry was performed as we have described (9).
Quantitative real-time polymerase chain reaction
Frozen LV tissue was ground under liquid nitrogen, and RNA was extracted with RNeasy Universal Mini kit (Qiagen, Hilden, Germany). Reverse transcription was performed using the High Capacity RNA-to-cDNA kit (Invitrogen, Carlsbad, California). Quantitative real-time polymerase chain reaction was performed using TaqMan reagents and probes for collagens Col1a1 and Col3a1 (Invitrogen). Relative mRNA expression was normalized to 18s using the ΔΔCT method.
Statistical analysis
Results are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, California). The statistical significance between 2 means was determined using Student’s t-test, whereas differences among groups was determined using analysis of variance with the Bonferroni adjustment for multiple pairwise comparisons. The statistical significance of differences within the same group was determined via paired Student's t-test. The slope of the phosphocreatine (PCr)/ATP versus RPP was calculated as: (PCr/ATP high work demand − PCr/ATP low work demand)/(RPP at high work demand − RPP low work demand). This was done for each individual heart, and ordinary 1-way analysis of variance with Tukey's multiple comparison test was performed to compare the groups. A value of p < 0.05 was considered statistically significant.
Results
SAC/VAL and VAL dosing
The dose of SAC/VAL was based on a pilot study showing that a dose of 100 mg/kg/day inhibited neprilysin activity by at least 80% (Figure 1A). In order to provide an amount of VAL equivalent to that in the selected dose of SAC/VAL, VAL was administered in a dose (50 mg/kg/day) that is 50% by weight of the SAC/VAL dose. This dose of VAL inhibits the vasopressor response to exogenous angiotensin by 80% (17). The concentrations of SAC/VAL and VAL in the drinking water were calculated on the basis of the measured water intake per animal in each treatment group. Using this approach, the actual doses of SAC/VAL and VAL were maintained very close to the target doses of 100 and 50 mg/kg/day, respectively, over the 16-week course of the study (Figure 1B).
Figure 1.
Neprilysin Inhibition and Drug Dosing
(A) Neprilysin activity pre- and post-treatment with SAC/VAL (100 mg/kg/day) for 7 days in mice fed a HFHS diet. ∗p < 0.05 versus pre-treatment. (B) Actual doses of VAL and SAC/VAL achieved over the 16-week course of the study. Doses of SAC/VAL and VAL were adjusted on the basis of water consumption to achieve target doses of 100 and 50 mg/kg/day, respectively. HFHS = high-fat, high-sucrose; MNA, methoxy-2-naphthlamine; SAC/VAL = sacubitril/valsartan; VAL = valsartan.
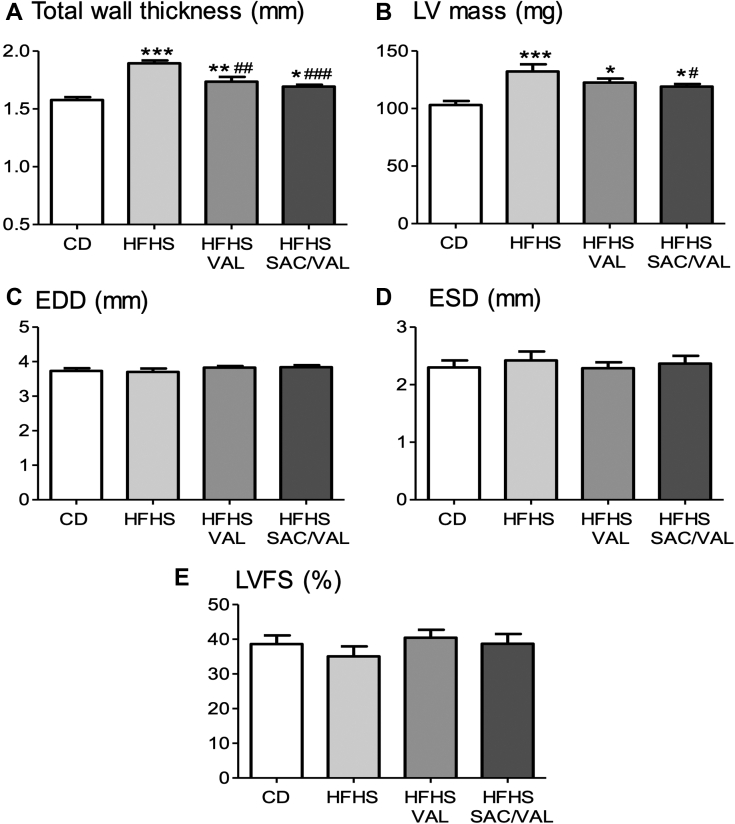
SAC/VAL and VAL attenuate LV hypertrophy in HFHS-fed mice
As we have previously shown (9), the HFHS-fed mice developed LV hypertrophy as reflected by increases in LV wall thickness, mass, and weight (Figures 2A and 2B, Table 1). VAL and SAC/VAL attenuated diet-induced LV hypertrophy to a similar extent. LV end-diastolic dimension was not affected by the HFHS diet, and was not affected by VAL or SAC/VAL (Figure 2C). LV end-systolic dimension and LV fractional shortening were not affected by diet, and neither was affected by VAL or SAC/VAL (Figures 2D and 2E).
Figure 2.
Effect of Treatment on Cardiac Structure and Systolic Function
Shown are echocardiographic measurements in mice fed a control (CD) or HFHS diet for 16 weeks with concurrent treatment with placebo, VAL, or SAC/VAL. (A) LV total wall thickness. (B) LV mass. (C) LV end-diastolic dimension (EDD). (D) LV end-systolic dimension (ESD). (E) LV fractional shortening (LVFS). Values are mean ± SEM; n = 12. ∗p < 0.05 versus CD; ∗∗p < 0.01 versus CD; ∗∗∗p < 0.001 versus CD; #p < 0.05 versus HFHS group; ##p < 0.01 versus HFHS group; ###p < 0.001 versus HFHS group. LV = left ventricular; other abbreviations as in Figure 1.
Table 1.
Body and Organ Weights
| CD | HFHS | HFHS/VAL | HFHS/SAC/VAL | p Value | |
|---|---|---|---|---|---|
| Body weight, g | 37.4 ± 2.0 | 49.6 ± 1.5‡ | 49.5 ± 2.4‡ | 51.0 ± 1.6‡ | <0.001 |
| TL, mm | 17.2 ± 0.8 | 17.7 ± 0.8 | 17.7 ± 0.8 | 17.5 ± 0.8 | 0.215 |
| Heart weight, mg | 141 ± 4 | 167 ± 5‡ | 152 ± 4 | 151 ± 3§ | <0.001 |
| Heart weight/TL, mg/mm | 8.18 ± 0.78 | 9.44 ± 0.03† | 8.58 ± 0.83 | 8.63 ± 0.83 | <0.01 |
| LV weight, mg | 100 ± 4 | 120 ± 4† | 105 ± 4 | 107 ± 3 | <0.01 |
| LV weight/TL, mg/mm | 5.83 ± 0.79 | 6.76 ± 0.88† | 5.89 ± 0.83§ | 6.12 ± 0.72§ | <0.01 |
| Lung weight, wet/dry | 1.20 ± 0.15 | 1.21 ± 0.19 | 1.22 ± 0.18 | 1.21 ± 0.17 | 0.649 |
| Liver weight, wet/dry | 1.75 ± 0.34 | 1.87 ± 0.33∗ | 1.78 ± 0.30 | 1.78 ± 0.25 | <0.05 |
Values are mean ± SEM. n = 12. Mice were sacrificed after 16 weeks on treatment, and organs were weighed and LV was prepared for microscopy.
CD = control diet; HFHS = high-fat, high-sucrose diet; LV = left ventricle; SAC/VAL = sacubitril/valsartan; TL = tibia length; VAL = valsartan.
p < 0.05 vs. CD;
p < 0.01 vs. CD;
p < 0.001 vs. CD;
p < 0.05 vs. HFHS.
SAC/VAL, but not VAL, prevents diastolic dysfunction in HFHS-fed mice
LV diastolic function was assessed by transmitral and tissue Doppler echocardiography. As we previously showed (9), transmitral flow velocity (E/A ratio) was decreased in HFHS-fed mice. Treatment with VAL did not affect the E/A ratio, whereas treatment with SAC/VAL prevented the HFHS diet–induced decrease (Figure 3A). Likewise, wall motion during diastole (Em) was decreased by HFHS diet (Figure 3B). VAL had no effect on Em, whereas SAC/VAL prevented the diet-induced decrease. As expected, E/Em was increased by HFHS diet, indicative of an increase in left atrial pressure. VAL did not significantly affect the E/Em ratio, whereas SAC/VAL prevented the diet-induced increase (Figure 3C).
Figure 3.
Effect of Treatment on LV Diastolic Function
Shown are the Doppler echocardiographic measurements made at 16 weeks of treatment as per Figure 2. (A) Ratio of peak early (E) to late (A) mitral inflow velocity (E/A). (B) Myocardial peak early diastolic velocity (Em). (C) Ratio of E/Em. Values are mean ± SEM; n = 12. ∗p < 0.05 versus CD; ∗∗p < 0.01 versus CD; ∗∗∗p < 0.001 versus CD; ###p < 0.001 versus HFHS; ††p < 0.01 versus HFHS/VAL group; †††p < 0.001 versus HFHS/VAL group. Abbreviations as in Figures 1 and 2.
Effects of SAC/VAL and VAL on cardiac reserve in isolated beating hearts
In hearts from CD-fed mice, LV systolic pressure appropriately increased with increased work demand, but failed to increase in HFHS hearts, and was not affected by treatment with VAL or SAC/VAL (Table 2). LV EDP was elevated during increased work demand in the HFHS-fed mice and the rise was prevented by both VAL and SAC/VAL. Developed pressure increased with increased work demand in CD hearts, but not in HFHS hearts, and was not affected by treatment with VAL or SAC/VAL. As we have previously shown (15), the RPP is decreased in HFHS-fed mice. In HFHS hearts, RPP at high work demand was increased by both SAC/VAL and VAL.
Table 2.
Isolated Beating Heart Function and Energetics
| CD |
HFHS |
HFHS/VAL |
HFHS/SAC/VAL |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | High Workload | Baseline | High Workload | Baseline | High Workload | Baseline | High Workload | |
| Systolic pressure, mm Hg | 104 ± 3.4 | 126 ± 3.5 | 102 ± 5.5 | 95 ± 3.0∗ | 93 ± 2.5 | 98 ± 3.0 | 101 ± 4.1 | 103 ± 4.2 |
| EDP, mm Hg | 9.4 ± 0.3 | 12 ± 0.3 | 9.4 ± 0.4 | 17.6 ± 1.4∗ | 8.25 ± 0.2 | 11.9 ± 0.7† | 9.1± 0.2 | 12.4 ± 1.0† |
| Developed pressure, mm Hg | 94 ± 3.2 | 114 ± 3.3 | 92 ± 5.6 | 77 ± 3.0∗ | 85 ± 2.4 | 86 ± 2.7∗ | 92 ± 4.2 | 91 ± 4.4∗ |
| Heart rate, paced, beats/min | 450 | 600 | 450 | 600 | 450 | 600 | 450 | 600 |
| RPP 103, mm Hg/min | 42.4 ± 1.4 | 68.3 ± 1.9 | 41.5 ± 2.5 | 46.2 ± 1.8∗ | 38.2 ± 1.1 | 51.4 ± 1.6∗‡ | 41.6 ± 1.9 | 54.6 ± 2.7∗‡ |
| PCr, mmol/l | 20 ± 0.6 | 14.1 ± 0.7 | 15.8 ± 0.4∗ | 11.8 ± 0.4∗ | 15.7± 0.4∗ | 12.4 ± 0.3 | 15.9 ± 0.3∗ | 13.2 ± 0.6 |
| ATP, mmol/l | 10 ± 0.1 | 9.4 ± 0.3 | 10 ± 0.2 | 9.7 ± 0.4 | 10 ± 0.3 | 9.9 ± 0.2 | 10 ± 0.2 | 9.32 ± 0.3 |
| PCr/ATP | 2.0 ± 0.1 | 1.51 ± 0.1 | 1.59 ± 0.1∗ | 1.22 ± 0.1∗ | 1.58 ± 0.1∗ | 1.26 ± 0.1∗ | 1.59 ± 0.1∗ | 1.41 ± 0.1 |
Values are mean ± SEM, unless otherwise indicated. n = 4 to 6. LV function and high-energy phosphates (31P nuclear magnetic resonance spectroscopy) were measured simultaneously in an isolated retrograde-perfused Langendorff heart preparation.
ATP = adenosine triphosphate; EDP = end-diastolic pressure; PCr = phosphocreatine; RPP = rate × pressure product; other abbreviations as in Table 1.
p < 0.05 vs. CD;
p < 0.05 vs. HFHS;
p < 0.05 vs. the respective baseline value.
Effects of SAC/VAL and VAL on energetics in the beating heart
High-energy phosphates were measured by 31P NMR spectroscopy performed simultaneously with the hemodynamics depicted in Table 2. As expected, adenosine triphosphate (ATP) levels were maintained at both work levels and were not affected by diet or treatment group. Likewise, pH was unaffected by work demand, diet group, or treatment group (data not shown). Phosphocreatine (PCr) and PCr normalized for ATP (PCr/ATP) are measures of the energy reserve that typically decrease with increased work demand (Table 2, Figure 4A). In HFHS (vs. CD) hearts, PCr and PCr/ATP were lower at baseline and decreased to lower values with high work demand, and neither was significantly affected by SAC/VAL or VAL (Figure 4A). The slope of the change in PCr/ATP relative to RPP with increased work demand provides an index of the energetic cost of the increase in contractile performance. The slope of the decline in PCr/ATP relative to RPP increased in HFHS versus CD hearts, and this increase was significantly attenuated by both SAC/VAL and VAL (Figure 4).
Figure 4.
Effect of Treatment on Myocardial Energetic Response to an Increase in Work Demand
High-energy phosphates were measured in the beating heart by 31P nuclear magnetic resonance (NMR) spectroscopy at 16 weeks. (A) The relationship between phosphocreatine (PCr) normalized to adenosine triphosphate (ATP) (PCr/ATP) versus work demand as measured by the rate × pressure product (RPP). (B) Mean slopes of the relationship between PCr/ATP and RPP in A. Values are mean ± SEM; n = 4 to 6. ∗p < 0.05 versus CD; #p < 0.05 versus HFHS. Abbreviations as in Figures 1 and 2.
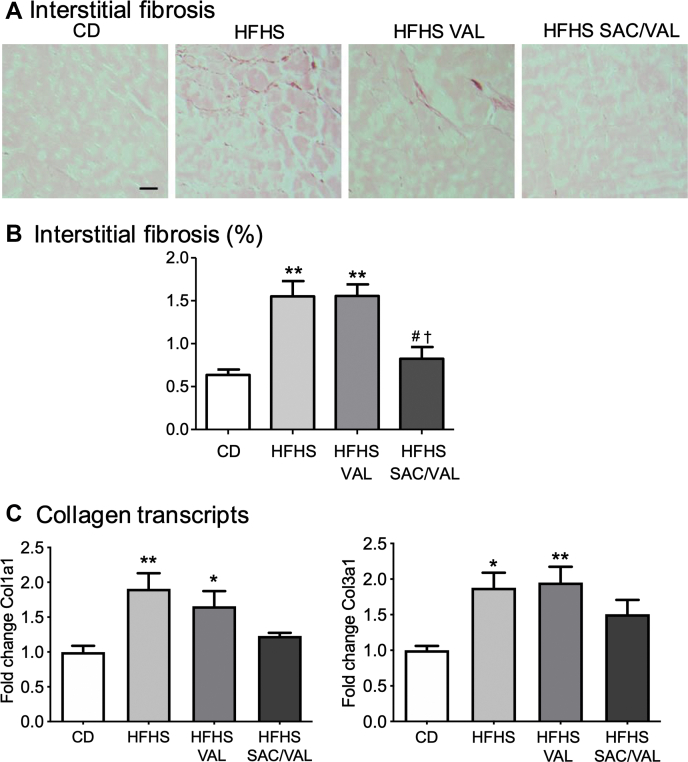
Comparative effects of SAC/VAL and VAL on myocyte hypertrophy and interstitial fibrosis
As we have previously shown (9), myocyte cross-sectional area was increased in HFHS-fed mice. HFHS diet–induced myocyte hypertrophy was inhibited to a similar extent by VAL and SAC/VAL (Figure 5). Interstitial fibrosis assessed by Picrosirius red staining was increased in HFHS-fed mice (Figures 6A and 6B). Treatment with SAC/VAL, but not VAL, prevented the HFHS diet–induced increase in interstitial fibrosis. Likewise, the levels of mRNA for collagens 1a1 and 3a1 were increased in HFHS hearts, and the increases were prevented by SAC/VAL, but not VAL (Figure 6C).
Figure 5.
Effect of Treatment on Cardiac Myocyte Size
Cardiac myocyte cross-sectional area was assessed at 16 weeks of treatment as per Figure 2. (A) Representative photomicrographs of LV myocardium stained by hematoxylin and eosin (scale bar = 20 μm). (B) Mean changes in myocyte cross-sectional area. Values are mean ± SEM; n = 4. ∗p < 0.05 versus CD; #p < 0.05 versus HFHS. Abbreviations as in Figures 1 and 2.
Figure 6.
Effect of Treatment on Myocardial Interstitial Fibrosis
(A) Representative photomicrographs of myocardium stained with Picrosirius red to assess interstitial fibrosis (scale bar = 25 μm). (B) Mean changes in interstitial fibrosis. (C) Relative mRNA expression of collagen 1a1 and 3a1, respectively. Values are mean ± SEM; n = 3 to 8. ∗p < 0.05 versus CD; ∗∗p < 0.05 versus CD; #p < 0.05 versus HFHS; †p < 0.05 versus HFHS/VAL group. Abbreviations as in Figures 1 and 2.
Effects of SAC/VAL and VAL on myocardial oxidative stress in HFHS-fed mice
4-Hydroxy-2-nonenal (HNE), a product of lipid peroxidation that reflects oxidative stress in the myocardium, was increased in HFHS-fed mice (Figure 7). Treatment with VAL and SAC/VAL decreased myocardial oxidative stress; however, the decrease was greater with SAC/VAL.
Figure 7.
Effect of Treatment on Myocardial Oxidative Stress
(A) Representative photomicrographs of immune-histochemical staining for 4-hydroxy-2-nonenal (HNE) at 16 weeks. HNE is shown in red, nuclei in blue (scale bar = 25 μm). (B) Mean levels of myocardial HNE. Values are mean ± SEM; n = 3. ∗p < 0.05 versus CD; ∗∗p < 0.01 versus CD; #p < 0.05 vs HFHS; ##p < 0.01 versus HFHS; †p < 0.05 versus HFHS/VAL group. Abbreviations as in Figures 1 and 2.
Discussion
This study provides new insight regarding the cardiac effects of SAC/VAL in a mouse model of MHD, and in particular, the differential effects of SAC/VAL versus a matched dose of VAL. The most notable difference was that SAC/VAL, but not VAL, improved diastolic function. In addition, SAC/VAL, but not VAL, prevented myocardial interstitial fibrosis and caused a greater decrease in myocardial oxidative stress. By contrast, SAC/VAL and VAL attenuated myocardial hypertrophy to a similar degree and caused similar improvements in cardiac energetics. Taken together, these findings indicate that SAC/VAL exerts favorable effects on the MHD phenotype in this model of diet-induced obesity and suggests that neprilysin inhibition exerts beneficial effects on diastolic function, interstitial fibrosis, and myocardial oxidative stress beyond those of angiotensin receptor blockade alone.
The hallmarks of MHD in obese individuals are diastolic dysfunction with preserved systolic function and LV hypertrophy (6,7,18). The HFHS-fed mouse develops obesity that is associated with diastolic dysfunction, myocardial hypertrophy, and interstitial fibrosis (9), thereby closely mimicking the human phenotype. In addition, in both humans with MHD and HFHS-fed mice, there is evidence of increased myocardial oxidative stress and impaired energetics that may contribute to the pathophysiology of diastolic dysfunction (19, 20, 21).
SAC/VAL prevents LV diastolic dysfunction and interstitial fibrosis, and attenuates hypertrophy in MHD
The most striking effect of SAC/VAL in this study was prevention of diastolic dysfunction. By contrast, VAL had no effect, suggesting that this effect of SAC/VAL cannot be attributed to angiotensin receptor blockade, but rather, is mediated by neprilysin inhibition. Relatively little is known about the effects of SAC/VAL on diastolic function, and in particular, about the effects on diastolic function in MHD or models of HFpEF. In 1 study, SAC/VAL improved LV relaxation in spontaneously hypertension rats with heart failure induced by myocardial ischemia/reperfusion (22).
A second striking finding was that SAC/VAL prevented the development of interstitial fibrosis, whereas VAL had no significant effect. We are not aware of prior studies in which the antifibrotic effects of SAC/VAL or neprilysin inhibition were examined in a model of obesity-related MHD. An antifibrotic effect has been observed in rodent models of heart failure characterized by systolic dysfunction including pressure overload (23), myocardial infarction (24), doxorubicin-induced dilated cardiomyopathy (25), chronic kidney disease (26), isoproterenol cardiotoxicity (27), and streptozotocin-induced systolic heart failure (28). In patients with HFrEF SAC/VAL decreased profibrotic biomarkers, suggesting that SAC/VAL may reduce fibrotic signaling (29). The differential effect of SAC/VAL versus VAL on fibrosis strongly suggests that this action is mediated, in large part, by neprilysin inhibition. Interstitial fibrosis in the HFHS hearts was associated with increased expression of transcripts for collagens 1 and 3, which was prevented by SAC/VAL, but not VAL, thus supporting the histological findings and suggesting that the effect of SAC/VAL is due, at least in part, to a decrease in collagen synthesis.
Both SAC/VAL and VAL inhibited myocardial hypertrophy by about 50%, and were associated with decreases in myocyte cross-sectional area. SAC/VAL and VAL have been shown to inhibit myocardial hypertrophy to a similar degree in a variety of disease models including chronic kidney disease (26) and streptozotocin-induced systolic dysfunction (28). In other models, SAC/VAL was superior to VAL. For example, in a mouse model of pressure overload-induced heart failure SAC/VAL decreased cardiac hypertrophy, whereas the molar equivalent dose of VAL had no effect (23). In patients with essential hypertension SAC/VAL decreased LV mass index more than the ARB olmesartan (30). The similar antihypertrophic effects of VAL and SAC/VAL in the current study suggest that angiotensin receptor blockade is primarily responsible for the antihypertrophic effect of SAC/VAL.
Antioxidant effects of VAL and SAC/VAL
Both SAC/VAL and VAL decreased myocardial oxidative stress as reflected by the oxidized lipid HNE, but the effect of SAC/VAL was more marked. The mechanism responsible for a decrease in oxidative stress remains to be elucidated and might reflect an antioxidant activity and/or a decrease in reactive oxygen species (ROS) production secondary to other consequences of the drug such as improved cardiac and/or mitochondrial function. An antioxidant effect of ARB inhibition is not surprising because it is known that a variety of neurohormones including angiotensin exert pro-oxidant effects (31). Conversely, in cardiac myocytes, natriuretic peptides exert an antioxidant effect that has been attributed to inhibition of NADPH oxidases (32). The greater effect of SAC/VAL may thus reflect the additive antioxidant effects of ARB inhibition and increased natriuretic peptide stimulation. Mitochondria are another important source of ROS in the failing heart (33) and are a major source of ROS in our mice with MHD (21). The beneficial effects of SAC/VAL and VAL on myocardial energetics suggest that an improvement in mitochondrial function may have contributed to a decrease in ROS production. In mice with heart failure induced by the cardiac-specific activation of NADPH oxidase, the beneficial effects of SAC/VAL on hypertrophy, oxidative damage, and interstitial fibrosis were generally similar (34). The increased effectiveness of SAC/VAL versus VAL to decrease oxidative stress and fibrosis in HFHS-fed mice may reflect a greater role for mitochondria as a source of ROS in MHD.
Effects on myocardial energetics
In patients with obesity-related MHD, there is evidence of myocardial energetic dysfunction as assessed by measurement of high-energy phosphates using 31P NMR spectroscopy (19): The severity of energetic impairment was ameliorated by weight loss and correlated with the severity of LV diastolic dysfunction (19,20). Similarly, we previously used 31P NMR spectroscopy to demonstrate energetic dysfunction in mice fed a HFHS diet for 4 months (16). In the current study, we confirmed that HFHS diet for 4 months leads to a significant impairment in cardiac reserve, as reflected by failure to appropriately increase RPP with increased work demand. The impairment in cardiac reserve was associated with a steeper decline in the relationship between energy reserve (PCr/ATP) and RPP when work demand was increased. The decrease in cardiac reserve (RPP) was improved by both SAC/VAL and VAL. Likewise, for both SAC/VAL and VAL, the energetics of increased work demand were improved as reflected by improvement in the PCr/ATP-to-RPP relationship (Figure 4B). There are several potential mechanisms for improved energetics with SAC/VAL and VAL. Cardiac myocyte hypertrophy and interstitial fibrosis can impair the delivery of nutrient substrates to the mitochondria, particularly under states of increased work demand. In addition, as we previously showed in HFHS-fed mice, oxidative stress leads to oxidative post-translational modification of mitochondrial proteins leading to impaired function (35). Although the beneficial effect of SAC/VAL tended to be greater than that of VAL, this did not reach significance, suggesting that a substantial portion of the beneficial effect of SAC/VAL on cardiac reserve and energetics can be attributed to VAL.
Study limitations
Although it is reasonable to conclude that the differential effects of SAC/VAL versus VAL are due to neprilysin inhibition, we cannot determine the neprilysin substrate(s) responsible. A major effect of neprilysin inhibition is an increase in circulating and tissue levels of natriuretic peptides (3,36), which would be expected to increase the generation of cyclic guanosine monophosphate (GMP) in cardiac myocytes and fibroblasts. Cyclic GMP may have several targets that can affect cardiac structure and function. We (37) and others (38,39) have shown that increasing cyclic GMP by activating natriuretic peptide receptors is a potent inhibitor of hypertrophy in cardiac myocytes and of collagen production in fibroblasts in vitro. Cyclic GMP also enhances the activity of protein kinase G (PKG), which may decrease myocyte stiffness by phosphorylation of titin (40,41). In addition, natriuretic peptides can exert antioxidant effects that oppose the effects of ROS, thus potentially affecting mitochondrial function and energetics that are involved in the regulation of myocyte relaxation. Of note, neprilysin has several other potentially important peptide substrates that may contribute to the effects of SAC/VAL, including substance P, bradykinin, adrenomedullin, calcitonin-related peptide, beta-endorphin, glucagon-like peptide-1, angiotensin I and II, and endothelin (42). It will be important for future studies to determine whether non-natriuretic peptide neprilysin substrates contribute to the beneficial effects of SAC/VAL observed here, thereby suggesting new therapeutic avenues.
Conclusions
In this model of obesity-related MHD, SAC/VAL exerts favorable effects on diastolic function, interstitial fibrosis, and oxidative stress that exceed those of VAL and are thus attributable to neprilysin inhibition. Taken together, these findings suggest that in MHD, neprilysin inhibition contributes importantly to the beneficial effects of SAC/VAL. In the PARAGON (Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction) trial, which was performed in patients with HFpEF of diverse etiologies and phenotypes, SAC/VAL did not meet the primary endpoint of hospitalization for heart failure and death, although there was a noteworthy positive trend (43). Because the HFHS model used here has a high degree of fidelity for patients with obesity and metabolic syndrome, our findings suggest that SAC/VAL may have particular benefit in patients who have or are at increased risk of developing HFpEF due to MHD.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study shows that in mice with metabolic heart disease due to obesity, diabetes, and/or metabolic syndrome, SAC/VAL improves diastolic function, interstitial fibrosis, and oxidative stress due to inhibition of neprilysin.
TRANSLATIONAL OUTLOOK: This study suggests that patients with or at risk of developing metabolic heart disease may be a subgroup of patients with HFpEF that would be benefited by treatment with SAC/VAL.
Footnotes
Supported by funds from a Novartis investigator-initiated trial (SAC/VAL696BUSNC22T) to Dr. Colucci; National Institutes of Health grants HL-064750 (Dr. Colucci) and K08 HL123744 (Dr. Panagia); and an American Heart Association Fellow-to-Faculty Award 15FTF25890062 (Dr. Luptak). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Borlaug B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Ruiz I. Dissecting the benefits of sacubitril-valsartan for heart failure. Nat Rev Cardiol. 2020;17:71. doi: 10.1038/s41569-019-0322-y. [DOI] [PubMed] [Google Scholar]
- 4.McMurray J.J., Packer M., Desai A.S. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 5.Reddy Y.N.V., Lewis G.D., Shah S.J. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX trial ancillary study. Mayo Clin Proc. 2019;94:1199–1209. doi: 10.1016/j.mayocp.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Ayalon N., Gopal D.M., Mooney D.M. Preclinical left ventricular diastolic dysfunction in metabolic syndrome. Am J Cardiol. 2014;114:838–842. doi: 10.1016/j.amjcard.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopal D.M., Ayalon N., Wang Y.C. Galectin-3 is associated with stage B metabolic heart disease and pulmonary hypertension in young obese patients. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y.C., Liang C.S., Gopal D.M. Preclinical systolic and diastolic dysfunctions in metabolically healthy and unhealthy obese individuals. Circ Heart Fail. 2015;8:897–904. doi: 10.1161/CIRCHEARTFAILURE.114.002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin F., Siwik D.A., Luptak I. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1766. doi: 10.1161/CIRCULATIONAHA.111.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tadic M., Cuspidi C. Obesity and heart failure with preserved ejection fraction: a paradox or something else? Heart Fail Rev. 2019;24:379–385. doi: 10.1007/s10741-018-09766-x. [DOI] [PubMed] [Google Scholar]
- 11.Willard J.R., Barrow B.M., Zraika S. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia. 2017;60:701–708. doi: 10.1007/s00125-016-4172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin F., Siwik D.A., Pimentel D.R. Cytosolic H2O2 mediates hypertrophy, apoptosis, and decreased SERCA activity in mice with chronic hemodynamic overload. Am J Physiol Heart Circ Physiol. 2014;306:H1453–H1463. doi: 10.1152/ajpheart.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luptak I., Yan J., Cui L., Jain M., Liao R., Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116:901–909. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- 14.Luptak I., Balschi J.A., Xing Y., Leone T.C., Kelly D.P., Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–2346. doi: 10.1161/CIRCULATIONAHA.105.534594. [DOI] [PubMed] [Google Scholar]
- 15.Luptak I., Qin F., Sverdlov A.L. Energetic dysfunction is mediated by mitochondrial reactive oxygen species and precedes structural remodeling in metabolic heart disease. Antioxid Redox Signal. 2019;31:539–549. doi: 10.1089/ars.2018.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luptak I., Sverdlov A.L., Panagia M. Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J Mol Cell Cardiol. 2018;116:106–114. doi: 10.1016/j.yjmcc.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y.H., Xu J., Yang X.P., Yang F., Shesely E., Carretero O.A. Effect of ACE inhibitors and angiotensin II type 1 receptor antagonists on endothelial NO synthase knockout mice with heart failure. Hypertension. 2002;39:375–381. doi: 10.1161/hy02t2.102796. [DOI] [PubMed] [Google Scholar]
- 18.de las Fuentes L., Brown A.L., Mathews S.J. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007;28:553–559. doi: 10.1093/eurheartj/ehl526. [DOI] [PubMed] [Google Scholar]
- 19.Rider O.J., Francis J.M., Ali M.K. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation. 2012;125:1511–1519. doi: 10.1161/CIRCULATIONAHA.111.069518. [DOI] [PubMed] [Google Scholar]
- 20.Rider O.J., Francis J.M., Tyler D., Byrne J., Clarke K., Neubauer S. Effects of weight loss on myocardial energetics and diastolic function in obesity. Int J Cardiovasc Imaging. 2013;29:1043–1050. doi: 10.1007/s10554-012-0174-6. [DOI] [PubMed] [Google Scholar]
- 21.Sverdlov A.L., Elezaby A., Qin F. Mitochondrial reactive oxygen species mediate cardiac structural, functional, and mitochondrial consequences of diet-induced metabolic heart disease. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trivedi R.K., Polhemus D.J., Li Z. Combined angiotensin receptor-neprilysin inhibitors improve cardiac and vascular function via increased NO bioavailability in heart failure. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke R.M., Lighthouse J.K., Mickelsen D.M., Small E.M. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.118.005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Lueder T.G., Wang B.H., Kompa A.R. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8:71–78. doi: 10.1161/CIRCHEARTFAILURE.114.001785. [DOI] [PubMed] [Google Scholar]
- 25.Xia Y., Chen Z., Chen A. LCZ696 improves cardiac function via alleviating Drp1-mediated mitochondrial dysfunction in mice with doxorubicin-induced dilated cardiomyopathy. J Mol Cell Cardiol. 2017;108:138–148. doi: 10.1016/j.yjmcc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Suematsu Y., Jing W., Nunes A. LCZ696 (sacubitril/valsartan), an angiotensin-receptor neprilysin inhibitor, attenuates cardiac hypertrophy, fibrosis, and vasculopathy in a rat model of chronic kidney disease. J Card Fail. 2018;24:266–275. doi: 10.1016/j.cardfail.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi T., Nakamura K., Miura D. Effect of LCZ696, a dual angiotensin receptor neprilysin inhibitor, on isoproterenol-induced cardiac hypertrophy, fibrosis, and hemodynamic change in rats. Cardiol J. 2019;26:575–583. doi: 10.5603/CJ.a2018.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suematsu Y., Miura S., Goto M. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail. 2016;18:386–393. doi: 10.1002/ejhf.474. [DOI] [PubMed] [Google Scholar]
- 29.Zile M.R., O'Meara E., Claggett B. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019;73:795–806. doi: 10.1016/j.jacc.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 30.Schmieder R.E., Wagner F., Mayr M. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur Heart J. 2017;38:3308–3317. doi: 10.1093/eurheartj/ehx525. [DOI] [PubMed] [Google Scholar]
- 31.Bendall J.K., Cave A.C., Heymes C., Gall N., Shah A.M. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 32.Laskowski A., Woodman O.L., Cao A.H. Antioxidant actions contribute to the antihypertrophic effects of atrial natriuretic peptide in neonatal rat cardiomyocytes. Cardiovasc Res. 2006;72:112–123. doi: 10.1016/j.cardiores.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Dai D.F., Johnson S.C., Villarin J.J. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorrentino A., Steinhorn B., Troncone L. Reversal of heart failure in a chemogenetic model of persistent cardiac redox stress. Am J Physiol Heart Circ Physiol. 2019;317:H617–H626. doi: 10.1152/ajpheart.00177.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sverdlov A.L., Elezaby A., Behring J.B. High fat, high sucrose diet causes cardiac mitochondrial dysfunction due in part to oxidative post-translational modification of mitochondrial complex II. J Mol Cell Cardiol. 2015;78:165–173. doi: 10.1016/j.yjmcc.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gori M., D'Elia E., Senni M. Sacubitril/valsartan therapeutic strategy in HFpEF: clinical insights and perspectives. Int J Cardiol. 2019;281:158–165. doi: 10.1016/j.ijcard.2018.06.060. [DOI] [PubMed] [Google Scholar]
- 37.Calderone A., Thaik C.M., Takahashi N., Chang D.L., Colucci W.S. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horio T., Nishikimi T., Yoshihara F., Matsuo H., Takishita S., Kangawa K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension. 2000;35:19–24. doi: 10.1161/01.hyp.35.1.19. [DOI] [PubMed] [Google Scholar]
- 39.Nishikimi T., Maeda N., Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Hamdani N., Franssen C., Lourenco A. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6:1239–1249. doi: 10.1161/CIRCHEARTFAILURE.113.000539. [DOI] [PubMed] [Google Scholar]
- 41.Kruger M., Kotter S., Grutzner A. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 42.D'Elia E., Iacovoni A., Vaduganathan M., Lorini F.L., Perlini S., Senni M. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail. 2017;19:710–717. doi: 10.1002/ejhf.799. [DOI] [PubMed] [Google Scholar]
- 43.Solomon S.D., McMurray J.J.V., Anand I.S. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]